Abstract
Rationale & Objective:
Uromodulin is released by tubular epithelial cells into the serum and lower levels are associated with more severe interstitial fibrosis and tubular atrophy (IF/TA). Low serum uromodulin (sUMOD) levels are associated with mortality and cardiovascular disease. However, little is known about the association of sUMOD with long-term kidney outcomes in older adults, a population with a high prevalence of IF/TA.
Study Design:
Case-cohort study and case-control
studySetting & Participants:
Random subcohort (n=933) and additional cases of end-stage kidney disease and kidney function decline (≥30% decline in estimated glomerular filtration rate (eGFR)) during follow-up of the Cardiovascular Health Study (CHS).
Predictor:
sUMOD Outcomes: ESKD (n=14) from the random subcohort and all additional ESRD cases from outside the random subcohort (n=39) during follow-up (10 years, case-cohort study); kidney function decline of ≥30% eGFR at 9 years of follow-up in individuals with repeated eGFR assessments from the random subcohort (n=56) as well as additional cases (n=123). 224 participants from the random subcohort served as controls (case-control study).
Analytical Approach:
Modified multivariable Cox regression for ESRD and multivariable logistic regression for kidney function decline. Both analyses adjusted for demographics, eGFR, urinary albumin-creatinine ratio, and other kidney disease progression risk factors.
Results:
Mean age of the random subcohort was 78 years, 40% were male, 15% black. Mean sUMOD level was 127±64 ng/ml and eGFR 63±19 ml/min/1.73 m2. In multivariable analysis, each 1 SD higher sUMOD was associated with a 63% lower risk of ESRD (HR, 0.37; 95% CI, 0.14–0.95). In demographic adjusted analyses of kidney function decline, each 1 SD higher sUMOD was associated with a 25% lower odds of kidney function decline (OR, 0.75; 95% CI, 0.60–0.95); after multivariable adjustment, the association was attenuated and no longer significant (OR, 0.88; 95% CI, 0.68–1.14).
Limitations:
Possibility of survival bias in the kidney function decline analysis.
Conclusions:
Higher levels of sUMOD may identify elderly persons at reduced risk for ESRD.
Keywords: Uromodulin, sUMOD, Tamm-Horsfall-Protein, tubular function, chronic kidney disease (CKD), end-stage renal disease (ESRD), cardiovascular disease (CVD), mortality, estimated glomerular filtration (eGFR), kidney function decline, renal end point
Introduction
Chronic kidney disease (CKD) is a major public health problem especially in the elderly population, with approximately 15–20% of US Americans >65 years affected (1). CKD is associated with increased risk for cardiovascular disease and mortality (2); therefore understanding risk factors for CKD progression and development of end-stage renal disease (ESRD) are important.
Estimation of glomerular filtration rate (eGFR) is problematic in elderly persons because the influence of non-renal factors on serum concentrations of creatinine (3). Beyond glomerular filtration, serum creatinine concentrations are influenced by muscle mass, and aging is strongly linked with muscle atrophy and frailty, rending serum creatinine particularly insensitive to losses in kidney function in older persons. In addition, markers of glomerular filtration do not reflect the extent of interstitial fibrosis and tubular atrophy (IF/TA), which is highly prevalent in the elderly population, and has been shown to strongly associate with adverse renal outcomes such as ESRD and kidney function decline in young patients with IgA nephropathy (4, 5) and the general CKD population (6, 7).
However, ascertainment of renal histology is not typically feasible in clinical practice, as biopsies are invasive and not without risk (8). Non-invasive biomarkers to assess tubular health are needed. Uromodulin may be a marker of tubular function; it is expressed only in the thick ascending limb of the loop of Henle and the collecting duct, and then released into both the tubular lumen and the blood (9–11). Serum uromodulin (sUMOD) has been associated with tubular atrophy in CKD (12) and correlates inversely with eGFR (13–15).
A recent study demonstrated that lower levels of sUMOD were associated with incident CKD in patients that underwent coronary angiography (16). sUMOD was also associated with long-term graft survival in kidney transplant recipients (17). However, data are limited on the relation between sUMOD and kidney function decline and development of end-stage renal disease (ESRD) especially in older community-living individuals.
We therefore examined the association of sUMOD with ESRD and kidney function decline in the Cardiovascular Health Study (CHS), a large community-based cohort of older adults.
Methods
Study Participants
CHS is an observational, community-based cohort study, that was started in 1989 and included men and women aged ≥65 years from 4 U.S. communities: Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA (18). A total of 5,888 Medicare eligible participants were enrolled including 5,201 Caucasians and 687 African Americans. All gave informed consent for participation, and local institutional review boards approved the study methods. The baseline examination included a medical history, physical examination, laboratory testing, and assessment for the presence of CVD. Participants were seen for yearly study visits until 1998–1999 and interviewed by telephone between study visits. After 1998–1999, participants were contacted by telephone every 6 months until another study visit in 2005–2006.
Study Design
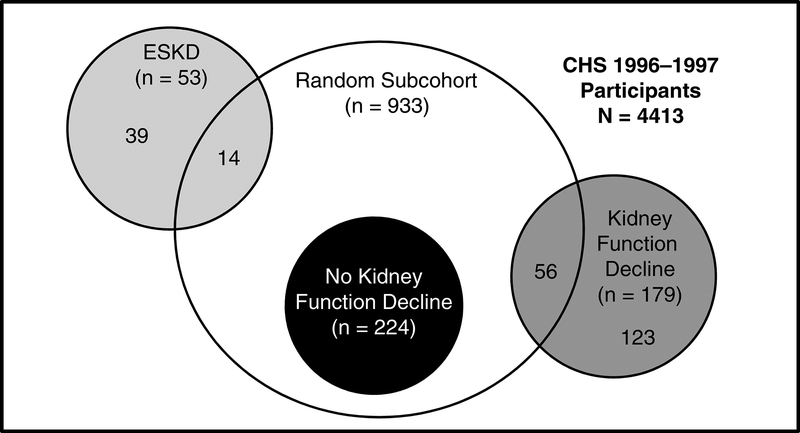
Figure 1 illustrates the sampling method for this study. The baseline visit for this study was 1996–1997 because this was the first time urine was collected in CHS, and adjustment for levels of albuminuria is essential in studies of kidney function decline. A random subcohort of 960 individuals was selected from the total of 4413 who attended this visit; 933 of these individuals had frozen samples available for sUMOD measurement.
