Supplemental Digital Content is available in the text.
Keywords: intensive care unit follow-up clinics, peer support, post-intensive care syndrome
Objectives:
To understand from the perspective of patients who did, and did not attend ICU recovery programs, what were the most important components of successful programs and how should they be organized.
Design:
International, qualitative study.
Setting:
Fourteen hospitals in the United States, United Kingdom, and Australia.
Patients:
We conducted 66 semi-structured interviews with a diverse group of patients, 52 of whom had used an ICU recovery program and 14 whom had not.
Interventions:
None.
Measurements and Main Results:
Using content analysis, prevalent themes were documented to understand what improved their outcomes. Contrasting quotes from patients who had not received certain aspects of care were used to identify perceived differential effectiveness. Successful ICU recovery programs had five key components: 1) Continuity of care; 2) Improving symptom status; 3) Normalization and expectation management; 4) Internal and external validation of progress; and 5) Reducing feelings of guilt and helplessness. The delivery of care which achieved these goals was facilitated by early involvement (even before hospital discharge), direct involvement of ICU staff, and a focus on integration across traditional disease, symptom, and social welfare needs.
Conclusions:
In this multicenter study, conducted across three continents, patients identified specific and reproducible modes of benefit derived from ICU recovery programs, which could be the target of future intervention refinement.
Patients recovering from critical illness have substantial problems—new, exacerbated, and preexisting (1–4). Despite the absence of randomized clinical trial-based evidence of efficacy, a number of post-ICU clinics and peer support groups have been, and are being established internationally (5–8). Patients and families continue to attend and engage with such ICU recovery programs, suggesting that both clinicians and patients perceive them as beneficial.
The underlying physiology and psychology of critical illness survivorship is complex and multi-causal (9–11). As such, it has been challenging to integrate reductionist scientific discovery into effective interventions which support patient outcomes (12–14). We adopted another strategy to identify potentially promising facets on interventions to accelerate ICU recovery. Rather than working from a problem-oriented, needs-based approach, we evaluated what patients believed to have been helpful to them during ICU recovery.
We therefore conducted a qualitative study of patients who had, and who had not, attended ICU recovery programs internationally. Our goal was to understand what the most highly beneficial components of an ICU recovery program were from a patient perspective. Although not initially sought in the interviews, we also identified the converse—what patients stated was missing from their recovery, or ineffective in the interventions evaluated.
MATERIALS AND METHODS
Setting and Ethical Approval
The study design and protocol were approved by the Western Health Low Risk Human Research Ethics Panel (Australia), the Vanderbilt University Institutional Review Board (U.S. coordinating site), and the South West (Cornwall and Plymouth) Research Ethics Committee for all U.K. sites.
A review of the literature showed that there was not enough evidence with which to create reliable, closed-ended surveys to gather data. We therefore chose qualitative inquiry (particularly semi-structured individual interviews) to investigate patient experience.
Participants, Sampling, and Recruitment
Sites involved in this study were part of the Society of Critical Care Medicine’s (SCCM’s) THRIVE program. THRIVE was established by the SCCM in 2015 to bring together critical care clinicians to improve patient outcomes after critical illness. Two learning collaboratives, peer support and post ICU clinics, were established via THRIVE and comprised member sites recruited over 4 years. The researchers of this study were involved in both collaboratives.
Within the THRIVE collaboratives, six general models of peer support are utilized and represented within this study (15). All programs involved in the THRIVE ICU follow-up clinic collaborative utilize a multidisciplinary team approach.
Patients attending ICU recovery programs were asked to participate in the study by professionals facilitating them. Additional study participants were recruited via social media in the United States, which included patients who had and had not been part of THRIVE programs. Inclusion criteria were as follows: patients older than 18 years, who had a critical care experience, and had adequate English language to participate. Exclusion criteria were as follows: ongoing severe neurologic/cognitive impairment, or continued inpatient status in a hospital or rehabilitation setting. We did explicitly include patients with cognitive impairment with capacity. We conducted stratified purposive sampling to promote sociodemographic and geographic diversity in the sample and sought patients from various centers, educational backgrounds, with different employment status. We sought to understand the different time points in the recovery trajectory and recruited patients at different timeframes across the patient journey. As we were attempting to fully understand the complexities of ICU recovery, we purposively recruited individuals who had not participated in a THRIVE program via social media. This step helped contextualize and understand delivered benefits for patients who had received interventions across the THRIVE sites. All patients who were approached by the pathways detailed above agreed to participate.
