Abstract
Objective
The purpose of the present study was to characterize the composition of the salivary microbiota and quantify salivary levels of inflammation‐related proteins (neutrophil gelatinase‐associated lipocalin [NGAL] and transferrin) in patients with psoriasis and compare data to those obtained in patients with periodontitis and orally healthy controls, respectively.
Materials and methods
Stimulated saliva samples from patients with psoriasis (n = 27), patients with periodontitis (n = 58), and orally healthy controls (n = 52) were characterized by means of next‐generation sequencing of the 16S rRNA gene. Salivary levels of NGAL and transferrin were quantified using immunoassays.
Results
Linear discriminant effect size analysis showed that 52 (22 psoriasis‐associated and 30 periodontitis‐associated) and 21 (8 psoriasis‐associated and 13 orally healthy control‐associated) bacterial taxa differentiated the salivary microbiota in patients with psoriasis from that of patients with periodontitis and orally healthy controls, respectively. Significantly lower mean salivary levels of NGAL (psoriasis: 996 [std. error 320], periodontitis: 2,072 [295], orally healthy controls: 2,551 [345] ng/ml, p < .0001) and transferrin (psoriasis: 4.37 [0.92], periodontitis: 7.25 [0.88], orally healthy controls: 10.02 [0.94] ng/ml, p < .0001) were identified in patients with psoriasis.
Conclusions
Psoriasis associates with characteristics of the salivary microbiota and salivary levels of inflammation‐related proteins, which are different from characteristics in patients with periodontitis and orally healthy controls, respectively.
Keywords: inflammation, microbiota, periodontitis, psoriasis, saliva
1. INTRODUCTION
Psoriasis is a chronic inflammatory disease, with a prevalence of approximately 2% in the western world (Nestle, Kaplan, & Barker, 2009). The pathobiological mechanisms underlying psoriasis are not fully understood, but the driving factor is presumably aberrant activation of both the innate and the adaptive immune system, which causes characteristic local inflammatory reactions in the skin as well as systemic low‐grade inflammation with increased circulating levels of inflammatory cytokines (Dowlatshahi, Voort, Arends, & Nijsten, 2013; Mahil, Capon, & Barker, 2015; Nestle et al., 2009). Periodontitis is a chronic inflammatory disease of the tooth supporting tissues, which affects as much as 50% of the adult population in the western world (Eke, Dye, Wei, Thornton‐Evans, & Genco, 2012). The hallmark of periodontitis is a chronic inflammatory reaction in the periodontal tissue that is driven, in part, by responses directed at the oral microbiota (Bartold & Van Dyke, 2017).
Several cross‐sectional studies have shown a higher prevalence of periodontitis and increased periodontal disease activity in patients with psoriasis, as compared to orally healthy controls (Egeberg, Mallbris, Gislason, Hansen, & Mrowietz, 2017; Lazaridou et al., 2013; Preus, Khanifam, Kolltveit, Mørk, & Gjermo, 2010; Sharma, Raman, & Pradeep, 2015; Skudutyte‐Rysstad, Slevolden, Hansen, Sandvik, & Preus, 2014; Üstün et al., 2013; Woeste, Graetz, Gerdes, & Mrowietz, 2019). Recently, these findings were confirmed in a systematic review and meta‐analysis, which reported a significant psoriasis‐associated risk of periodontitis (Ungprasert, Wijarnpreecha, & Wetter, 2017). Conversely, patients with psoriasis appear to have an increased risk of periodontitis (Keller & Lin, 2012; Nakib, Han, Li, Joshipura, & Qureshi, 2013). Collectively, these studies indicate a bi‐directional relationship between periodontitis and psoriasis, with one disease increasing risk of the other, and vice versa. Although shared inflammatory mechanisms may contribute to this relationship, the biological mechanisms behind these findings remain unclear.
Saliva is the fluid of the oral cavity, which besides being critical to maintenance of oral homeostasis harbors various biological substances such as the salivary microbiota and inflammatory markers (Lynge Pedersen & Belstrom, 2019). The presence of untreated periodontitis has been shown to alter the composition of the salivary microbiota (Belstrom et al., 2017) and increase salivary levels of inflammatory proteins such interleukin (IL)‐1β and matrix metalloproteinase (MMP)‐8 (Lee, Chen, Tu, Wu, & Chang, 2018; Liukkonen, Gürsoy, Pussinen, Suominen, & Könönen, 2016; Sorsa et al., 2016). Thus, the composition of the salivary microbiota and salivary levels of inflammatory markers have been suggested as a proxy of oral and systemic health status (Yoshizawa et al., 2013). While psoriasis associates with periodontitis, it is not known whether psoriasis directly impacts these salivary markers of oral homeostasis.
The purpose of the present study was therefore to characterize the composition of the salivary microbiota in patients with psoriasis. Furthermore, as inflammatory mechanisms including neutrophils and presumably iron metabolism play important roles in psoriasis (Rocha‐Pereira et al., 2004; Schon, Broekaert, & Erpenbeck, 2017) we aimed to quantify salivary levels of the inflammation‐related proteins, neutrophil gelatinase‐associated lipocalin (NGAL) and transferrin in patients with psoriasis. We compared these data to those in patients with periodontitis and orally healthy controls, respectively, to test the hypothesis that psoriasis is linked with characteristics of the salivary microbiota and salivary levels of inflammation‐related proteins, which are different from characteristics in patients with periodontitis and oral healthy individuals, respectively.
2. METHODS
2.1. Study population
In the period from October 2018 to May 2019, a total of 142 individuals were screened for enrollment in the study. Patients with psoriasis were recruited at the Department of Dermatology, Herlev and Gentofte Hospital, whereas patients with periodontitis and orally healthy controls were recruited at the Department of Odontology, University of Copenhagen. General inclusion criteria were age > 18 years and ≥20 remaining natural teeth. General exclusion criteria included pregnancy, breast‐feeding, and use of any systemic antibiotics 3 months prior to study participation. Patients with psoriasis were excluded if they had treatment‐requiring periodontitis, and patients with periodontitis were excluded if they had psoriasis. Accordingly, 13 patients with psoriasis were excluded as they had treatment‐requiring periodontitis, and samples from 7 patients with psoriasis were excluded as they did not pass initial salivary sample quality control due to insufficient amount of bacterial DNA. Consequently, samples from 27 patients with psoriasis, 58 patients with periodontitis, and 52 orally healthy controls were included in the study. The study was performed in accordance with the Helsinki declaration, and all participants signed an informed consent prior to participation. The study was approved by the regional ethical committee (H‐18013543) and reported to the local data authorization of the Faculty of Health and Medical Sciences, University of Copenhagen.
