Supplemental Digital Content is available in the text.
Keywords: chronic critical illness, cohort study, persistent critical illness, postdischarge mortality, prolonged mechanical ventilation
Objectives:
We aimed to understand the prevalence, timing of onset, resource use, and long-term outcomes of patients who developed persistent critical illness in a national dataset.
Design:
Retrospective cohort. Using a physiologic risk adjustment model from ICU admission, we examined the relative ability of acute (related to reason for ICU presentation) and antecedent (demographics, comorbidities) characteristics to discriminate hospital mortality models. Persistent critical illness was defined as the point during an ICU stay when, at the population-level, patients’ acute diagnoses and physiologic disturbance are no longer more accurate at discriminating who survives than are baseline demographics and comorbidity. We examined the change across ICU stay in the relative discrimination of those characteristics, and short-term (in-hospital and 30 d after admission) and medium-term (90 d after admission) survival. Finally, we analyzed the changes in the population definition of persistent critical illness over time.
Setting:
Patients admitted as level 3 to Scottish ICUs between 2005 and 2014.
Patients:
Seventy-two–thousand two-hundred fifty-three adult level 3 ICU admissions in 23 ICUs across Scotland.
Interventions:
None.
Measurements and Main Results:
The onset of persistent critical illness, occurs at an average of 5.0 days (95% CI, 3.9–6.4 d) across this dataset. The crossing point increased across the decade, by an average of 0.36 days (95% CI, 0.22–0.50 d) per year. In this dataset, 24,425 (33.8%) remained in the ICU long enough to meet this greater than 5-day definition of persistent critical illness. The care of such patients involved 72.3% ICU days used by any level 3 patient; 46.5% of all Scottish ICU bed-days were after day 5. Although rates of 30 days after admission survival rose dramatically during the decade under study, these rates were similar for those with shorter or longer ICU stays, as were the rates of 90-day survival among those who survived at least 30 days.
Conclusions:
Persistent critical illness occurred in one in three ICU patients in Scotland. These minority of patients accounted for disproportionate hospital resources but did not have worse 30- or 90-day postadmission survival.
One reason why patient might fail to either promptly recover or quickly die of critical illness has been termed persistent critical illness (PerCI)—the development of cascading new organ failures that render the ICU patient’s original reason for requiring critical care (1–4) less prognostically important. It has been hypothesized that the onset of PerCI, at a population-level, can be identified as the point during an ICU stay when patients’ acute diagnoses and physiologic disturbance on ICU presentation are no longer more accurate at discriminating who will live from who will die than are patients’ baseline demographics and comorbidity (5, 6).
Using this definition, the population-onset of critical illness was found to occur after ICU day 10 in Australia and New Zealand during 2000–2014 and after ICU day 9 in Alberta, Canada, during 2012–2014 (5, 6). Such patients who become persistently critically ill (5% in Australia and New Zealand and 16% Alberta meet this population-level definition) accounted for large numbers of ICU bed-days (33% and 54%, respectively).
The generalizability of these findings to other health systems is unclear. Australia, New Zealand, and Alberta are considered to have relatively high numbers of critical care beds relative to both population and other hospital beds. In contrast, many systems operate with fewer critical care beds, or with different organizing principles. The United Kingdom is often considered a system with fewer critical care beds and uses a level 2 (1:2 nurse/patient ratio) and level 3 (1:1 nurse/patient ratio) distinction which is less common in Australia, New Zealand, and Canada. The prevalence, timing of onset, resource use, and outcomes of PerCI across such a system are not yet known.
Therefore, we examined 10 years of prospectively collected data in Scotland (Scottish Intensive Care Society Audit Group [SICSAG] database). We used a complete, nationally linked data set from 2005 to 2014 across all 23 public ICUs, who input data on each individual admission into this database. We closely replicated methods used in Australia, New Zealand, and Alberta to allow comparability, while also providing, for the first time, information on change in the population-onset of PerCI over time, and post-ICU survival.