Data Collection
A semi-structured interview schedule was used (Supplementary File 1, Supplemental Digital Content 1, http://links.lww.com/CCX/A156), with prompting questions. Questions were generated by examining previous literature and through iterative discussion with the research group (J.M., L.M.B., K.J.H., C.M.S.) (3, 5–7, 10–12). Previous literature was examined in relation to the challenges following critical care and the feasibility of developing interventions in this area. All researchers, alongside patient representatives, discussed the interview schedule to ensure consistency. We also invited experts in qualitative research, who were outside the direct research team, to comment on the interview schedule structure and content. Some of the interviewers were known to the participants through their role in direct clinical care. However, interviewers did not interview patients who provided direct clinical care for. Interviews were undertaken by four researchers (J.M., L.M.B., E.H., J.J.). All researchers undertaking the interviews had extensive experience in qualitative methodology and undertaking interviews of this type. Patients were given the opportunity to ask any questions about the process and content of the study before the interview began. At the beginning of each interview, the researcher also explained their professional background and their role in the project. Data were audio-recorded and transcribed verbatim. Interviews were undertaken via telephone and lasted between 20 minutes and 1 hour. No repeat interviews were undertaken. Patients were recruited until data saturation was achieved as decided by the primary analysis team. Interviews with non-THRIVE participants did not include questions about recovery programs; instead questions discussed what was missing from recovery and how the recovery pathway could have been enhanced.
Data Analysis and Rigor
The study design used a thematic content analysis based on the framework by Miles and Huberman (16).
Five key steps were included in the data analysis process (Supplementary File 2, Supplemental Digital Content 2, http://links.lww.com/CCX/A157). First, the primary analysis team (J.M., L.M.B., T.J.I., K.J.H., C.M.S.) undertook preliminary sweeps of the data to familiarize themselves with the content and develop initial coding. No preset or a priori codes were utilized. Second, the team built two coding frameworks, one based around what the challenges of recovery were, and the second based around what the ideal model of ICU recovery looked like. At this stage, any differences in the data generated were examined; for example, international differences and diversity between different age groups. Third, the initial coding was grouped under key themes related to these frameworks and iteratively checked across the interview transcripts. Fourth, three researchers (J.M., L.M.B., T.J.I.) defined and classified the key themes. Finally, the primary analysis team reviewed the conceptual models created and extracted quotations to support the thematic analysis. The lead researchers (J.M., L.M.B., K.J.H., C.M.S.) had monthly meetings to discuss any issues related to study conduct and analysis. An audit trail was uploaded onto a shared, secured site for all researchers involved in the analysis. Member checking was also undertaken pre and post analysis of the data.
The Consolidated Reporting of Qualitative Research checklist (17) was used for this study.
Descriptive statistics were used to present patient demographics. Age is presented as a median with an associated interquartile range (IQR).
RESULTS
Patient Characteristics
Demographic characteristics are summarized in Table 1. Sixty-six patients were interviewed; 13 from four hospitals (19.7%) in the United Kingdom, three from Australia (4.5%) (single site), and 36 from nine hospitals (54.6%) in the United States. Fourteen patients (21.2%) received no ICU recovery program; all of these patients came from the United States. Data from this group were used to identify perceived benefits distinctive to ICU recovery programs. Interviews took place between July 2018 and February 2019. The participating sites and details of the ICU recovery programs which are delivered in these sites are presented in Supplementary File 3 (Supplemental Digital Content 3, http://links.lww.com/CCX/A158).
TABLE 1.