2.2. Clinical examination and case definitions
Periodontal examination was performed in all patients with psoriasis by the same examiner (JME), and plaque, bleeding on probing (BOP), probing pocket depth (PPD), and clinical attachment level (CAL) were recorded at six sites per tooth (third molars excluded). Periodontitis was defined as BOP ≥ 25% of total sites, with minimum two teeth with clinical attachment level ≥ 4 mm and a minimum two teeth with PPD ≥ 6 mm (Kongstad et al., 2013), corresponding to stage 3 using the newly presented classification of periodontitis (Papapanou et al., 2018).
2.3. Collection of saliva samples
Stimulated saliva samples were collected between 8.00 a.m. and 15.00 p.m. Monday–Friday. The saliva samples were collected at least two hours after self‐performed oral hygiene, and before any dental treatment to avoid bleeding and contamination. Two types of stimulated saliva samples were collected. First, a Salivette (Sarstedt) was used. The swab from the Salivette was placed in the mouth of each of the participants. The swab was removed after 2 min and stored in the suspended insert of the Salivette. Two Salivette saliva samples were collected from each participant. Next, a paraffin‐stimulated saliva sample was collected as previously described (Bardow et al., 2014). The saliva was collected in plastic cups and split into two aliquots, which were transferred to Eppendorf tubes (Eppendorf Nordic). The Salivettes and the Eppendorf tubes were stored at –80° Celsius degrees until analysis.
2.4. Sequencing and data analysis
Bacterial 16S rRNA gene‐targeted amplicon sequencing was performed using a custom dual‐index protocol, as previously described (Kozich, Westcott, Baxter, Highlander, & Schloss, 2013). Custom 16S primers were used to amplify the V1‐V3 regions of the 16S rRNA gene, which were designed to provide the best coverage of the 16S gene while maintaining high sensitivity. Libraries were prepared using a 22‐cycle PCR, which reduces chimera formation (unless otherwise noted). Purification of final PCR products was done using Ampure XP beads, pooled in equal amounts, and gels were purified by the QIAGEN MinElute Gel Extraction Kit (Qiagen). Quantification of pooled purified libraries was performed using the NEBNext Library Quant Kit for Illumina (Illumina). The Illumina® MiSeq was used for sequencing of final libraries with a v2 reagent kit (500 cycles) at a 10 p.m. loading concentration with >20% PhiX spike‐in. The DADA2 R package (Callahan et al., 2016) was used to identify and quantify amplicon sequencing reads on the FastQC files obtained after demultiplexing with the Illumina MiSeq software, and low‐quality sequences were removed using FastQC. Results of FastQC were compiled using MultiQC (Ewels, Magnusson, Lundin, & Käller, 2016). Trimmed and filtered reads were processed through the denoizing, concatenating read1 and read2 with a 10N spacer, and chimera removal steps of DADA2 to identify and quantify true amplicon sequence variants (ASV) present in the sample. Taxonomy of the identified ASVs was assigned using the RDP classifier algorithm (Wang, Garrity, Tiedje, & Cole, 2007) implemented in the DADA2 package with a training dataset developed at The Forsyth Institute and based on the Extended Human Oral Microbe Database (eHOMD) (Chen et al., 2010).
2.5. Analyses of NGAL and transferrin
The samples were thawed at RT, spun down at 2000G for 5 min, and transferred to standard 13‐mm analysis tubes. NGAL was analyzed on the Siemens Vista 1500 platform (Siemens) using a NGAL Test assay (cat. nr. ST001RA), the NGAL Calibrator Kit (cat. nr. ST002CA), and the NGAL Control Kit (cat. nr ST003CA) all from Bioporto (Bioporto Diagnostics). Samples with NGAL concentration over 3,000 ng/ml were diluted 1:4, 1:9, or 1:19 in isotonic NaCl and reanalyzed. Transferrin concentration in saliva was measured on the Vista 1500 platform using the TRF Flex reagent in urine mode, as the transferrin concentration in saliva is low. The detection limit of NGAL and transferrin was 25 ng/ml and 2.46 mg/L, respectively. In case of measurements below detection limit, the value was set conservatively as the detection limit.
2.6. Statistics
Since no previous data were available on salivary levels of NGAL and transferrin or the salivary microbiota in patients with psoriasis, the sample size was calculated using the clinical periodontal parameter, mean PPD. Based on findings from previous studies (Belstrøm et al., 2018; Üstün et al., 2013), the expected PPD in the periodontitis group was 3.4 ± 1.0 mm and the expected PPD in the psoriasis group was 2.8 mm. With fixed values of α = 0.05 and β = 0.10, the calculated sample size was n = 116, with 58 individuals in each group. Thus, the number of healthy controls was also set to n = 58. All parameters tested were checked for normality. Data which followed a Gaussian distribution were compared using t test, chi‐square test, and ANOVA, whereas non‐parametric data were compared by Mann–Whitney U test, Friedman test, and Kruskal–Wallis H test with p < .05 considered as statistically significant. The core salivary microbiota was defined as bacterial genera and species present with a mean relative abundance >1% across all samples. The salivary microbiota were characterized and compared by means of predominant genera and species, relative abundance, α‐diversity (Shannon index), principal component analysis, and linear discriminant effect size analysis (Segata et al., 2011). Data on relative abundance were corrected for multiple dependent associations using Benjamini–Hochberg correction (Hochberg & Benjamini, 1990). All statistics were computed with MeV (Saeed et al., 2006) and GraphPad Prism (GraphPad).
3. RESULTS
3.1. Background and clinical data
Background data of the study population are detailed in Table 1. A comparable age and gender distribution was observed between the three groups. A significantly higher number of current smokers were identified in the periodontitis group (p < .05), whereas a significantly higher proportion of patients with psoriasis reported to attend regular dental care, as compared to periodontitis patients and healthy controls (p < .05). A limited proportion of the study population reported concurrent cardiovascular disease or diabetes. Patients with psoriasis were in good periodontal condition as expressed by number of teeth (mean: 26, range: 21–28), PPD (mean: 3.0, range: 2.4–3.4), and CAL (mean: 2.8, range: 2.0–3.9), despite having relatively high percentages of BOP (mean: 63, range: 26–99) and plaque (mean: 82, range: 37–100).
Table 1.