MATERIALS AND METHODS
All data were anonymized and part of routinely collected data across Scotland. As a result, ethics approval was sought and deemed not necessary. Approvals were obtained from SICSAG as well as the Scottish National Data Governance Body (Privacy Board Committee, Information Services Division).
Variables
The SICSAG dataset included prospective entry of physiologic variables including admitting specialty, age, gender, Acute Physiology and Chronic Health Evaluation (APACHE) II scoring, length of stay (LOS), length of mechanical ventilation, cardiovascular support, renal replacement therapy, and ICU survival and hospital survival. This dataset was then linked to the Scottish death records dataset.
All patients over the age of 16 years and who were level 3 patients were included in the analysis. Level 3 patients, per the U.K. Intensive Care Society’s definition, require multiple organ support or invasive respiratory support. Within the U.K. context, level 3 patients require a critical care environment in the acute hospital setting. Within this dataset, all patients had at least 1 day of level 3 care, although not necessarily their first ICU day. Each distinct admission to the ICU was included in the data as we are not able to distinguish “readmissions” from first visits; note that this is different from prior analyses, which have excluded patients with previous ICU stays from the denominator at risk for developing PerCI (4–7).
Demographic data, diagnoses, and risk-adjustment data were obtained from the SICSAG database. Additional information about the organization of Scottish critical care services is available at http://www.sicsag.scot.nhs.uk.
Statistical Analysis
All statistical analyses were carried out in R Version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria), using Multivariate Adaptive Regression Splines (MARS) models from the Earth R package Version 4.7.0 (https://CRAN.R-project.org/package=earth) and summary statistics from the Tangram R package Version 0.6.2 (https://CRAN.R-project.org/package=tangram) (8).
For simple comparison statistics, Kruskal-Wallis tests were applied to all numeric comparisons and Pearson chi-square tests to all categorical covariates with a p value of 0.05 considered significant.
Conceptually, the goal of the primary analyses was to identify the population-level crossing point at which acute characteristics no longer better discriminate who will live from who will die than do antecedent characteristics. In order to do so, we used an objective, automated approach to build the best possible risk-of-hospital-death prediction model using each of the two sets of variables separately. Then we ran the two separate models on the patients still in the ICU at a given LOS and compared their discrimination in those patients as the area under the receiver operating characteristic curve (AUROC, which is equivalent to the C-statistic).
More specifically, initially, a full logistic MARS model was built combining both the acute and antecedent covariates, enabling a complete risk of “hospital” mortality to be calculated across all patients. Note that the MARS model automatically allowed nonlinearities in continuous variables. All included variables are shown in Appendix A (Supplemental Digital Content 1, http://links.lww.com/CCX/A159); the exact nonlinear weightings are calculated through this process internally. The full dataset was categorized into hospital risk greater than 66% (high), between 33% and 66% (moderate), and less than 33% (low) groups, as had been done in the past, and displays in-ICU mortality.
For the primary analyses, we focused on “hospital” mortality, since that is less susceptible to variation driven by ICU bed pressures. The main data set was then split into 28 reduced sets based on ICU LOS, each split containing only those patients still in the ICU on or after the given LOS. A 100-fold stratified cross-validation process was then applied to each of these 28 LOS splits creating 100 randomly chosen datasets for each LOS split. This cross-validation process divided each of the 100 created datasets into a set of two thirds, one third, training and validation datasets using a stratified approach based on hospital mortality. This randomly chose patients to be in the training and validation sets such that the overall proportion of hospital mortality remained equal to the proportion in the original complete LOS dataset. The cross-validation processes also ensured that a patient was only included in a single validation cohort across any of the 100 random divisions.
To maximize the likelihood of finding a good set of models, imputation was then applied to each of the new training and validation datasets in line with that in previous applications of this technical process (6). All continuous acute physiologic predictors’ single imputation with normal value substitution was performed. For patients with missing ages, the median cohort age was used. Finally, for all categorical variables, the missing values were made explicit by adding in a new category, “Missing,” to account for this. The imputation process was applied to each of the cross-validation splits to offset any random bias introduced by the imputation procedures and the cross-validation sampling process.
