Abstract
Objective
To conduct a systematic literature review and meta-analysis to estimate the incidence of anaemia, leucopoenia, neutropenia and thrombocytopenia associated with MTX plus folic acid among patients with rheumatic diseases.
Methods
We searched MEDLINE, PubMed and EMBASE through August 2016 for all randomized controlled clinical trials with a MTX monotherapy arm. We excluded randomized controlled clinical trials for cancer and included only double-blind studies that reported on haematologic adverse events. Studies were excluded if patients did not receive folic acid or leucovorin supplementation. Full text articles were assessed by two independent reviewers. Incidence estimates were calculated using random-effects models.
Results
Of 1601 studies identified, 30 (1.87%) were included, representing 3858 patients; all had RA. Seventeen trials reported on anaemia (n = 2032), 17 reported on leucopoenia (n = 2220), 16 reported on neutropenia (n = 2202) and 12 reported on thrombocytopenia (n = 1507). The incidence for any anaemia was 2.55% (95% CI 0.60–5.47%), any leucopoenia 1.17% (95% CI 0.16–2.80%), any neutropenia 1.77% (95% CI 0.33–4.00%), and any thrombocytopenia 0.19% (95% CI 0.00–0.86%). Four cases of severe anaemia were reported, as defined by authors, along with three cases of severe neutropenia. No cases of severe leucopoenia, severe thrombocytopenia or pancytopenia were reported.
Conclusion
Cytopenias are an uncommon side effect of low-dose MTX with folic acid supplementation among RA patients. Further research is needed to reach a more precise estimate.
Keywords: Methotrexate, rheumatoid arthritis, anaemia, leucopoenia, neutropenia, thrombocytopenia, meta-analysis
Rheumatology key messages
Incidence of anaemia was ∼2.55%, of leucopenia was ∼1.17%, of neutropenia was ∼1.77%, of thrombocytopenia was ∼0.19%.
No cases of pancytopenia were reported across 30 trials representing 3858 individuals.
Folic acid supplementation and modern clinical guidelines may account for the infrequency of cytopenias.
Introduction
Now a cornerstone in the treatment of RA, MTX was first used in oncology to treat haematologic cancers. MTX and its metabolites inhibit dihydrofolate reductase, which is required for the production of nucleic acids. Even at low dosages (7.5–25 mg weekly) used for rheumatic diseases, MTX can slow or halt the maturation of haematopoietic cells and reduce blood cell counts across all cell lineages [1, 2]. MTX recommendations from rheumatology societies suggest complete blood counts every 2–3 months as a necessary part of clinical monitoring [3–5] .
In the 1990s, folic and folinic acid (FA) supplementation became part of standard practice as a means of combating cytopenias and other MTX-associated adverse events. However, since the widespread use of FA, the incidence of cytopenias among patients taking low-dose MTX has not been re-examined. Such data may improve the rationale for evidence-based monitoring guidelines for MTX.
We conducted a systematic literature review and meta-analysis to estimate the incidence of anaemia, leucopoenia, neutropenia and thrombocytopenia associated with MTX plus FA supplementation among patients with rheumatic diseases enrolled in randomized controlled clinical trials.
Methods
We searched PubMed, MEDLINE and EMBASE databases through August 2016 without specifying a start date and using the search terms ‘methotrexate’ and either ‘pancytopenia’, ‘anaemia’, ‘leukopenia’, ‘thrombocytopenia’ or ‘drug-related side effects and adverse reactions’. We excluded studies with ‘neoplasms’ as a Medical Subject Heading (MeSH). Full PubMed search criteria are available in the online supplement (Supplementary Tables S1 and S2, available at Rheumatology online). We reviewed all resulting records and removed those without abstracts available in English. From these abstracts we included only double-blind randomized controlled clinical trials in adults. We excluded abstracts from oncology studies and included only those studies that studied inflammatory diseases. As folic and FA supplementation became part of standard practice in the late 1990s, studies published prior to the year 2000 were excluded if they did not specify that all subjects were taking FA supplementation. Those published after the year 2000 were considered to include FA supplementation for all patients unless otherwise specified.
We manually reviewed the reference sections of all included papers for additional studies that met our selection criteria. We then reviewed full-text articles and included only those with a MTX monotherapy arm. Studies that allowed concomitant use of other synthetic DMARDs (e.g. hydroxychloroquine) with MTX were excluded. Concomitant use of glucocorticoids was allowed. Only articles that included reports of the occurrence and type of haematologic adverse events were included.
Study characteristics, adverse event data, and risk of bias assessment were carried out by two independent authors (K.V. and D.H.S.) using a predefined data abstraction form, which is available in the online supplement. Risk of bias was assessed according to the Cochrane Collaboration guidelines for random sequence generation and allocation concealment (selection bias), blinding of participants and researchers (performance bias), blinding of outcome assessment (detection bias), study retention (attrition bias), and selective or incomplete reporting (reporting bias) [6]. Discrepancies were resolved through discussion.