Background data of the study groups
| Psoriasis (n = 27) | Periodontitis (n = 58) | Orally healthy controls (n = 52) | |
|---|---|---|---|
| Gender male/female | 16/11 | 33/25 | 27/25 |
| Age (mean, range) years | 55.3 (38–74) | 53.4 (38–76) | 54.8 (40–80) |
| Smoking status (Non‐/former/current smoker) | 15/10/2* | 20/15/23 | 30/16/6* |
| Heart disease (Y/N) | 0/27 | 5/53 | 8/44 |
| Diabetes (Y/N) | 2/25 | 4/54 | 4/48 |
| Regular dental care (Y/N) | 23/4* | 17/41 | 28/24 |
p < .05.
3.2. Sequence metadata
A total number of 137 samples passed quality control with a mean (range) of 33,591 (8,278–105,689) sequences per samples. In general, 99.8 (96.2–100.0)% of the generated sequences could be identified at genus level, whereas 98.0% (93.7–99.8) of the sequences could be identified at species level. In total, 477 different taxa were identified. A comparable percentage of genus‐level and species‐level identifications were observed in all groups studied.
3.3. Core salivary microbiota in psoriasis, periodontitis, and oral health
The composition of the predominant bacterial genera and species identified is presented in Figure 1a‐b. The four most predominant bacterial genera identified in all three study groups, which constituted approximately 70% of the salivary microbiota, were Streptococcus, Prevotella, Veillonella, and Neisseria. At species level, the most predominant bacterial species was Prevotella melalogenica followed by Streptococcus salivarius. In general, the most predominant bacterial species identified were Streptococcus, Prevotella, and Veillonella. No significant differences were observed in α‐diversity and relative abundance of predominant genera or predominant species in the three groups. Furthermore, principal component analysis revealed a completely random distribution of saliva samples from patients with psoriasis, patients with periodontitis, and healthy controls (Figure 1c).
Figure 1.
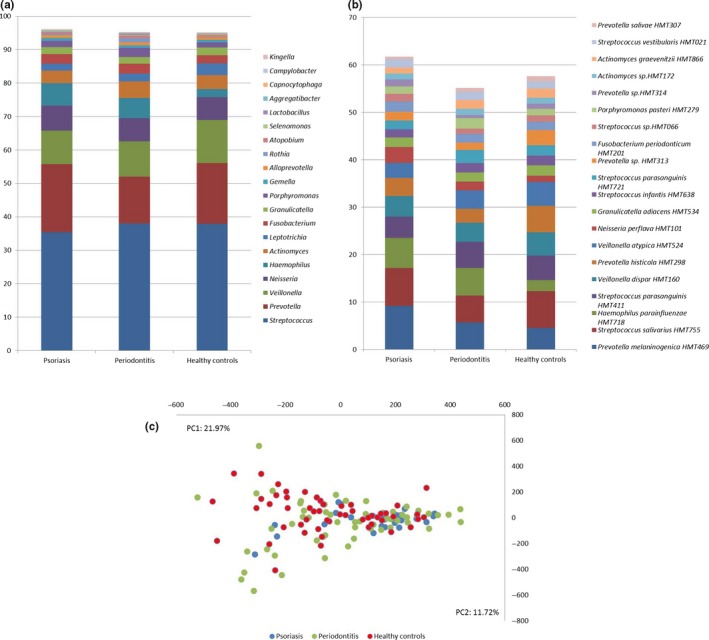
Core salivary microbiota. (a) Relative abundance of predominant bacterial genera expressed as % of total sequences. (b) Relative abundance of predominant bacterial species expressed as % of total sequences. (c) Principal component analysis with x (PC1) and y‐axis (PC2) as the two most decisive components collectively accounting for 33.7% of the variation. Sample denotation: psoriasis: blue, periodontitis: green, and oral health: red
3.4. Relative abundance and linear discriminant effect size analysis of salivary microbiota
A total of 18 bacterial species were identified with a significantly different relative abundance in saliva samples from patients with psoriasis, patients with periodontitis, and oral healthy controls (Table 2, adjusted p < .001). A significantly higher relative abundance of specific proposed periodontal pathogens, such as Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Prevotella intermedia, and Filifactor alocis, was identified in saliva samples from patients with periodontitis. Pairwise comparisons using linear discriminant effect size analysis showed that 52 (22 psoriasis‐associated and 30 periodontitis‐associated; Figure 2a) taxa differentiated patients with psoriasis from patients with periodontitis, 21 (8 psoriasis‐associated and 13 orally healthy control‐associated; Figure 2b) taxa differentiated patients with psoriasis from orally healthy controls, and 78 (42 periodontitis‐associated and 36 orally healthy control‐associated; Figure 2c) taxa differentiated patients with periodontitis from orally healthy controls, respectively.
Table 2.
Bacterial taxa with significantly different relative abundance
| Psoriasis | Periodontitis | Orally healthy controls | p‐value | ||||
|---|---|---|---|---|---|---|---|
| RA | Presence | RA | Presence | RA | Presence | ||
| Rothia mucilaginosa HMT681 | 0.330686 | 96.15385 | 1.064432422 | 100 | 0.051519542 | 88.46154 | 1.00E‐11 |
| Mycoplasma faucium HMT606 | 0.002838 | 3.846154 | 0.075078827 | 72.41379 | 0.004729376 | 19.23077 | 3.93E‐10 |
| Tannerella forsythia HMT613 | 0.011631 | 38.46154 | 0.07827513 | 82.75862 | 0.007009139 | 38.46154 | 2.74E‐08 |
| Peptostreptococcaceae [XI][G‐6] nodatum HMT694 | 0.000757 | 3.846154 | 0.021276436 | 55.17241 | 0.00195116 | 7.692308 | 6.52E‐08 |
| Porphyromonas gingivalis HMT619 | 0.000000 | 0 | 0.049856888 | 50 | 0.000804377 | 5.769231 | 1.22E‐07 |
| Filifactor alocis HMT539 | 0.012395 | 15.38462 | 0.135676421 | 63.7931 | 0.015349738 | 21.15385 | 2.65E‐07 |
| Rothia dentocariosa HMT587 | 0.022593 | 23.07692 | 0.091595477 | 65.51724 | 0.003578482 | 17.30769 | 7.87E‐07 |
| Bacteroidaceae [G‐1] HMT272 | 0.005529 | 15.38462 | 0.001228553 | 6.896552 | 0.015004592 | 50 | 7.49E‐06 |
| Treponema sp.HMT237 | 0.010219 | 15.38462 | 0.045178271 | 50 | 0.002571879 | 11.53846 | 8.10E‐06 |
| Mogibacterium timidum HMT042 | 0.030495 | 46.15385 | 0.106152765 | 82.75862 | 0.026568563 | 53.84615 | 1.34E‐05 |
| Fusobacterium nucleatum HMT200 | 0.066781 | 42.30769 | 0.257576702 | 77.58621 | 0.07180377 | 48.07692 | 3.15E‐05 |
| Treponema denticola HMT584 | 0.009736 | 23.07692 | 0.040443669 | 48.27586 | 0.001278036 | 9.615385 | 4.14E‐05 |
| Fretibacterium fastidiosum HMT363 | 0.005070 | 19.23077 | 0.032599761 | 53.44828 | 0.003339819 | 17.30769 | 4.14E‐05 |
| Prevotella intermedia HMT643 | 0.034083 | 30.76923 | 0.244041221 | 62.06897 | 0.031503929 | 23.07692 | 5.05E‐05 |
| Peptostreptococcaceae [XI][G‐5] saphenum HMT759 | 0.011885 | 11.53846 | 0.076931378 | 53.44828 | 0.007156812 | 23.07692 | 5.14E‐05 |
| Porphyromonas endodontalis HMT273 | 0.090996 | 61.53846 | 0.319207693 | 87.93103 | 0.084759485 | 69.23077 | 6.89E‐05 |
| Fretibacterium sp.HMT360 | 0.004001 | 19.23077 | 0.024301347 | 51.72414 | 0.002661645 | 15.38462 | 7.59E‐05 |
| Peptostreptococcaceae [XI][G‐4] HMT369 | 0.001159 | 11.53846 | 0.008681835 | 44.82759 | 0.001356973 | 9.615385 | 1.60E‐04 |
Abbreviation: RA, relative abundance.