Patient Demographics
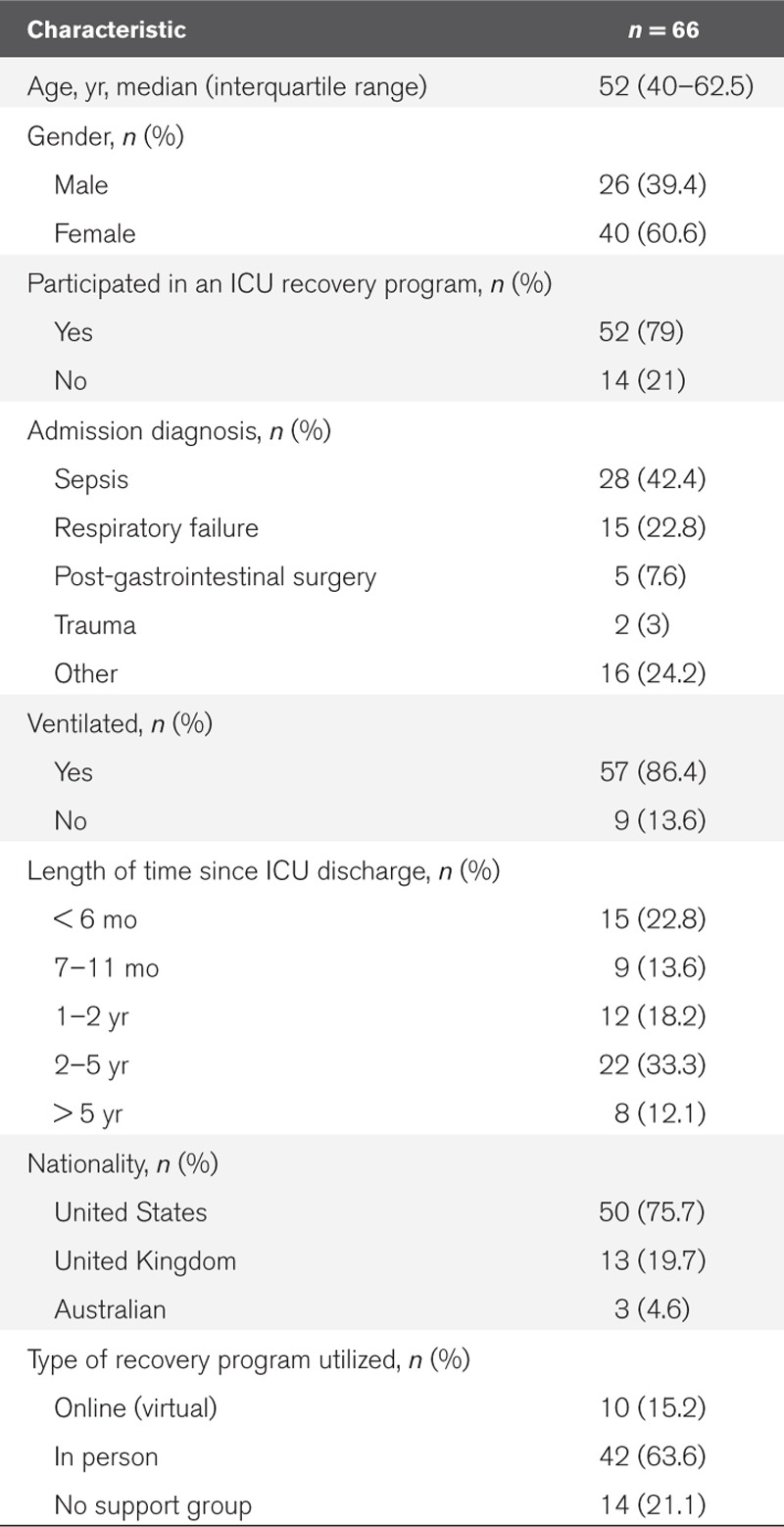
The median age of patients was 52 years (IQR, 40–62.5 yr), 40 were female (60.1%) and 86.4% were ventilated (n = 57) during their critical care stay. Almost half of the patients were admitted to critical care with a diagnosis of sepsis (42.4%; n = 28) (Table 1).
Key Components of ICU Recovery
Patients in ICU recovery programs identified at least five processes by which these programs improved outcomes: 1) Continuity of care; 2) Improving symptom status; 3) Normalization and expectation management; 4) Internal and external validation of progress; and 5) Reducing feelings of guilt and helplessness (Fig. 1). Illustrative quotes for these areas of benefit are presented in Table 2. Of note, some topics discussed crossed over different themes presented. We have deliberately chosen this approach to ensure clarity with our presentation: however, our theoretical figure (Fig. 1) demonstrates this overlap.
TABLE 2.