Figure 1: Patient sampling for the outcomes end-stage kidney disease (ESKD) and kidney function decline (i.e. ≥30% reduction in estimated glomerular filtration rate from baseline to the follow-up visit 2005–06).
Participants were sampled at the year 9 visit of the Cardiovascular Health Study (CHS). The unshaded circle represents the random subcohort (serving as control for the case-cohort study on ESRD), the light gray circle the cases for the case-cohort study on ESRD, the dark gray circle the cases for the study on CKD progression that returned to follow-up in 2005/2006 and had creatinine measurement at this visit, the black circle the controls for the study on CKD progression that also returned to follow-up in 2005/2006 and had creatinine measurement at this visit.
We studied the association of sUMOD and ESRD using a case-cohort design. This design includes a subcohort randomly selected from the entire cohort. In addition, it includes all cases of ESRD that occurred in participants from outside the random subcohort we selected. This subsampling technique enables an unbiased evaluation of the hazard for an outcome in a cohort without the need to sample the entire cohort (19, 20).
To analyze the association of sUMOD with kidney function decline, we conducted a case-control study. For this analysis we used the same random subcohort we already selected for the ESRD analysis. Within this subcohort every participant that attended a follow-up visit in 2005–06 and had stored serum samples was categorized into cases vs. controls (see definitions of cases and controls below). Furthermore, we supplemented the group of cases with all additional cases from outside of the random subcohort that represented to the 2005–06 follow-up visit.
As defining kidney function decline requires returning and giving blood for creatinine measurement at the 2005–06 visit, and as many individuals had died in the interim, we required cases and controls to have survived, returned to the 2005–06 visit, and have cystatin C measured.
Exposure
Serum samples were obtained at the 1996–1997 study visit and stored at −70 °C until they were thawed for measurement in singlicate. sUMOD measurements were performed at the University of Cincinnati Children’s Hospital Medical Center, using a commercial ELISA (Euroimmun Medizinische Labordiagnostika AG, Lübeck, Germany) as described previously based on the manufacturer’s instructions (13). This assay is based on a colorimetric sandwich immunoassay using a polyclonal antibody against human UMOD as the capture antibody and a biotinylated polyclonal antibody against human UMOD as the detection antibody. Validation data of the ELISA are: intra-assay coefficient of variation (CV) 1.8–3.2%, inter-assay CV 6.6–7.8%, mean linearity recovery 97%, lower limit of detection (LOD) 2.0 ng/ml.
Outcomes
To assess the outcome of ESRD, we merged CHS data with Centers for Medicare and Medicaid Services (CMS) claims data until 06/2009. We used the ESRD eligibility flag for fee for service Medicare that begins on the first day of the fourth month after dialysis initiation. A prior linkage of CHS data with United States Renal Data System (USRDS) had been conducted through 2003 (21), and when compared with the USRDS data the CMS linkage had 70.1% sensitivity (95% CI, 59.4%–79.5%) and 99.9% specificity (95% CI, 99.8%-99.9%) for ESRD. This approach has been used previously (22).
Kidney function decline was defined as a reduction of ≥30% of eGFR from baseline (1996–1997 visit) to the 2005–2006 visit. This threshold was chosen based on published literature, demonstrating that eGFR decline of ≥30% is associated with higher incidence of ESRD and mortality (23, 24). In a sensitivity analysis, we repeated the analysis defining the outcome as an eGFR decline ≥40% (25). eGFR was estimated using the cystatin C-based CKD Epidemiology Collaboration (CKD-EPI) equation (26). Frozen serum samples stored at −70 °C from both visits were available for cystatin C measurements. Measurements were performed at the CHS Core Laboratory in 2006 and 2008, respectively. A particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, now Siemens Healthcare Diagnostics Inc, Deerfield, Illinois) with a BN II nephelometer (BNII, Siemens Healthcare Diagnostics Inc, Munich, Germany) was used. An earlier study has shown that this assay is stable through several freeze-thaw cycles (27). The intra-assay coefficients of variation (CV) ranged between 2.0–2.8% in both measurements, the inter-assay CVs ranged from 2.3 to 3.1 %.
We chose cystatin C instead of creatinine to estimate eGFR due to lack of standardization of the creatinine assay between the two study visits.
Covariates
For the multivariable models, we utilized data on socio-demographic variables (age, sex, race, clinical site, educational status), body-mass-index (BMI), markers of kidney function (eGFR, urinary albumin-creatinine-ratio (UACR)), cardiovascular disease (CVD) risk factors (prevalent diabetes (defined by use of hypoglycemic agents, fasting plasma glucose >126 mg/dl or non-fasting glucose ≥200 mg/dl), self-reported smoking status, systolic blood pressure, serum cholesterol, C-reactive protein), pharmacologic therapy (lipid lowering therapy, antihypertensive medication) and prevalent heart failure (HF) or CVD (i.e. myocardial infarction and/or stroke) at baseline.
Statistical Analysis
For the ESRD analysis, we described the population in the random subcohort overall and across sUMOD quartiles using mean and standard deviation for continuous variables and percentages for binary and categorical variables. We compared the distribution of variables across sUMOD quartiles using χ2-test for categorical variables and a linear trend for continuous variables. We evaluated the association of sUMOD with eGFR using univariable linear regression analysis. Multivariable Cox regression models were used to examine the association of sUMOD with ESRD, using LinYing weighting to accommodate the case-cohort design (28). Post-stratification on age, gender, eGFR, and UACR using generalized Lin-Ying estimator Borgan II did not notably improve the precision of the hazard ratio estimates (29). Generalized additive models were used to graphically study the functional form of sUMOD with splines. We found no departures from linearity.
