Abstract
Objectives
Abatacept, a biologic DMARD, was associated with respiratory adverse events in a small subgroup of RA patients with chronic obstructive pulmonary disease (COPD) in a trial. Whether this potential risk is specific to abatacept or extends to all biologics and targeted synthetic DMARDs (tsDMARDs) is unclear. We assessed the risk of adverse respiratory events associated with biologic and tsDMARDs compared with conventional synthetic DMARDs (csDMARDs) among RA patients with concomitant COPD in a large, real-world cohort.
Methods
We used a prevalent new-user design to study RA patients with COPD in the US-based MarketScan databases. New users of biologic DMARDs and/or tsDMARDs were matched on time-conditional propensity scores to new users of csDMARDs. Adverse respiratory events were estimated using Cox models comparing current use of biologic/tsDMARDs with csDMARDs.
Results
The cohort included 7424 patients initiating biologic/tsDMARDs and 7424 matched patients initiating csDMARDs. The adjusted hazard ratio of hospitalized COPD exacerbation comparing biologic/tsDMARD vs csDMARD was 0.76 (95% CI: 0.55, 1.06), while it was 1.02 (95% CI: 0.82, 1.27) for bronchitis, 1.21 (95% CI: 0.92, 1.58) for hospitalized pneumonia or influenza and 0.99 (95% CI: 0.87, 1.12) for outpatient pneumonia or influenza. The hazard ratio of the combined end point of COPD exacerbation, bronchitis and hospitalized pneumonia or influenza was 1.04 (95% CI: 0.89, 1.21).
Conclusion
In this large, real-world comparative safety study, biologic and tsDMARDs, including abatacept, were not associated with an increased risk of adverse respiratory events when compared with csDMARDs in patients with RA and COPD.
Rheumatology key messages
Abatacept has been suspected to increase adverse respiratory complications in patients with RA and concomitant COPD.
It is unclear whether this potential risk extends to all biologic DMARDs.
This study finds no increased risk with biologic DMARDs, including abatacept, compared with conventional synthetic DMARDs.
Introduction
RA is a chronic systemic inflammatory disease with an overall cumulative prevalence rate of 0.5%, an age-standardized incidence rate of 65.6 (95% CI: 63.7, 67.5) per 100 000 and an age-standardized all-cause mortality of 8.3 deaths per 1000, ∼1.5 times higher than the general population [1]. Patients with RA are at an increased risk of infection when compared with the general population [2–4]. Respiratory infections are the most common type of infection in RA, accounting for almost 50% of RA hospital admissions for infection [5]. The incidence rates of hospitalized infections and hospitalized pneumonia in RA have been reported to have ranges of 1.41–3.53 and 0.27–1.31 per 100 patient-years, respectively [6]. Immune dysfunction due to the disease process as well as the use of corticosteroids and immunosuppressive drugs targeting key components of immunity have been implicated in this increased risk [7–9]. Comorbidities may also contribute to the increased risk of infection. Chronic obstructive pulmonary disease (COPD) is a common comorbidity in RA, affecting up to 10% of patients [10]. This is similar to the rate of COPD in the general population, estimated to be 8.12% in the USA in 2010 [11]. Patients with COPD are also at higher risk of respiratory infections [12, 13].
Abatacept is a selective co-stimulation modulator of T cell activation used to treat RA. It was shown to be safe and effective in the one-year ASSURE randomized trial [14]. However, in subgroup analyses, serious adverse respiratory events including COPD exacerbation, worsening of COPD, bronchitis and pneumonia were observed more often in RA patients with COPD receiving abatacept compared with placebo. However, the subgroup of RA patients with COPD included only 54 patients. A large observational study conducted to address this question, including over 1800 patients with RA and concomitant COPD who initiated treatment with abatacept, found no increase in the risk of adverse respiratory events compared with other biologic disease-modifying anti-rheumatic drugs (DMARDs) [15]. However, the potential risk of adverse respiratory events could have been obscured in the comparison between abatacept and other biologic DMARDs if the risk is associated with the entire class of biologic DMARDs.
We therefore undertook this study to assess the risk of adverse respiratory events associated with biologic DMARDs, including abatacept, compared with conventional synthetic DMARDs (csDMARDs), among RA patients with COPD, in a real-world observational setting.