Quotations to Illustrate Key Mechanisms of Benefit
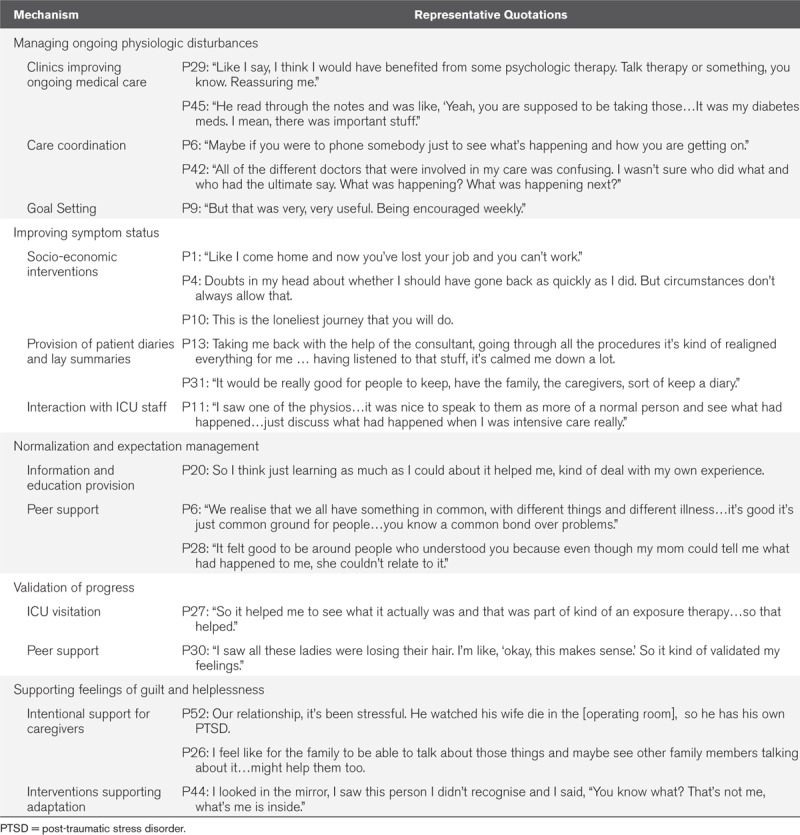
Figure 1.
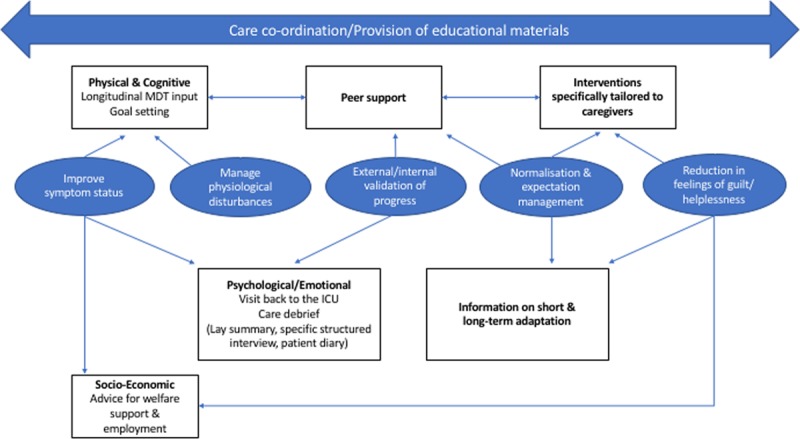
Components of the optimal ICU recovery program. MDT = multi-disciplinary team.
Continuity of Care
Patients described several ways in which clinicians in post-ICU programs improved their physiologic management in the domains of traditional medical care. Meeting such ongoing need for treatment of incompletely resolved medical problems, still present after hospital discharge, was essential to providing sufficient medical stability to allow benefits from other interventions.
P45: “He read through the notes and was like, ‘Yeah, you are supposed to be taking those…It was my diabetes meds. I mean, there was important stuff.”
Many of the ongoing physiologic problems discussed by patients were often hindered by fragmented care, true across respondents from all three nations. Patients speculated that had someone been available to coordinate their care, their recovery would have been more rapid, and symptoms would be managed more effectively; this was discussed in almost every interview:
P3: “[My care] just drops off and then you are sitting there playing a waiting game and you ring and no one knows what’s going on and you’re just waiting, waiting, waiting.”
P43: “I think it would be nice if there was some central main doctor that would be the liaison for all the other various people that you hear from the medical field…if you have a go to person…”
Patients described the potential benefit of starting an ICU recovery program during the hospital stay and then having intermittent contact before any in-person visit—ideally from someone serving as a central coordinator:
P6: “Maybe if you were to phone somebody just to see what’s happening and how you are getting on.”
Improving Symptom Status
The management, treatment, and prevention of the new and ongoing symptoms related to ICU recovery was felt to be fundamental to improving functional trajectories. These symptoms were not just physiologic in nature. Patients also discussed the manifestation of emotional and social symptoms of post-intensive care syndrome. To effectively manage symptoms, there was an emphasis on care delivery which linked health and social care. Patients reported that such care benefited them in several ways.