A logistic MARS model with backward step pruning and interactions was then fit to each of the imputed training datasets. This step removes nonsignificant variables using Akaike information criterion. These models are essentially a logistic regression with stepwise parameter selection allowing for nonlinear trends in the continuous covariates via hinge function splines. Allowing for nonlinearity within the modeling process enables a better fit due to the complex nature of most physiologic processes. Day 1 physiologic and chronic health scores were used in all analyses.
A number of assessment metrics (accuracy, agreement, and discrimination) for each of the model fits were then produced. For each of these statistics, the median as well as the upper and lower 95th percentiles were then calculated across each of the 100-folds to produce a summary of the statistic. The summaries of the discrimination, measured by the AUROC, were then extracted and plotted per day in the given LOS for each acute or chronic model fit. As a sensitivity analysis, this was replicated using only the acute and antecedent risk scores from the day 1 cohort.
The crossing point as well as upper and lower confidence bounds for the crossing point of the acute and antecedent models was solved using a minimization routine (9). This process resulted in two risk models of hospital mortality: one utilizing antecedent covariate and one with the acute covariates. We define the onset of PerCI—consistent with past publications—as the point at which the acute model is no longer better discriminates than the antecedent model.
The yearly crossing point was calculated using the same method as above, with stratification of the dataset by year.
Mortality at 30 and 90 days after admission was also examined among hospital survivors. Of note, we also examined two previously published definitions of PerCI in this analysis (day 8 and 10), in addition to the definition established within Scotland (5, 6).
RESULTS
There were 72,253 adult level 3 ICU admissions in 23 ICUs in Scotland during 2005–2014. Overall, 18,067 died (25.0%) as an inpatient. Thirty-day survival (counting from ICU admission) increased dramatically during the time under study (65.1% in 2005 vs 80.9% in 2013). Level 3 patients’ median age was 61 (interquartile range [IQR], 47–71), and median APACHE II score was 18 (IQR, 11–24). The most common specific diagnoses were bacterial pneumonia, cardiac or respiratory arrest, gastrointestinal perforation or rupture, and self-inflicted overdose (Table 1).
TABLE 1.
Characteristics of the Cohort