For the kidney function decline analysis, we described the sample comparing the participants with vs. without kidney function decline with mean and standard deviation for continuous variables and percentages for binary and categorical variables. We used multivariable logistic regression to estimate odds ratios for this outcome.
We evaluated a series of adjusted models for both outcomes: unadjusted, model 1 adjusted for baseline demographic/clinical parameters (age, sex, race, clinical site, BMI and educational status); model 2 added eGFR and UACR; model 3 added diabetes, smoking status, SBP, serum cholesterol, C-reactive protein, lipid lowering therapy, antihypertensive medication use, and prevalent HF and CVD. For the ESRD analysis, we evaluated a potential interaction with age, sex and baseline eGFR in the fully adjusted model. Interactions between continuous variables were defined by a product of the variables. Estimates with p-values <0.05 were considered statistically significant. All analyses were conducted using R, version 3.5.1 (R Core Team (2018), Vienna, Austria).
Results
Population characteristics
In the random subcohort (n=933), the mean age was 78±5 years, 40% were male, 15% were black, eGFR was 63±19 ml/min/1.73 m2, and UACR was 14±4 mg/g (Table 1). The mean serum concentration of sUMOD was 127±64 ng/ml; values ranged from 6 to 634 ng/ml. Participants of the random subcohort were slightly younger and had a lower prevalence of diabetes, hypertension, and heart failure compared to the rest of the participants, but were comparable by sex, race, eGFR and UACR (Table S1). Individuals in the lower sUMOD quartiles of the random subcohort were older, were more likely to be male and Caucasian, had lower eGFR, higher CRP and UACR and had a higher prevalence of diabetes, hypertension, and CVD (Table 1). Each ng/ml higher sUMOD was associated with a 1.51 ml/min/1.73 m2 (95% CI, 1.32–1.71) higher eGFR in univariate linear regression analysis.
Table 1:
Baseline characteristics of participants in the random subcohort stratified by quartiles of sUMOD
| Total Cohort (N=933) |
sUMOD Quartile | p-value | ||||
|---|---|---|---|---|---|---|
| 1 (n=234) | 2 (n=233) | 3 (n=233) | 4 (n=233) | |||
| sUMOD | ||||||
| range (ng/ml) | - | ≤ 83.3 | > 83.3 – 118.4 | > 118.4 – 162.3 | > 162.3 | |
| Mean (ng/ml) | 127.2 (63.6) |
57.5 (17.4) | 100.4 (9.9) | 138.6 (13.6) |
212.4 (51.2) |
|
| Demographics | ||||||
| Age (ys) | 78.1 (4.8) | 79.5 (5.33) | 78.4 (4.95) | 77.4 (4.3) | 77.2 (4.2) | <0.001 |
| Male | 39.7 | 48.7 | 41.6 | 38.2 | 30.0 | <0.001 |
| Black Race | 15.3 | 12.0 | 11.6 | 19.3 | 18.5 | 0.03 |
| Site | 0.2 | |||||
| Wake Forest | 23.2 | 17.1 | 27.0 | 24.9 | 23.6 | |
| UC Davis | 28.5 | 26.9 | 28.3 | 30.5 | 28.3 | |
| Johns Hopkins | 21.8 | 27.4 | 21.0 | 18.5 | 20.2 | |
| Pittsburgh | 26.6 | 28.6 | 23.6 | 26.2 | 27.9 | |
| Laboratory Measures | ||||||
| eGFR <60 ml/min/1.73 m2 | 41.7 | 69.7 | 45.1 | 31.8 | 20.2 | <0.001 |
| eGFR (ml/min/1.73 m2) | 63.4 (18.6) | 49.9 (19.4) | 63.0 (15.7) | 68.0 (15.6) | 72.7 (15.0) | <0.001 |
| UACR>30 mg/g | 20.5 | 33.9 | 19.6 | 15.7 | 11.8 | <0.001 |
| log2 (UACR in mg/g) | 3.8 (1.9) | 4.5 (2.3) | 3.8 (1.8) | 3.5 (1.6) | 3.3 (1.7) | <0.001 |
| Fasting glucose (mg/dl) | 107.4 (34.6) |
113.1 (35.7) |
104.2 (23.4) |
108.1 (39.0) |
104.3 (37.5) |
0.03 |
| log2 (CRP in mg/dl) | 1.3 (1.6) | 1.6 (1.6) | 1.3 (1.6) | 1.3 (1.6) | 1.0 (1.6) | <0.001 |
| Total cholesterol (mg/dl) | 201.4 (38.8) |
196.6 (43.3) |
201.8 (37.8) |
203.3 (38.1) |
204.0 (35.5) |
0.04 |
| Serum albumin (g/dl) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 0.2 |
| CVD risk factors and prevalent CVD | ||||||
| Systolic BP (mmHg) | 137.0 (21.0) |
138.3 (21.7) |
136.3 (21.6) |
136.4 (17.1) |
137.0 (23.0) |
0.6 |
| Diastolic BP (mmHg) | 69.8 (11.0) | 67.5 (13.4) | 69.8 (10.1) | 70.7 (9.9) | 71.1 (9.9) | <0.001 |
| BMI (kg/m2) | 26.9 (4.7) | 27.7 (5.1) | 26.9 (4.9) | 26.8 (4.2) | 26.3 (4.4) | 0.001 |
| Diabetes | 25.3 | 19.7 | 12.0 | 14.2 | 9.4 | 0.002 |
| Hypertension | 60.0 | 71.1 | 60.3 | 59.7 | 48.9 | <0.001 |
| Heart failure | 9.2 | 17.5 | 8.6 | 6.0 | 4.7 | <0.001 |
| CVD | 17.5 | 26.9 | 18.5 | 12.4 | 12.0 | <0.001 |
| Medication Use | ||||||
| Lipid lowering | 12.0 | 13.2 | 12.4 | 10.3 | 12.0 | 0.8 |
| Antihypertensive | 55.4 | 73.5 | 59.2 | 52.8 | 36.1 | <0.001 |
| Lifestyle factors | ||||||
| Smoking | 0.3 | |||||
| Current | 7.4 | 5.2 | 7.4 | 9.7 | 7.5 | |
| Former | 41.6 | 46.5 | 43.0 | 39.2 | 37.4 | |
| Never | 51.0 | 48.3 | 49.4 | 51.1 | 55.1 | |
Continuous variables are presented as mean +/−standard deviation, categorical variables as percentage of total population. Cardiovascular Disease is defined as history of myocardial infarction and/or stroke prior to baseline assessment.