P52: “Well, I was diagnosed with PTSD…I think linking people up with community resources, not just the medical aspect, but for social, emotional.”
P37: “I was having quite a bit of anxiety and depression…They talked to me about coping mechanisms. Then also put me on medication to help me with that. I also had a social worker come and talk to me. It was just like every area that I needed help with; the ICU team were able to provide.”
Patients reported benefit in the creation of accurate, factual memories related to ICU, which served to reduce perceived signs and symptoms of post-traumatic stress disorder with the aim of improving emotional symptoms which has manifested during recovery. This was achieved by having questions about the ICU answered directly by ICU staff and using structured summaries such as patient diaries or simple patient journey “letters”:
P52: “[with your ICU] FAQ sheet…you get your discharge summary…this is how many litres of blood you got, how many this…something clear that you don’t have to try figure out through reading records.”
P13: “I felt very vulnerable…Taking me back with the help of the consultant, going through all the procedures it’s kind of realigned everything for me…having listened to that stuff, it’s calmed me down a lot.”
Self-management of persistent symptoms played a critical role for patients and was an important component of successful ICU recovery programs, but patients reported that feasible targets (goals of care) were essential. Successful ICU recovery programs helped patients set and meet goals for their own care. Such repeated setting, meeting, and then resetting of goals was considered important for developing patients’ own intrinsic motivation and sense of self-efficacy to take back control of their own life. Longitudinal feedback from centralized staff was felt to help this:
P19: “They let you set goals. When do you want you get out of here? Every Monday they’d come in and say, ‘did your goal change?’. Well my goal initially was to be out for my daughter’s graduation. They’re like, ‘well, that’s pushing it, but we will get it done’.”
Patients valued a sense that things were being done on their behalf by the ICU recovery programs. This could include active listening and ongoing referral to other services, or having symptoms actively managed (i.e., physiotherapy for physical weakness). Patients were less enthusiastic when there was no apparent follow-up to questionnaires being administered and this appeared to be an ineffective component of some recovery programs:
P42: “I didn’t feel much different…It was just checking in with me…other than that; I don’t think it had a big influence on me.”
Normalization and Expectation Management
Patients perceived value in ICU recovery programs when they were able to understand what to expect next in the illness trajectory, this allowed them to shape to plans for recovery based on what was feasible:
P25: “I always want more information. I found that the more information I could get…It was a way to calm myself; it was a way to move to the rational, away from the irrational. I found that helpful.”
Conversely, a lack of anticipatory guidance, often seen in those who had not received any recovery program, led to a sense of frustration, anxiety, and strain. As such, expectation management was described as a key component of recovery programs:
P43: The challenges were not knowing what I would ever be able to do again.
In addition to education and information provision, patients described peer support as an important component of ICU recovery which helped expectation management. Further, peer support reduced feelings of social isolation and helped set realistic goals:
P30: “I saw all these ladies were losing their hair. I’m like, ‘okay, this makes sense.’ So it kind of validated my feelings.”
This was highlighted further by those who had not attended ICU recovery programs, thus these important mechanisms of recovery appeared to be missing:
P38: “I would absolutely want a support group. I would want to be able to meet with other people and find out what they have been through.”
It was noted that peer support was not effective for all patients, either as an in-person or virtual strategy; this subgroup articulated that individual sessions should be available to reduce the psychologic sequelae of social isolation:
P42: For me, the groups setting isn’t really…I’m not really comfortable speaking in a group setting, but if there had been a one to one, that would have been helpful.
Validation of Progress
Patients described value in recognizing their own individual progress (internal validation). External validation of this progress also provided reassurance and improved self-esteem. For example, a return visit to the ICUs and meeting with nurses reframed one patient’s perspective and reenergized their commitment to doing the work of rehabilitation:
P15: “All five of them said how well I looked and that I’d done well. I realised that yeah, I had done extremely well, which was good for me mentally.”
Similarly, for those patients who had not had this opportunity, there appeared to be an important facet missing from recovery:
P20: “There should be some option to go back, or to remain connected with people there…even if you don’t need any clinical help beyond that, just to plan to come back and be able to reconnect with your experience.”
