Abstract
Infants’ social‐cognitive skills first develop within the parent–infant relationship, but large differences between parents exist in the way they approach and interact with their infant. These may have important consequences for infants’ social‐cognitive development. The current study investigated effects of maternal sensitive and intrusive behavior on 6‐ to 7‐month‐old infants’ ERP responses to a socio‐emotional cue that infants are often confronted with from an early age: emotional prosody in infant‐directed speech. Infants may differ in their sensitivity to environmental (including parenting) influences on development, and the current study also explored whether infants’ resting frontal asymmetry conveys differential susceptibility to effects of maternal sensitivity and intrusiveness. Results revealed that maternal intrusiveness was related to the difference in infants’ ERP responses to happy and angry utterances. Specifically, P2 amplitudes in response to angry sounds were less positive than those in response to happy sounds for infants with less intrusive mothers. Whether this difference reflects an enhanced sensitivity to emotional prosody or a (processing) preference remains to be investigated. No evidence for differential susceptibility was found, as infant frontal asymmetry did not moderate effects of sensitivity or intrusiveness.
1. INTRODUCTION
Parents provide the earliest social environment an individual comes into contact with, and infants’ social‐cognitive skills first develop within the parent–infant relationship (Bowlby, 1969; Hrdy, 1999). Studies on infant cognition often focus on typical development, disregarding individual differences. However, large differences between parents exist in the way they approach and interact with their infant and infants too may vary in their responses and interactive behavior, and these may have important and lasting consequences for infants’ social‐cognitive development. The current study investigates effects of maternal interactive behavior in one domain of infant socio‐cognitive development: infants’ neural processing of emotional prosody in infant‐directed speech (IDS).
Two important dimensions along which parental behaviors toward infants and children vary are sensitivity and intrusiveness (e.g., Bakermans‐Kranenburg, Van IJzendoorn, & Juffer, 2003; Belsky & Fearon, 2002; Egeland, Pianta, & O’Brien, 1993). Sensitive parents provide a responsive, supportive, reliable, clear and consistent social environment. They recognize and balance the infant's needs for both care and protection on the one hand and exploration of his/her physical and social surroundings on the other. Parental sensitivity has long been recognized as an important determinant of secure attachment relationships (e.g., Bakermans‐Kranenburg et al., 2003; De Wolff & Van IJzendoorn, 1997) and has been shown to foster cognitive and social development (Belsky & Fearon, 2002; Feldman, Eidelman, & Rotenberg, 2004). Intrusiveness is most readily observed during parent–infant play and refers to the parent's tendency to control the situation physically and/or verbally, to behave demandingly or negatively toward the infant, and to interfere with or override the infant's activities rather than follow the infant's “lead” (Biringen & Robinson, 1991; Carlson & Harwood, 2003; Egeland et al., 1993). Parental intrusiveness has been associated with a more negative development into early childhood, both in the socio‐emotional domain and in terms of more general cognitive development (e.g., Egeland et al., 1993).
Infant cognitive development is tightly intertwined with the development of the neural pathways underlying cognition (Dehaene‐Lambertz & Spelke, 2015; Johnson, 2003). In the current study, we therefore investigate effects of maternal sensitivity and intrusiveness on the neural underpinnings of socio‐cognitive development of 6‐ to 7‐month‐old infants using event‐related potentials (ERPs). We focus on the neural processing of a specific socio‐emotional cue that infants are confronted with often and from an early age: emotional prosody in IDS. Infants’ ERP responses to sounds, including speech, reflect the degree and efficiency of information processing in the brain and are characterized by a broad, fronto‐centrally distributed positive wave that is usually called P2 (Coch & Gullick, 2012; Kraus et al., 1993; Kushnerenko et al., 2002; Novak, Kurtzberg, Kreuzer, & Vaughan, 1989). The current study thus focuses on infants’ neural processing of prosodic information from IDS as reflected in the P2 component of the ERP. Because previous studies of infants’ neural activity suggest that the capacity to process different types of emotional prosody in language develops around the age of 6 or 7 months (Grossmann, Oberecker, Koch, & Friedericki, 2010), we observed infants in this age range.
When speaking to infants, most adults readily adapt the way they talk. In English, IDS, sometimes referred to as “motherese,” is characterized by simplified sentence structures, a higher pitch, greater variation in pitch, longer pauses, and a slower rate of speech compared to adult‐directed speech (Fernald, 1992; Grieser & Kuhl, 1988). In other languages, other phonological, lexical, and/or grammatical features may be involved and the difference between the infant‐ and adult‐directed speech registers may be more or less pronounced (Fisher & Tokura, 1996). In French, the language our infant participants were exposed to, IDS is characterized by higher maximum pitch, a greater pitch range, shorter utterances, and longer pause durations (Fernald et al., 1989). The use of emotionally positive IDS is a distinctive element of positive mother–infant interactions (Rosenblum, Dayton, & McDonough, 2006), and exposure to IDS may also facilitate language learning (e.g., Cooper & Aslin, 1990; Fernald, 1985; Nelson, Hirshpasek, Jusczyk, & Cassidy, 1989). As an element of positive emotional communication, IDS can thus be considered a potent linguistic and socio‐emotional signal. If maternal sensitivity and intrusiveness influence the development of social cognition, including the neural processing of socio‐emotional information, differences in infants’ neural response to IDS can be expected depending on their mothers’ level of sensitivity and intrusiveness. The relationship between maternal sensitivity and intrusiveness on the one hand and infants’ neural responses to emotional prosody on the other have not been examined before. Several hypotheses are possible. Infants of sensitive mothers probably experience high degrees of IDS (as an element of positive mother–infant interaction). This may facilitate infants’ processing of (emotional) prosody, enhancing neural responses, reflected in more positive P2 amplitudes. As infants of sensitive mothers are likely exposed more often to emotionally positive than negative communication, effects might be stronger for positive than negative prosody. Infants of more intrusive mothers, by contrast, experience higher levels of negative, controlling, and demanding parental behavior (see, e.g., Biringen & Robinson, 1991; Egeland et al., 1993) that may include negative speech (Rosenblum et al., 2006). Although positive mother–infant interactions (including emotionally positive speech) also occur in these dyads, infants of more intrusive mothers are likely exposed to higher levels of emotionally negative IDS than infants of less intrusive mothers. This may enhance infants’ differentiation between or processing preferences for positive versus negative emotional prosody, in which case maternal intrusiveness is expected to relate to larger differences in infants’ P2 amplitudes to positive versus negative prosody. Alternatively, if exposure to a particular type of prosody simply enhances its processing, or if the relative novelty of negative prosody for infants of less intrusive mothers plays a role, maternal intrusiveness is expected to relate to smaller differences in infants’ P2 responses to positive versus negative prosody. In sum, we expect both maternal sensitivity and intrusiveness to be related to infants’ ERP responses to IDS. We specifically expect maternal sensitivity to relate to increased responses (i.e., more positive P2 amplitudes) to positive prosody and expect maternal intrusiveness to relate to differential responding to positive and negative prosody (i.e., the difference in P2 amplitudes in response to positive and negative prosody).
Importantly though, not all infants may be equally sensitive to their mothers’ parenting behaviors. Thus, maternal interactive behavior may affect infants’ neural processing of emotional prosody (as reflected in their ERPs) more for some infants than for others. Interest in individual variation in the way infants and children respond to external influence is growing rapidly. Over the last years, the emphasis has shifted from a model of environmental risk and resilience (i.e., a focus on individual differences in susceptibility to negative environments) to a model of differential susceptibility (i.e., individual differences in susceptibility to both positive and negative environmental influence). Differential susceptibility theory posits that, due to their genetic make‐up, some individuals are more affected by their (social) environment than others (Belsky, Bakermans‐Kranenburg, & Van IJzendoorn, 2007; Ellis, Boyce, Belsky, Bakermans‐Kranenburg, & Van IJzendoorn, 2011). Indeed, results of a variety of studies are consistent with this idea, and dopaminergic and serotonergic genes that may convey differential susceptibility have been identified (see Bakermans‐Kranenburg & Van IJzendoorn, 2015; Van IJzendoorn, Belsky, & Bakermans‐Kranenburg, 2012 for meta‐analytic evidence). However, the analysis of genetic material is time‐consuming and expensive and surrounded by ethical concerns. In addition, susceptibility may not be conveyed by a single gene, but rather by many, and obtaining an endophenotype for susceptibility would therefore be worthwhile.