Methods
Study design
We designed a prevalent new-user study in a cohort consisting of new users of biologic and csDMARDs matched on time-conditional propensity scores [16]. This design allows the inclusion of subjects who switched from csDMARD to biologic DMARDs and ensures a more comprehensive assessment of drug safety.
Data source
Data from two large US administrative databases were used in this study. The Truven MarketScan Commercial database is a US administrative claims database with patient information dating back to 2006. The database provides detailed information regarding over 70 million privately insured patients younger than 65 years, from >150 employers and 20 health plans. The MarketScan Medicare Supplemental Database covers patients over 65 years of age receiving Medicare coverage in the USA. This database includes data on ∼6 million patients including demographics, drug information, enrolment information, etc. also dating back to 2006.
Study population
First, we created a base cohort of all patients with an RA diagnosis, identified from outpatient and inpatient physician codes, and a prescription for a biologic or csDMARD between January 2007 and December 2015. The algorithm to identify RA in administrative databases, using outpatient and inpatient physician codes (ICD-9 714.xx, ICD-10 M05, M06, M08) and prescription records for drugs used to treat RA, has been validated [17]. We then identified every prescription for which the patient had at least 180 days of prior medication insurance coverage, was 50 years of age or more, had at least two diagnoses of RA in the prior 180 days and had a prior COPD diagnosis. COPD was defined by the presence of at least one outpatient or inpatient diagnosis (ICD-9 codes 491.x, 492.x, or 496.x or ICD-10 codes J41-J44) in the patient’s history any time between 2006 and cohort entry.
Prescriptions were then divided into those for biologic and csDMARDs. To identify new use of a biologic DMARD prescription, we excluded those with a prescription for a biologic drug in the prior 180 days and those with prescriptions for more than one biologic drug [18]. To identify new use of a csDMARD prescription, we excluded those with a biologic DMARD prescription in the prior 180 days, with a prescription for the same csDMARD in the prior 180 days or with two csDMARDs. Finally, new users of biologic DMARDs, as defined by episodes of new use, were matched 1 : 1 with new users of csDMARDs on time-conditional propensity scores (described below) [16].
Exposure definition
Exposure to DMARDs was defined using the National Drug Code (NDC) for dispensed medications and procedure codes for injection or infusion. The biologic DMARDs included abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, rituximab, tocilizumab. Tofacitinib citrate is a synthetic DMARD, but because of its targeted mechanism of action, was included with the biologic DMARDs. The csDMARDs included auranofin, aurothioglucose, gold salt, hydroxychloroquine, leflunomide, methotrexate and sulfasalazine. Patients were considered to be current users of DMARD for a 60-day period from the date of the dispensed prescription. Thus, patients were considered continuously exposed if the 60-day duration of one prescription overlapped with the date of the next prescription. We used an as-treated definition of exposure, where patients were censored at the date of treatment discontinuation.
Outcomes
The primary outcomes of interest were: (i) severe COPD exacerbation requiring hospitalization, (ii) bronchitis (identified by a diagnosis for bronchitis from an outpatient physician visit and a prescription for a respiratory antibiotic on the same day or within 2 days of the diagnosis), (iii) severe pneumonia or influenza (denoted as pneumonia/influenza) requiring hospitalization, (iv) pneumonia/influenza identified from outpatient diagnoses, and (v) a combined outcome consisting of (i), (ii) and (iii).
Covariates
The covariates included age, sex, comorbidities and medications identified during the 180-day baseline period. Co-morbidities were identified using diagnosis codes for inpatient and outpatient encounters, which included hospitalized infections, hypertension, diabetes, cancer (including lymphoma), peripheral vascular disease and cardiovascular disease (ischaemic heart disease, other heart disease, cerebrovascular disease and chronic rheumatic heart disease), as well as joint replacement. Medications included non-steroidal anti-inflammatory drugs (NSAIDs), oral and injection corticosteroids, cholesterol lowering medications, oral antidiabetic agents, insulin, ACE inhibitors, angiotensin II receptor blockers, beta-blockers, calcium channel blockers, diuretics, proton-pump inhibitors, narcotics, antidepressants, antipsychotics, benzodiazepines, anticonvulsants and antiparkinsonian drugs. Other immunosuppressants, including azathioprine, cyclophosphamide, penicillamine, tacrolimus, ciclosporin, everolimus and mycophenolate, were included, as was the presence of prior hospitalizations. COPD-specific covariates included a prior asthma diagnosis, COPD exacerbation requiring hospitalization and medications for COPD such as inhaled corticosteroids, long-acting β2-agonists, long-acting muscarinic antagonists, short-acting β2-agonists, ipratropium bromide, theophylline and antibiotics used for respiratory infections.