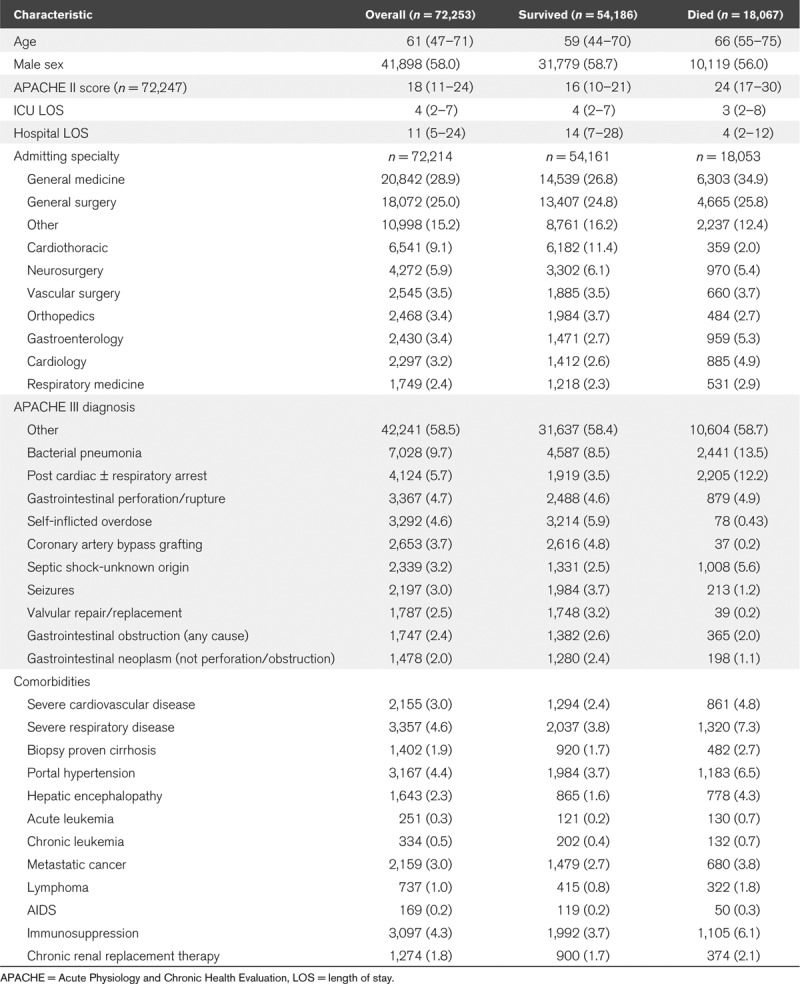
Trajectories of Risk of Death
When patients were divided into higher (> 66% risk of death), medium (34–66%), and lower (≤ 33%) risk according to the predicted hospital mortality from the initial combined MARS model, there were (as expected) substantial differences in early mortality among the groups. (Fig. 1; note these initial descriptive data show ICU mortality). However, among those still in the ICU, those initially in the higher risk group rapidly became less likely to die with each additional day in the ICU—declining from 73.9% in-ICU mortality among all 7,013 higher risk patients to 43.6% in-ICU mortality by day 5 (of 1,963 high-risk patients still in the ICU at day 5, 856 died thereafter) and 38.6% by day 10 (of 1,001 initially higher risk patients still in the ICU on day 10, 386 died thereafter). Indeed, among those who were in the ICU from this initially higher risk group, in-ICU mortality declined to 35% by day 28 (of 171 still in the ICU). In contrast, in the large lower-risk population of 51,698 patients admitted, ICU mortality was 8%, but rose to 13.3% still in ICU on day 10 (of 8,903 initially low-risk patients still in the ICU on day 10, 1,180 died thereafter), plateauing at that level thereafter. Among those who died in the hospital, the median time to death was 3 days (IQR, 1–11 d), decreasing from 4 days (IQR, 1–12 d) in 2005 to 3.5 days (IQR, 1–10 d) in 2013.
Figure 1.
ICU mortality for patients. Unadjusted ICU mortality, stratified by patients’ overall probability of death at admission. Probability of death is based on their ICU admission total predicted risk of death from a logistic regression including both the acute and antecedent risk scores. The numbers of patients between groups are unbalanced as they were chosen for illustrative purposes to span clinically relevant ranges of patients and comparability with past work.
Population-Level Onset of PerCI
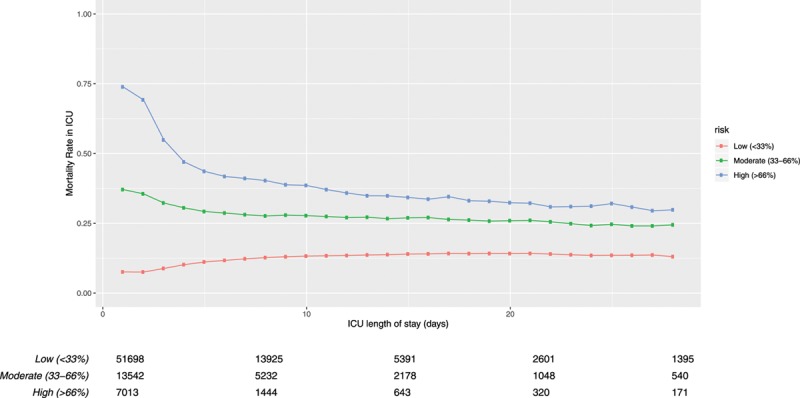
On ICU presentation, characteristics of acute physiology were substantially more effective than were antecedent characteristics at discriminating patients who would and would not die in the hospital. However, the discriminative ability of day 1 acute characteristics fell rapidly, and by ICU day 5.0 (95% CI, 3.9–6.4 d) was not superior to that of antecedent characteristics. (Fig. 2; note that these primary analytic data use in-hospital mortality) When this analysis was replicated in a sensitivity analysis using the only the acute and antecedent risk weights from day 1, the cross-over was at day 4.6 (95% CI, 3.9–5.4 d).