With such concerns in mind, various studies have focused on temperamental characteristics (e.g., Gilissen, Bakermans‐Kranenburg, Van IJzendoorn, & Van der Veer, 2008; Groeneveld, Vermeer, & Linting, 2010; Pluess & Belsky, 2009). A recent meta‐analysis suggests that in infancy negative emotionality may be a marker for susceptibility, with infants higher in negative emotionality being more susceptible to environmental influences (Slagt, Semon Dubas, Decović, & Van Aken, 2016). Infants’ emotional tendencies may have a neural basis in resting asymmetric frontal brain activity (i.e., the difference in activity of the left and right frontal cortex). Asymmetric activity of frontal brain regions has been linked to emotional valence as well as to approach‐withdrawal motivation. Greater activity of left compared to right frontal areas is associated with a tendency for positive emotionality and approach, whereas greater activity of the right than the left frontal cortex is associated with a tendency for negative emotionality and behavioral withdrawal (see Harmon‐Jones, Gable, & Peterson, 2010 for a review). The association of right frontal asymmetry with negative emotionality and withdrawal as well as the role of right frontal asymmetry in differential reactivity to an experimental intervention (childhood parenting experiences and administered oxytocin affected prosocial behavior only in those showing right frontal asymmetry; Huffmeijer, Alink, Tops, Bakermans‐Kranenburg, & Van IJzendoorn, 2012) suggests that right frontal asymmetry may play a role in differential susceptibility.
Frontal asymmetry can be measured easily and non‐invasively using EEG. Differences in power within the EEG alpha band (8–12 Hz in adults, 6–9 Hz in infants [Marshall, Bar‐Haim, & Fox, 2002]) over the right and left frontal cortex are widely used to quantify asymmetric frontal brain activity in adults, children, and infants (e.g., Coan & Allen, 2004; Davidson & Fox, 1989; Fox, Henderson, Rubin, Calkins, & Schmidt, 2001). Greater alpha power is related to deactivation of the underlying cortical tissue (Cook, O’Hara, Uijtdehaage, Mandelkern, & Leuchtner, 1998; Laufs et al., 2003). Therefore, greater alpha power over left compared to right frontal areas reflects greater activity of the right compared to left frontal cortex. Conversely, greater alpha power over right than left frontal areas reflects relatively greater activity of the left frontal cortex. Although research investigating the neural origins of asymmetric electrocortical activity is scarce, the available evidence suggests that EEG measures of frontal asymmetry reflect activity in dorsolateral prefrontal cortex (Pizzagalli, Sherwood, Henriques, & Davidson, 2005; see also Davidson, 2004), involved in the processing of reward‐related information for goal‐directed behavior (Kobayashi, Lauwereyns, Koizumi, Sakagami, & Hikosaka, 2002; Wallis & Miller, 2003) and supporting the integration of cognitive and emotional material (Gray, Braver, & Raichle, 2002; Herrington et al., 2005).
The main aim of the current study is to investigate effects of maternal sensitivity and intrusiveness on infants’ ERP responses to emotional prosody. We expect maternal sensitivity to be associated with increased P2 responses to positive prosody in particular and maternal intrusiveness to relate to differential responding (i.e., differences in P2 amplitudes) to positive and negative prosody. The secondary aim is to explore whether infant frontal asymmetry conveys differential susceptibility by studying its moderating role in the association between maternal interactive behavior and infants’ P2 responses to emotional prosody in IDS. Because social‐cognitive capacities may be partially heritable (e.g., Anokhin, Golosheykin, & Heath, 2010; Scourfield, Martin, Lewis, & McGuffin, 1999), we take mothers’ ability for emotion recognition into account in all analyses.
2. METHOD
2.1. Participants
A total of 33 infants and their mothers participated in two experimental sessions. Another 10 mother–infant pairs participated in the first session only (as infants neural activity was recorded during the second session, these 10 pairs are not included in the current analyses). Participation was rewarded with a diploma for the infant. Complete datasets were available for 22 of the 33 mother–infant pairs (one infant's EEG was not recorded due to technical error, for one infant data collection was stopped within 1 min because the infant did not tolerate the cap, ERPs were unavailable for three infants because of a poor connection of the mastoid electrodes, two infants provided insufficient artifact‐free baseline and ERP data, three babies provided insufficient artifact‐free ERP data only, and one baby provided insufficient artifact‐free baseline data only). All 22 infants (11 boys; 14 first‐borns) were born term (37–42 weeks), and none had a known history of hearing problems, ear infections, or a family history of hearing and language problems. Ten infants were exposed only to French, whereas 12 were exposed to both French and other languages (including Bulgarian, Chinese, English, Greek, Kabyle, Spanish, Vietnamese, and Wolof). Thirteen were regularly exposed to some form of non‐parental care (daycare center or babysitter). Infants were approximately 6 months old (24–28 weeks, M = 25.95, SD = 1.21) at the time the first session took place. The second session took place 3–9 weeks later (M = 4.73, SD = 1.28; infant age: 28–36 weeks, M = 30.82, SD = 1.79). Mothers were between 26 and 43 years old (M = 34, SD = 4.6). The study was conducted according to the guidelines laid down in the Declaration of Helsinki (Tokyo, 2004). Written informed consent was obtained from a parent or guardian for each child before any assessment or data collection. All procedures involving human subjects in this study were approved by the “Comité d'éthique de la recherche en santé” (CERES) of Paris Descartes University.
2.2. Procedure
Informed consent was obtained from parents at the start of the first session. The first session took place at the Laboratoire Psychologie de la Perception (Paris Descartes University & CNRS) and consisted of a 15‐min observation of mother–infant interaction (see details below), after which mothers completed the Reading the Mind in the Eyes Test (RMET). The second session took place at the Robert Debré Hospital (Paris, France). During the second session, babies’ brain activity was measured using both EEG and NIRS (see details below). The NIRS data will be reported elsewhere. Brain activity was recorded first during a 1‐ to 3‐min baseline period and then while babies listened to directives pronounced with a happy or an angry prosody by a female adult. Throughout the recording session, infants were seated on their mother's lap and watched an experimenter silently building towers with foam blocks in a standardized sequence to maintain the infant's attention and avoid excessive movement. After the second session, infants received a diploma for participation.
2.3. Observations
Mothers and infants were videotaped while they played together for 15 min in a small room. A play‐mat was placed within the room and several toys were laid out on it. Mother–infant pairs could position themselves as they wished. This is essential to the observation, as the way mothers regulate their position and distance to the infant and when and how mothers touch and pick up their infant is informative with respect to sensitivity and intrusiveness. Mothers were instructed to play with their infant as they normally would. After 8 min of free play, mothers were given a short questionnaire (containing questions about the infant's development and language environment) to complete while the observation of mother–infant interaction continued. The purpose of this experimental intervention was to observe whether and, if yes, how an interfering task changes maternal sensitivity and intrusion. This second part of the observation is less suited to our current purposes, so we used only the first 8 min, that is, free play, in our current analyses. Results from the second part of the observation session will be analyzed and reported elsewhere. The observation sessions were videotaped. The recordings were coded offline for sensitivity and intrusiveness by an expert coder (MJBK) using the Ainsworth scales (Ainsworth, Bell, & Stayton, 1974; 9‐point scales). Higher scores reflect more sensitivity and more intrusiveness, respectively.