Abbreviations: sUMOD=serum uromodulin, eGFR=estimated glomerular filtration rate, CRP=C-reactive protein, UACR=urinary albumin-creatinine ratio, CVD=cardiovascular disease, BP=blood pressure, BMI=body mass index.
sUMOD and ESRD
During the median follow-up period of 9.8 (IQR, 0.3–13.0) years, 53 participants experienced ESRD. Fourteen of these were from the sub-cohort, the remainder were sampled outside the sub-cohort (Figure 1). One patient with ESRD was excluded because of missing blood sample for sUMOD measurement.
Incidence rates of ESRD in the random subcohort were 1.71 per 1000 person years of follow-up. Of the 14 events that occurred in the random subcohort, 11 (78.6%) were in the lowest sUMOD quartile, and 1, 2, and 0 cases occurred in the other three ascending quartiles. In univariate analysis, each SD (63.6 ng/ml) higher sUMOD was associated with a 94% lower hazard (HR, 0.06; 95% CI, 0.03–0.11) of ESRD (Table 2). In multivariate Cox regression analysis, each SD higher sUMOD was associated with a 63% lower hazard of ESRD (HR, 0.37; 95% CI, 0.14–0.95). We did not observe any relevant interaction with age (p=0.4), sex (p=0.2) or eGFR (p=0.2).
Table 2:
Associations of sUMOD with ESKD
| HR per 1-SD higher sUMODa | |
|---|---|
| Univariate | 0.06 (0.03–0.11) |
| Model 1b | 0.04 (0.02–0.09) |
| Model 2c | 0.42 (0.17–1.01) |
| Model 3d | 0.37 (0.14–0.95) |
There were 14 cases in the subcohort and 39 cases outside of the subcohort.
Abbreviations: SD=standard deviation; eGFR=estimated glomerular filtration rate; UACR=urinary albumincreatinine-ratio; ESKD, end-stage kidney disease.
1 SD=63.6 ng/ml; values in parentheses are 95% confidence intervals
adjusted for demographics (age, sex, race, clinic site, body-mass index, level of education)
adjusted as in model 1 + eGFR & log2(UACR)
adjusted as in model 2 + diabetes, smoking status, systolic blood pressure, serum cholesterol, log2(serum C-reactive protein), lipid-lowering medication, antihypertensive medication, prevalent heart failure and cardiovascular disease (CVD) at baseline
sUMOD and eGFR decline
A total of 1876 participants returned for the follow up visit in 2005–2006. In general, these participants were slightly younger, were more likely to be female, had higher eGFR and lower ACR, and had a lower prevalence of diabetes, HF and CVD at the baseline visit compared to the total cohort at year 9 (Table 1 and Table S2). We included all participants from the random subcohort (n=280; 56 with and 224 without an eGFR decline ≥30% since baseline) and all additional cases from outside the random subcohort (n=123), i.e. a total of 179 participants with an eGFR ≥30% and 224 without (Table 3). There were no significant differences between the 280 individuals from the random subcohort and the 1596 participants not from the random subcohort (Table S2). Comparing participants with and without ≥30% eGFR decline, those with kidney function decline had lower sUMOD levels (125 vs.144 ng/ml, p=0.003) but similar eGFR (67 vs. 69 ml/min/1.73 m2, p=0.2, Table 3). UACR and SBP were higher in those with kidney function decline.