Figure 2.
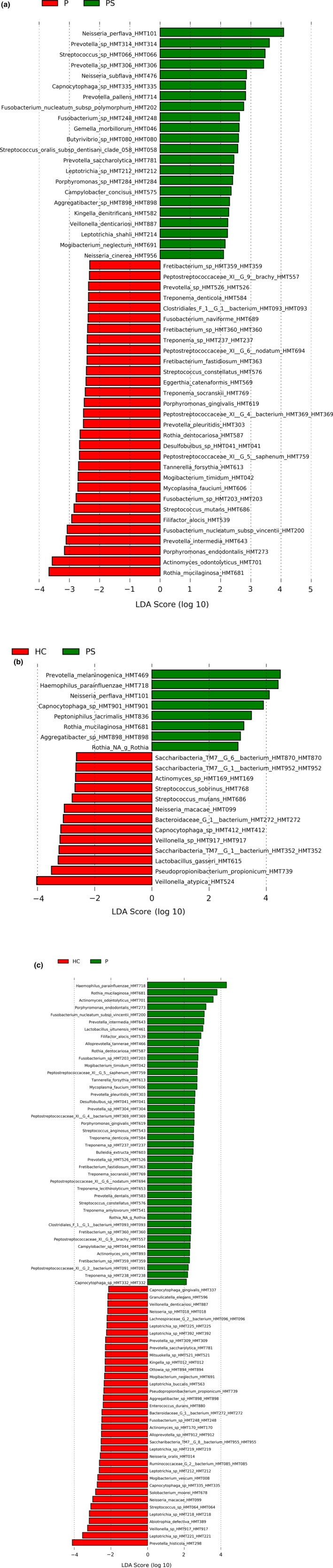
Linear discriminant effect size analysis. Linear discriminant analysis score expressed as (log 10) of significant bacterial taxa. (a) Psoriasis (PS) versus periodontitis (P). (b) Psoriasis (PS) versus healthy controls (HC). (c) Periodontitis (P) versus healthy controls (HC)
3.5. Salivary NGAL and transferrin levels
All samples were recorded with NGAL levels above detection limit, whereas transferrin levels were below detection limit in 30 samples.
A significantly lower mean salivary level of NGAL (996 [std. error 320] ng/ml) was recorded in patients with psoriasis, as compared to patients with periodontitis (2,072 [295] ng/ml) and orally healthy controls (2,551 [345] ng/ml, p < .0001).
Salivary transferrin levels were also significantly lower in patients with psoriasis (4.37 [0.92] ng/ml), as compared to patients with periodontitis (7.25 [0.88] ng/ml) and orally healthy controls (10.02 [0.94] ng/ ml, p < .0001).
4. DISCUSSION
The purpose of the present study was to characterize the composition of the salivary microbiota and measure salivary levels of NGAL and transferrin in patients with psoriasis, and compare these data to the characteristics in patients with periodontitis and orally healthy controls.
We found that psoriasis was associated with specific characteristics of the salivary microbiota, which were different from patients with periodontitis and orally healthy controls (Figure 2a‐b, Table 2). Furthermore, patients with psoriasis had significantly lower salivary NGAL and transferrin levels than patients with periodontitis and orally healthy controls. These data suggest that psoriasis impacts oral homeostasis and the significance of reduced levels salivary levels of NGAL and transferrin in psoriasis clearly also merits further study.
In the present study, 29% of the patients with psoriasis were excluded as they had treatment‐requiring periodontitis. This finding is in line with increased risk of periodontitis found in other studies (Skudutyte‐Rysstad et al., 2014; Woeste et al., 2019). In a recently published paper, the prevalence of periodontitis in a Danish cohort ranged from 9.2% to 31.0% depending on the periodontal classification used (Kongstad, Enevold, Christensen, Fiehn, & Holmstrup, 2017). Thus, data from the present study support a psoriasis‐associated risk of periodontitis. Notably, all patients with psoriasis, who were excluded based on the presence of treatment‐requiring periodontitis, reported to attend regular dental care, as compared to 54% of orally healthy controls, indicating that patients with psoriasis might be prone to periodontitis despite having regular dental care.
The core salivary microbiota identified in patients with psoriasis could not be differentiated from patients with periodontitis and orally healthy controls (Figure 1a‐c). However, 18 bacterial species were identified with a significantly different relative abundance in the three groups studies (Table 2). Notably, the majority of these bacterial species, including the proposed periopathogen P. gingivalis, were identified with higher relative abundance in patients with periodontitis. Salivary levels of P. gingivalis have been reported to associate with periodontitis (Damgaard et al., 2019), and previous studies have shown a positive correlation of salivary and subgingival levels of P. gingivalis (Belstrøm et al., 2018; Nickles, Scharf, Röllke, Dannewitz, & Eickholz, 2017) suggesting that the salivary microbiota reflects local bacterial alterations associated with periodontitis.