2.4. Reading the mind in the eyes test
As an independent measure of mothers’ ability to recognize emotions, mothers completed a computerized version of a French translation (Prevost et al., 2013) of the RMET. The RMET measures the ability to infer others’ emotional mental state, a capacity central to both theory of mind and empathy (Baron‐Cohen, Wheelwright, Hill, Raste, & Plumb, 2001). The French RMET consisted of 36 items, each showing a gray‐scale photograph of the eye region of a face (presented in the center of the computer screen) surrounded by four emotion words. Participants’ task was to select the emotion that best described what the person in the photograph felt by clicking on the corresponding word with the mouse. For each participant, we computed the percentage of correct responses. Items 13 and 19 were excluded, because they were answered incorrectly by more than 75% of all 43 participants who completed the RMET (item 13:84% incorrect, item 19:79% incorrect; all other items ≥49% correct).
2.5. EEG
Infants’ EEG was recorded from 12 electrodes (located at F3, Fz, F4, C3, C4, P3, Pz, P4, O1, O2, left mastoid, and right mastoid) with a Cz reference and a ground placed at FPz. The EEG was filtered with a 0.01–100 Hz passband range and digitized at 500 Hz using a Brain Vision ActiChamp amplifier and PyCorder software (Brain Products GmbH). Impedances were kept below 50 kΩ as much as possible. Further processing of the EEG was done offline using Brain Vision Analyzer 2 (Brain Products GmbH). Infants sat on their mothers’ lap during the entire recording. Mothers were instructed to try and keep the baby's hands away from the wires, but otherwise not to interfere or interact with the infant and to remain silent throughout the recording procedure.
2.5.1. Task and stimuli
Infants’ EEG was recorded during a 1‐ to 3‐min (M = 127 s) baseline period and then while they listened to sounds. During the baseline period, the experimenter silently built different types of towers with foam blocks (e.g., stacking all the blocks in a one‐block‐wide tower, building a pyramid, making a two‐block wide tower, stacking the blocks into two towers slightly apart from each other) in a standardized sequence to maintain the infant's attention and avoid excessive movement. There was no auditory stimulation during the baseline.
After the baseline period, infants were presented with a maximum of 16 blocks of utterances spoken with a happy or an angry prosody. The utterances were 24 simple directives (e.g., Viens [Come], Regarde [Look], Aide‐moi [Help me]) that were rated as semantically neutral, and hence potentially compatible with both an angry and a happy prosody. To rate the inherent valence of the utterances, seven adult French native speakers were presented with a written list of 46 simple directives and rated each utterance on a 7‐point scale ranging from “very negative” to “very positive.” We selected only those utterances that had an average rating larger than 3 and smaller than 5, and for which the extreme scores of 1 and 7 were not selected by any rater. A female French native speaker then recorded each of the 24 utterances three times with an angry prosody and three times with a happy prosody. Sound files for each utterance were edited using Audacity 2.1.0 software. A hard limiter (−10 dB) was applied to approximately equalize the volume of all sounds, and a linear fade over the first 100 ms (0 to −10 dB) of the sound was applied to approximately match onset envelopes. We restricted the editing to these two operations to avoid interfering with the emotional prosody. Finally, for each of the 24 utterances a happy and an angry version that best matched each other in duration were selected for inclusion in the experiment. The mean duration of the selected utterances was 768 ms (SD = 145), with no difference between happy and angry utterances (angry: 459–994 ms, M = 757, SD = 147; happy: 477–992 ms, M = 780, SD = 146; t(23) = −1.36, p = .19).
Utterances were presented through speakers located behind the infant, in blocks of eight utterances of the same category (happy or angry), for a maximum of 16 blocks (eight per condition for a total of 128 utterances). Recording was stopped when the baby became too fussy or the mother wished to discontinue the experiment. Blocks were presented in quasi‐random order, with the restriction that the same condition (happy or angry) could not be repeated more than once. There was a silent period lasting 20.0, 22.5 or 25.0 s (duration selected randomly) after each block of utterances to facilitate NIRS recordings (data presented elsewhere).
Sound volume was kept identical across infants. Within a block, utterances were presented at an average rate of one per two seconds (trial duration 1,925–2,075 ms, varying randomly). Thus, depending on the length of the utterance and the randomly selected trial duration, the inter‐stimulus interval could vary between 931 and 1,616 ms. The utterances presented within each block were selected randomly without replacement from the full list of 24 utterances. The same utterance could not be repeated within the same block.
The experimenter continued to silently build towers of foam blocks in a standardized sequence throughout the experiment to maintain the infant's attention and avoid excessive movement. The experimenter avoided eye contact with both baby and mother throughout the experiment.
2.5.2. Data processing: Baseline frontal asymmetry
EEG data collected during the baseline period were bandpass filtered between 0.3 and 30 Hz (highpass: −3 dB, 12 dB/octave; lowpass: −3 dB, 48 dB/octave) and segmented into 1 s segments with 0.5 s overlap. Baseline data obtained from the electrodes placed at the mastoids were discarded (the mastoid electrodes were included to function as a reference in ERP computation only and have no function in the measurement of frontal asymmetry). Next, following standard practice in EEG/ERP research, segments and channels affected by artifacts were rejected (see, e.g., Luck, 2015). An automatically aided artifact rejection was performed: segments were automatically rejected if the difference between the minimum and maximum voltage in the segment exceeded 200 µV for at least three channels (if such an artifact only occurred on one or two channels, the individual channels were removed from the segment), and additional segments were rejected if visual inspection revealed an artifact that was not detected by the automatic algorithm (this was sometimes the case with small eye movements or blinks that could be seen, especially on the frontal channels). A short‐term Fourier transform (1.00 Hz resolution, 100% Hanning window) was computed to obtain power values (μV2) for the remaining segments (see below for the number of segments included in the analysis). Power values were averaged across all segments and then averaged across the frequency range of 6–9 Hz to obtain measures of power within the alpha band (the 6–9 Hz range is considered the infant equivalent of the 8–12 Hz adult alpha band; Marshall et al., 2002). To normalize data distribution, the natural logarithm (ln) of these values was computed. As a measure of frontal asymmetry, values obtained at F3 (i.e., alpha activity over the left frontal cortex) were subtracted from values obtained at F4 (i.e., alpha activity over the right frontal cortex): ln(F4)–ln(F3). A zero value on this measure thus represents no frontal alpha asymmetry, whereas more negative values result from greater alpha power over left compared to right frontal cortex and therefore represent greater relative right frontal cortical activity. Similarly, more positive values represent greater relative left frontal cortical activity.
An individual infant's data were considered adequate for inclusion in the analyses if two criteria were met: (a) The infant provided at least 59 artifact‐free segments (corresponding to 30 s; see, e.g.,Brooker, Canen, Davidson, & Goldsmith, 2017; Howarth, Fettig, Curby, & Bell, 2016 for similar criteria), and (b) at least 30% of the total number of segments were artifact‐free. The 22 infants that constitute the final sample provided on average 203 artifact‐free segments (SD = 63, range: 76–302).
2.5.3. Data processing: ERPs
EEG data collected while the infants listened to the utterances were bandpass filtered between 0.3 and 30 Hz (highpass: −3 dB, 12 dB/octave; lowpass: −3 dB, 48 dB/octave) and rereferenced to the average activity at left and right mastoids. Segments extending from 200 ms before to 600 ms after the onset of each utterance were extracted. Then, following standard practice in ERP research to discard data contaminated by artifacts (see, e.g., Luck, 2015), an automatically aided artifact rejection was performed as follows: Segments were automatically rejected if the difference between the minimum and maximum voltage in the segment exceeded 160 µV for at least three channels (if such an artifact only occurred on one or two channels, the individual channels were removed from the segment). Additional segments were rejected if visual inspection revealed an artifact that was not detected by the automatic algorithm (this was sometimes the case with small eye movements or blinks that could be seen, especially on the frontal channels). The remaining segments were averaged per condition, and a 200 ms pre‐stimulus baseline was subtracted from all data points.