Statistical analysis
Descriptive statistics were used to describe the baseline characteristics of the cohort, stratified by new users of biologic and csDMARDs. A time-conditional propensity score of biologic DMARD initiation relative to csDMARD initiation was developed for each patient based on the time-dependent values of the covariates, estimated using a proportional hazards regression model. The propensity score derivation included demographic, comorbidities and medications listed above, present at or during the 6-month period prior to the exposure set date. The time-conditional propensity score allows patients who switched from a csDMARD to a biologic DMARD to be matched to a similar patient who was on a csDMARD at the same time point in the treatment course and switched to another csDMARD. Thus, each new user of a biologic DMARD was matched to a new user of csDMARD with the closest time-conditional propensity score and on the number of different csDMARDs in the last 6 months. To satisfy the assumption of positivity, the biologic DMARD users whose time-conditional propensity score value did not lie within the range of the propensity scores of the corresponding exposure set were excluded [16].
Cohort follow-up was from cohort entry date, namely the date of new biologic DMARD use and of matched new csDMARD use, until the date of the event of interest, treatment discontinuation, end of enrolment in the database, or end of data collection (31 December 2015), whichever occurred first. The Cox proportional hazard regression model was used to provide an estimate of the hazard ratio of each outcome associated with current use of biologic DMARDs relative to current use of csDMARDs, adjusted for the confounders using the propensity score matching. Covariates that were not included in the propensity score, namely prior hospitalizations, hospitalized infections, joint replacement, injection corticosteroids and other immunosuppressants, as well as those found to be imbalanced between the groups, were adjusted for in the regression model.
Several sensitivity analyses were performed. We first extended the baseline period to 1 year and varied the 60-day time window used to define continuous exposure from 30 to 90 days. We also performed stratified analyses by recent csDMARD use, and by degree of COPD severity, defined as prescriptions for triple therapy (long-acting muscarinic antagonists, long-acting β2-agonists and inhaled corticosteroids) in the baseline period. Finally, a sensitivity analysis was performed evaluating short-term effect of the drugs using an as-treated analysis, where the follow-up was limited to one year after treatment initiation, as in the ASSURE randomized trial [14]. Statistical analyses were conducted using SAS, Version 9.4 (SAS Institute, Inc., Cary, NC, USA) and R (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/). The study was approved by the Research Ethics Board of the Jewish General Hospital, Montreal, Canada (no. 2019-1473).
Results
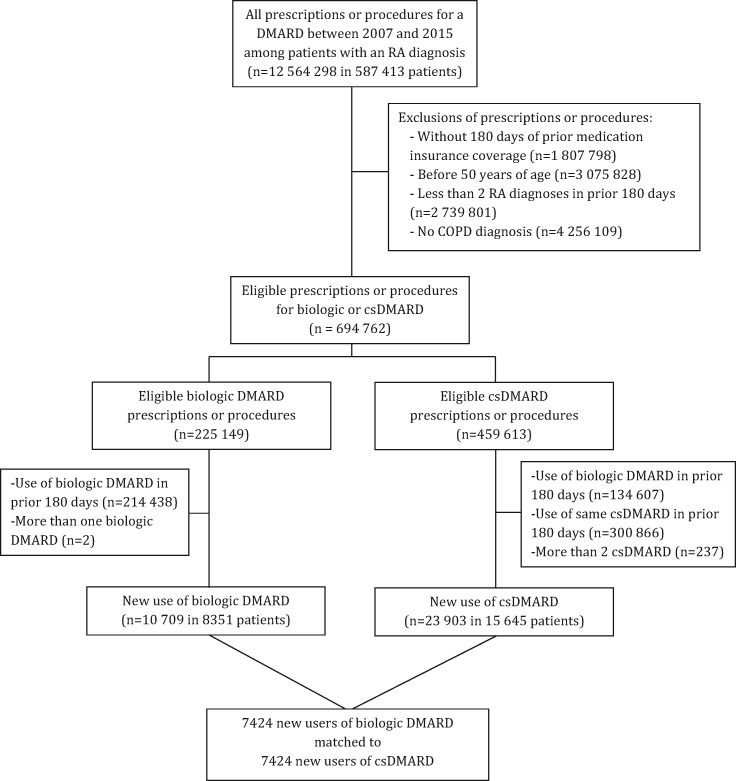
The base cohort included 587 413 subjects with RA with a DMARD prescription or procedure between 2007 and 2015. After applying inclusion and exclusion criteria, the cohort included 8351 patients who initiated a biologic DMARD and 15 645 who initiated a csDMARD (Fig. 1). After one-to-one propensity score matching and trimming from non-overlap, the study cohort included 7424 patients who initiated biologic DMARD therapy and 7424 matched patients who initiated a csDMARD.