Figure 2.
Predictiveness of acute (ICU presentation) and antecedent characteristics for hospital mortality. Shaded areas are 95% CIs. Appendix 1 (Supplemental Digital Content 1, http://links.lww.com/CCX/A159) contains all characteristics. ROC AUC = area under the receiver operating characteristic curve.
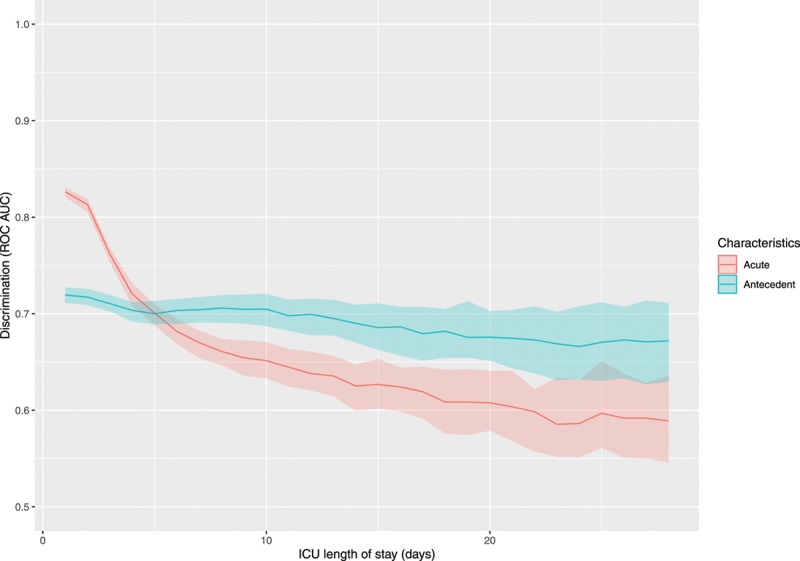
However, there was evidence of substantial change over time in the cross-over (Fig. 3). In 2005 data analyzed alone, the cross-over point was day 5.3 (95% CI, 2.6–6.0 d). In contrast, in 2013 data analyzed alone, the estimated cross-over point was at day 6.9 (95% CI, 4.1–10.0 d). Smoothing over all years, there was evidence that the cross-over point defining the population prevalence of PerCI became progressively delayed across this decade, increasing on average by 0.36 days (95% CI, 0.22–0.50 d) per year during the period under study.
Figure 3.
Estimated Crossing points (d) stratified by year.
Of all level 3 ICU patients, 24,425 (33.8%) remained in the ICU long enough to meet the time-averaged 5-day definition of PerCI—that is, they remained in ICU for greater than 5 days. The care of such patients involved 342,202 of the 473,145 (72.3%) ICU days used by any level 3 patient during that decade. Indeed, 46.5% of all Scottish ICU bed occupancies are used by patients after 5 days. Similar high-levels of use were present for other population-level cutoffs. Of all level 3 ICU patients, 17,698 remained in the ICU for 8 or more days, using 298,937 (63.2%) ICU days; 32.6% of all Scottish ICU bed occupancies are used by patients who reach 8-day landmark. Of all level 3 ICU patients, 13,423 remained in the ICU for 10 or more days, using 262,763 (55.5%) ICU days; 27.2% of all Scottish ICU bed occupancies are used by patients who reached 10 days of admission.
Patients who went on to develop PerCI in Scotland had several differences than those who did not (Appendix B, Supplemental Digital Content 2, http://links.lww.com/CCX/A160). Patients who went on to develop PerCI, defined by an ICU LOS of greater than 5 days, were more acutely physiologically deranged on ICU presentation (APACHE II score of 20 vs 16; p < 0.01), and somewhat more likely to be admitted with general medical or surgical diagnoses. Although statistically significant, there were few clinically meaningful differences in the rates of APACHE II severe comorbidities. Similar results were obtained using an 8- or 10-day cutoff. Despite the relatively modest differences between the groups on any given characteristic, the best-fitting MARS model had an accuracy of 74.2% (95% CI, 73.5–74.7%) and an area under the curve (AUC) discrimination of 0.79 (95% CI, 0.78–0.8) at day 5, 79.7% (95% CI, 79.2–80.1%) and an AUC discrimination of 0.79 (95% CI, 0.78–0.8) at day 8, and 83.5% (95% CI, 83.2–83.9%) and an AUC discrimination of 0.78 (95% CI, 0.77–0.79) at day 10.