An individual infant's data were considered adequate for inclusion in the analyses if three criteria were met: (a) Data were collected from at least three blocks of utterances per condition, (b) the infant provided at least 10 artifact‐free segments per condition (see, e.g., Peltola, Leppänen, Mäki, & Hietanen, 2009 for use of the same criterion), and (c) at least 25% of the total number of segments were artifact‐free. The 22 infants that constitute the final sample provided on average 49 artifact‐free segments (SD = 17; angry: 10–47, M = 24, SD = 9; happy: 10–45, M = 25, SD = 9).
The grandaverage ERPs were characterized by a positive wave starting shortly after 100 ms and lasting throughout the ERP, that appeared larger over frontal and central compared to parietal areas and was absent at occipital electrode sites (see Figure 1). Similar fronto‐central positivities have been observed in infants’ ERPs to auditory stimuli in previous studies and are typically termed P2 (Kraus et al., 1993; Novak et al., 1989; see also Coch & Gullick, 2012). We initially quantified this P2 as the mean amplitude in the 200‐600 ms post‐stimulus window at each frontal, central and parietal electrode site separately (F3, Fz, F4, C3, Cz, C4, P3, Pz, and P4). A preliminary repeated‐measures ANOVA with anteriority (frontal vs. central vs. posterior) and laterality (left vs. center vs. right) as independent variables and the P2 amplitude averaged across happy and angry conditions as dependent variable revealed an effect of anteriority, F(1.47, 26.40) = 53.02, p < .01, = 0.74 (Greenhouse–Geisser corrected because of a sphericity violation). Post hoc comparisons with LSD correction for multiple comparisons confirmed that amplitudes were larger at frontal (M = 9.54, SE = 1.51) and central (M = 8.84, SE = 1.40) compared to parietal (M = 3.09, SE = 1.19) sites (both ps < .01; no significant difference between frontal and central sites, p = .18). There was no significant effect of laterality (F[2, 36] = 2.53, p = .09) and no significant anteriority*laterality interaction (F[4, 72] = 0.67, p = .61). We therefore computed the averaged P2 amplitude across frontal and central sites (i.e., F3, Fz, F4, C3, Cz, and C4) in response to happy and angry sounds for use in our final analyses.
Figure 1.
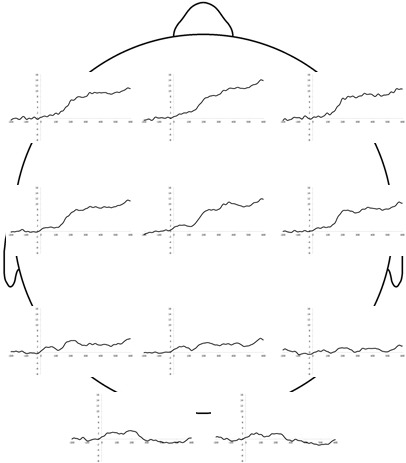
Grandaveraged event‐related potentials (ERPs), averaged across prosody types (happy and angry). Infants’ ERPs to utterances pronounced with a happy or angry prosody were categorized by a large fronto‐central positivity (P2)
2.6. Analyses
Statistical analyses were performed using IBM SPSS 23 software. In a first set of preliminary analyses, we tested the correlations between all maternal variables (sensitivity, intrusiveness, and RMET performance), as well as between maternal sensitivity and intrusiveness on the one hand and infants’ frontal asymmetry on the other.
We tested the main hypotheses in a second set of analyses. Specifically, a repeated‐measures ANCOVA with P2 amplitude as the dependent variable, condition (happy vs. angry) as a categorical independent variable, maternal sensitivity and maternal intrusiveness as continuous independent variables, and maternal RMET scores as a covariate was used to test whether maternal behavior was associated with infants’ ERP responses to happy and angry utterances. To test whether infants’ frontal asymmetry moderated any associations between ERP amplitudes and maternal variables, a second repeated‐measures ANCOVA was performed with infant frontal asymmetry as an additional continuous independent variable. The analysis included interaction terms between frontal asymmetry and maternal interactive behavior (i.e., sensitivity and intrusiveness). A significant interaction with frontal asymmetry would be evidence for moderation and potentially differential susceptibility.
3. RESULTS
3.1. Preliminary analyses
All variables (maternal sensitivity, maternal intrusiveness, maternal RMET scores, infant frontal asymmetry, infants’ P2 amplitudes to happy sounds, infants’ P2 amplitudes to angry sounds) were approximately normally distributed (standardized |skewness| < 3 for all variables, standardized |kurtosis| < 3 for all variables except P2 amplitude to happy sounds [standardized kurtosis = 3.31]) without outliers (i.e., all |z| < 3.29). Mothers’ sensitivity and intrusiveness varied across almost the entire range of the scale (Sensitivity: 2.0–9.0, M = 4.88, SD = 2.14; Intrusiveness: 1.5–8.0, M = 5.30, SD = 2.07). On average, mothers answered 75% of RMET items correctly (53%–97%, SD = 11%). Maternal sensitivity was significantly correlated with intrusiveness (r = −.57, p < .01) and with the RMET score (r = .44, p < .05), but the percentage of shared variance was low enough (r2 = .32 and r2 = .19 respectively) to include all variables in the same analysis. Maternal intrusiveness and the RMET score were not significantly related (r = −.20, p > .10). Importantly, maternal sensitivity, intrusiveness, and RMET scores were not significantly related to infant's frontal asymmetry (all |r|s ≤ .37, ps > .05). Descriptive statistics for ERP variables and frontal asymmetry are presented in Table 1.
Table 1.
Descriptive statistics of event‐related potential (ERP) variables and infant frontal asymmetry
| Mean | SD | |
|---|---|---|
| Frontal asymmetry | 0.09 | 0.13 |
| P2 amplitude happy | 9.72 | 7.78 |
| P2 amplitude angry | 8.57 | 6.75 |
3.2. ERPs: associations with maternal variables
The repeated‐measures ANCOVA with condition (happy vs. angry), and maternal sensitivity, intrusiveness, and RMET performance as independent variables revealed a significant interaction effect of condition and maternal intrusiveness on infants’ P2 amplitudes, F(1, 18) = 5.23, p = .04, = 0.23. As can be seen in Figure 2, maternal intrusiveness related to the difference in infants’ P2 amplitudes in response to happy and angry utterances (happy—angry) in such a way that ERP amplitudes in response to angry utterances were increasingly smaller than those in response to happy utterances for infants with less intrusive mothers. There were no significant main effects of condition, maternal sensitivity, maternal intrusiveness, and maternal RMET performance, or interactions between condition and maternal sensitivity and RMET performance (all Fs ≤ 2.46, ps > .10; see Table 2 for an overview of all statistics).
Figure 2.
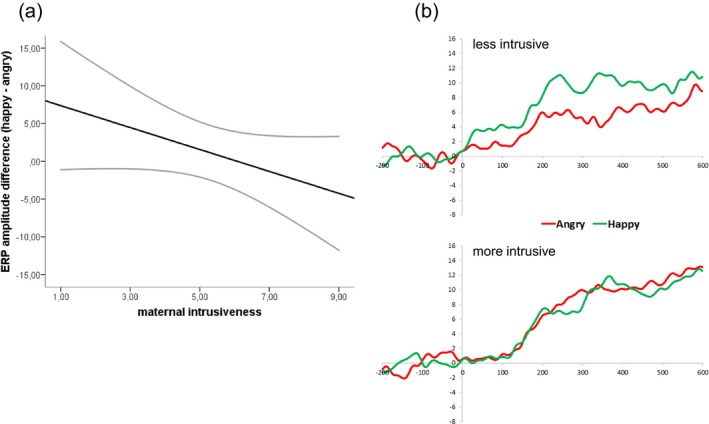
Illustration of the relation between maternal intrusiveness and infants’ event‐related potential (ERP) responses to utterances pronounced with a happy and angry prosody. ERP amplitudes within the 200–600 ms post‐stimulus interval (P2) in response to happy utterances become increasingly more positive than those in response to angry utterances for infants with less intrusive mothers. (a) Regression line illustrating the relation between maternal intrusiveness and infants’ ERP amplitudes in response to utterances pronounced with a happy versus angry prosody (happy–angry). (b) Grandaveraged ERPs, averaged across frontal and central electrode locations for infants of less intrusive (top panel) and more intrusive mothers (bottom panel). Groups were created based on a median split for displaying purposes only
Table 2.