Fig 1.
Flowchart of study cohort selection
COPD: chronic obstructive pulmonary disease.
The initiators of biologic DMARD and their propensity score-matched initiators of csDMARDs were generally well balanced on all baseline covariates (Table 1). Imbalances were observed for methotrexate, hydroxychloroquine and leflunomide, factors that were further adjusted for. There were 69% of the patients who had recently used csDMARDs during the baseline period. The majority of drugs in the biologic DMARD group were etanercept (29%), adalimumab (22%), rituximab (15%), infliximab (13%) and abatacept (10%), while the majority of drugs in the csDMARD group were methotrexate (41%), hydroxychloroquine (35%), leflunomide (19%) and sulfasalazine (14%) (Supplementary Table S1, available at Rheumatology online).
Table 1.
Characteristics of patients with RA and COPD initiating biologic or conventional synthetic DMARD
| Biologic DMARD | csDMARD | Standardized difference | |
|---|---|---|---|
| Number of subjects | 7424 | 7424 | |
| Age at cohort entry, mean (s.d.), years | 64.7 (9.3) | 66.6 (9.9) | −0.1926 |
| 50–59 | 2476 (33.4) | 2063 (27.8) | 0.1210 |
| 60–69 | 2751 (37.1) | 2579 (34.7) | 0.0483 |
| 70–79 | 1588 (21.4) | 1869 (25.2) | −0.0896 |
| 80+ | 609 (8.2) | 913 (12.3) | −0.1353 |
| Women, n (%) | 5174 (69.7) | 5202 (70.1) | −0.0082 |
| Prior DMARD use | 5083 (68.5) | 5083 (68.5) | 0.0000 |
| Comorbidities in baseline period | |||
| Hospitalization for COPD | 113 (1.5) | 108 (1.5) | 0.0056 |
| Hospitalization for other infection | 469 (6.3) | 540 (7.3) | −0.0380 |
| Any hospitalization | 1602 (21.6) | 1603 (21.6) | −0.0003 |
| Asthma | 1216 (16.4) | 1238 (16.7) | −0.0080 |
| Hypertension | 3634 (48.9) | 3925 (52.9) | −0.0785 |
| Ischaemic heart disease | 1385 (18.7) | 1483 (20.0) | −0.0334 |
| Cerebrovascular disease | 569 (7.7) | 622 (8.4) | −0.0263 |
| Chronic rheumatic heart disease | 134 (1.8) | 135 (1.8) | −0.0010 |
| Other form of heart disease | 1889 (25.4) | 2083 (28.1) | −0.0591 |
| Diabetes | 1616 (21.8) | 1610 (21.7) | 0.0020 |
| Malignancy | 717 (9.7) | 809 (10.9) | −0.0408 |
| Chronic kidney disease | 466 (6.3) | 553 (7.4) | −0.0464 |
| Peripheral vascular disease | 877 (11.8) | 926 (12.5) | −0.0202 |
| Joint replacement | 415 (5.6) | 360 (4.8) | 0.0333 |
| RA medications in baseline period | |||
| Methotrexate | 3528 (47.5) | 2174 (29.3) | 0.3818 |
| Hydroxychloroquine | 1464 (19.7) | 2163 (29.1) | −0.2205 |
| Leflunomide | 1124 (15.1) | 614 (8.3) | 0.2149 |
| Sulfasalazine | 533 (7.2) | 529 (7.1) | 0.0021 |
| Auranofin | 5 (0.1) | 12 (0.2) | −0.0279 |