Subsequent Outcomes
In Scotland, there were no clinically meaningful differences in short-term or medium-term rates of survival between those who did and did not develop PerCI. For example, of the 47,828 patients who were in ICU for less than equal to 5 days, 35,892 survived (75%) to hospital discharge whilst 18,294 (74.9%) of the longer staying patients survived the hospital. The rates of 30 days after ICU admission survival were likewise similar (35,461/47,828 [74.1%] vs 19,010/24,425 [77.8%]), as were the rates of 90-day survival after ICU admission (34,327/47,828 [71.8%] vs 17,711/24,425 [72.5%]). Similar results were true for 30-day survival (counting from ICU admission) using the cutoff of day 8 (74.5% vs 78.5%) and day 10 (74.6% vs 79.4%), and this lack of difference in survival as a function of ICU LOS was true when analyzing data over time as well. In contrast, there was marked variation over time in 30-day survival among those who stayed at least 5 days (62.5% in 2005 vs 80.1% in 2013), 8 days (62.7% in 2005 vs 80.9% in 2013), and 10 days (74% in 2005 vs 81.6% in 2013).
DISCUSSION
PerCI has been defined, at the population level as the point during an ICU stay when patients’ acute diagnoses and physiologic disturbance on ICU presentation are no longer more accurate at discriminating who will live from who will die than are patients’ baseline demographics and comorbidity. PerCI is quite common in Scotland. The onset of PerCI is earlier in Scotland than in Australia, New Zealand, and Alberta. The timepoint on onset changed in Scotland contemporaneous with a major expansion of ICU capacity and investment in the National Health Service. Yet by either the Scottish population transition point (either after ICU day 5, or the more recent transition point of day 8) or the Australian/New Zealand population transition point (after ICU day 10), caring for patients with PerCI accounts for one-quarter to nearly one-half of the day-to-day work of Scottish ICUs. Despite the quite high short-term mortality of these persistently critically ill patients (25%), their subsequent 30- and 90-day mortality if discharged alive was high but by no means uniformly fatal.
These data extend the emerging story of PerCI in several ways. First, they replicate the core finding of past work that there is a point in the ICU stay, for which prognostic relevance of the driver for ICU admission diminishes—and that this transition occurs at a clinically meaningful timepoint and happens commonly (4–7). The bedside clinical implication of this finding is not that it should be used to prognosticate for any given patient; instead, it has been argued, the clinical implication of this finding may be that there are groups of patients are at increased risk of iatrogenesis, and that iatrogenesis may be caused by decision-making anchoring on the patient’s diagnosis and prognosis as defined at ICU admission, rather than updating to reflect the patients’ dynamically changing status (10). These data show a relatively rapid increasing statistical discriminatory capacity of admission characteristics in comparison to other populations in which this phenomenon has been examined (7, 11).
Second, these results show an important and previously undescribed change in the population cross-over defining critical illness in the context of a rapidly changing healthcare system. It has been unclear to what extent PerCI represents an unfolding of pathology in patients over time, and to what extent its onset is modifiable by changes in care. These data suggest—but do not prove—that the onset of PerCI may be modified by care practices and/or the organization of critical care services. There is an urgent need to understand to what specific practices drive this change, and whether PerCI is more common at higher quality hospitals (representing an unfortunate “legacy of critical illness”) or instead represent another measure of imperfectly delivered care (12).