Outcomes of the repeated‐measures ANCOVA examining effects of condition (happy vs. angry), maternal sensitivity, intrusiveness, and RMET performance on infants’ P2 amplitudes, revealing a significant interaction effect of condition and intrusiveness on P2 amplitude
| F(1, 18) | p |
|
||
|---|---|---|---|---|
| Condition | 1.09 | .31 | 0.06 | |
| Sensitivity | 0.00 | .98 | 0.00 | |
| Intrusiveness | 0.14 | .71 | 0.01 | |
| RMET performance | 0.14 | .71 | 0.01 | |
| Condition*Sensitivity | 2.46 | .13 | 0.12 | |
| Condition*Intrusiveness | 5.23 | .04 | 0.23 | |
| Condition*RMET performance | 0.28 | .60 | 0.02 |
To test whether effects of maternal interactive behavior on infants’ ERPs were moderated by infants’ frontal asymmetry, we added infants’ frontal asymmetry and the interactions between frontal asymmetry and maternal intrusiveness and between frontal asymmetry and maternal sensitivity as independent variables to the ANCOVA (all independent variables: condition [happy vs. angry], maternal sensitivity, maternal intrusiveness, maternal RMET performance, infant frontal asymmetry, infant frontal asymmetry*maternal intrusiveness, infant frontal asymmetry*maternal sensitivity). No evidence for moderation was obtained, as the interaction between condition, frontal asymmetry and maternal intrusiveness was not significant, F(1, 15) = 0.24, p = .63, with a negligible effect size, = 0.02. The two‐way interactions between frontal asymmetry and maternal intrusiveness (F[1, 15] = 0.29, p = .60, = 0.02) and between frontal asymmetry and sensitivity (F[1, 15] = 0.04, p = .84, = 0.00) were not significant either, and neither was the three‐way interaction between condition, frontal asymmetry and sensitivity (F[1, 15] = 0.68, p = .42, = 0.04).
4. DISCUSSION
The current study investigated whether maternal interactive behavior, specifically maternal sensitivity and intrusiveness in mother–infant interaction, is related to infants’ neural responses to positive and negative prosody in IDS. As was expected, there was a relation between maternal behavior and infants’ ERP responses to emotional prosody. Specifically, intrusiveness predicted differential responses to happy and angry prosody, with infants of less intrusive mothers showing less positive P2 amplitudes to angry than to happy utterances. Such differentiation in P2 amplitudes may be indicative of infants’ capacity to distinguish between different types of prosody or, alternatively, may reflect an attentional or processing preference for happy compared to angry prosody. Preferences may also potentially be related to the relative novelty of angry speech, and negative, demanding interaction in general, for infants of less intrusive mothers (see, e.g., Biringen & Robinson, 1991; Egeland et al., 1993). Unfortunately, it is not possible to distinguish between these alternative explanations using the current data. Future research employing other (additional) methods will be necessary to shed light on this issue. In particular, researchers may attempt to measure infants’ preferences at the behavioral level or monitor infants’ emotional responses to emotionally positive and negative communication. Nevertheless, the current study constitutes an important contribution to existing literature about infants’ social‐cognitive development. Research focusing on the development of emotional speech perception in infancy is scarce, in general, and usually focused on typical development rather than individual differences. Studies investigating how variations in parenting behaviors contribute to infant and child attachment and social development more generally have traditionally favored behavioral measures, although interest in the underlying neuro‐cognitive processes is growing rapidly. The current study contributes to bridging this gap by showing, for the first time, that maternal intrusiveness in mother–infant play predicts infants’ neural differentiation of happy and angry speech.
Maternal sensitivity, on the contrary, was not significantly related to infants’ ERPs. Sensitivity in play situations includes processes such as support for the infant's exploration, following the infant's lead in play, and joint attention, which assist the infant's behavioral organization and regulation (Bigelow et al., 2010; Power, 1985; Spanglar, Schieche, Ilg, Maier, & Ackermann, 1994). Perhaps insensitivity as observed in the current play setting, for example showing a lack of support, a lack of interest in the infant, or simply very little interaction with the infant, does not capture the kind of socio‐emotional behavior that would interfere with the developing capacity to process the emotional aspects of speech and/or the establishment of processing biases. Future studies could focus on elucidating which specific parental behaviors constituting the broad dimensions of sensitivity and intrusiveness are associated with infants’ processing of the emotional aspects of speech (prosody) as well as other types of social and emotional information.
Although maternal interactive behavior was associated with infants’ neural processing of prosody, we did not obtain evidence for differential susceptibility to such effects, that is, no significant moderation of effects of intrusiveness or sensitivity by infants’ frontal asymmetry. The negligible effect sizes might suggest a true absence of effects rather than a failure to detect effects of moderate size due to the relatively small sample size and associated limited power. However, several caveats deserve attention before concluding that frontal asymmetry does not convey differential susceptibility. First of all, it may be possible that consequences of differential susceptibility are not visible yet at the age of the current sample (infants were only about 7 months at the time of the ERP assessment), at least in the domain of affective speech processing which is still developing at this age (Grossmann et al., 2010). It may take more time for differences to become observable. Longitudinal studies, assessing the relationships between maternal behavior, infant frontal asymmetry, and infants’ processing of affective speech and/or other socio‐emotional stimuli at multiple points in time may shed light on this issue.
Second, challenges related to frontal asymmetry measurement in infants may also play a role. We argued that frontal asymmetry measured in the absence of external stimulation may serve as a differential susceptibility marker. Emotion‐eliciting stimuli are well known to modulate frontal asymmetry (see, e.g., Coan & Allen, 2004; Davidson, 2004; Harmon‐Jones et al., 2010). It is difficult, however, to obtain a truly emotionally neutral resting baseline assessment from infants. Infants are unfamiliar with the experimental situation and researcher, which may elicit emotions, such as fear, associated with withdrawal and right frontal asymmetry, because it is impossible to explain the situation to the infant. Also, the mother is usually present, and it is necessary to provide some form of engaging, positive stimulation (associated with approach) to maintain infants’ attention and avoid excessive movement. Besides the conditions under which frontal asymmetry is measured, the quantification of infant frontal asymmetry itself warrants attention. Although reasonable arguments have been provided for the equivalence of the 6–9 Hz frequency band in infants to adult alpha (Marshall et al., 2002), comparatively little is known about infants’ and children's frontal asymmetry. For example, previous studies have found power in the 8–12 Hz alpha band in adults to be related to deactivation of cortical tissue (Cook et al., 1998; Laufs et al., 2003), but to the best of our knowledge, no comparable evidence is available for infants. Also, the frequency composition of the EEG changes over the course of development. However, whether and how such developmental change affects frontal asymmetry remains unclear (Saby & Marshall, 2012). Studies investigating the stability of frontal asymmetry throughout childhood have observed limited stability (Howarth, et al., 2016; Vuga, Fox, Cohn, Kovacs, & George, 2008), suggesting that developmental changes take place. Finally, with respect to the relation between frontal asymmetry and approach‐withdrawal motivation and behavior, expected associations between frontal asymmetry and its theorized behavioral antecedents and consequences have not always been found (see, e.g., Peltola et al., 2014).