Using linear discriminant effect size analysis, we identified unique characteristics of the salivary microbiota in psoriasis, as 52 (22 psoriasis‐associated and 30 periodontitis‐associated; Figure 2a) and 21 (8 psoriasis‐associated and 13 health‐associated; Figure 2b) taxa differed in psoriasis as compared to periodontitis and orally healthy controls, respectively. To the best of our knowledge, this is the first study to characterize the salivary microbiota in patients with psoriasis. On the other hand, several studies have demonstrated an impact of diabetes on the composition of the oral microbiota (Wang et al., 2019; Xiao et al., 2017), and two recent studies reported that periodontal bone loss in patients with rheumatoid arthritis was associated with alterations of the oral microbiota (Corrêa et al., 2019, 2016). In addition, dysbiosis of the salivary microbiota has been linked with inflammatory bowel disease (Xun, Zhang, Xu, Chen, & Chen, 2018). Thus, accumulated evidence suggests that systemic diseases, as exemplified in the current study by psoriasis, might impact the composition of the salivary microbiota.
We found significantly lower salivary levels of NGAL and transferrin in patients with psoriasis, as compared to patients with periodontitis and orally healthy controls, respectively. To the best of our knowledge, salivary levels of these inflammation‐related proteins have not previously been reported in patients with psoriasis. However, a study from 2015 showed higher salivary levels of pro‐inflammatory cytokines, including tumor necrosis factor‐α, IL‐1β, transforming growth factor‐1β, and monocyte chemoattractant protein‐1 in patients with psoriasis as compared to orally healthy controls (Ganzetti et al., 2015). Therefore, psoriasis seems to associate with increased levels of inflammatory markers in saliva and it is remarkable that in our study patients with psoriasis had significantly lower levels of salivary NGAL as compared to patients with periodontitis and orally healthy controls, respectively. NGAL is an inflammation‐related protein, and increased levels of NGAL in the circulation and urine have been intensively investigated as a marker of acute kidney injury (Hjortrup, Haase, Treschow, Møller, & Perner, 2015). Also, in periodontitis a positive correlation has been found between urinary levels of NGAL and the severity of periodontitis (Nakajima et al., 2019). Notably, in a study from 1996, increased expression of NGAL was identified in gingival tissue and saliva from patients with periodontitis (Westerlund et al., 1996), and a recent report showed that experimental gingivitis caused an increase in salivary levels of NGAL (Morelli et al., 2014). Furthermore, higher NGAL levels in local samples, that is, gingival crevicular fluid (GCF), were reported in patients with periodontitis (Pradeep, Nagpal, Karvekar, & Patnaik, 2016). The present salivary NGAL data are therefore intriguing and warrant further study, but it is remarkable that conflicting data also exist for other salivary inflammatory markers in periodontitis, for example, with increased (Lee et al., 2018) or similar (Moura et al., 2017) salivary levels of IL‐1β compared to healthy controls. While the main contributor to salivary proteins is the circulation, with proteins shed from local oral surfaces playing a lesser role (Lynge Pedersen & Belstrom, 2019), disease‐specific mechanisms are likely to be involved, for example, with periodontitis‐dependent increased expression of inflammatory mediators in the inflamed oral tissue potentially being countered by increased local tissue binding or other interactions that contribute to unpredictability of salivary levels of these molecules. It would therefore be recommendable to include blood and GCF samples in future studies on salivary levels of inflammation‐related proteins.
Also, salivary transferrin levels were lower in patients with psoriasis compared to patients with periodontitis and orally healthy controls, respectively. Transferrin is a “negative” inflammation‐related protein, and inflammation is associated with a decrease in circulating transferrin levels. Accordingly, several studies have reported that periodontal treatment is associated with an increase in blood transferrin levels, albeit that to our knowledge, there is no evidence available on salivary transferrin levels (Fang et al., 2015; Shirmohamadi et al., 2016).
Some limitations apply to the present study, primarily the relatively low number of included patients with psoriasis. However, as significant differences in the salivary microbiota and salivary levels of NGAL and transferrin, respectively, were observed, the number of participants may have been sufficient. Furthermore, the severity of psoriasis and current treatment regimen was not recorded but we recently found that similar patients with psoriasis from the recruiting hospital had a psoriasis area and severity index (PASI) score of approximately 10 suggestive of severe disease (Ahlehoff et al., 2011). Moreover, as blood samples and local samples from the oral cavity, for example, GCF, were not collected, it was not possible to compare salivary, local, and circulating levels of NGAL and transferrin. In addition, the higher amount of current smokers in the periodontitis group may have influenced data on the salivary microbiota, as smoking status has been reported to influence the composition of the subgingival microbiota (Mason et al., 2015). However, previous studies have shown that the impact of smoking on the salivary microbiota is probably not as pronounced (Belstrøm, Fiehn, et al., 2014; Belstrøm, Holmstrup, et al., 2014). Finally, the prevalence of cardiovascular disease and diabetes in patients with psoriasis was in the present study somewhat lower than expected, which might be explained by the fact that cardiovascular disease and diabetes was self‐reported and therefore not based on data from a medical examination.
In conclusion, data from the present study suggest that psoriasis is associated with characteristics of the salivary microbiota and salivary levels of inflammation‐related proteins, which is different from that of patients with periodontitis and orally healthy controls. More studies are needed to shed further light on the mechanisms underlying the association between psoriasis and periodontitis.
CONFLICT OF INTEREST
The authors have stated explicitly that there are no conflicts of interest in connection with this article. This study was supported financially by the Independent Research Council Denmark, the Danish Foundation for Mutual Efforts in Dental Care, and The Danish Dental Association. Daniel Belstrøm and Josefine Maria Eiberg contributed equally to this manuscript. PRH is supported by unrestricted grant from the LEO Foundation, a Borregaard Clinical Scientist Fellowship from the Novo Nordisk Foundation, and a clinical academic group grant from the Greater Copenhagen Health Science Partners.
AUTHOR CONTRIBUTION
Daniel Belstrøm (DB), Peter Riis Hansen (PRH), and Claus Antonio Juel Jensen (CAJJ) designed the study. Josefine Maria Eiberg (JME) and Lone Skov recruited the patients. JME performed the clinical examinations and collected the samples. Christian Enevold, Maria Grande, and CAJJ performed the molecular analysis. DB did the statistical analysis. DB and PRH wrote the first draft of the manuscript, which was critically revised by all authors. All authors approved the final version of the paper.