The primary outcomes were the occurrence of any of the following: anaemia, leucopoenia, neutropenia and thrombocytopenia. Each was defined according to the Outcome Measures in Rheumatoid Arthritis Clinical Trial (OMERACT) standards [7] or per report of the trial authors. Leucopoenia and neutropenia were considered independent of one another.
Random-effects models were examined for the estimate of the incidence for each outcome. We included articles in the analyses of each cytopenia if they reported the occurrence or absence of the four main cytopenias; each study was included in one to four analyses. The incidence was calculated as the number of positive cases divided by the total sample size of relevant studies. The arcsine square root transformation was used to stabilize the weights of estimates. Estimates are represented using a diamond. The pattern of the diamond represents the estimated effect size and the width of the diamond reflects the precision of the estimate. The incidence was then estimated for each cytopenia among a subgroup of studies that included MTX-naïve patients. The meta-analysis was analysed and graphed using R-3.4.3 (https://cran.r-project.org) with the ‘meta’ package.
Study heterogeneity was tested using the Cochran’s Q and I2. I2 is the percentage of observed total variation across studies due to real heterogeneity, a value of 0% indicates no observed heterogeneity, and larger values show increasing heterogeneity. The Cochran’s Q is the weighted sum of squares on a standardized scale. The 0.10 is used as a cut-off for significance of presence of heterogeneity. Possible publication bias was investigated graphically using funnel plot and Egger’s weighted regression statistic with a P-value <0.05 indicating significant publication bias [8].
Results
PubMed, MEDLINE and EMBASE searches yielded 1601 records (Fig. 1) . After excluding records without abstracts available in English, 1211 abstracts were reviewed; 28 of those met inclusion criteria and were reviewed as full text articles. After review of the reference sections of these 28 articles, another 79 studies were identified. Forty-four full text articles were excluded because they did not report on haematologic adverse events. Another four were excluded for reporting haematologic abnormalities in aggregate without specifying the type of cytopenia. Thirty studies were included in our analyses.
Fig. 1.
Study selection process per PRISMA guidelines
The included studies represented 3858 patients randomized to MTX monotherapy. In 26 studies (n = 3278), subjects took MTX plus a placebo or a biologic disease modifying anti-rheumatic drug (bDMARD). Two studies (n = 224) randomized subjects to MTX or leflunomide monotherapy. Three studies (n = 356) assigned subjects to MTX plus FA or placebo. Though we did not search or filter by disease, all included trials studied RA; no other conditions were represented.
Seventeen trials reported rates of anaemia (n = 2032), 12 reported on thrombocytopenia (n = 1507), 17 reported on leucopoenia (n = 2220), 16 reported on neutropenia (n = 2202), and five reported on lymphopenia (n = 324). Among the 22 studies (n = 2926) that reported MTX doses, the mean dose of MTX was 15.4 (s.d. 4.5) mg/week, with a maximum dose of 30 mg/week (Table 1). Seventeen studies (n = 2666) reported on the percentage of subjects using corticosteroids, with 41.9% of subjects using corticosteroids. Study duration ranged from 12–62 weeks with a mean of 31 (s.d. 16) weeks. Fifteen studies reported on the route of MTX administration, of which ten used oral MTX only and five used oral or parenteral MTX.
Table 1.