The latter two points are especially relevant with respect to differential susceptibility, as differences in susceptibility are believed to be caused by stable (ultimately genetically determined) characteristics (e.g., Belsky et al.., 2007; Ellis et al.., 2011) and hypotheses regarding frontal asymmetry as a susceptibility factor rely in part on the association with withdrawal‐related tendencies. Further research focusing on frontal asymmetry, including potential consequences of the development of the EEG frequency composition for frontal asymmetry and/or its quantification, is thus badly needed. Finally, although there is some evidence for genetic influences on frontal asymmetry (Anokhin, Heath, & Meyers, 2006; Bismark et al., 2010; Gao, Tuvblad, Raine, Lozano, & Baker, 2009; Gatt et al., 2010), studies of frontal asymmetry as a susceptibility factor, whether in infants, older children or adults, should also directly address its relation with genetic profiles and temperamental characteristics associated with differential susceptibility.
Future studies may also take other limitations of the current study into account. Although 33 mother–infant pairs participated in the current study and the attrition rate of 30% is not uncommon for infant ERP research (see, e.g., Stets, Stahl, & Reid, 2012), the final sample was rather small, providing limited power especially when exploring the combined effects of both maternal interactive behavior and frontal asymmetry. Replication of our findings among larger samples will thus be necessary. Furthermore, the limited editing of sound files for the current study caused a less than perfect match between happy and angry utterances on characteristics such as pitch, as well as some variation between utterances in the onset of sound waves, which may have led to some temporal smearing of the early part of the ERP (rendering the examination of early effects of prosody impossible). Although limiting the amount of editing was necessary to maximize differences between happy and angry speech, future studies may find ways to reduce this variation. In addition, although happy and angry utterances clearly differed in prosody and all researchers (as well as the native speaker who recorded the utterances) agreed on the emotion displayed, the utterances were not rated for emotion by independent raters. Thus, subtle differences in, for example, emotional intensity between happy and angry utterances may have gone unnoticed, and obtaining independent judgments of the emotional prosody is advisable for future studies.
In conclusion, maternal interactive behavior was associated with 7‐month‐old infants’ processing of happy and angry prosody in IDS. Specifically, infants of less intrusive mothers showed larger differences in their P2 responses to happy versus angry utterances. Whether increased differentiation reflects an enhanced capacity to distinguish between happy and angry speech or a processing preference remains to be investigated. Regardless of the need for further study, our findings make an important contribution to emerging knowledge about the role of parenting in infants’ social‐cognitive development and highlight the usefulness of ERP measures for investigating individual differences in infancy. The current study provided no evidence for differential susceptibility conveyed by infants’ frontal asymmetry. However, studies validating measures of infant frontal asymmetry are needed before definite conclusions can be drawn. Longitudinal studies investigating the combined effects of maternal behavior and infant frontal asymmetry as well as other potential susceptibility factors on infants’ processing of socio‐emotional stimuli at multiple points in time will be needed to conclusively examine whether, when, and why infants vary in their sensitivity to effects of maternal behavior on their developing socio‐emotional information processing.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest with regard to the funding source for this study.
ACKNOWLEDGMENTS
RH was supported by a Marie Curie Intra‐European Fellowship, Grant number 626598. MJBK was supported by research awards from the Netherlands Organization for Scientific Research (VICI grant no. 453‐09‐003) and the European Research Council (ERC AdG 669249), and JG acknowledges the support of the LABEX EFL (ANR‐10‐LABX‐0083), a Fyssen Foundation Startup Grant, an Emergence(s) Programme Grant from the City of Paris, a Human Frontiers Science Program Young Investigator Grant (RGY‐0073‐2014), and an ANR grant (nr. ANR‐15‐CE37‐0009‐01).
Huffmeijer R, Bakermans‐Kranenburg MJ, Gervain J. Maternal intrusiveness predicts infants’ event‐related potential responses to angry and happy prosody independent of infant frontal asymmetry. Infancy. 2020;25:246–263. 10.1111/infa.12327
Footnotes
Both criteria needed to be met. Note that the duration of the baseline could vary from 1 to 3 min, corresponding to 119–359 segments. Thus, it is possible for an individual baby to provide at least 59 artifact‐free trials without meeting the 30% criterion (e.g., 59 artifact‐free segments out of a total of 359 amount to only 16%). Similarly, it is possible for 30% of segments from an individual baby to be artifact‐free without meeting the 59 segments criterion (e.g., if 30% of a total of 119 segments are artifact‐free, that amounts to only 36 segments).
REFERENCES
- Ainsworth, M. D. S. , Bell, S. M. , & Stayton, D. J. (1974). Infant mother attachment and social development: Socialization as a product of reciprocal responsiveness to signals In Richards M. P. M. (Ed.), The integration of a child into the social world (pp. 99–135). Cambridge, UK: Cambridge University Press. [Google Scholar]
- Anokhin, A. P. , Golosheykin, S. , & Heath, A. C. (2010). Heritability of individual differences in cortical processing of facial affect. Behavioral Genetics, 40, 178–185. 10.1007/s10519-010-9337-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anokhin, A. P. , Heath, A. C. , & Myers, E. (2006). Genetic and environmental influences on frontal EEG asymmetry: A twin study. Biological Psychology, 71(3), 289–295. 10.1016/j.biopsycho.2005.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans‐Kranenburg, M. J. , & Van IJzendoorn, M. H. (2015). The hidden efficacy of interventions: Gene × environment experiments from a differential susceptibility perspective. Annual Review of Psychology, 66, 11.1–11.29. 10.1146/annurev-psych-010814-015407 [DOI] [PubMed] [Google Scholar]
- Bakermans‐Kranenburg, M. J. , van IJzendoorn, M. H. , & Juffer, F. (2003). Less is more: Meta‐analyses of sensitivity and attachment interventions in early childhood. Psychological Bulletin, 129(2), 195–215. 10.1037/0033-2909.129.2.195 [DOI] [PubMed] [Google Scholar]
- Baron‐Cohen, S. , Wheelwright, S. , Hill, J. , Raste, Y. , & Plumb, I. (2001). The “Reading the Mind in the Eyes” test revised version: A study with normal adults, and adults with Asperger Syndrome or high‐functioning Autism. Journal of Child Psychology and Psychiatry, 42(2), 241–251. 10.1111/1469-7610.00715 [DOI] [PubMed] [Google Scholar]
- Belsky, J. , Bakermans‐Kranenburg, M. J. , & van IJzendoorn, M. H. (2007). For better and for worse: Differential susceptibility to environmental influences. Current Directions in Psychological Science, 16(6), 300–304. 10.1111/j.1467-8721.2007.00525.x [DOI] [Google Scholar]
- Belsky, J. , & Fearon, R. M. P. (2002). Early attachment security, subsequent maternal sensitivity, and later child development: Does continuity in development depend upon continuity of caregiving? Attachment and Human Development, 4(3), 361–387. 10.1080/14616730210167267 [DOI] [PubMed] [Google Scholar]