Table 3:
Baseline characteristics of participants included in the kidney function decline outcome analysis
| Total (N=403) | Kidney function decline <30% (n=224) | Kidney Function Decline ≥30% (n=179) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (ys) | 76.4 (3.6) | 76.5 (3.9) | 76.4 (3.3) | 0.9 |
| Male | 39.0 | 37.5 | 40.8 | 0.6 |
| Black Race | 14.6 | 13.8 | 15.6 | 0.7 |
| Site | 0.2 | |||
| Wake Forest | 20.6 | 17.0 | 25.1 | |
| UC Davis | 33.3 | 33.5 | 33.0 | |
| Johns Hopkins | 19.4 | 21.9 | 16.2 | |
| Pittsburgh | 26.8 | 27.7 | 25.7 | |
| Laboratory Measures | ||||
| sUMOD (ng/ml) | 135.6 (63.9) | 143.8 (67.5) | 125.4 (57.6) | 0.003 |
| eGFR <60 ml/min/1.73 m2 | 31.3 | 29.9 | 33.0 | 0.6 |
| eGFR (ml/min/1.73 m2) | 68.0 (17.5) | 69.1 (17.2) | 66.6 (17.7) | 0.2 |
| UACR>30 mg/g | 11.0 | 6.1 | 16.8 | 0.002 |
| log2(UACR) (mg/g Cr) | 3.4 (1.6) | 3.0 (1.2) | 3.7 (1.9) | <0.001 |
| Fasting glucose (mg/dl) | 104.5 (31.7) | 100.8 (24.6) | 109.1 (38.5) | 0.01 |
| log2(CRP) (mg/dl) | 1.3 (1.5) | 1.1 (1.5) | 1.7 (1.6) | <0.001 |
| Total cholesterol (mg/dl) | 201.2 (36.9) | 204.8 (35.4) | 196.8 (38.2) | 0.03 |
| Serum albumin (g/dl) | 3.8 (0.3) | 3.8 (0.3) | 3.8 (0.3) | 0.2 |
| CVD risk factors and prevalent CVD | ||||
| Systolic BP (mmHg) | 136.8 (19.7) | 133.8 (18.8) | 140.6 (20.1) | <0.001 |
| Diastolic BP (mmHg) | 70.5 (11.2) | 70.4 (10.4) | 70.6 (12.3) | 0.9 |
| BMI (kg/m2) | 27.7 (4.5) | 27.2 (4.4) | 28.3 (4.6) | 0.02 |
| Diabetes mellitus | 12.7 | 8.0 | 18.4 | 0.003 |
| Hypertension | 58.9 | 52.5 | 66.9 | 0.005 |
| Heart failure | 6.0 | 5.4 | 6.7 | 0.7 |
| CVD | 11.7 | 8.9 | 15.1 | 0.08 |
| Medication Use | ||||
| Lipid lowering | 14.6 | 12.1 | 17.9 | 0.1 |
| Antihypertensive | 52.9 | 47.8 | 59.2 | 0.03 |
| Lifestyle factors | ||||
| Smoking | 0.7 | |||
| Current | 7.8 | 6.8 | 9.0 | |
| Former | 40.7 | 40.6 | 40.7 | |
| Never | 51.5 | 52.5 | 50.3 |
Continuous variables are presented as mean +/−standard deviation, categorical variables as percentage of total population. Kidney function decline is defined a decrease in estimated glomerular filtration rate ≥30% from baseline to the follow-up visit. CVD is defined as history of myocardial infarction and/or stroke prior to baseline assessment. Abbreviations: sUMOD=serum uromodulin, eGFR=estimated glomerular filtration rate, CRP=C-reactive protein, UACR = urinary albumin-creatinine ratio, CVD=cardiovascular disease, BP=blood pressure, BMI=body mass index.
In univariate analysis, each SD (63.9 ng/ml) higher sUMOD was associated with significantly lower odds (odds ratio (OR), 0.73; 95% CI, 0.59–0.91) for kidney function decline. In multivariate Cox regression analysis, the lower odds per each SD higher sUMOD persisted after adjusting for demographics (OR, 0.75; 95% CI, 0.60–0.95) (Table 4). After further adjustment for eGFR, UACR, and CVD risk factors this result was attenuated and no longer significant (OR, 0.88; 95% CI, 0.68–1.14).
Table 4:
Associations of serum uromodulin with kidney function decline (≥30% decrease in eGFR)
| HR per 1-SD higher sUMODa | |
|---|---|
| Univariate | 0.73 (0.59–0.91) |
| Model 1b | 0.75 (0.60–0.95) |
| Model 2c | 0.81 (0.63–1.04) |
| Model 3d | 0.88 (0.68–1.14) |
There were 179 cases and 224 controls
Kidney function decline is defined a reduction of estimated glomerular filtration rate ≥30% from baseline (1996–1997) for the follow-up visit (2005–2006). Abbreviations: SD=standard deviation; eGFR=estimated glomerular filtration rate; UACR=urinary albumin-creatinine-ratio.
1 SD=63.9 ng/ml; values in parentheses are 95% confidence intervals
adjusted for demographics (age, sex, race, clinic site, body-mass index, level of education)
adjusted as in model 1 + eGFR & log2(UACR)
adjusted as in model 2 + diabetes, smoking status, systolic blood pressure, serum cholesterol, log2(serum C-reactive protein), lipid-lowering medication, antihypertensive medication, prevalent heart failure and cardiovascular disease (CVD) at baseline
When we defined an eGFR decline ≥40% as the outcome variable in the 403 participants selected for the original analysis (i.e. eGFR decline ≥30%), 24 (43%) of the 56 cases from the random subcohort had an eGFR decline ≥40%, whereas 68 (55%) of the 123 additional cases from outside of the random subcohort had eGFR decline ≥40%. sUMOD was associated with the kidney function decline in univariate analysis, however the association was attenuated in the multivariable analysis (Table S3).
Discussion
Our results demonstrate that, in a community-living cohort of older adults, lower sUMOD is associated with development of ESRD independently of eGFR, UACR, and cardiovascular and CKD risk factors. In demographic-adjusted analysis, sUMOD was also associated with kidney function decline; however, after full adjustment this relationship was attenuated and rendered no longer statistically significant.
Whereas urinary uromodulin is thought to have physiological roles in terms of the prevention of urinary tract infection and kidney stone formation (30–35), the function of sUMOD is less-well known, and it remains unclear whether secretion of uromodulin into the blood is only a byproduct of uromodulin expression in the tubular cells. However, serum levels of UMOD decrease in CKD and are directly correlated with eGFR (13, 14); in line with this, we also found a strong association of sUMOD and eGFR. In contrast, uUMOD concentrations are only weakly correlated with eGFR (36, 37). sUMOD may therefore be a better measure of tubular mass and integrity given that sUMOD levels are also directly correlated with tubular pathology (38). sUMOD levels may also have fewer analytic concerns than uUMOD. uUMOD levels show significant intra- and inter- individual variability (9) and uUMOD filaments are modified by duration of storage and freezing (30, 39). In addition, centrifugation and vortex mixing influenc the amount of detectable uUMOD (40). The latter measurement shortcomings have not been reported for sUMOD.