Characteristics of included trials
| Author | Year | Setting | Region | Comparator drug | MTX mono, n | Mean Dose of MTX, mg/week | Route of MTX | Folate or Leucovorin | % Taking steroids | Length of trial, weeks | Frequency of CBCs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Atsumi [9] | 2016 | Multi | Asia | Certolizumab | 157 | 11.6 | Oral | — | 18.50% | 52 | — |
| Bathon [10] | 2000 | Multi | N. America | Etanercept | 217 | 19.0 | Oral | Folic, 1mg/day | 18.90% | 52 | 2 weeks, 1 month, 6, 8, 10, 12 months |
| Chen [11] | 2013 | Multi | Asia | Anbainuo | 131 | 16.7 | Oral | — | 27.50% | 24 | — |
| Chen [12] | 2016 | Multi | Asia | Anbainuo | 119 | — | — | — | — | 12 | Weeks 2, 6, 12 |
| Cohen [13] | 2002 | Multi | N. America, Asia | Anakinra | 74 | 16.3 | — | — | 66.20% | 24 | Weeks 0, 1, 2, 4, 8, 12, 16, 20, 24 |
| Emery [14] | 2009 | Multi | Global | Golimumab | 160 | 19.1 | — | — | 68.10% | 24 | — |
| Genovese [15] | 2013 | Multi | N. America | Tabalumab | 36 | 16.8 | — | — | — | 24 | — |
| Genovese [16] | 2013 | Multi | Europe | Tabalumab | 34 | 12 | — | — | 41% | 24 | — |
| Genovese [17] | 2013 | Multi | Global | Sekukinumab | 50 | — | — | — | 16 | Week 2, 4, 8, 16 | |
| Genovese [18] | 2014 | Multi | N. America, Europe | Olokizumab | 44 | — | — | — | — | 12 | — |
| Genovese [19] | 2015 | Multi | Global | Sarilumab | 427 | 15.6 | Oral | Folic | 63.30% | 62 | Weeks 2, 4, 6, 8, 10, 12, 16, 20, 24, 28, 36, 42, 50 |
| Griffith [20] | 2000 | Single | Europe | Placebo Folate | 38 | 13.35 | — | Folic, 5mg/day | 23.68% | 52 | Every 3 months |
| Hu [21] | 2009 | Multi | Asia | Etanercept | 120 | 12.5 | Oral | — | 28.33% | 24 | Weeks 2, 4, 8, 12, 16, 20, 24 |
| Huizinga [22] | 2013 | Multi | Global | Sarilumab | 52 | 16.9 | — | Folic | — | 12 | Every 2 weeks |
| Jaimes-Hernández [23] | 2012 | Single | N. America | Leflunomide | 42 | 10 | Oral | — | — | 52 | Weeks 4, 8, 16, 24, 32, 40, 48, 52 |
| Jones [24] | 2010 | Multi | Global | Tocilizumab | 284 | — | Oral | — | 47% | 24 | — |
| Keystone [25] | 2008 | Multi | Global | Certolizumab | 199 | 13.4 | — | — | — | 52 | Weeks 1, 2, 4, 6, 8, 10, 12, 14, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52 |
| Kremer [26] | 2011 | Multi | Global | Tocilizumab | 393 | 15.0 | Oral, PAR | — | 11.50% | 52 | Weeks 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 52 |
| Kremer [27] | 2012 | Multi | Global | Tofacitinib | 99 | 17.0 | Oral, PAR | Folic | 44.90% | 24 | Weeks 2, 4, 6, 8, 12, 16, 20, 24 |
| Maini [28] | 2000 | Multi | N. America, Europe | Infliximab | 88 | 15 | Oral, PAR | Folic | 64% | 30 | — |
| Shiroky [29] | 1993 | Multi | N. America | PBO Leucovorin | 44 | — | Oral | Folinic, 2.5-5mg/day | — | 52 | Every 3 weeks |
| Smolen [30] | 2008 | Multi | Global | Tocilizumab | 204 | 14.8 | — | Folic | 54% | 24 | Weeks 2, 4, 6, 8, 12, 14, 16, 20, 24, 28, 32 |
| Strand [31] | 1999 | Multi | N. America | Leflunomide | 182 | — | Oral | Folic | 52.70% | 52 | — |
| Tanaka [32] | 2011 | Multi | Asia | Tofacitinib | 28 | 8.1 | — | Folic | 71.40% | 12 | Weeks 1, 2, 4, 8, 12 |
| Tanaka [33] | 2016 | Multi | Asia | Baricitinib | 49 | — | — | — | — | 12 | — |
| van der Heijde [34] | 2013 | Multi | Global | Tofacitinib | 160 | — | — | — | — | 12 | — |
| van Ede [35] | 2001 | Multi | Europe | PBO Folate | 133 | 18.0 | Oral | Folic, 1 mg/day | — | 48 | Every 3 weeks |
| 141 | 16.4 | Oral | Folinic, 2.5mg/week | ||||||||
| Weinblatt [36] | 1999 | Multi | N. America | Etanercept | 30 | 18 | Oral, PAR | Folic or Folinic | 70% | 24 | Days 1, 8, 15 weeks 4, 8, 12, 16, 20, 24 |
| Weinblatt [37] | 2003 | Multi | N. America | Adalimumab | 62 | 16.5 | Oral, SC | Folic | — | 24 | — |
| Weinblatt [38] | 2015 | Multi | Global | Clazakizumab | 61 | 17.5 | — | Folic | — | 24 | Weeks 1, 2, 4, 8, 12, 16, 20 |
CBC, complete blood count; Multi, multicentre; —, not specified; N. America, North America; PAR, parenteral; PBO, placebo; SC, subcutaneous.