A further important component of ICU recovery programs was the validation patients’ feelings. Many were repeatedly told that they were lucky to be alive and struggled to come to terms with not resuming previous roles:
P36: “When I woke up I was still really upset about my hair loss, my weight loss, being off my hormones and stuff, but I kept getting people and I still do, who say, ‘At least you aren’t dead.' I feel like that kind of overlooks…yes I’m glad to be alive, but also I have these other problems that people are just kind of ignoring.”
Supporting Feelings of Guilt and Helplessness
Intentional, specific rehabilitation for caregivers was sought by patients; this relieved common feelings of guilt. This guilt related to what patients perceived they had “put their relatives through”—both during the critical illness as well as with their ongoing care needs following discharge. Those who had received no recovery program also described challenges when no support was available for family members. In some cases, this had a negative impact on wellbeing:
P13: “[My wife] was with me in the hospital every day…she was really instrumental in my recovery. What I have realised is what I have been through physically, she’s been through mentally as well. It’s been really hard on her.”
Some patients also felt helpless in relation to their symptoms and “new normal.” Centering interventions on adaptations to the challenges associated with recovery was seen as useful to reduce these feelings. For example, one patient discussed how an ICU recovery program had given support with adaptations:
P2: “So I felt fairly dependent upon someone…So I felt pretty trapped in that I couldn’t just hop on the bus and go shopping, because I didn’t have the physical capability of doing that…for me it was proving I am a lot stronger that I thought and your programme realising my strengths and your programme…the knowledge and meeting other people that were going through the same thing.”
DISCUSSION
Patients reported that ICU recovery programs improved care by: treating ongoing physiologic problems; improving symptom status; normalizing their experience and helping them manage their expectations; internally and externally validating their progress in recovery; and reducing feelings of guilt. To our knowledge, this is one of the first international studies which has moved beyond documenting patient needs in this area (18). It also identified certain recurrent design features that patients believed made it possible for these benefits to be delivered, including early involvement (even before hospital discharge), direct involvement of ICU staff, and a focus on integration across traditional disease, symptom, and social welfare needs (Fig. 1).
Beyond the specific components, improvements in transitions and coordination of care throughout the acute recovery period were discussed in almost every interview. Patients discussed significant incidents and challenges they had encountered as a result of fragmented and disjointed care across the recovery pathway, as they have in other studies (19–21). Previous research has focused on the use of generic rehabilitation specialists during the acute hospitalization recovery period (22). The patients interviewed here reported needs for help with more holistic reintegration of the multiple aspects of their life than for the specific focus on muscle strength or joint mobility, which is the focus of much rehabilitation. For example, patients reported the need for integrating health and social care process and linking acute practice with community practitioners; they repeatedly proposed a care coordinator who could facilitate this. Such a coordinator, which has shown benefit in other disease pathways such as chronic obstructive pulmonary disease, may reduce the high number of unscheduled readmissions to acute care and the poor social outcomes which are seen in this patient group (23–27).
The key components noted by patients were delivered through a variety of specific organizational forms. Debate has emerged in the literature about the staffing of ICU recovery programs (28, 29). The present research suggests patients perceived direct benefit from involvement of staff from the ICU alongside specialties related to rehabilitation. ICU staff could answer specific questions about the ICU stay and contextualize functional improvements; this helped reduce distressing memories and thoughts about the critical care stay itself and helped patients understand individual progress. Recent evidence also demonstrates that this process may also facilitate tangible improvements in the critical care environment (30).
This research does not detract from the challenges of obtaining the funding and infrastructure required to establish ICU recovery programs (31, 32). Furthermore, there is still no proof to the efficacy of delivering care that provides any or all the program components identified by patients (12), nor that other aspects of the health system might not meet patient need—although these patients certainly did not perceive alternative sources of support as adequate. However, this study raises caution about some models. Patients sought active rather than passive interventions; ICU recovery programs which were perceived primarily to screen for issues, rather than to treat them, seemed ineffective and indeed frustrating from the perspective of patients already facing a wide array of new problems and providers. Clinicians must reflect critically on intervention development and what the purpose of their service is, if we are to demonstrate measurable changes in patient outcomes.