There are several potential explanations for the associations of sUMOD with ESRD and kidney function decline. First, lower sUMOD may reflect the degree of IF/TA, which in turn is associated with kidney disease progression (4–6, 41, 42). Indeed, a prior study has demonstrated that higher levels of sUMOD are associated with lower levels of IF/TA (12), suggesting that sUMOD might be a non-invasive biomarker for IF/TA. Second, sUMOD may identify individuals with reduced capacity to defend against tubular function insults such as AKI (43) and ischemia (44) or maintain homeostasis in systems related to tubular function such as acid-base-balance, which in turn are risk factors for ESRD and kidney function decline (45–47). This is supported by research showing that uromodulin knockout mice develop more severe AKI after ischemia reperfusion injury than wild-type animals (43, 48). The latter can be explained by the immune-modulating effect of uromodulin, which ameliorates acute tubular necrosis both in the ascending limb of Henle and neighboring tubular segments. Likewise, uromodulin is thought to also have an overall immune-modulating effect both in the circulation and the renal interstitium (43), which is supported by clinical studies demonstrating the inverse association of sUMOD with inflammatory parameters (49, 50).
Major strengths of this study are the use of a well-characterized cohort with detailed ascertainment of exposures, covariates and outcomes. We believe this is the first study to evaluate community-living older persons, which has an increased risk for ESRD and earlier stages of CKD, and a high prevalence of IF/TA compared to the general population. This type of cohort reduces the risk of confounding from genetic mutations in the UMOD gene as these patients develop CKD and ESRD in earlier phases of life. Limitations include a storage time of the serum samples >10 years. This might have led to some degree of degradation of sUMOD, although no major impact of storage time on sUMOD levels has been previously documented. If there was degradation of sUMOD in the sample, misclassification of the exposure variable may have occurred and results would be diluted to the null. Our ESRD outcome assessment has a very high specificity but only modest sensitivity, so it is possible we missed some ESRD cases within our cohort. The number of ESRD outcomes is relatively small, which may have limited statistical power. However, in general with an effective sample size of 53 events, a power of 80% and a two-sided type 1 error rate at 5%, the minimal detectable HR in a Cox regression analysis is 0.68 or lower per SD higher continuous exposure. In our fully adjusted model the HR is 0.37, so we believe the statistical power was adequate. Our kidney progression analyses included those who survived to give blood samples at the 2005–06 visit, by necessity, as serum cystatin C concentrations at follow-up were required to assess CKD progression. These results may limit the generalizability to the oldest old (mean age of 85 years at follow-up). Likewise, our results might be confounded by survival bias because neither participants who died before the 2005–06 visit nor participants who did not return to this visit were included in our study. Furthermore, there is a chance of misclassification bias in the kidney function decline analysis and thereby a reduction in statistical power, because the actual time from baseline to follow-up ranged between 8.2 and 9.5 years. Likewise, the sensitivity of our CMS linkage analysis has a sensitivity of 70% to identify ESRD cases, which might lead to misclassification bias, i.e. biasing our results towards the null. However, since we detected a significant association between sUMOD and ESRD, the true association might be even more pronounced. No information on the reason for censoring other than death is available, so we cannot rule out that censoring was in part informative. However, due to the large number of covariates in our multivariable model, we think the bias possibly introduced because of informative censoring is likely of less importance. We noted significant attenuation when adjusting for eGFR and ACR given that these variables are correlated with sUMOD and associated with ESRD, but also raising some concern about residual confounding. This may be particularly true for our cohort since older adults have a higher number of comorbidities. Although we cannot rule out residual confounding we attempted to adjust for all relevant factors associated with kidney function decline (51, 52). We acknowledge that due to the observational design of our study we cannot rule out reverse causality, i.e. reduced eGFR leading to a decline in sUMOD levels over time; however the longitudinal nature of the study makes this less likely. Last, we did not adjust our analysis for genetic variants that have been shown to modify sUMOD levels. However, studies evaluating the association of sUMOD with mortality and CKD were not significantly different after adjustment for SNPs (16, 53).
In conclusion, we demonstrate that sUMOD is independently associated with ESRD, but not eGFR decline ≥30% in a general community cohort of the elderly. In view of the inaccuracy of GFR estimation from creatinine in the elderly, the lack of correlation of IF/TA with eGFR, and the association of IF/TA with prognosis as well as drug handling sUMOD may be a useful measure to be assessed in the geriatric population (54, 55). Future studies should attempt to reproduce these results, and if consistent, focus on the value of markers of IF/TA, including sUMOD, in addition to measures of glomerular filtration in assessing risk stratification for ESRD.
Supplementary Material
Table S1: Baseline characteristics of the total CHS cohort at year 1996–1997, as well as the subgroups with and without sUMOD measurement.
Table S2: Baseline characteristics of participants from the random subcohort with an eGFR available at 2005–2006 visit, compared to the remaining patients that returned to this visit.
Table S3: Associations of sUMOD with kidney function decline ≥ 40%.
Acknowledgements:
A full list of the principal CHS investigators and institutions can be found at http://www.chs-nhlbi.org/pi .
Support: This work was supported by grants from the National Institutes of Aging (NIA) R01AG 027002 (to MJS and MGS) and from the National Institute of Diabetes Digestive and Kidney Diseases (NIDDK) R01 DK098234 (to JHI and MGS). The Cardiovascular Health Study was supported by contracts HHSN268201200036C, HHSN268200800007C, and N01HC-85079 through N01HC-85086, N01HC-35129, N01HC-15103, N01HC-55222, N01HC-75150, N01HC-54133, and N01-HC85239 and grant U01 HL080295 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contributions from the National Institute of Neurological Disorders and Stroke. Additional support was provided by R01AG023629 from the NIA. PD was supported by National Institutes of Health grant P50 DK096418. PSG is supported by National Institutes of Health training grant 5 T32 DK007777–13. The funders of this study have approved the manuscript prior to submission, but did not play a major role in the study design, collection, analysis, interpretation of the data or writing the report.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplementary Material Descriptive Text for Online Delivery
Financial Disclosure: The authors declare that they have no relevant financial interests.
Peer Review: Received _______. Evaluated by 2 external peer reviewers and a statistician, with direct editorial input from a Statistics/Methods Editor and an International Editor, who served as Acting Editor-in-Chief. Accepted in revised form February 28, 2019. The involvement of an Acting Editor-in-Chief was to comply with AJKD’s procedures for potential conflicts of interest for editors, described in the Information for Authors & Journal Policies.