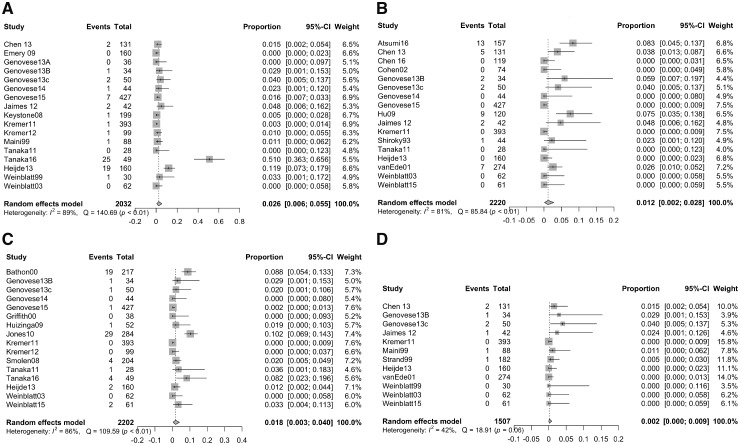
We analysed the five cytopenia separately, and each analysis included only the studies that reported that type of cytopenia as having occurred or not. A total of 64 cases of anaemia were reported across 17 studies (n = 2032; see Fig. 2A). The incidence of any anaemia by a random effects model was 2.55% (95% CI 0.60–5.47%). Of these cases, 18 were mild (27.7%), seven were moderate (10.8%), three were severe (4.6%), one was life threatening (1.5%), and 35 were of unknown severity (53.8%).
Fig. 2.
Incidences of (A) anaemia, (B) leucopoenia, (C) neutropenia and (D) thrombocytopenia
Forest plot showing incidence of each cytopenia across all studies that reported on its presence or absence at any point during the study. The grey squares represent the weight of each study, and the black bars show the 95% CIs. The grey diamond shows the incidence.
Forty-one cases of leucopoenia occurred across 17 studies (n = 2220; see Fig. 2B); one case was reported as mild (2.4%), and severity was not reported for the remaining 40 cases. The incidence of any leucopoenia was 1.17% (95% CI 0.16–2.80%). Among 16 studies (n = 2202), there were 65 cases of neutropenia (see Fig. 2C). The incidence of neutropenia was 1.77% (95% CI 0.33–4.00%). Twenty-nine cases were reported as mild neutropenia (44.6%), nine were moderate (13.8%), three were severe (4.6%), none were life threatening (0%), and 24 (36.9%) were not characterized by severity.
Thrombocytopenia was seen in eight cases across 12 studies (n = 1507; see Fig. 2D), with two cases being reported as mild (25%) and the other six cases being of unspecified severity. The incidence of any thrombocytopenia was 0.19% (95% CI 0.00–0.86%). No studies reported any cases of pancytopenia.
Six studies included only MTX-naïve subjects [9, 10, 14, 29, 31, 35]. Among this subgroup (n = 1034), one study reported no occurrences of anaemia [14] (n = 160) and one reported 19 cases of neutropenia [10](n = 217). Three studies [9, 29, 35] (n = 475) reported a total of 21 cases of leucopoenia. The incidence of leucopoenia among this MTX-naïve subgroup was 4.16% (95% CI 1.01–8.97%). One case of thrombocytopenia was reported between two studies [31, 35] (n = 456) in the MTX-naïve subgroup; the incidence of thrombocytopenia among this subgroup was 0.13% (95% CI 0.00–1.07%). Forest plots for the leucopoenia and thrombocytopenia subanalyses are shown in Fig. 3A and B.
Fig. 3.
Incidences of (A) leucopoenia and (B) thrombocytopenia in studies that included MTX-naïve subjects
Forest plot showing incidence of (A) leucopoenia and (B) thrombocytopenia among studies that included MTX-naïve subjects. The grey squares represent the weight of each study, and the black bars show the 95% CIs. The grey diamond shows the incidence.
Significant heterogeneity existed across studies for all cytopenias. The I2 was 89% for anaemia (P < 0.01), 81% for leucopoenia (P < 0.01), 86% for neutropenia (P < 0.01) and 42% for thrombocytopenia (P = 0.06). Cochran’s Q was 140.69 (P < 0.01) for anaemia, 85.84 (P < 0.01) for leucopoenia, 109.59 (P < 0.01) for neutropenia and 18.91 (P = 0.06) for thrombocytopenia.
As double-blind studies, all studies had a low risk of performance and detection bias. Twenty-three studies did not describe their method of random sequence generation, and 19 studies did not report their strategy for allocation concealment. Eight studies experienced loss to follow-up and did not report on adverse events for all subjects. Another eight studies only reported on serious adverse events or events that led to study discontinuation. Risk of bias assessment is detailed in Fig. 4.
Fig. 4.
Cochrane risk of bias assessment
Author assessment of the risk of bias for each included study.
