Abstract
At the time this article was written, the World Health Organization had declared a global pandemic due to the novel coronavirus disease 2019, the first pandemic since 2009 H1N1 influenza A. Emerging respiratory pathogens are a common trigger of acute surge events—the extreme end of the healthcare capacity strain spectrum in which there is a dramatic increase in care demands and/or decreases in care resources that trigger deviations from normal care delivery processes, reliance on contingencies and external resources, and, in the most extreme cases, nonroutine decisions about resource allocation. This article provides as follows: 1) a conceptual introduction and approach to healthcare capacity strain including the etiologies of patient volume, patient acuity, special patient care demands, and resource reduction; 2) a framework for considering key resources during an acute surge event—the “four Ss” of preparedness: space (beds), staff (clinicians and operations), stuff (physical equipment), and system (coordination); and 3) an adaptable approach to and discussion of the most common domains that should be addressed during preparation for and response to acute surge events, with an eye toward combating novel respiratory viral pathogens.
Keywords: capacity strain, coronavirus disease 2019, pandemics, preparedness and response
At the time this article was written, the World Health Organization (WHO) had declared a global pandemic due to the novel coronavirus disease 2019 (COVID-19) (1), the first pandemic since 2009 H1N1 influenza A. Emerging respiratory pathogens are a common trigger of acute surge events—the extreme end of the healthcare capacity strain spectrum in which there is a dramatic increase in care demands and/or decreases in care resources that trigger deviations from normal care delivery processes, reliance on contingencies and external resources, and, in the most extreme cases, nonroutine decisions about resource allocation. Novel respiratory viral pandemics remain the greatest threat to massive societal disruption (2), and in the past 2 decades, three novel coronaviruses have made the jump from animals to humans and caused significant morbidity and mortality: severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus, and now SARS-CoV-2 causing COVID-19 (3). This article provides as follows: an introduction to the concept of healthcare capacity strain; a framework for considering key resources during an acute surge event; and an adaptable approach to and discussion of the most common domains that should be addressed during preparation for acute surge events, with an eye toward combating novel respiratory viral pathogens.
CONCEPTUAL MODEL OF HEALTHCARE CAPACITY STRAIN
Healthcare capacity strain is a clinical operations concept that describes threats to the ability of a given care delivery unit—a care team, ward, unit, hospital, or health system—to provide high-quality care, or at least the standard of care, to all patients who require medical attention at a specific point in time or over a specific duration of time (4).
Healthcare capacity strain can occur due to numerous patient and system factors (5–11), but can be summarized to come from one or more of the following four factors (Fig. 1): 1) increased patient volume, 2) increased patient acuity, 3) special patient care demands, and 4) resource reduction. Patient volume can take the form of prevalence (i.e., occupancy) and frequency (i.e., turnover). Increased patient acuity—such as patients who require ICU admission and invasive mechanical ventilation—can cause strain by either an increase in acuity among the same patient volume (e.g., an influenza season with higher virulence but the same prevalence) or increased per-patient acuity paired with increased patient volume (e.g., a novel viral epidemic that leads to a new population of critically ill patients on top of the normal respiratory viral season burden). Special patient care demands include the added burdens of personal protective equipment (PPE), isolation precautions logistics, staff safety and wellbeing, and other factors, either dependent or independent of acuity, which may require a significant increase in per-patient resource utilization. For example, a single noncritically ill patient under investigation for Ebola virus disease is likely to use far greater resources than a much larger number of patients requiring droplet precautions during a routine respiratory viral season at the same hospital (G.L. Anesi, unpublished observations, 2020). Finally, resource reduction can take the form of either an inability to augment resources to match increases in patient volume, acuity, or special care demands, or an absolute reduction in resources such as due to local or distant infrastructure disruption. Most strain events operate via multiple of these pathways simultaneously, in a way that is at least additive and may be synergistic: a severe respiratory virus that brings both more and sicker patients who require isolation precautions and PPE shortages; a natural disaster that brings a surge in trauma and a concomitant loss of local operational healthcare facilities.
Figure 1.