| Aurothioglucose | 0 (0.0) | 0 (0.0) | |
| Gold salt | 6 (0.1) | 4 (0.1) | 0.0104 |
| Other medications in baseline period | |||
| Oral corticosteroids | 4948 (66.6) | 4785 (64.5) | 0.0462 |
| Injection corticosteroids | 428 (5.8) | 362 (4.9) | 0.0396 |
| Other immunosuppressants | 305 (4.1) | 126 (1.7) | 0.1440 |
| Inhaled corticosteroids | 1618 (21.8) | 1747 (23.5) | −0.0415 |
| Long acting β2-agonist | 1421 (19.1) | 1537 (20.7) | −0.0391 |
| Long-acting muscarinic antagonist | 825 (11.1) | 909 (12.2) | −0.0352 |
| Short-acting β2-agonist | 1883 (25.4) | 2046 (27.6) | −0.0498 |
| Ipratropium | 532 (7.2) | 589 (7.9) | −0.0291 |
| Theophylline | 65 (0.9) | 78 (1.1) | −0.0179 |
| Antibiotics used in respiratory infections | 4395 (59.2) | 4369 (58.8) | 0.0071 |
| NSAIDs | 2802 (37.7) | 2738 (36.9) | 0.0178 |
| Cholesterol-lowering medication | 2678 (36.1) | 2825 (38.1) | −0.0410 |
| ACE inhibitors | 1543 (20.8) | 1650 (22.2) | −0.0351 |
| Angiotensin II receptor blockers | 1321 (17.8) | 1308 (17.6) | 0.0046 |
| Diuretics | 2674 (36.0) | 2847 (38.3) | −0.0482 |
| Calcium channel blockers | 1609 (21.7) | 1713 (23.1) | −0.0336 |
| Beta-blockers | 2188 (29.5) | 2364 (31.8) | −0.0514 |
| Insulin | 458 (6.2) | 394 (5.3) | 0.0371 |
| Oral anti-diabetics | 992 (13.4) | 975 (13.1) | 0.0068 |
| Proton-pump inhibitors | 2967 (40.0) | 2849 (38.4) | 0.0326 |
| Narcotics | 4688 (63.1) | 4391 (59.1) | 0.0821 |
| Antidepressant | 2846 (38.3) | 2694 (36.3) | 0.0423 |
| Antipsychotics | 210 (2.8) | 191 (2.6) | 0.0158 |
| Benzodiazepines | 1736 (23.4) | 1737 (23.4) | −0.0003 |
| Anticonvulsants | 1129 (15.2) | 1162 (15.7) | −0.0123 |
| Antiparkinsonian | 269 (3.6) | 302 (4.1) | −0.0231 |
ACE: angiotensin converting enzyme; COPD: chronic obstructive pulmonary disease; csDMARD: conventional synthetic DMARD; n: number.
In the patients who initiated a biologic DMARD, the incidence rate (IR) of severe COPD exacerbation was 1.8 per 100 person-years, while it was 4.9 for bronchitis, 3.2 for hospitalized pneumonia or influenza, and 14.7 for outpatient pneumonia or influenza (Table 2). Among the matched patients who initiated a csDMARD, the IR of severe COPD exacerbation was 2.5 per 100 person-years, while it was 5.0 for bronchitis, 3.1 for hospitalized pneumonia or influenza, and 16.1 for outpatient pneumonia or influenza. For the combined end point of severe COPD exacerbation, bronchitis and hospitalized pneumonia or influenza, there were 386 events in the biologic DMARD group (IR 9.8 per 100 person-years) and 352 events in the csDMARD group (IR 10.3 per 100 person-years).
Table 2.