The strengths of this study include its international, multicenter approach to understanding the optimal recovery program for ICU survivors. There are limitations to these data. Patients who did not attend post ICU programs were recruited through a pathway that still involves some degree of self-designation as needing help, for example, via participation in online ICU chat rooms. Therefore, this subgroup may not be representative of all ICU survivors. The post-ICU programs reported were part of an international collaborative; programs run in isolation may have different effects, as such this may have biased our data. However, each site was using different models of care and this may actually enhance the reproducibility of these results. We have used contemporary qualitative methods, including specific approaches to enhance reproducibility, such as a rigorous analytical process across an international team alongside extensive member checking. Nonetheless, other interpretations may be possible. Finally, we have limited in-patient data on the patient cohort included in this study, which may limit our contextual understanding of individual patients involved.
CONCLUSIONS
This international study has established what the potential efficacious components of successful ICU recovery programs are, and how they could be implemented in future comparative trials. Future work should focus developing innovative approaches to provide such care in a demonstrably cost-effective way.
ACKNOWLEDGMENTS
We would like to acknowledge and thank the following individuals who collectively contributed to the review of the original grant application: Dr. Daniela Lamas, Ms. Kate Cranwell, Dr. Craig French, and Dr. Carol Hodgson. We would also like to acknowledge the wider THRIVE steering group within the Society of Critical Care Medicine.
Supplementary Material
Footnotes
Drs. Haines and Sevin contributed equally to this work as co-senior authors.
Drs. McPeake, Mikkelsen, Iwashyna, Haines, and Sevin involved in conception and design of the study. Drs. McPeake, Boehm, Iwashyna, Haines, and Sevin involved in data extraction and primary analysis of the study. Drs. McPeake, Boehm, and Iwashyna involved in analysis and interpretation of the study. All authors involved in drafting and revising the article for important intellectual content.
Supplemental digital content is available for this article. Direct URL citations appear in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by grant from the Society of Critical Care Medicine (SCCM). The scientific questions, analytic framework, data collection, and analysis were undertaken independently of the funder. The SCCM Council reviewed the article and offered input prior to finalization.
The authors do not necessarily represent the views of the U.S. government or the Department of Veterans Affairs.
Drs. McPeake’s, Boehm’s, Hibbert’s, Bastin’s, Johnson’s, Montgomery-Yates’s, Quasim’s, Haines’s, and Sevin’s institutions received funding from the Society of Critical Care Medicine. Dr. McPeake’s, Dr. Quasim’s, and Mrs. MacTavish’s institutions received funding from the Health Foundation (United Kingdom). Drs. Boehm’s (K12 HL137943) and Hope’s institutions received funding from the American Association of Critical-Care Nurses and the National Heart, Lung, and Blood Institute. Drs. Boehm, Hope, and Jackson received support for article research from the National Institutes of Health. Dr. Iwashyna disclosed government work (K12 HL138039). The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Griffith DM, Salisbury LG, Lee RJ, et al. ; RECOVER Investigators. Determinants of health-related quality of life after ICU: Importance of patient demographics, previous comorbidity, and severity of illness. Crit Care Med. 2018; 46:594–601 [DOI] [PubMed] [Google Scholar]
- 2.Iwashyna TJ, Ely EW, Smith DM, et al. Long term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010; 304:1787–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matte A, et al. Functional disability five years after ARDS. N Engl J Med. 2011; 364:1293–304 [DOI] [PubMed] [Google Scholar]
- 4.McPeake J, Mikkelsen ME, Quasim T, et al. Return to employment after critical illness and its association with psychosocial outcomes. A systematic review and meta-analysis. Ann Am Thorac Soc. 2019; 16:1304–1311 [DOI] [PubMed] [Google Scholar]