The publication bias of the primary outcomes was assessed using visual examination of funnel plots and Egger’s weighted regression statistic (P = 0.084 for anaemia, P = 0.073 for leucopoenia, P = 0.654 for neutropenia and P < 0.001 for thrombocytopenia [Fig. 5A–C]), which indicated a potential publication bias for thrombocytopenia. Trim-and-fill results suggested that 13 more studies would be needed to achieve a symmetry funnel plot, as shown in Fig. 5D.
Fig. 5.
Funnel plots for (A) anaemia, (B) leucopoenia, (C) neutropenia and (D) thrombocytopenia
Risk of publication bias analysis for each type of cytopenia.
Discussion
MTX has long been known to increase the risk of cytopenias, but the incidence of haematologic abnormalities among patients taking low-dose MTX in the era of FA supplementation has remained poorly defined. We conducted the first systematic review and meta-analysis to our knowledge to estimate the incidence of cytopenias among RA patients taking low-dose MTX with FA. We identified 30 double-blind randomized clinical trials that reported on haematologic adverse events in such a population. The incidence was 2.55% (95% CI 0.60–5.47%) for anaemia, 1.17% (95% CI 0.16–2.80%) for leucopoenia, 1.77% (95% CI 0.33–4.00%) for neutropenia, and 0.19% (95% CI 0.00–0.86%) for thrombocytopenia.
While low red blood cell folate levels are associated with elevated rates of cytopenias in patients taking low-dose MTX [39, 40], FA supplementation has not been directly associated with fewer cases of cytopenias. Three meta-analyses have looked for a reduction in haematologic abnormalities during FA supplementation but failed to find any due to small sample sizes and inconsistent reporting [1, 41, 42]. Estimates of the incidence of haematologic abnormalities associated with low-dose MTX published prior to the widespread use of folic acid supplementation ranged from as low as 3% [43, 44] to as high as 10% [45, 46] combined across all cell lines. Our results are consistent with these lower estimates.
Current professional resources list the incidence of thrombocytopenia to be between 3–10%; however, these estimates are drawn from studies published prior to the widespread use of FA supplementation [39, 47, 48]. Other prior studies have estimated that thrombocytopenia occurs in about 4.1–4.7% [49, 50] of patients. In contrast, we found thrombocytopenia to be the least common cytopenia with an incidence of <1%. Thrombocytopenia appears to be a rare side effect of low-dose MTX in the era of FA supplementation.
Prior literature has reported that pancytopenia occurs in 1.4–2% [47, 51] of patients taking low-dose MTX for RA, while resources for physicians list the incidence of pancytopenia in this population to be between 1–3%. However, among 3858 patients represented in our analysis, no cases of pancytopenia were reported. It remains possible that cases of multiple line cytopenia or pancytopenia were reported as multiple events that each affected one cell line. Pancytopenia appears to be very rare in patients without prior risk factors who receive close clinical monitoring.
Several factors limited our analysis. First, few studies stated the precise definitions or the severity of the cytopenias that they reported, and definitions varied across studies. The high I2 values for each analysis point to significant heterogeneity across all studies. Second, doses of FA and corticosteroids varied and may have influenced the rates of cytopenias. Corticosteroids raise white blood cell counts and may decrease the rates of leucopoenia and neutropenia. All studies required FA supplementation, and most studies noted 10 mg prednisone daily as the maximum allowable dose of corticosteroids.
Third, the trials in our analysis included a relatively healthy subset of RA patients. For example, Atsumi and colleagues, Shiroky and colleagues, and Tanaka and colleagues set age limits of 65, 70 and 75 years, respectively [9, 29, 33], although about two-thirds of the included trials did not comment on an upper age limit. Kremer and colleagues and van der Heijde and colleagues required an estimated glomerular filtration rate of at least 50 ml/min and 40 ml/min, respectively, and Weinblatt and colleagues required a creatine level of <177 μmol/l [27, 34, 36]. However, most studies did not specify requirements for kidney function. Thus, the elderly and those with renal insufficiency were likely to be excluded from these studies and may be more likely to develop cytopenias.
Finally, most of the included studies compared MTX with a biologic, so to be eligible for these studies, patients must have tolerated MTX prior to the trial period without developing cytopenias. Our results reflect the rates of cytopenias in patients established on MTX and FA, but these rates may be higher in those initiating MTX.
In a subanalysis of MTX-naïve subjects, we found that the rate of leucopoenia was 4.16% (95% CI 1.01–8.97%) among MTX-naïve subjects, compared with 1.17% (95% CI 0.16–2.80%) among all subjects. Leucopoenia appears to be more common in those who recently started MTX, though more data would be needed to confirm this finding. The incidence of thrombocytopenia was 0.13% (95% CI 0.00–1.07%) among the MTX-naïve subgroup and 0.19% (95% CI 0.00–0.86%) among all subjects. Thrombocytopenia does not seem to be more common among MTX-naïve patients. More data would be needed to evaluate the frequency of cytopenias in patients newly started on MTX.