ACKNOWLEDGEMENTS
The authors wish to thank nurses and medical doctors at the Department of Dermatology and Allergy, Herlev and Gentofte Hospital for their help with recruitment of patients with psoriasis. Furthermore, Bioporto is acknowledged for providing the NGAL test essays. Sequencing was done by the Sequencing and Bioinformatics Core at the Forsyth Institute, Boston, USA.
Belstrøm D, Eiberg JM, Enevold C, et al. Salivary microbiota and inflammation‐related proteins in patients with psoriasis. Oral Dis. 2020;26:677–687. 10.1111/odi.13277
Belstrøm and Eiberg contributed equally to the present article.
DATA AVAILABILITY STATEMENT
Unrestricted access to all data including raw sequences will be granted upon request to the corresponding author (dbel@sund.ku.dk).
REFERENCES
- Ahlehoff, O. , Gislason, G. H. , Charlot, M. , Jørgensen, C. H. , Lindhardsen, J. , Olesen, J. B. , … Hansen, P. R. (2011). Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. Journal of Internal Medicine, 270(2), 147–157. 10.1111/j.1365-2796.2010.02310.x [DOI] [PubMed] [Google Scholar]
- Bardow, A. , Lykkeaa, J. , Qvist, V. , Ekstrand, K. , Twetman, S. , & Fiehn, N. E. (2014). Saliva composition in three selected groups with normal stimulated salivary flow rates, but yet major differences in caries experience and dental erosion. Acta Odontologica Scandinavica, 72(6), 466–473. 10.3109/00016357.2013.860621 [DOI] [PubMed] [Google Scholar]
- Bartold, P. M. , & Van Dyke, T. E. (2017). Host modulation: controlling the inflammation to control the infection. Periodontology 2000, 75(1), 317–329. 10.1111/prd.12169. [DOI] [PubMed] [Google Scholar]
- Belstrøm, D. , Constancias, F. , Liu, Y. , Yang, L. , Drautz‐Moses, D. I. , Schuster, S. C. , … Givskov, M. (2017). Metagenomic and metatranscriptomic analysis of saliva reveals disease‐associated microbiota in patients with periodontitis and dental caries. NPJ Biofilms and Microbiomes, 3, 23 10.1038/s41522-017-0031-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belstrøm, D. , Fiehn, N.‐E. , Nielsen, C. H. , Kirkby, N. , Twetman, S. , Klepac‐Ceraj, V. , … Holmstrup, P. (2014). Differences in bacterial saliva profile between periodontitis patients and a control cohort. Journal of Clinical Periodontology, 41(2), 104–112. 10.1111/jcpe.12190 [DOI] [PubMed] [Google Scholar]
- Belstrøm, D. , Grande, M. A. , Sembler‐Møller, M. L. , Kirkby, N. , Cotton, S. L. , Paster, B. J. , & Holmstrup, P. (2018). Influence of periodontal treatment on subgingival and salivary microbiotas. Journal of Periodontology, 89(5), 531–539. 10.1002/JPER.17-0377 [DOI] [PubMed] [Google Scholar]
- Belstrøm, D. , Holmstrup, P. , Nielsen, C. H. , Kirkby, N. , Twetman, S. , Heitmann, B. L. , … Fiehn, N.‐E. (2014). Bacterial profiles of saliva in relation to diet, lifestyle factors, and socioeconomic status. Journal of Oral Microbiology, 6(1), 23609 10.3402/jom.v6.23609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan, B. J. , McMurdie, P. J. , Rosen, M. J. , Han, A. W. , Johnson, A. J. , & Holmes, S. P. (2016). DADA2: High‐resolution sample inference from Illumina amplicon data. Nature Methods, 13(7), 581–583. 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, T. , Yu, W. H. , Izard, J. , Baranova, O. V. , Lakshmanan, A. , & Dewhirst, F. E. (2010). The Human Oral Microbiome Database: a web accessible resource for investigating oral microbe taxonomic and genomic information. Database (Oxford), 2010:baq013 10.1093/database/baq013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa, J. D. , Fernandes, G. R. , Calderaro, D. C. , Mendonça, S. M. S. , Silva, J. M. , Albiero, M. L. , … Graves, D. T. (2019). Oral microbial dysbiosis linked to worsened periodontal condition in rheumatoid arthritis patients. Scientific Reports, 9(1), 8379 10.1038/s41598-019-44674-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrêa, J. D. , Saraiva, A. M. , Queiroz‐Junior, C. M. , Madeira, M. F. M. , Duarte, P. M. , Teixeira, M. M. , … da Silva, T. A. (2016). Arthritis‐induced alveolar bone loss is associated with changes in the composition of oral microbiota. Anaerobe, 39, 91–96. 10.1016/j.anaerobe.2016.03.006 [DOI] [PubMed] [Google Scholar]
- Damgaard, C. , Danielsen, A. K. , Enevold, C. , Massarenti, L. , Nielsen, C. H. , Holmstrup, P. , & Belstrøm, D. (2019). Porphyromonas gingivalis in saliva associates with chronic and aggressive periodontitis. Journal of Oral Microbiology, 11(1), 1653123 10.1080/20002297.2019.1653123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlatshahi, E. A. , van der Voort, E. A. , Arends, L. R. , & Nijsten, T. (2013). Markers of systemic inflammation in psoriasis: A systematic review and meta‐analysis. The British Journal of Dermatology, 169(2), 266–282. 10.1111/bjd.12355 [DOI] [PubMed] [Google Scholar]
- Egeberg, A. , Mallbris, L. , Gislason, G. , Hansen, P. R. , & Mrowietz, U. (2017). Risk of periodontitis in patients with psoriasis and psoriatic arthritis. Journal of the European Academy of Dermatology and Venereology, 31(2), 288–293. 10.1111/jdv.13814 [DOI] [PubMed] [Google Scholar]
- Eke, P. I. , Dye, B. A. , Wei, L. , Thornton‐Evans, G. O. , & Genco, R. J. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. Journal of Dental Research, 91(10), 914–920. 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- Ewels, P. , Magnusson, M. , Lundin, S. , & Käller, M. (2016). MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics, 32(19), 3047–3048. 10.1093/bioinformatics/btw354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, F. , Wu, B. , Qu, Q. , Gao, J. , Yan, W. , Huang, X. , … Liu, Y. (2015). The clinical response and systemic effects of non‐surgical periodontal therapy in end‐stage renal disease patients: A 6‐month randomized controlled clinical trial. Journal of Clinical Periodontology, 42(6), 537–546. 10.1111/jcpe.12411 [DOI] [PubMed] [Google Scholar]