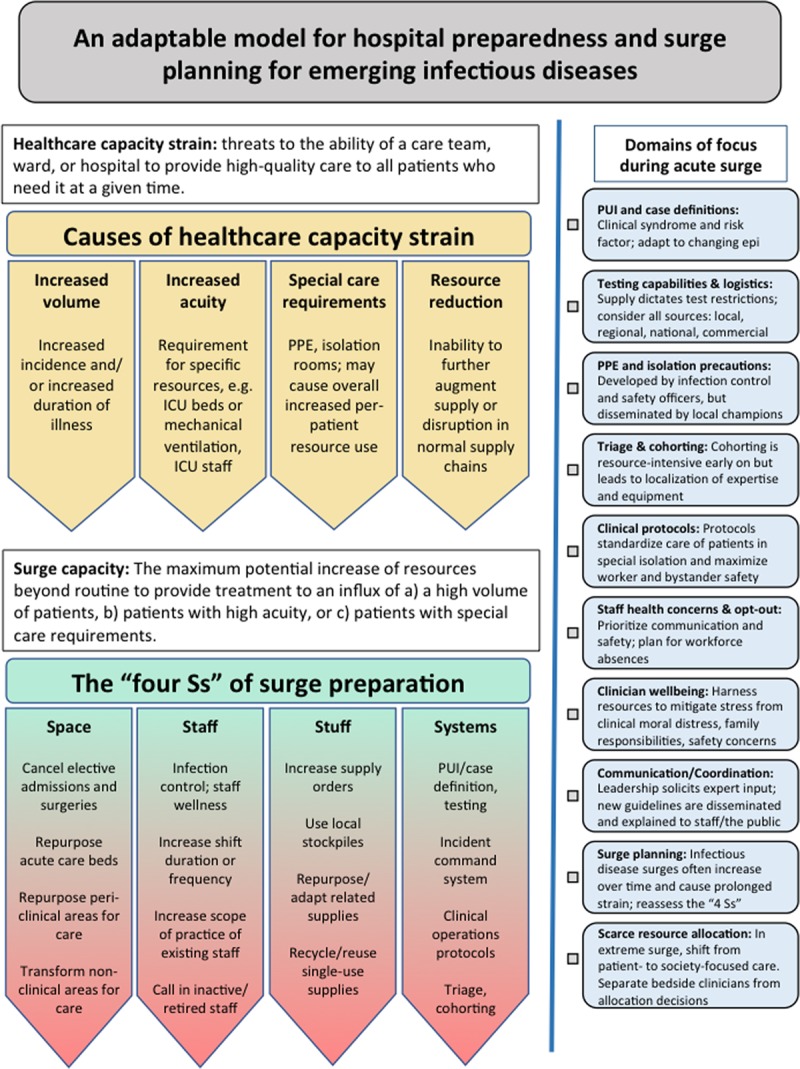
A conceptual and adaptable approach to hospital preparedness for acute surge events due to emerging infectious diseases. Healthcare capacity strain occurs due to increased patient volume, increased patient acuity, special patient care demands, and/or resource reduction. Preparedness and response strategies to combat acute surge events must address the “four Ss”: space (beds), staff (clinicians and operations), stuff (physical equipment), and system (coordination) (Surge capacity themes from Toerper et al [16]). PPE = personal protective equipment.
Healthcare capacity strain also exists along a spectrum (Fig. 2). The center of the spectrum includes the phenomena of random variation (6), where, for example, 1 day happens to be busier than the day prior and the day after, or semi-predictable variations that still fall within fairly normal operating processes, such as the Monday morning emergency department rush from people who did not want to come in over the weekend (12), summer weekend night spikes in traumas (13), or typical influenza and respiratory viral seasons. One extreme of the healthcare capacity spectrum is what may be called “chronic” or “static” strain, and is the situation under which care is delivered in resource-limited settings, domestically and globally, where there is a baseline, persistent mismatch in the supply and demand of care resources, on top of which acute insults may additionally be added (5). The other extreme of the healthcare capacity spectrum is an “acute surge event,” in which there is a dramatic increase in care demands and/or decrease in care resources that trigger deviations from normal care delivery processes, reliance on contingencies and external resources, and, in the most extreme cases, nonroutine decisions about resource allocation. Etiologies of acute surge events include epidemics and pandemics from emerging pathogens, natural disasters, attacks on the public, and primary infrastructure loss (14).
Figure 2.