- Bigelow, A. E. , MacLean, K. , Proctor, J. , Myatt, T. , Gillis, R. , & Power, M. (2010). Maternal sensitivity throughout infancy: Continuity and relation to attachment security. Infant Behavior and Development, 33, 50–60. 10.1016/j.infbeh.2009.10.009 [DOI] [PubMed] [Google Scholar]
- Biringen, Z. , & Robinson, J. (1991). Emotional availability in mother‐child interactions: A reconceptualization for research. American Journal of Orthopsychiatry, 61(2), 258 10.1037/h0079238 [DOI] [PubMed] [Google Scholar]
- Bismark, A. W. , Moreno, F. A. , Stewart, J. L. , Towers, D. N. , Coan, J. A. , Oas, J. , … Allen, J. J. B. (2010). Polymorphisms of the HTR1a allele are linked to frontal brain electrical asymmetry. Biological Psychology, 83(2), 153–158. 10.1016/j.biopsycho.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowlby, J. (1969). Attachment and loss, Vol. 1. Attachment. Harmondsworth, UK: Penguin Books. [Google Scholar]
- Brooker, R. J. , Canen, M. J. , Davidson, R. J. , & Goldsmith, H. H. (2017). Short‐ and long‐term stability of alpha asymmetry in infants: Baseline and affective measures. Psychophysiology, 54(8), 1100–1109. 10.1111/psyp.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson, V. J. , & Harwood, R. L. (2003). Attachment, culture, and the caregiving system: The cultural patterning of everyday experiences among Anglo and Puerto Rican mother‐infant pairs. Infant Mental Health Journal, 24(1), 53–73. 10.1002/imhj.10043 [DOI] [Google Scholar]
- Coan, J. A. , & Allen, J. J. B. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67(1–2), 7–49. 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Coch, D. , & Gullick, M. (2012). Event‐related potentials and development In Luck S. J., & Kappenman E. S. (Eds.), The Oxford handbook of event‐related potential components (H17). (pp. 475–512). Oxford, UK: Oxford University Press. [Google Scholar]
- Cook, I. A. , O’Hara, R. , Uijtdehaage, S. H. J. , Mandelkern, M. , & Leuchtner, A. F. (1998). Assessing the accuracy of topographic EEG mapping for determining local brain function. Electroencephalography and Clinical Neurophysiology, 107, 408–414. 10.1016/S0013-4694(98)00092-3 [DOI] [PubMed] [Google Scholar]
- Cooper, R. P. , & Aslin, R. N. (1990). Preference for infant‐directed speech in the first months after birth. Child Development, 61(5), 1584–1595. 10.1111/j.1467-8624.1990.tb02885.x [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. (2004). What does the prefrontal cortex “do” in affect: Perspectives on frontal EEG asymmetry research. Biological Psychology, 67, 219–233. 10.1016/j.biopsycho.2004.03.008 [DOI] [PubMed] [Google Scholar]
- Davidson, R. J. , & Fox, N. A. (1989). Frontal brain asymmetry predicts infants response to maternal separation. Journal of Abnormal Psychology, 98(2), 127–131. 10.1037/0021-843x.98.2.127 [DOI] [PubMed] [Google Scholar]
- De Wolff, M. S. , & Van IJzendoorn, M. H. (1997). Sensitivity and attachment: A meta‐analysis on parental antecedents of infant attachment. Child Development, 68(4), 571–591. 10.2307/1132107 [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz, G. , & Spelke, E. S. (2015). The infancy of the human brain. Neuron, 88, 93–109. 10.1016/j.neuron.2015.09.026 [DOI] [PubMed] [Google Scholar]
- Egeland, B. , Pianta, R. , & O’Brien, M. A. (1993). Maternal intrusiveness in infancy and child maladaptation in early school years. Development and Psychopathology, 5, 359–370. 10.1017/S0954579400004466 [DOI] [Google Scholar]
- Ellis, B. J. , Boyce, W. T. , Belsky, J. , Bakermans‐Kranenburg, M. J. , & Van IJzendoorn, M. H. (2011). Differential susceptibility to the environment: An evolutionary‐neurodevelopmental theory. Development and Psychopathology, 23(1), 7–28. 10.1017/S0954579410000611 [DOI] [PubMed] [Google Scholar]
- Feldman, R. , Eidelman, A. I. , & Rotenberg, N. (2004). Parenting stress, infant emotion regulation, maternal sensitivity, and the cognitive development of triplets: A model for parent and child influences in a unique ecology. Child Development, 75(6), 1774–1791. 10.1111/j.1467-8624.2004.00816.x [DOI] [PubMed] [Google Scholar]
- Fernald, A. (1985). Four‐month‐old infants prefer to listen to motherese. Infant Behavior and Development, 8, 181–195. 10.1016/S0163-6383(85)80005-9 [DOI] [Google Scholar]
- Fernald, A. (1992). Meaningful melodies in mothers' speech to infants In Papoušek H., & Jürgens U. (Eds.), Nonverbal vocal communication: Comparative and developmental approaches (pp. 262–282). New York, NY: Cambridge University Press. [Google Scholar]
- Fernald, A. , Taeschner, T. , Dunn, J. , Papousek, M. , de Boysson‐Bardies, B. , & Fukui, I. (1989). A cross‐language study of prosodic modifications in mothers’ and fathers’ speech to preverbal infants. Journal of Child Language, 16(3), 477–501. 10.1017/s0305000900010679 [DOI] [PubMed] [Google Scholar]
- Fisher, C. , & Tokura, H. (1996). Acoustic cues to grammatical structure in infant‐directed speech: Cross‐linguistic evidence. Child Development, 67(6), 3192–3218. 10.1111/j.1467-8624.1996.tb01909.x [DOI] [PubMed] [Google Scholar]
- Fox, N. A. , Henderson, H. A. , Rubin, K. H. , Calkins, S. D. , & Schmidt, L. A. (2001). Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development, 72(1), 1–21. 10.1111/1467-8624.00262 [DOI] [PubMed] [Google Scholar]
- Gao, Y. , Tuvblad, C. , Raine, A. , Lozano, D. I. , & Baker, L. A. (2009). Genetic and environmental influences on frontal EEG asymmetry and alpha power in 9–10‐year‐old twins. Psychophysiology, 46(4), 787–796. 10.1111/j.1469-8986.2009.00815.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt, J. M. , Nemeroff, C. B. , Schofield, P. R. , Paul, R. H. , Clark, C. R. , Gordon, E. , & Williams, L. M. (2010). Early life stress combined with serotonin 3A receptor and brain‐derived neurotrophic factor valine 66 to methionine genotypes impacts emotional brain and arousal correlates of risk for depression. Biological Psychiatry, 68(9), 818–824. 10.1016/j.biopsych.2010.06.025 [DOI] [PubMed] [Google Scholar]
- Gilissen, R. , Bakermans‐Kranenburg, M. J. , van IJzendoorn, M. H. , & van der Veer, R. (2008). Parent‐child relationship, temperament, and physiological reactions to fear‐inducing film clips: Further evidence for differential susceptibility. Journal of Experimental Child Psychology, 99(3), 182–195. 10.1016/j.jecp.2007.06.004 [DOI] [PubMed] [Google Scholar]
- Gray, J. R. , Braver, T. S. , & Raichle, M. E. (2002). Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America, 99(6), 4115–4120. 10.1073/pnas.062381899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieser, D. L. , & Kuhl, P. K. (1988). Maternal speech to infants in a tonal language – Support for universal prosodic features in motherese. Developmental Psychology, 24(1), 14–20. 10.1037/0012-1649.24.1.14 [DOI] [Google Scholar]
- Groeneveld, M. G. , Vermeer, H. J. , van IJzendoorn, M. H. , & Linting, M. (2010). Stress, cortisol and well‐being of caregivers and children in home‐based child care: A case for differential susceptibility. Child: Care, Health and Development, 38(2), 251–260. 10.1111/j.1365-2214.2010.01194.x [DOI] [PubMed] [Google Scholar]