- Ganzetti, G. , Campanati, A. , Santarelli, A. , Pozzi, V. , Molinelli, E. , Minnetti, I. , … Offidani, A. (2015). Involvement of the oral cavity in psoriasis: Results of a clinical study. The British Journal of Dermatology, 172(1), 282–285. 10.1111/bjd.13201 [DOI] [PubMed] [Google Scholar]
- Hjortrup, P. B. , Haase, N. , Treschow, F. , Møller, M. H. , & Perner, A. (2015). Predictive value of NGAL for use of renal replacement therapy in patients with severe sepsis. Acta Anaesthesiologica Scandinavica, 59(1), 25–34. 10.1111/aas.12427 [DOI] [PubMed] [Google Scholar]
- Hochberg, Y. , & Benjamini, Y. (1990). More powerful procedures for multiple significance testing. Statistics in Medicine, 9(7), 811–818. 10.1002/sim.4780090710 [DOI] [PubMed] [Google Scholar]
- Keller, J. J. , & Lin, H. C. (2012). The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. The British Journal of Dermatology, 167(6), 1338–1344. 10.1111/j.1365-2133.2012.11126.x [DOI] [PubMed] [Google Scholar]
- Kongstad, J. , Ekstrand, K. , Qvist, V. , Christensen, L. B. , Cortsen, B. , Grønbæk, M. , … Fiehn, N.‐E. (2013). Findings from the oral health study of the Danish Health Examination Survey 2007–2008. Acta Odontologica Scandinavica, 71(6), 1560–1569. 10.3109/00016357.2013.776701 [DOI] [PubMed] [Google Scholar]
- Kongstad, J. , Enevold, C. , Christensen, L. B. , Fiehn, N. E. , & Holmstrup, P. (2017). Impact of periodontitis case criteria: A cross‐sectional study of lifestyle. Journal Periodontology, 88(6), 602–609. 10.1902/jop.2017.160426 [DOI] [PubMed] [Google Scholar]
- Kozich, J. J. , Westcott, S. L. , Baxter, N. T. , Highlander, S. K. , & Schloss, P. D. (2013). Development of a dual‐index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Applied and Environmental Microbiology, 79(17), 5112–5120. 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaridou, E. , Tsikrikoni, A. , Fotiadou, C. , Kyrmanidou, E. , Vakirlis, E. , Giannopoulou, C. , … Ioannides, D. (2013). Association of chronic plaque psoriasis and severe periodontitis: A hospital based case‐control study. Journal of European Academy of Dermatology and Venereology, 27(8), 967–972. 10.1111/j.1468-3083.2012.04615.x [DOI] [PubMed] [Google Scholar]
- Lee, C. H. , Chen, Y. W. , Tu, Y. K. , Wu, Y. C. , & Chang, P. C. (2018). The potential of salivary biomarkers for predicting the sensitivity and monitoring the response to nonsurgical periodontal therapy: A preliminary assessment. Journal of Periodontal Research, 53(4), 545–554. 10.1111/jre.12544 [DOI] [PubMed] [Google Scholar]
- Liukkonen, J. , Gürsoy, U. K. , Pussinen, P. J. , Suominen, A. L. , & Könönen, E. (2016). Salivary concentrations of Interleukin (IL)‐1beta, IL‐17A, and IL‐23 vary in relation to periodontal status. Journal of Periodontology, 87(12), 1484–1491. 10.1902/jop.2016.160146 [DOI] [PubMed] [Google Scholar]
- Lynge Pedersen, A. M. , & Belstrom, D. (2019). The role of natural salivary defences in maintaining a healthy oral microbiota. Journal of Dentistry, 80(Suppl 1), S3–S12. 10.1016/j.jdent.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Mahil, S. K. , Capon, F. , & Barker, J. N. (2015). Genetics of psoriasis. Dermatologic Clinics, 33(1), 1–11. 10.1016/j.det.2014.09.001 [DOI] [PubMed] [Google Scholar]
- Mason, M. R. , Preshaw, P. M. , Nagaraja, H. N. , Dabdoub, S. M. , Rahman, A. , & Kumar, P. S. (2015). The subgingival microbiome of clinically healthy current and never smokers. The ISME Journal, 9(1), 268–272. 10.1038/ismej.2014.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli, T. , Stella, M. , Barros, S. P. , Marchesan, J. T. , Moss, K. L. , Kim, S. J. , … Offenbacher, S. (2014). Salivary biomarkers in a biofilm overgrowth model. Journal of Periodontology, 85(12), 1770–1778. 10.1902/jop.2014.140180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura, M. F. , Navarro, T. P. , Silva, T. A. , Cota, L. O. M. , Soares Dutra Oliveira, A. M. , & Costa, F. O. (2017). Periodontitis and endothelial dysfunction: periodontal clinical parameters and levels of salivary markers interleukin‐1beta, tumor necrosis factor‐alpha, matrix metalloproteinase‐2, tissue inhibitor of metalloproteinases‐2 complex, and nitric oxide. Journal of Periodontology, 88(8), 778–787. 10.1902/jop.2017.170023 [DOI] [PubMed] [Google Scholar]
- Nakajima, M. , Hosojima, M. , Tabeta, K. , Miyauchi, S. , Yamada‐Hara, M. , Takahashi, N. , … Yoshie H. (2019). beta 2‐microglobulin and neutrophil gelatinase‐associated lipocalin, potential novel urine biomarkers in periodontitis: A Cross‐Sectional Study in Japanese. International Journal of Dentistry, 2019, 1394678 10.1155/2019/1394678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakib, S. , Han, J. , Li, T. , Joshipura, K. , & Qureshi, A. A. (2013). Periodontal disease and risk of psoriasis among nurses in the United States. Acta Odontologica Scandinavica, 71(6), 1423–1429. 10.3109/00016357.2013.766360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestle, F. O. , Kaplan, D. H. , & Barker, J. (2009). Psoriasis. The New England Journal of Medicine, 361(5), 496–509. 10.1056/NEJMra0804595 [DOI] [PubMed] [Google Scholar]
- Nickles, K. , Scharf, S. , Röllke, L. , Dannewitz, B. , & Eickholz, P. (2017). Comparison of two different sampling methods for subgingival plaque: Subgingival paper points or mouthrinse sample? Journal of Periodontology, 88(4), 399–406. 10.1902/jop.2016.160249 [DOI] [PubMed] [Google Scholar]