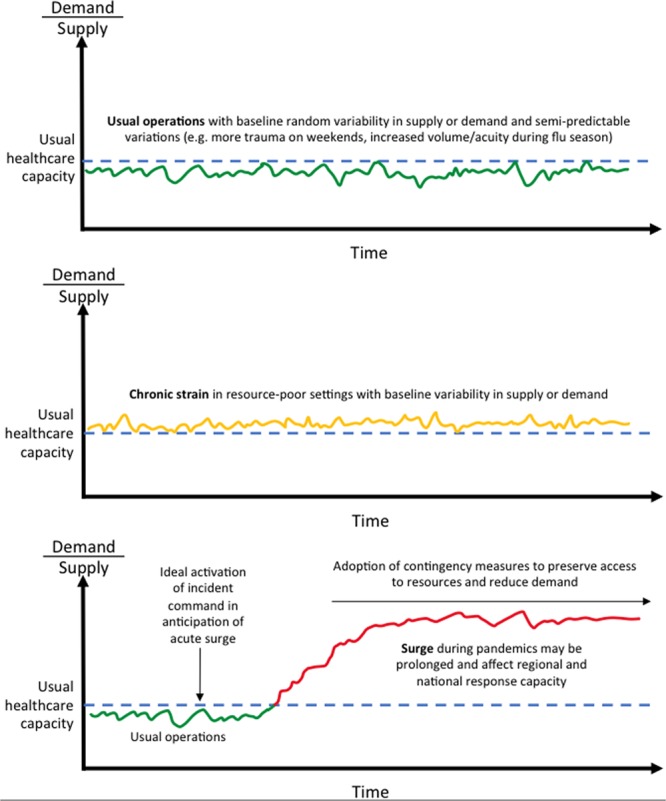
The spectrum of healthcare capacity strain. Random variation and semi-predictable variations can exist in well-resourced settings or on top of a baseline of static strain in resource-limited settings. Acute surge events are defined by a dramatic increase in care demands and/or decrease in care resources that trigger deviations from normal care delivery processes, reliance on contingencies and external resources, and, in the most extreme cases, nonroutine decisions about resource allocation. Etiologies of acute surge events include epidemics and pandemics from emerging pathogens, natural disasters, attacks on the public, and primary infrastructure loss.
ACUTE SURGE EVENTS AND A FRAMEWORK FOR SURGE PREPARATION
During acute surge events, hospitals and health systems must consider their own surge capacity, which is usually defined as the maximum potential increase of resources beyond routine to provide treatment to the sudden unexpected influx of a large number of patients (16). We suggest expansion of the definition of surge capacity to include in addition: 1) preparations for the sudden unexpected influx of any number of patients with a) higher acuity and/or b) special care requirements and 2) preparations for a sudden reduction in resources (i.e., infrastructure loss, supply chain disruption). Although the term “acute surge” is used, these conditions may then be present for weeks or months in a pandemic.
The preparedness canon has long cited the “four Ss” of surge planning: space, staff, stuff, and system (Fig. 1) (16). Space refers to the physical spaces for patient care—bed capacity. Threats to space include as follows: more patients; higher acuity patients that require specialized spaces (e.g., ICU beds); special care requirements (e.g., airborne infection isolation rooms [AIIRs, aka negative pressure rooms]); and infrastructure loss. Methods for increasing bed capacity, roughly in order of increasing disruption to usual practice patterns, include as follows: canceling elective surgeries and admissions; diverting patients to lower-acuity hospitals (i.e., trauma patients to nontrauma centers); repurposing licensed beds (e.g., using wards beds for ICU care); opening unlicensed beds in or near clinical areas (e.g., operating rooms, procedure suites, doubling rooms, hallway beds); opening unlicensed beds in nonclinical areas (e.g., lobbies, multipurpose and educational spaces, surge tents); and reverse triage in which there is a systematic initiation of earlier-than-normal discharges beginning with the least acute patients.
Staff refers to the personnel involved in care delivery and hospital operations. Threats to staff include as follows: more and higher acuity patients who require appropriately trained clinical staff (e.g., critical care) and supportive hospital operations personnel; staff reductions due to infection, injury, quarantine, family care duties (e.g., school closures, sick or injured relatives); care avoidance for personal health reasons (e.g., pregnancy, immunosuppression); and inability to travel to work. Methods for maintaining and increasing staff capacity include as follows: optimal infection control practices; limiting or canceling staff travel; extending shifts of and bringing in off-duty usual staff; deploying nonusual staff by closeness of related training (e.g., using hospitalists to care for critically ill patients); calling in previously trained but inactive staff (e.g., researchers, retirees); deploying unrelatedly trained or nonclinical staff with just-in-time task-specific training and tiered supervision; and accepting external staff of varying degrees of related training without clinical privileges.