At the same time, artifactual differences resulting from differences in data cannot be fully excluded. For example, all population-based analyses use timing of ICU admission as an approximation for timing of onset of critical illness and thereby onset of time at risk for developing PerCI. If Scottish patients tend to present to ICU later in their illness (despite adjustment for APACHE II score), and access to ICUs has changed during the time period under study, this would create the appearance of earlier onset via a mechanism that is the inverse of lead-time bias. Similarly, limitations of this dataset prevent us from excluding patients with previous critical illness in the same or prior hospitalizations, which would have similar impacts, or with preexisting neuromuscular disease. Finally, it may be that patients who will die within the hospital die more quickly in some systems than others (13, 14); yet we find a median time to death of 3 days (IQR, 1–11 d) compared with 2.3 days (IQR, 0.9–5.7 d) in Australia and New Zealand (R. Bellomo, unpublished data, 2020) or 8.5 days (IQR, 2.0–25 d) in Alberta (S. Bagshaw, unpublished data, 2020).
A third contribution of these Scottish data is to, for the first time, measure the postdischarge mortality of patients with PerCI as defined here. Australia/New Zealand data were unable to measure this. It is has been proposed that patients with PerCI might be at high risk for futile care; if care for the persistently critically ill were futile, we would expect near uniform death in the weeks to months after hospital discharge. Instead, while mortality is certainly high and ongoing, the majority of patients who survive hospital discharge are still alive 90 days after admission (3). The contribution to such later postdischarge mortality attributable to PerCI needs to be defined in future work; it was found to be quite high for sepsis, but rather more modest for acute hypoxemic respiratory failure (15, 16).
These data have limitations beyond those mentioned above. Most importantly, specific care practices that mediate these outcomes have not been identified in this initial work. Candidate care practices have been identified by a recent systematic review and will require novel data collection to measure (17). Second, we replicated past work in operationalizing the measurement of onset of critical illness (our target concept) as the timing of ICU admission, and the duration of critical illness (our target concept) as the time in level 2 or level 3 ICU care. To the extent that a hardy reluctance to seek care, widely available rapid response teams, or differences between Scottish and Australian/New Zealand/Alberta definition of ICU exist, those may introduce interpretive challenges. Third, PerCI is one concept in the family of concepts that are lumped together under the label of chronic critical illness; measuring other concepts may produce other results (2). Fourth, we have not determined whether the transition from acute to PerCI can be determined at an individual-patient level, a task for which machine learning approaches might be particularly suitable.
CONCLUSIONS
In conclusion, patients cared for in 23 Scottish ICUs over a decade often manifested the onset of PerCI when defined on a population basis using methods comparable to past work. These data showed more rapid onset of patients with PerCI, that the timing of onset at the population-level changed across the decade understudy, and that patients after the onset account for a substantial amount of the work of these ICUs. While inpatient mortality was high, postdischarge mortality was not uniformly terminal. Mechanistic insights and new care practices that may drive better outcomes for these patients are urgently needed and could free up substantial ICU resources for other patients.
ACKNOWLEDGMENTS
We thank Scottish Intensive Care Society Audit Group and the ICUs of Scotland.
Footnotes
Drs. Shaw, McPeake, and Quasim acquired the data, designed the study, drafted the original article, and revised the article for significant content. Drs.Viglianti, Pilcher, and Bellomo interpreted revised the article for significant content. Dr. Bagshaw acquired the data and revised the article for significant content. Dr. Iwashyna designed the study, interpreted the data analyses, and revised the article for significant content.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Dr. McPeake’s institution received funding from The Institute of Healthcare Improvement studies Institute (PD-2019-02-16) and the Health Foundation. This work was supported by grants T32 HL7749-25 (to Dr. Viglianti), K12 HL138039 (to Dr. Iwashyna) from the National Institutes of Health (NIH) and National Heart, Lung, and Blood Institute. Dr. Viglianti received support for article research from the NIH. Dr. Bagshaw received funding from Baxter and CNA Diagnostics. Dr. Iwashyna disclosed government work. Dr. Quasim’s institution received funding from the Health Foundation. The remaining authors have disclosed that they do not have any potential conflicts of interest.
This work does not represent the official position of the U.S. Government or the Department of Veterans Affairs.