Our study had several important strengths. First, by including only double-blind randomized trials, we minimized the risks of selection and performance bias. Second, subjects enrolled in a clinical trial receive laboratory monitoring at regular intervals, which allows for the consistent detection of cytopenias. Third, the use of FA supplementation by all subjects in all included studies agrees with current practice. Finally, we meta-analyzed 30 studies total, which gave our analyses a relatively robust sample size.
In conclusion, cytopenias are uncommon among patients with RA taking low-dose MTX with FA supplementation. In patients established on MTX, anaemia, leucopoenia and neutropenia occur much less frequently than previously estimated. Thrombocytopenia is a rare side effect of low-dose MTX, occurring far less frequently than previously thought. Similarly, pancytopenia is far less common than previously estimated, which could be due to the universal use of FA supplementation and the establishment of clear contraindications to MTX due to the risk of pancytopenia (e.g. renal failure, active infections, concomitant use of sulfamethoxazole-trimethoprim). Given the low incidence of cytopenias among patients with no other risk factors taking low-dose MTX, current monitoring guidelines might be reconsidered.
Funding: This work was supported by the National Institutes of Health [NIH-HL119718, NIH-AR072577].
Disclosure statement: D.H.S. receives research support from institutional contracts with Abbvie, Amgen, Genentech and Pfizer. He also serves as an epidemiologic consultant to Corrona. Both other authors have no conflicts of interest.
Supplementary Material
References
- 1. Whittle SL, Hughes RA.. Folate supplementation and methotrexate treatment in rheumatoid arthritis: a review. Rheumatology 2003;43:267–71. [DOI] [PubMed] [Google Scholar]
- 2. Mori S, Hidaka M, Kawakita T. et al. Factors associated with myelosuppression related to low-dose methotrexate therapy for inflammatory rheumatic diseases. PloS One 2016;11:e0154744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh JA, Saag KG, Bridges L Jr. et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res 2016;68:1–26. [DOI] [PubMed] [Google Scholar]
- 4. Ledingham J, Gullick N, Irving K. et al. BSR and BHPR guideline for the prescription and monitoring of non-biologic disease-modifying anti-rheumatic drugs. Rheumatology 2017;6:865–8. [DOI] [PubMed] [Google Scholar]
- 5. Duarte AC, Santos-Faria D, Gonçalves MJ. et al. Portuguese recommendations for the use of methotrexate in rheumatic diseases - 2016 update. Acta Reumatol Port 2017;42:127–40. [PubMed] [Google Scholar]
- 6. Higgins JP, Altman DG, Gøtzsche PC. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Woodworth T, Furst DE, Alten R. et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: the Rheumatology Common Toxicity Criteria v. 2.0. J Rheumatol 2007;34:1401–14. [PubMed] [Google Scholar]
- 8. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions: Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. http://handbook.cochrane.org.
- 9. Atsumi T, Yamamoto K, Takeuchi T. et al. The first double-blind, randomised, parallel-group certolizumab pegol study in methotrexate-naive early rheumatoid arthritis patients with poor prognostic factors, C-OPERA, shows inhibition of radiographic progression. Ann Rheum Dis 2016;75:75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bathon JM, Martin RW, Fleischmann RM. et al. A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 2000;343:1586–93. [DOI] [PubMed] [Google Scholar]
- 11. Chen XX, Dai Q, Huang AB. et al. A multicenter, randomized, double-blind clinical trial of combination therapy with Anbainuo, a novel recombinant human TNFRII: fc fusion protein, plus methotrexate versus methotrexate alone or Anbainuo alone in Chinese patients with moderate to severe rheumatoid arthritis. Clin Rheumatol 2013;32:99–108. [DOI] [PubMed] [Google Scholar]
- 12. Chen XX, Li ZG, Wu HX. et al. A randomized, controlled trial of efficacy and safety of Anbainuo, a bio-similar etanercept, for moderate to severe rheumatoid arthritis inadequately responding to methotrexate. Clin Rheumatol 2016;35:2175–83. [DOI] [PubMed] [Google Scholar]
- 13. Cohen S, Hurd E, Cush J. et al. Treatment of rheumatoid arthritis with anakinra, a recombinant human interleukin‐1 receptor antagonist, in combination with methotrexate: results of a twenty‐four–week, multicenter, randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 2002;46:614–24. [DOI] [PubMed] [Google Scholar]