Stuff refers to the physical equipment required to deliver care and support care delivery. Threats to stuff include as follows: more and higher acuity patients who require a higher volume of routine equipment (e.g., mechanical ventilators); supply and demand limitations for medications (e.g., sedation medications or antibiotics); increased turnover times for existing equipment (e.g., CT scanners) due to need for adequate disinfection between patients; and special care requirements (e.g., PPE). Methods for increasing stuff include as follows: increasing supply orders, although supply chains are often overwhelmed or interrupted; utilizing local stockpiles; conservation (e.g., reducing inefficient and nonessential use); substitution or adaptation (e.g., repurposing related supplies); and reuse of supplies typically intended for single use.
The fourth S of preparedness—system—refers to the planning and leadership to operationalize and optimize a response effort. During usual operations, hospitals have multifaceted systems that dictate triage and flow of inpatients and hospital equipment through the hospital to meet dynamic demand. During an acute surge event, a centralized incident commander should oversee these systems. This incident command system will continually assess the demands on resources posed by all facets of the healthcare system and with input from clinical and operations leadership, provide targeted recommendations to alter operations to conserve or free up resources for the response. The incident commander will communicate frequently with other local and regional healthcare systems, as well as state and/or national authorities to allow for real-time tracking of a pandemic, the institution of a coordinated response to maximize local or regional capacity early on in the surge, and to mobilize state or national support as the surge continues. Systems are strengthened by: situational awareness of current resources; frequent data tracking during normal operations to provide early warnings of a surge; established early triggers for activation of incident command and institution of surge plans; and preexisting lines for communication between local and regional healthcare entities for early mobilization of resources.
AN ADAPTABLE APPROACH TO HOSPITAL PREPAREDNESS
The following sections of preparedness advice, while not exhaustive, cover the domains that occupy the majority of mental energy and time for individuals coordinating the response to an acute surge event at a specific facility. They provide the points not to miss, the challenges that are likely to arise, and paths to surmounting said barriers (Fig. 1).
Person Under Investigation and Case Definitions
For transmissible pathogens, such as respiratory viruses, that pose a risk to bystander patients and healthcare workers, significant attention must be paid to the screening definition for a person under investigation (PUI), which patients should undergo diagnostic testing, and how cases are declared. Centers for Disease Control and Prevention (CDC) and WHO PUI and case definitions are a starting point but are rarely as clear-cut as desired. PUI definitions frequently begin with the combination of a compatible clinical syndrome and a relevant travel history or direct contact with a suspected or known infection. This definition can change rapidly when regional epidemiology changes as an outbreak spreads, including most dramatically when community transmission—infections with no known specific sick contact—are observed locally or within a hospital. Key factors that influence protocols for diagnostic testing and case declarations include testing availability, testing turnaround time, regional epidemiology, and pathogen-specific factors such as transmissibility, virulence, and PPE requirements.
Testing Capabilities and Logistics
Testing capabilities are central to clinical operations during an infectious surge event. Identifying confirmed cases allows appropriate isolation practices that limit spread to other patients, healthcare workers, visitors, and bystanders, a major tool in “bending the curve” of an epidemic or pandemic. Nearly as important, a negative test allows reallocation of PPE and isolation rooms, thereby conserving resources, and bringing sidelined clinicians back into the workforce. For an emerging pathogen, testing is often first available from centralized governmental public health labs such as the CDC or state departments of health. We recommend hospitals simultaneously pursue all available avenues of testing for a novel pathogen—federal, state, local, commercial, and in-house—as supply (of tests or testing components), demand, and regulatory barriers may be unpredictable. If demand for tests exceeds supply, centralized testing centers may enact restrictions, but local hospitals may have to further restrict which patients are referred for testing. This scarce resource allocation can significantly impact PPE utilization, in-facility transmission, and staff safety and anxiety. Iterative deployment of testing algorithms that are applied by frontline clinical staff is a priority.