- 5.McPeake J, Shaw M, Iwashyna TJ, et al. Intensive Care Syndrome: Promoting Independence and Return to Employment (InS:PIRE). Early evaluation of a complex intervention. PLoS One. 2017; 12:e0188028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sevin CM, Bloom SL, Jackson JC, et al. Comprehensive care of ICU survivors: Development and implementation of an ICU recovery center. J Crit Care. 2018; 46:141–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakhru RN, Davidson JF, Bookstaver RE, et al. Physical function impairment in survivors of critical illness in an ICU recovery clinic. J Crit Care. 2018; 45:163–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haines KJ, Holdsworth C, Cranwell K, et al. Development of a peer support model using experience-based co-design to improve critical care recovery. Crit Care Explor. 2019; 1:e0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prescott HC, Angus DC. Enhancing recovery from sepsis: A review. JAMA. 2018; 319:62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wade DM, Howell DC, Weinman JA, et al. Investigating risk factors for psychological morbidity three months after intensive care: A prospective study. Crit Care. 2012; 16:R192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marra A, Pandharipande PP, Girard TD, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit Care Med. 2018; 46:1393–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schofield-Robinson OJ, Lewis SR, Smith AF, et al. Follow-up service for improving long-term outcomes in intensive care (ICU) survivors. Cochrane Database Syst Rev. 2018; 11:1465–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuthbertson BH, Rattray J, Campbell MK, et al. ; PRaCTICaL study group. The practical study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: A pragmatic randomised controlled trial. BMJ. 2009; 339:b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt K, Worrack S, Von Korff M, et al. ; SMOOTH Study Group. Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: A randomized clinical trial. JAMA. 2016; 315:2703–2711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McPeake J, Hirshberg EL, Christie LM, et al. Models of peer support to remediate post-intensive care syndrome: A report developed by the Society of Critical Care Medicine Thrive International Peer Support Collaborative. Crit Care Med. 2019; 47:e21–e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles MB., Huberman AM. Qualitative Data Analysis: A Sourcebook of New Methods. 1984Thousand Oaks, CA: Sage [Google Scholar]
- 17.Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): A 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007; 19:349–357 [DOI] [PubMed] [Google Scholar]
- 18.King J, O’Neill B, Ramsay P, et al. Identifying patients’ support needs following critical illness: A scoping review of the qualitative literature. Crit Care. 2019; 23:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown SM, Bose S, Banner-Goodspeed V, et al. Approaches to addressing post-intensive care syndrome among intensive care unit (ICU) survivors: A narrative review. Ann Am Thorac Soc. 2019; 16:947–956 [DOI] [PubMed] [Google Scholar]
- 20.Donaghy E, Salisbury L, Lone NI, et al. Unplanned early hospital readmission among critical care survivors: A mixed methods study of patients and carers. BMJ Qual Saf. 2018; 27:915–927 [DOI] [PubMed] [Google Scholar]
- 21.Lim WC, Black N, Lamping D, et al. Conceptualizing and measuring health-related quality of life in critical care. J Crit Care. 2016; 31:183–193 [DOI] [PubMed] [Google Scholar]
- 22.Walsh TS, Salisbury LG, Merriweather JL, et al. ; RECOVER Investigators. Increased hospital-based physical rehabilitation and information provision after intensive care unit discharge: The RECOVER randomized clinical trial. JAMA Intern Med. 2015; 175:901–910 [DOI] [PubMed] [Google Scholar]
- 23.McPeake J, Iwashyna TJ, Devine H, et al. Peer support to improve recovery following critical care discharge: A case-based discussion. Thorax. 2017; 72:856–858 [DOI] [PubMed] [Google Scholar]
- 24.McPeake JM, Henderson P, Darroch G, et al. Social and economic problems of ICU survivors identified by a structured social welfare consultation. Crit Care. 2019; 23:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lone NI, Lee R, Salisbury L, et al. Predicting risk of unplanned hospital readmission in survivors of critical illness: A population-level cohort study. Thorax. 2019; 74:1046–1054 [DOI] [PubMed] [Google Scholar]
- 26.Prescott HC, Langa KM, Liu V, et al. Increased 1-year healthcare use in survivors of severe sepsis. Am J Respir Crit Care Med. 2014; 190:62–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lainscak M, Kadivec S, Kosnik M, et al. Discharge coordinator intervention prevents hospitalizations in patients with COPD: A randomized controlled trial. J Am Med Dir Assoc. 2013; 14:450.e1–e6 [DOI] [PubMed] [Google Scholar]
- 28.Meyer J, Brett SJ, Waldmann C. Should ICU clinicians follow patients after ICU discharge? Yes. Intensive Care Med. 2018; 44:1539–1541 [DOI] [PubMed] [Google Scholar]
- 29.Sevin CM, Jackson JC. Post-ICU clinics should be staffed by ICU clinicians. Crit Care Med. 2019; 47:268–272 [DOI] [PubMed] [Google Scholar]
- 30.Haines KJ, Sevin CM, Hibbert E, et al. Key mechanisms by which post-ICU activities can improve in-ICU care: Results of the international THRIVE collaboratives. Intensive Care Med. 2019; 45:939–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haines KJ, McPeake J, Hibbert E, et al. Barriers and facilitators to implementing ICU follow-up clinics and peer support groups. Crit Care Med. 2019; 47:1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herridge MS. Long-term outcomes after critical illness: Past, present, future. Curr Opin Crit Care. 2007; 13:473–475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