- Papapanou, P. N. , Sanz, M. , Buduneli, N. , Dietrich, T. , Feres, M. , Fine, D. H. , … Tonetti, M. S. (2018). Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri‐Implant Diseases and Conditions. Journal of Periodontology., 89(Suppl 1), S173–S182. 10.1002/JPER.17-0721 [DOI] [PubMed] [Google Scholar]
- Pradeep, A. R. , Nagpal, K. , Karvekar, S. , & Patnaik, K. (2016). Levels of lipocalin‐2 in crevicular fluid and tear fluid in chronic periodontitis and obesity subjects. Journal of Investigative and Clinical Dentistry, 7(4), 376–382. 10.1111/jicd.12165 [DOI] [PubMed] [Google Scholar]
- Preus, H. R. , Khanifam, P. , Kolltveit, K. , Mørk, C. , & Gjermo, P. (2010). Periodontitis in psoriasis patients: A blinded, case‐controlled study. Acta Odontologica Scandinavica, 68(3), 165–170. 10.3109/00016350903583678 [DOI] [PubMed] [Google Scholar]
- Rocha‐Pereira, P. , Santos‐Silva, A. , Rebelo, I. , Figueiredo, A. , Quintanilha, A. , & Teixeira, F. (2004). The inflammatory response in mild and in severe psoriasis. The British Journal of Dermatology, 150(5), 917–928. 10.1111/j.1365-2133.2004.05984.x [DOI] [PubMed] [Google Scholar]
- Saeed, A. I. , Bhagabati, N. K. , Braisted, J. C. , Liang, W. , Sharov, V. , Howe, E. A. , … Quackenbush, J. (2006). TM4 microarray software suite. Methods in Enzymology, 411, 134–193. 10.1016/S0076-6879(06)11009-5 [DOI] [PubMed] [Google Scholar]
- Schon, M. P. , Broekaert, S. M. , & Erpenbeck, L. (2017). Sexy again: The renaissance of neutrophils in psoriasis. Experimental Dermatology, 26(4), 305–311. 10.1111/exd.13067 [DOI] [PubMed] [Google Scholar]
- Segata, N. , Izard, J. , Waldron, L. , Gevers, D. , Miropolsky, L. , Garrett, W. S. , & Huttenhower, C. (2011). Metagenomic biomarker discovery and explanation. Genome Biology, 12(6), R60 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma, A. , Raman, A. , & Pradeep, A. R. (2015). Association of chronic periodontitis and psoriasis: Periodontal status with severity of psoriasis. Oral Diseases, 21(3), 314–319. 10.1111/odi.12271 [DOI] [PubMed] [Google Scholar]
- Shirmohamadi, A. , Chitsazi, M. T. , Faramarzi, M. , Salari, A. , Naser, A. F. , & Pashazadeh, N. (2016). Effect of non‐surgical periodontal treatment on transferrin serum levels in patients with chronic periodontitis. Journal of Dental Research Dental Clinics, Dental Prospects, 10(3), 169–175. 10.15171/joddd.2016.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skudutyte‐Rysstad, R. , Slevolden, E. M. , Hansen, B. F. , Sandvik, L. , & Preus, H. R. (2014). Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health, 14, 139 10.1186/1472-6831-14-139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorsa, T. , Gursoy, U. K. , Nwhator, S. , Hernandez, M. , Tervahartiala, T. , Leppilahti, J. , Mäntylä, P. (2016). Analysis of matrix metalloproteinases, especially MMP‐8, in gingival creviclular fluid, mouthrinse and saliva for monitoring periodontal diseases. Periodontology 2000, 70(1), 142–163. 10.1111/prd.12101 [DOI] [PubMed] [Google Scholar]
- Ungprasert, P. , Wijarnpreecha, K. , & Wetter, D. A. (2017). Periodontitis and risk of psoriasis: A systematic review and meta‐analysis. Journal of the European Academy of Dermatology and Venereology, 31(5), 857–862. 10.1111/jdv.14051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Üstün, K. , Sezer, U. , Kısacık, B. , Şenyurt, S. Z. , Özdemir, E. Ç. , Kimyon, G. , … Onat, A. M. (2013). Periodontal disease in patients with psoriatic arthritis. Inflammation, 36(3), 665–669. 10.1007/s10753-012-9590-y [DOI] [PubMed] [Google Scholar]
- Wang, Q. , Garrity, G. M. , Tiedje, J. M. , & Cole, J. R. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73(16), 5261–5267. 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, R.‐R. , Xu, Y.‐S. , Ji, M.‐M. , Zhang, L. I. , Li, D. , Lang, Q. , … Liu, B.‐C. (2019). Association of the oral microbiome with the progression of impaired fasting glucose in a Chinese elderly population. Journal of Oral Microbiology, 11(1), 1605789 10.1080/20002297.2019.1605789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund, U. , Ingman, T. , Lukinmaa, P.‐L. , Salo, T. , Kjeldsen, L. , Borregaard, N. , … Sorsa, T. (1996). Human neutrophil gelatinase and associated lipocalin in adult and localized juvenile periodontitis. Journal of Dental Research, 75(8), 1553–1563. 10.1177/00220345960750080601 [DOI] [PubMed] [Google Scholar]
- Woeste, S. , Graetz, C. , Gerdes, S. , & Mrowietz, U. (2019). Oral health in patients with psoriasis‐A prospective study. Journal of Investigative Dermatology, 139(6), 1237–1244. 10.1016/j.jid.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Xiao, E. , Mattos, M. , Vieira, G. H. A. , Chen, S. , Corrêa, J. D. , Wu, Y. , … Graves, D. T. (2017). Diabetes enhances IL‐17 expression and alters the oral microbiome to increase its pathogenicity. Cell Host & Microbe, 22(1), 120–128. 10.1016/j.chom.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun, Z. , Zhang, Q. , Xu, T. , Chen, N. , & Chen, F. (2018). Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Frontiers in Microbiology, 9, 1136 10.3389/fmicb.2018.01136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa, J. M. , Schafer, C. A. , Schafer, J. J. , Farrell, J. J. , Paster, B. J. , & Wong, D. T. (2013). Salivary biomarkers: Toward future clinical and diagnostic utilities. Clinical Microbiology Reviews, 26(4), 781–791. 10.1128/CMR.00021-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Unrestricted access to all data including raw sequences will be granted upon request to the corresponding author (dbel@sund.ku.dk).