- 14. Emery P, Fleischmann RM, Moreland LW. et al. Golimumab, a human anti–tumor necrosis factor α monoclonal antibody, injected subcutaneously every four weeks in methotrexate‐naive patients with active rheumatoid arthritis: twenty‐four-week results of a phase III, multicenter, randomized, double‐blind, placebo‐controlled study of golimumab before methotrexate as first‐line therapy for early‐onset rheumatoid arthritis. Arthritis Rheum 2009;60:2272–83. [DOI] [PubMed] [Google Scholar]
- 15. Genovese MC, Lee E, Satterwhite J. et al. A phase 2 dose-ranging study of subcutaneous tabalumab for the treatment of patients with active rheumatoid arthritis and an inadequate response to methotrexate. Ann Rheum Dis 2013;72:1453–60. [DOI] [PubMed] [Google Scholar]
- 16. Genovese MC, Bojin S, Biagini IM. et al. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: a phase II, randomized, placebo‐controlled trial. Arthritis Rheum 2013;65:880–9. [DOI] [PubMed] [Google Scholar]
- 17. Genovese MC, Durez P, Richards HB. et al. Efficacy and safety of secukinumab in patients with rheumatoid arthritis: a phase II, dose-finding, double-blind, randomised, placebo controlled study. Ann Rheum Dis 2013;72:863–9. [DOI] [PubMed] [Google Scholar]
- 18. Genovese MC, Fleischmann R, Furst D. et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised Phase IIb study. Ann Rheum Dis 2014;73:1607–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Genovese MC, Fleischmann R, Kivitz AJ. et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol 2015;67:1424–37. [DOI] [PubMed] [Google Scholar]
- 20. Griffith SM, Fisher J, Clarke S. et al. Do patients with rheumatoid arthritis established on methotrexate and folic acid 5 mg daily need to continue folic acid supplements long term? Rheumatology 2000;39:1102–9. [DOI] [PubMed] [Google Scholar]
- 21. Hu D, Bao C, Chen S. et al. A comparison study of a recombinant tumor necrosis factor receptor: fc fusion protein (rhTNFR: fc) and methotrexate in treatment of patients with active rheumatoid arthritis in China. Rheumatol Int 2009;29:297–303. [DOI] [PubMed] [Google Scholar]
- 22. Huizinga TW, Fleischmann RM, Jasson M. et al. Sarilumab, a fully human monoclonal antibody against IL-6Rα in patients with rheumatoid arthritis and an inadequate response to methotrexate: efficacy and safety results from the randomised SARIL-RA-MOBILITY Part A trial. Ann Rheum Dis 2014;73:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jaimes-Hernández J, Meléndez-Mercado CI, Mendoza-Fuentes A, Aranda-Pereira P, Castañeda-Hernández G.. [Efficacy of leflunomide 100 mg weekly compared to low dose methotrexate in patients with active rheumatoid arthritis: double blind, randomized clinical trial]. [Article in Spanish]. Reumatol Clin 2012;8:243–9. [DOI] [PubMed] [Google Scholar]
- 24. Jones G, Sebba A, Gu J. et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: the AMBITION study. Ann Rheum Dis 2010;69:88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Keystone E, van der Heijde D, Mason D. et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty‐two–week, phase III, multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group study. Arthritis Rheum 2008;58:3319–29. [DOI] [PubMed] [Google Scholar]
- 26. Kremer JM, Blanco R, Brzosko M. et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate responses to methotrexate: results from the double‐blind treatment phase of a randomized placebo‐controlled trial of tocilizumab safety and prevention of structural joint damage at one year. Arthritis Rheum 2011;63:609–21. [DOI] [PubMed] [Google Scholar]
- 27. Kremer JM, Cohen S, Wilkinson BE. et al. A phase IIb dose‐ranging study of the oral JAK inhibitor tofacitinib (CP‐690, 550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum 2012;64:970–81. [DOI] [PubMed] [Google Scholar]
- 28. Maini R, St Clair EW, Breedveld F. et al. Infliximab (chimeric anti-tumour necrosis factor a monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. Lancet 1999;354:1932–9. [DOI] [PubMed] [Google Scholar]
- 29. Shiroky JB, Neville C, Esdaile JM. et al. Low‐dose methotrexate with leucovorin (folinic acid) in the management of rheumatoid arthritis: results of a multicenter randomized, double‐blind, placebo‐controlled trial. Arthritis Rheum 1993;36:795–803. [DOI] [PubMed] [Google Scholar]
- 30. Smolen JS, Beaulieu A, Rubbert-Roth A. et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008;371:987–97. [DOI] [PubMed] [Google Scholar]