Associations between study endpoints and biologic compared with conventional synthetic DMARD
| Initial treatment | Number of patients | Number of events | Person- years | Rate per 100 person-years | Crude matched HR | Adjusted a HR (95% CI) |
|---|---|---|---|---|---|---|
| Severe COPD exacerbation | ||||||
| Biologic DMARD | 7424 | 74 | 4058 | 1.8 | 0.74 | 0.76 (0.55, 1.06) |
| csDMARD | 7424 | 90 | 3533 | 2.5 | 1.00 | 1.00 (reference) |
| Bronchitis | ||||||
| Biologic DMARD | 7424 | 195 | 4000 | 4.9 | 1.00 | 1.02 (0.82, 1.27) |
| csDMARD | 7424 | 175 | 3483 | 5.0 | 1.00 | 1.00 (reference) |
| Severe pneumonia/influenza | ||||||
| Biologic DMARD | 7424 | 130 | 4042 | 3.2 | 1.06 | 1.21 (0.92, 1.58) |
| csDMARD | 7424 | 109 | 3530 | 3.1 | 1.00 | 1.00 (reference) |
| Outpatient pneumonia/influenza | ||||||
| Biologic DMARD | 7424 | 575 | 3855 | 14.9 | 0.96 | 0.99 (0.87, 1.12) |
| csDMARD | 7424 | 537 | 3335 | 16.1 | 1.00 | 1.00 (reference) |
| Combined respiratory endpointb | ||||||
| Biologic DMARD | 7424 | 386 | 3927 | 9.8 | 0.98 | 1.04 (0.89, 1.21) |
| csDMARD | 7424 | 352 | 3414 | 10.3 | 1.00 | 1.00 (reference) |
Adjusted for age, injection corticosteroids, other immunosuppressants, prior hospitalization, prior hospitalized infection, prior joint replacement, methotrexate, hydroxychloroquine and leflunomide. bIncludes the first of severe COPD exacerbation, severe pneumonia or influenza, or bronchitis. csDMARD: conventional synthetic DMARD; COPD: chronic obstructive pulmonary disease; HR: hazard ratio.
The adjusted hazard ratio of attaining a first severe COPD exacerbation in patients starting a biologic DMARD vs a csDMARD was 0.76 (95% CI: 0.55, 1.06), while for bronchitis it was 1.02 (95% CI: 0.82, 1.27), for hospitalized pneumonia or influenza it was 1.21 (95% CI: 0.92, 1.58) and for outpatient pneumonia or influenza it was 0.99 (95% CI: 0.87, 1.12). For the combined end point of severe COPD exacerbation, bronchitis and hospitalized pneumonia or influenza, the hazard ratio was 1.04 (95% CI: 0.89, 1.21).
Sensitivity analyses based on the as-treated approach are shown in Table 3 for the combined end point and in Supplementary Tables S2–S5 available at Rheumatology online for severe COPD exacerbation, bronchitis, hospitalized pneumonia or influenza, and outpatient pneumonia or influenza. Globally, results were consistent with the main analysis.
Table 3.
Sensitivity analyses for the combined respiratory end point
| Initial treatment | Number of patients | Number with event | Person-years | Rate per 100 person-year | Crude HR | Adjusted a HR (95% CI) |
|---|---|---|---|---|---|---|
| One-year baseline period | ||||||
| Biologic DMARD | 5806 | 314 | 3373 | 9.3 | 0.90 | 0.95 (0.81, 1.12) |
| csDMARD | 5806 | 304 | 2846 | 10.7 | 1.00 | 1.00 (reference) |
| Current use defined by 30-day exposure | ||||||
| Biologic DMARD | 7424 | 136 | 1233 | 11.0 | 0.99 | 1.00 (0.77, 1.29) |
| csDMARD | 7424 | 133 | 1209 | 11.0 | 1.00 | 1.00 (reference) |
| Current use defined by 90-day exposure | ||||||
| Biologic DMARD | 7424 | 541 | 5726 | 9.4 | 0.96 | 1.02 (0.90, 1.16) |
| csDMARD | 7424 | 522 | 5228 | 10.0 | 1.00 | 1.00 (reference) |
| No recent DMARD use | ||||||
| Biologic DMARD | 2341 | 106 | 1011 | 10.5 | 1.15 | 1.16 (0.87, 1.55) |
| csDMARD | 2341 | 81 | 853 | 9.5 | 1.00 | 1.00 (reference) |
| Recent DMARD use | ||||||
| Biologic DMARD | 5083 | 280 | 2916 | 9.6 | 0.93 | 1.00 (0.83, 1.20) |
| csDMARD | 5083 | 271 | 2561 | 10.6 | 1.00 | 1.00 (reference) |
| Severe COPD patient | ||||||
| Biologic DMARD | 436 | 39 | 215 | 18.2 | 0.99 | 0.99 (0.62, 1.56) |
| csDMARD | 462 | 40 | 211 | 19.0 | 1.00 | 1.00 (reference) |
| Mild COPD patient | ||||||
| Biologic DMARD | 6988 | 347 | 3713 | 9.3 | 0.99 | 1.05 (0.89, 1.23) |
| csDMARD | 6962 | 312 | 3203 | 9.7 | 1.00 | 1.00 (reference) |
| Follow-up limited to 1 year | ||||||
| Biologic DMARD | 7424 | 331 | 3074 | 10.8 | 1.00 | 1.06 (0.90, 1.25) |
| csDMARD | 7424 | 307 | 2811 | 10.9 | 1.00 | 1.00 (reference) |
Adjusted for age, injection corticosteroids, other immunosuppressants, prior hospitalization, prior hospitalized infection, prior joint replacement, methotrexate, hydroxychloroquine and leflunomide. COPD: chronic obstructive pulmonary disease; csDMARD: conventional synthetic DMARD; HR: hazard ratio.