Prior Presentation: Aspects of this work were presented at the Kongress für Nephrologie 2018.
References
- 1.Murphy D, McCulloch CE, Lin F, Banerjee T, Bragg-Gresham JL, Eberhardt MS, et al. Trends in Prevalence of Chronic Kidney Disease in the United States. Annals of internal medicine. 2016;165(7):473–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney international. 2011;79(12):1331–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clinical journal of the American Society of Nephrology : CJASN. 2008;3(2):348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppo R, Lofaro D, Camilla RR, Bellur S, Cattran D, Cook HT, et al. Risk factors for progression in children and young adults with IgA nephropathy: an analysis of 261 cases from the VALIGA European cohort. Pediatr Nephrol. 2017;32(1):139–50. [DOI] [PubMed] [Google Scholar]
- 5.Coppo R, Troyanov S, Bellur S, Cattran D, Cook HT, Feehally J, et al. Validation of the Oxford classification of IgA nephropathy in cohorts with different presentations and treatments. Kidney international. 2014;86(4):828–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenbaum JL, Mikail M, Wiedmann F. Further correlation of renal function with kidney biopsy in chronic renal disease. The American journal of the medical sciences. 1967;254(2):156–60. [DOI] [PubMed] [Google Scholar]
- 7.Yamanouchi M, Hoshino J, Ubara Y, Takaichi K, Kinowaki K, Fujii T, et al. Clinicopathological predictors for progression of chronic kidney disease in nephrosclerosis: a biopsy-based cohort study. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association European Renal Association. 2018. [DOI] [PubMed] [Google Scholar]
- 8.Whittier WL. Complications of the percutaneous kidney biopsy. Advances in chronic kidney disease. 2012;19(3):179–87. [DOI] [PubMed] [Google Scholar]
- 9.Devuyst O, Olinger E, Rampoldi L. Uromodulin: from physiology to rare and complex kidney disorders. Nature reviews Nephrology. 2017;13(9):525–44. [DOI] [PubMed] [Google Scholar]
- 10.Iorember FM, Vehaskari VM. Uromodulin: old friend with new roles in health and disease. Pediatric nephrology (Berlin, Germany). 2014;29(7):1151–8. [DOI] [PubMed] [Google Scholar]
- 11.Scolari F, Izzi C, Ghiggeri GM. Uromodulin: from monogenic to multifactorial diseases. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(8):1250–6. [DOI] [PubMed] [Google Scholar]
- 12.Prajczer S, Heidenreich U, Pfaller W, Kotanko P, Lhotta K, Jennings P. Evidence for a role of uromodulin in chronic kidney disease progression. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association European Renal Association. 2010;25(6):1896–903. [DOI] [PubMed] [Google Scholar]
- 13.Steubl D, Block M, Herbst V, Nockher WA, Schlumberger W, Satanovskij R, et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine. 2016;95(10):e3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Risch L, Lhotta K, Meier D, Medina-Escobar P, Nydegger UE, Risch M. The serum uromodulin level is associated with kidney function. Clinical chemistry and laboratory medicine. 2014;52(12):1755–61. [DOI] [PubMed] [Google Scholar]
- 15.Fedak D, Kuzniewski M, Fugiel A, Wieczorek-Surdacka E, Przepiorkowska-Hoyer B, Jasik P, et al. Serum uromodulin concentrations correlate with glomerular filtration rate in patients with chronic kidney disease. Pol Arch Med Wewn. 2016;126(12):995–1004. [DOI] [PubMed] [Google Scholar]
- 16.Leiherer A, Muendlein A, Saely CH, Brandtner EM, Geiger K, Fraunberger P, et al. The value of uromodulin as a new serum marker to predict decline in renal function. Journal of hypertension. 2018;36(1):110–8. [DOI] [PubMed] [Google Scholar]
- 17.Bostom A, Steubl D, Garimella PS, Franceschini N, Roberts MB, Pasch A, et al. Serum Uromodulin: A Biomarker of Long-Term Kidney Allograft Failure. American journal of nephrology. 2018;47(4):275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Annals of epidemiology. 1991;1(3):263–76. [DOI] [PubMed] [Google Scholar]
- 19.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 20.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. Journal of clinical epidemiology. 1999;52(12):1165–72. [DOI] [PubMed] [Google Scholar]
- 21.Dalrymple LS, Katz R, Kestenbaum B, Shlipak MG, Sarnak MJ, Stehman-Breen C, et al. Chronic kidney disease and the risk of end-stage renal disease versus death. Journal of general internal medicine. 2011;26(4):379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ix JH, Biggs ML, Mukamal K, Djousse L, Siscovick D, Tracy R, et al. Urine Collagen Fragments and CKD Progression-The Cardiovascular Health Study. Journal of the American Society of Nephrology : JASN. 2015;26(10):2494–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, et al. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. Jama. 2014;311(24):2518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Inker LA, Matsushita K, Greene T, Willis K, Lewis E, et al. GFR decline as an end point for clinical trials in CKD: a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(6):821–35. [DOI] [PubMed] [Google Scholar]
- 25.Lambers Heerspink HJ, Tighiouart H, Sang Y, Ballew S, Mondal H, Matsushita K, et al. GFR decline and subsequent risk of established kidney outcomes: a meta-analysis of 37 randomized controlled trials. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(6):860–6. [DOI] [PubMed] [Google Scholar]
- 26.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clinical chemistry. 1997;43(6 Pt 1):1016–22. [PubMed] [Google Scholar]
- 28.Lin DY, Ying ZW. Cox regression with incomplete covariate measurements. Journal of the American Statistical Association. 1993;88 (424):1341–9. [Google Scholar]
- 29.Borgan O, Langholz B, Samuelsen SO, Goldstein L, Pogoda J. Exposure stratified case-cohort designs. Lifetime data analysis. 2000;6(1):39–58. [DOI] [PubMed] [Google Scholar]
- 30.Wiggins RC. Uromucoid (Tamm-Horsfall glycoprotein) forms different polymeric arrangements on a filter surface under different physicochemical conditions. Clinica chimica acta; international journal of clinical chemistry. 1987;162(3):329–40. [DOI] [PubMed] [Google Scholar]