- Grossmann, T. , Oberecker, R. , Koch, S. P. , & Friedericki, A. D. (2010). The developmental origins of voice processing in the human brain. Neuron, 65, 852–858. 10.1016/j.neuron.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon‐Jones, E. , Gable, P. A. , & Peterson, C. K. (2010). The role of asymmetric frontal cortical activity in emotion‐related phenomena: A review and update. Biological Psychology, 84, 451–462. 10.1016/j.biopsycho.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Herrington, J. D. , Mohanty, A. , Koven, N. S. , Fisher, J. E. , Stewart, J. L. , Banich, M. T. , … Heller, W. (2005). Emotion‐modulated performance and activity in left dorsolateral prefrontal cortex. Emotion, 5(2), 200–207. 10.1037/1528-3542.5.2.200 [DOI] [PubMed] [Google Scholar]
- Howarth, G. Z. , Fettig, N. B. , Curby, T. W. , & Bell, M. A. (2016). Frontal electroencephalogram asymmetry and temperament across infancy and early childhood: An exploration of stability and bidirectional relations. Child Development, 87(2), 465–476. https://doi.org/10.11/cdev.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrdy, S. B. (1999). Mother nature. New York, NY: Ballantine Books. [Google Scholar]
- Huffmeijer, R. , Alink, L. R. A. , Tops, M. , Bakermans‐Kranenburg, M. J. , & van IJzendoorn, M. H. (2012). Asymmetric frontal brain activity and parental rejection predict altruistic behavior: Moderation of oxytocin effects. Cognitive Affective and Behavioral Neuroscience, 12(2), 382–392. 10.3758/s13415-011-0082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, M. H. (2003). Development of human brain function. Biological Psychiatry, 54(12), 1312–1316. 10.1016/S0006-3223(03)00426-8 [DOI] [PubMed] [Google Scholar]
- Kobayashi, S. , Lauwereyns, J. , Koizumi, M. , Sakagami, M. , & Hikosaka, O. (2002). Influence of reward expectation on visuospatial processing in macaque lateral prefrontal cortex. Journal of Neurophysiology, 87, 1488–1498. 10.1152/jn.00472.2001 [DOI] [PubMed] [Google Scholar]
- Kraus, N. , McGee, T. , Carrell, T. , Sharma, A. , Micco, A. , & Nicol, T. (1993). Speech‐evoked cortical potentials in children. Journal of the American Academy of Audiology, 4, 238–248. [PubMed] [Google Scholar]
- Kushnerenko, E. , Ceponiene, R. , Balan, P. , Fellman, V. , Huotilainen, M. , & Näätänen, R. (2002). Maturation of the auditory event‐related potentials during the first year of life. NeuroReport, 13(1), 47–51. 10.1097/00001756-200201210-00014 [DOI] [PubMed] [Google Scholar]
- Laufs, H. , Krakow, K. , Sterzer, P. , Eger, E. , Beyerle, A. , Salek‐Haddadi, A. , & Kleinschmidt, A. (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America, 100, 11053–11058. 10.1073/pnas.1831638100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck, S. J. (2015). An introduction to the event‐related potential technique. Cambridge, MA: MIT press. [Google Scholar]
- Marshall, P. J. , Bar‐Haim, Y. , & Fox, N. A. (2002). Development of the EEG from 5 months to 4 years of age. Clinical Neurophysiology, 113, 1199–1208. 10.1016/S1388-2457(02)00163-3 [DOI] [PubMed] [Google Scholar]
- Nelson, D. G. K. , Hirshpasek, K. , Jusczyk, P. W. , & Cassidy, K. W. (1989). How the prosodic cues in motherese might assist language‐learning. Journal of Child Language, 16(1), 55–68. 10.1017/S030500090001343X [DOI] [PubMed] [Google Scholar]
- Novak, G. P. , Kurtzberg, D. , Kreuzer, J. , & Vaughan, H. G. (1989). Cortical responses to speech sounds and their formants in normal infants: Maturational sequence and spatiotemporal analysis. Electroencephalography and Clinical Neurophysiology, 73, 295–305. 10.1016/0013-4694(89)90108-9 [DOI] [PubMed] [Google Scholar]
- Peltola, M. J. , Bakermans‐Kranenburg, M. J. , Alink, L. R. A. , Huffmeijer, R. , Biro, S. , & Van IJzendoorn, M. H. (2014). Resting frontal asymmetry in children: Meta‐analyses of the effects of psychosocial risk factors and associations with internalizing and externalizing behavior. Developmental Psychobiology, 56(6), 1377–1389. 10.1002/dev.21223 [DOI] [PubMed] [Google Scholar]
- Peltola, M. J. , Leppänen, J. M. , Mäki, S. , & Hietanen, J. K. (2009). Emergence of enhanced attention to fearful faces between 5 and 7 months of age. Social Cognitive and Affective Neuroscience, 4, 134–142. 10.1093/scan/nsn046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli, D. A. , Sherwood, R. J. , Henriques, J. B. , & Davidson, R. J. (2005). Frontal brain asymmetry and reward responsiveness: A source localization study. Psychological Science, 16, 805–813. 10.1111/j.1467-9280.2005.01618.x [DOI] [PubMed] [Google Scholar]
- Pluess, M. , & Belsky, J. (2009). Differential susceptibility to rearing experience: The case of childcare. Journal of Child Psychology and Psychiatry, 50(4), 396–404. 10.1111/j.1469-7610.2008.01992.x [DOI] [PubMed] [Google Scholar]
- Power, T. G. (1985). Mother‐ and father‐infant play: A developmental analysis. Child Development, 56(6), 1514–1524. 10.2307/1130470 [DOI] [Google Scholar]
- Prevost, M. , Carrier, M. E. , Chowne, G. , Zelkowitz, P. , Joseph, L. , & Gold, J. (2013). The reading the mind in the eyes test: Validation of a French version and exploration of cultural variations in a multi‐ethnic city. Cognitive Neuropsychiatry, 19(3), 189–204. 10.1080/13546805.2013.823859 [DOI] [PubMed] [Google Scholar]
- Rosenblum, K. L. , Dayton, C. J. , & McDonough, S. (2006). Communicating feelings: Links between mothers’ representations of their infants, parenting, and infant emotional development In Mayseless O. (Ed.), Parenting representations: Theory, research, and clinical implications (pp. 109–148). New York, NY: Cambridge University Press. [Google Scholar]
- Saby, J. N. , & Marshall, P. J. (2012). The utility of EEG band power analysis in the study of infancy and early childhood. Developmental Neuropsychology, 37(3), 253–273. 10.1080/87565641.2011.614663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scourfield, J. , Martin, N. , Lewis, G. , & McGuffin, P. (1999). Heritability of social cognitive skills in children and adolescents. British Journal of Psychiatry, 175, 559–564. 10.1192/bjp.175.6.559 [DOI] [PubMed] [Google Scholar]
- Slagt, M. , Semon Dubas, J. , Deković, M. , & Van Aken, M. A. G. (2016). Differences in sensitivity to parenting depending on child temperament: A meta‐analysis. Psychological Bulletin, 142(10), 1068–1110. 10.1037/bul0000061 [DOI] [PubMed] [Google Scholar]
- Spanglar, G. , Schieche, M. , Ilg, U. , Maier, U. , & Ackermann, C. (1994). Maternal sensitivity as external organizer for biobehavioral regulation in infancy. Developmental Psychobiology, 27(7), 425–437. 10.1002/dev.420270702 [DOI] [PubMed] [Google Scholar]
- Stets, M. , Stahl, D. , & Reid, V. M. (2012). A meta‐analysis investigating factors underlying attrition rates in infant ERP studies. Developmental Neuropsychology, 37(3), 226–252. 10.1080/87565641.2012.654867 [DOI] [PubMed] [Google Scholar]
- van IJzendoorn, M. H. , Belsky, J. , & Bakermans‐Kranenburg, M. J. (2012). Serotonin transporter genotype 5HTTLPR as a marker of differential susceptibility? A meta‐analysis of child and adolescent gene‐by‐environment studies. Translational Psychiatry, 2, e147 10.1038/tp.2012.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuga, M. , Fox, N. A. , Cohn, J. F. , Kovacs, M. , & George, C. J. (2008). Long‐term stability of electroencephalographic asymmetry and power in 3 to 9 year‐old children. International Journal of Psychophysiology, 67, 70–77. 10.1016/j.ijpsycho.2007.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis, J. D. , & Miller, E. K. (2003). Neuronal activity in primate dorsolateral and orbital prefrontal cortex during performance of a reward preference task. European Journal of Neuroscience, 18, 2069–2081. 10.1046/j.1460-9568.2003.02922.x [DOI] [PubMed] [Google Scholar]