Discussion
In this large real-world US claims database study, which included over 7400 patients with RA and concomitant COPD who initiated treatment with a biologic DMARD, the risk of adverse respiratory events was not increased compared with a propensity-matched group of patients initiating treatment with a csDMARD. These adverse events included severe COPD exacerbation requiring hospitalization, bronchitis, as well as hospitalized and outpatient pneumonia or influenza. The risk was also not elevated for the combined end point of severe COPD exacerbation, bronchitis and hospitalized pneumonia or influenza.
This study provides important complementary observations to two studies investigating the safety of abatacept in RA. The ASSURE 1-year randomized trial compared abatacept (n = 959) vs placebo (n = 482) [14]. The subset of patients with COPD included 37 patients in the abatacept arm and 17 on placebo. Among those, serious adverse respiratory events were more common in the abatacept compared with the placebo group (10.8% vs 0%). However, the uneven number of RA patients with COPD exposed to abatacept and placebo, and the small number of serious adverse respiratory events (n = 1 for each of COPD exacerbation, worsening of COPD, bronchitis and pneumonia) contributed to considerable uncertainty around the association. Nevertheless, these data contributed to the current abatacept product label warning that ‘COPD patients may develop more frequent respiratory adverse events’. To examine this question more carefully, a large observational study including over 1800 patients with RA and concomitant COPD was undertaken. Using the same data sources as in this study, it found that the risk of adverse respiratory events in those treated with abatacept was not increased compared with other biologic DMARDs: hazard ratio 0.87 (95% CI: 0.68, 1.12) for the combined end point of hospitalized COPD exacerbation, bronchitis and hospitalized pneumonia or influenza [15]. However, the comparison between abatacept and other biologic DMARDs could have obscured the risk of respiratory adverse events if such risk was associated with the entire class of biologic DMARDs. For this reason, this study was undertaken to compare the risks of adverse respiratory events comparing biologic to csDMARDs. The data again do not support an increased risk of adverse respiratory events in RA patients with COPD treated with biologic compared with csDMARDs.
This study is not without limitations inherent to observational studies, in particular, information bias that can arise from misclassification of exposure or outcome. However, this bias was likely non-differential between the two study groups. Moreover, we used the age limit of 50 years to minimize misclassification of COPD and asthma diagnoses. Also, we cannot rule out the potential for residual confounding from unmeasured covariates such as disease severity, which are not captured in these databases. However, such confounders would have to be moderately prevalent, strongly predictive of outcome and likely to affect the treatment decision to prescribe a biologic or csDMARD to an RA patient with COPD. We cannot exclude such residual confounding due to smoking or steroid dosage.
On the other hand, the study design and sophisticated analytical plan represent important strengths of the study. Indeed, the prevalent new user design allowed the inclusion of subjects who switched from csDMARD to biologic DMARDs, thereby permitting a more comprehensive and realistic assessment of drug safety [16]. In addition, the use of time-conditional propensity scores allowed identification of comparable patients and minimization of confounding. Finally, the sensitivity analyses provided support for the robustness of the results. Another important strength of the study is the use of a large claims database. This allowed efficient identification a large number of patients with both RA and COPD, maximization of power to detect potential adverse effects, and real-world estimates of effects.
Biologic drugs have revolutionized the treatment of RA. However, safety remains an important consideration when initiating any treatment for this disease. COPD is a common comorbidity in RA and both conditions are associated with increased risk of respiratory complications. This study provides reassurance to physicians who treat RA patients with COPD that the risk of adverse respiratory events is not increased with biologic compared with csDMARDs.