- 31. Strand V, Cohen S, Schiff M. et al. Treatment of active rheumatoid arthritis with leflunomide compared with placebo and methotrexate. Arch Intern Med 1999;159:2542–50. [DOI] [PubMed] [Google Scholar]
- 32. Tanaka Y, Suzuki M, Nakamura H, Toyoizumi S, Zwillich SH; Tofacitinib Study Investigators. Phase II study of tofacitinib (CP‐690, 550) combined with methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate. Arthritis Care Res 2011;63:1150–8. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka Y, Emoto K, Cai Z. et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol 2016;43:504–11. [DOI] [PubMed] [Google Scholar]
- 34. van der Heijde D, Tanaka Y, Fleischmann R. et al. Tofacitinib (CP‐690, 550) in patients with rheumatoid arthritis receiving methotrexate: twelve‐month data from a twenty‐four–month phase III randomized radiographic study. Arthritis Rheum 2013;65:559–70. [DOI] [PubMed] [Google Scholar]
- 35. van Ede AE, Laan RF, Rood MJ. et al. Effect of folic or folinic acid supplementation on the toxicity and efficacy of methotrexate in rheumatoid arthritis: a forty‐eight–week, multicenter, randomized, double‐blind, placebo‐controlled study. Arthritis Rheum 2001;44:1515–24. [DOI] [PubMed] [Google Scholar]
- 36. Weinblatt ME, Kremer JM, Bankhurst AD. et al. A trial of etanercept, a recombinant tumor necrosis factor receptor: fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med 1999;340:253–9. [DOI] [PubMed] [Google Scholar]
- 37. Weinblatt ME, Keystone EC, Furst DE. et al. Adalimumab, a fully human anti–tumor necrosis factor α monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003;48:35–45. [DOI] [PubMed] [Google Scholar]
- 38. Weinblatt ME, Mease P, Mysler E. et al. The efficacy and safety of subcutaneous clazakizumab in patients with moderate‐to‐severe rheumatoid arthritis and an inadequate response to methotrexate: results from a multinational, phase IIb, randomized, double‐blind, placebo/active‐controlled, dose‐ranging study. Arthritis Rheumatol 2015;67:2591–600. [DOI] [PubMed] [Google Scholar]
- 39. Weinblatt ME, Fraser PA.. Elevated mean corpuscular volume as a predictor of hematologic toxicity due to methotrexate therapy. Arthritis Rheum 1989;32:1592–6. [DOI] [PubMed] [Google Scholar]
- 40. Andersen LS, Hansen EL, Knudsen JB. et al. Prospectively measured red cell folate levels in methotrexate treated patients with rheumatoid arthritis: relation to withdrawal and side effects. J Rheumatol 1997;24:830–7. [PubMed] [Google Scholar]
- 41. Shea B, Swinden MV, Tanjong Ghogomu E. et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev 2013;5:CD000951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shea B, Swinden MV, Ghogomu ET. et al. Folic acid and folinic acid for reducing side effects in patients receiving methotrexate for rheumatoid arthritis. J Rheumatol 2014;41:1049–60. [DOI] [PubMed] [Google Scholar]
- 43. Salliot C, van der Heijde D.. Long-term safety of methotrexate monotherapy in patients with rheumatoid arthritis: a systematic literature research. Ann Rheum Dis 2009;68:1100–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinblatt ME. Toxicity of low dose methotrexate in rheumatoid arthritis. J Rheumatol Suppl 1985. Dec;12:35–9. [PubMed] [Google Scholar]
- 45. Alarcón GS, Tracy IC, Blackburn WD Jr.. Methotrexate in rheumatoid arthritis: toxic effects as the major factor in limiting long-term treatment. Arthritis Rheum 1989;32:671–6. [DOI] [PubMed] [Google Scholar]
- 46. Hanrahan PS, Scrivens GA, Russel AS.. Prospective long term follow-up of methotrexate therapy in rheumatoid arthritis: toxicity, efficacy and radiological progression. Br J Rheumatol 1989;28:147–53. [DOI] [PubMed] [Google Scholar]
- 47. Gutierrez-Ureña S, Molina JF, García CO, Cuéllar ML, Espinoza LR.. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum 1996;39:272–6. [DOI] [PubMed] [Google Scholar]
- 48. Kremer JM. Major Side Effects of Low-Dose Methotrexate. UpToDate 2018. https://www.uptodate.com/contents/major-side-effects-of-low-dose-methotrexate (25 January 2019, date last accessed).
- 49. Franck H, Rau R, Herborn G.. Thrombocytopenia in patients with rheumatoid arthritis on long-term treatment with low dose methotrexate. Clin Rheumatol 1996;15:163–7. [DOI] [PubMed] [Google Scholar]
- 50.Methotrexate Injection: Professional. https://www.drugs.com/pro/methotrexate-injection.html (25 January 2019, date last accessed).
- 51. Williams HJ, Willkens RF, Samuelson CO. et al. Comparison of low-dose oral pulse methotrexate and placebo in the treatment of rheumatoid arthritis. Arthritis Rheum 1985;28:721–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.