Ethics approval and consent to participate: All data were anonymized and part of routinely collected data across Scotland. Approvals were obtained from Scottish Intensive Care Society Audit Group as well as the Scottish National Data Governance Body (Privacy Board Committee, Information Services Division).
Availability of data and material: The data that support the findings of this study are available from Scottish Intensive Care Society Audit Group database but restrictions apply to the availability of these data, which were used under a data use agreement for the current study, and so are not publicly available. Data related to this analysis are, however, available from the authors upon reasonable request and with permission of the Scottish Intensive Care Society.
REFERENCES
- 1.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Persistent critical illness characterised by Australian and New Zealand ICU clinicians. Crit Care Resusc. 2015; 17:153–158 [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Towards defining persistent critical illness and other varieties of chronic critical illness. Crit Care Resusc. 2015; 17:215–218 [PubMed] [Google Scholar]
- 3.Iwashyna TJ, Viglianti EM. Patient and population-level approaches to persistent critical illness and prolonged intensive care unit stays. Crit Care Clin. 2018; 34:493–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darvall JN, Boonstra T, Norman J, et al. Persistent critical illness: Baseline characteristics, intensive care course, and cause of death. Crit Care Resusc. 2019; 21:110–118 [PubMed] [Google Scholar]
- 5.Iwashyna TJ, Hodgson CL, Pilcher D, et al. Timing of onset and burden of persistent critical illness in Australia and New Zealand: A retrospective, population-based, observational study. Lancet Respir Med. 2016; 4:566–573 [DOI] [PubMed] [Google Scholar]
- 6.Bagshaw SM, Stelfox HT, Iwashyna TJ, et al. Timing of onset of persistent critical illness: A multi-centre retrospective cohort study. Intensive Care Med. 2018; 44:2134–2144 [DOI] [PubMed] [Google Scholar]
- 7.Viglianti EM, Kramer R, Admon AJ, et al. Late organ failures in patients with prolonged intensive care unit stays. J Crit Care. 2018; 46:55–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.R Core Team R: A Language and Environment for Statistical Computing. 2018, Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- 9.Brent RP. Algorithms for Minimization Without Derivatives. 1973, Englewood Cliffs, NJ: Prentice-Hall [Google Scholar]
- 10.Kajdacsy-Balla Amaral AC, Barros BS, Barros CC, et al. Nighttime cross-coverage is associated with decreased intensive care unit mortality. A single-center study. Am J Respir Crit Care Med. 2014; 189:1395–1401 [DOI] [PubMed] [Google Scholar]
- 11.Viglianti EM, Zajic P, Iwashyna TJ, et al. Neither vitamin D levels nor supplementation are associated with the development of persistent critical illness: A retrospective cohort analysis. Crit Care Resusc. 2019; 21:39–44 [PMC free article] [PubMed] [Google Scholar]
- 12.Sakusic A, Gajic O. Chronic critical illness: Unintended consequences of intensive care medicine. Lancet Respir Med. 2016; 4:531–532 [DOI] [PubMed] [Google Scholar]
- 13.Public Health England: Chapter 4: European Comparisons. Health Profile for England; 2017. Available at: https://www.gov.uk/government/publications/health-profile-for-england/chapter-4-european-comparisons. Accessed June 5, 2019. [Google Scholar]
- 14.McCartney G, Collins C, Walsh D, et al. Accounting for Scotland’s Excess Mortality: Towards a Synthesis. 2011. Available at: https://www.gcph.co.uk/assets/0000/1080/GLA147851_Hypothesis_Report__2_.pdf. Accessed June 5, 2019.
- 15.Prescott HC, Osterholzer JJ, Langa KM, et al. Late mortality after sepsis: Propensity matched cohort study. BMJ. 2016; 353:i2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prescott HC, Sjoding MW, Langa KM, et al. Late mortality after acute hypoxic respiratory failure. Thorax. 2018; 73:618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rose L, Istanboulian L, Allum L, et al. Patient and family centered actionable processes of care and performance measures for persistent and chronic critical illness: A systematic review. Crit Care Explor. 2019; 1:e0005. [DOI] [PMC free article] [PubMed] [Google Scholar]