Supplementary Material
Acknowledgements
M.H. is the recipient of a senior clinician-scientist award from the Fonds de recherche du Québec-Santé. S.S. is the recipient of the Distinguished James McGill Professorship award.
Funding: This research was funded in part by grants from the Canadian Institutes of Health Research (CIHR), the Canadian Foundation for Innovation (CFI), and Bristol-Myers-Squibb. The sponsors were not involved in the design and conduct of the study. S.S. acts as guarantor of this manuscript.
Disclosure statement: M.H. has participated in advisory board meetings for Boehringer-Ingelheim and received unrestricted research funding from Bristol-Myers-Squibb. S.S. has participated in advisory board meetings or as speaker or received research grants from Boehringer-Ingelheim and has participated in advisory board meetings or as speaker for AstraZeneca, Bayer, Boehringer‐Ingelheim, Bristol-Myers-Squibb and Novartis. T.S. and S.S. are employees of Bristol-Myers-Squibb.
References
- 1. Jean S, Hudson M, Gamache P. et al. Temporal trends in prevalence, incidence, and mortality for rheumatoid arthritis in Quebec, Canada: a population-based study. Clin Rheumatol 2017;36:2667–71. [DOI] [PubMed] [Google Scholar]
- 2. Doran MF, Crowson CS, Pond GR, O'Fallon WM, Gabriel SE.. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. [DOI] [PubMed] [Google Scholar]
- 3. Smitten AL, Choi HK, Hochberg MC. et al. The risk of hospitalized infection in patients with rheumatoid arthritis. J Rheumatol 2008;35:387–93. [PubMed] [Google Scholar]
- 4. Listing J, Gerhold K, Zink A.. The risk of infections associated with rheumatoid arthritis, with its comorbidity and treatment. Rheumatology 2013;52:53–61. [DOI] [PubMed] [Google Scholar]
- 5. Franklin J, Lunt M, Bunn D, Symmons D, Silman A.. Risk and predictors of infection leading to hospitalisation in a large primary-care-derived cohort of patients with inflammatory polyarthritis. Ann Rheum Dis 2007;66:308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Simon TA, Askling J, Lacaille D. et al. Infections requiring hospitalization in the abatacept clinical development program: an epidemiological assessment. Arthritis Res Ther 2010;12:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dixon WG, Suissa S, Hudson M.. The association between systemic glucocorticoid therapy and the risk of infection in patients with rheumatoid arthritis: systematic review and meta-analyses. Arthritis Res Ther 2011;13:R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Listing J, Strangfeld A, Kary S. et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005;52:3403–12. [DOI] [PubMed] [Google Scholar]
- 9. Ramiro S, Sepriano A, Chatzidionysiou K. et al. Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 2017;76:1101. [DOI] [PubMed] [Google Scholar]
- 10. Hyldgaard C, Bendstrup E, Pedersen AB. et al. Increased mortality among patients with rheumatoid arthritis and COPD: a population-based study. Respir Med 2018;140:101–7. [DOI] [PubMed] [Google Scholar]
- 11. Murray CJ, Atkinson C, Bhalla K. et al. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA 2013;310:591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sethi S. Infection as a comorbidity of COPD. Eur Respir J 2010;35:1209–15. [DOI] [PubMed] [Google Scholar]
- 13. Inghammar M, Engström G, Ljungberg B. et al. Increased incidence of invasive bacterial disease in chronic obstructive pulmonary disease compared to the general population—a population based cohort study. BMC Infect Dis 2014;14:163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weinblatt M, Combe B, Covucci A. et al. Safety of the selective costimulation modulator abatacept in rheumatoid arthritis patients receiving background biologic and nonbiologic disease-modifying antirheumatic drugs: a one-year randomized, placebo-controlled study. Arthritis Rheum 2006;54:2807–16. [DOI] [PubMed] [Google Scholar]
- 15. Suissa S, Hudson M, Dell'Aniello S. et al. Comparative safety of abatacept in rheumatoid arthritis with COPD: a real-world population-based observational study. Semin Arthritis Rheum 2019, doi:10.1016/j.semarthrit.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 16. Suissa S, Moodie EE, Dell'Aniello S.. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 2017;26:459–68. [DOI] [PubMed] [Google Scholar]
- 17. Kim SY, Servi A, Polinski JM. et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Research & Therapy 2011;13:R32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 2003;158:915–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.