- 31.Mutig K, Kahl T, Saritas T, Godes M, Persson P, Bates J, et al. Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner. The Journal of biological chemistry. 2011;286(34):30200–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bachmann S, Mutig K, Bates J, Welker P, Geist B, Gross V, et al. Renal effects of Tamm-Horsfall protein (uromodulin) deficiency in mice. American journal of physiology Renal physiology. 2005;288(3):F559–67. [DOI] [PubMed] [Google Scholar]
- 33.Serafini-Cessi F, Monti A, Cavallone D. N-Glycans carried by Tamm-Horsfall glycoprotein have a crucial role in the defense against urinary tract diseases. Glycoconjugate journal. 2005;22(7–9):383–94. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, et al. Progressive renal papillary calcification and ureteral stone formation in mice deficient for Tamm-Horsfall protein. American journal of physiology Renal physiology. 2010;299(3):F469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gudbjartsson DF, Holm H, Indridason OS, Thorleifsson G, Edvardsson V, Sulem P, et al. Association of variants at UMOD with chronic kidney disease and kidney stones-role of age and comorbid diseases. PLoS genetics. 2010;6(7):e1001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garimella PS, Katz R, Ix JH, Fried LF, Kritchevsky SB, Devarajan P, et al. Association of urinary uromodulin with kidney function decline and mortality: the health ABC study. Clinical nephrology. 2017;87(6):278–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garimella PS, Biggs ML, Katz R, Ix JH, Bennett MR, Devarajan P, et al. Urinary uromodulin, kidney function, and cardiovascular disease in elderly adults. Kidney international. 2015;88(5):1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webster AC, Nagler EV, Morton RL, Masson P. Chronic Kidney Disease Lancet; (London, England: ). 2017;389(10075):1238–52. [DOI] [PubMed] [Google Scholar]
- 39.Goodall AA, Marshall RD. Effects of freezing on the estimated amounts of Tamm--Horsfall glycoprotein in urine, as determined by radioimmunoassay. The Biochemical journal. 1980;189(3):533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youhanna S, Weber J, Beaujean V, Glaudemans B, Sobek J, Devuyst O. Determination of uromodulin in human urine: influence of storage and processing. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association European Renal Association. 2014;29(1):136–45. [DOI] [PubMed] [Google Scholar]
- 41.Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet; (London, England: ). 1968;2(7564):363–6. [DOI] [PubMed] [Google Scholar]
- 42.Bunnag S, Einecke G, Reeve J, Jhangri GS, Mueller TF, Sis B, et al. Molecular correlates of renal function in kidney transplant biopsies. J Am Soc Nephrol. 2009;20(5):1149–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El-Achkar TM, McCracken R, Liu Y, Heitmeier MR, Bourgeois S, Ryerse J, et al. Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury. American journal of physiology Renal physiology. 2013;304(8):F1066–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. American journal of physiology Renal physiology. 2008;295(2):F534–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Driver TH, Shlipak MG, Katz R, Goldenstein L, Sarnak MJ, Hoofnagle AN, et al. Low serum bicarbonate and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(4):534–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldenstein L, Driver TH, Fried LF, Rifkin DE, Patel KV, Yenchek RH, et al. Serum bicarbonate concentrations and kidney disease progression in community-living elders: the Health, Aging, and Body Composition (Health ABC) Study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;64(4):542–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al. Acute kidney injury increases risk of ESRD among elderly. Journal of the American Society of Nephrology : JASN. 2009;20(1):223–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.El-Achkar TM, McCracken R, Rauchman M, Heitmeier MR, Al-Aly Z, Dagher PC, et al. Tamm-Horsfall protein-deficient thick ascending limbs promote injury to neighboring S3 segments in an MIP-2-dependent mechanism. American journal of physiology Renal physiology. 2011;300(4):F999–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scherberich JE, Gruber R, Nockher WA, Christensen EI, Schmitt H, Herbst V, et al. Serum uromodulin-a marker of kidney function and renal parenchymal integrity. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association European Renal Association. 2018;33(2):284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jian L, Fa X, Zhou Z, Liu S. Functional analysis of UMOD gene and its effect on inflammatory cytokines in serum of essential hypertension patients. International journal of clinical and experimental pathology. 2015;8(9):11356–63. [PMC free article] [PubMed] [Google Scholar]
- 51.Young BA, Katz R, Boulware LE, Kestenbaum B, de Boer IH, Wang W, et al. Risk Factors for Rapid Kidney Function Decline Among African Americans: The Jackson Heart Study (JHS). American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;68(2):229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Archives of internal medicine. 2009;169(4):342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delgado GE, Kleber ME, Scharnagl H, Kramer BK, Marz W, Scherberich JE. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. Journal of the American Society of Nephrology : JASN. 2017;28(7):2201–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rule AD, Amer H, Cornell LD, Taler SJ, Cosio FG, Kremers WK, et al. The association between age and nephrosclerosis on renal biopsy among healthy adults. Annals of internal medicine. 2010;152(9):561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Putt TL, Duffull SB, Schollum JB, Walker RJ. GFR may not accurately predict aspects of proximal tubule drug handling. European journal of clinical pharmacology. 2014;70(10):1221–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Baseline characteristics of the total CHS cohort at year 1996–1997, as well as the subgroups with and without sUMOD measurement.
Table S2: Baseline characteristics of participants from the random subcohort with an eGFR available at 2005–2006 visit, compared to the remaining patients that returned to this visit.
Table S3: Associations of sUMOD with kidney function decline ≥ 40%.