PPE and Isolation Precautions
Optimal PPE is that which protects healthcare workers and other patients while not or minimally interfering with the standard of care. Factors that influence PPE practices include public health guidelines, local epidemiology, staff training and comfort, and availability. Education, dissemination, and training for PPE typically begins with infection control or safety officers, but because demand for training can easily overwhelm those individuals or departments, it is best propagated by local champions that take ownership over PPE training and adherence in their unit, and by adopting a teach-the-teacher approach so that rotating staff can feel confident they will have access to training or retraining on a rolling basis. Unit-based trained observers (i.e., doffing monitors) can be a very effective safety mechanism, particularly early in an infectious disease surge where PPE expertise is heterogeneous. Threats to a well-functioning approach to PPE include staff confusion and distrust, shortages, disagreements in public health guidelines, inconsistencies in internal training documents and policies including for handling PUI/test-pending status, and evolving epidemiology including documented nosocomial transmission.
Triage and Cohorting
Cohorting of patients—dedicating hospital units to only confirmed cases and/or PUIs—is both good practice for highly transmissible pathogens (17) and difficult for many hospitals to commit to from a clinical operations standpoint in the lead up to an acute surge event. Most hospitals do not have available or easily emptied hospital units to dedicate to what is often one or a small number of patients early on. The alternative to cohorting is admitting patients to scattered locations throughout the hospital, for example, to the one or two AIIRs on each floor. This leads to disparate patient locations that each require PPE and clinical up-training of local staff, and increases the volume of staff and bystander patients that may be exposed. In contrast, cohorting concentrates equipment and expertise in one or more dedicated locations, preserves PPE, and removes or reduces transmission risk from other areas of the hospital. Isolation unit teams become experts at PPE, clinical management of the given pathogen, and implementing clinical operations protocols newly designed to improve care and safety. Many hospitals that have experienced an infectious acute surge event convert to cohorting and reflect they wish they had done so sooner. Although it is hard to begin cohorting when there are only a small number of patients in-house for financial and logistical reasons, it is decidedly more difficult to transition to cohorting later, when a hospital has many patients spread across multiple units among their routine patients.
Clinical Protocols
When special isolation precautions are required, no clinical task is as it once was in terms of logistics, safety, or staff comfort. Beginning with the most common tasks required in routine bedside care, hospitals should create or adapt existing protocols to guide clinical management including, but not limited to: daily rounding; laboratory collection and handling; bedside procedures; travel to imaging; chest radiographs; clinical emergencies including rapid responses, intubations, and codes; patient visitors and communication; dialysis; and hospital discharge. In parallel with clinical operations, create or adapt guidelines or protocols for clinical management and be prepared for iterative updates as new experience and data emerge. In all of the above, have an eye toward healthcare worker and bystander patient safety. For respiratory viruses, for example, hospital or unit leadership will be asked how to handle routine but aerosol-producing procedures such as nebulized bronchodilators, sputum and tracheal aspirate sampling, bronchoscopy, high-flow nasal cannula, noninvasive ventilation, intubation, and extubation. Cardiopulmonary resuscitation is a particularly sensitive example of a procedure with high risk of exposure to a large number of staff, for which, in the context of a novel pathogen and/or severe resource shortages, hospital leadership will undoubtedly be asked about its continued safety. Involve the relevant stakeholders—bedside providers and nursing, radiology technicians, respiratory therapy—for insight and to obtain buy-in.
Staff Health Concerns and Opt-Out Policies
Infectious disease surges pose unique risks to staffing. Reassignment of staff at higher risk for complications or logistical concerns surrounding quarantine (e.g., sole caregiver) away from care of affected patients can and should be considered. Develop protocols for staff symptom monitoring and create mechanisms for expedited testing of staff to ensure adequate quarantine of those infected and timely return of staff who test negative to the workforce. Hospitals should determine how they will deal with staff who wish to opt-out of patient care, either due to personal discomfort with the risk of transmission, or—in the setting of extreme surge and resource reallocation—moral objections. Identify pools of volunteer staff early who are willing to care for affected patients, especially those who may need additional training to maintain standards of care. It cannot be understated to what degree staff with inadequately addressed concerns about their own health and safety can undermine a coordinated response effort.
Clinician Wellbeing
Healthcare workers experience multiple sources of stress during a surge. They must cope with increased volume and acuity of patients, moral distress due to resource scarcity and reallocation, concern for personal health and the risk of transmission to family, and juggling childcare or other family responsibilities with potential increased demands for their skills at work. Hospitals should provide staff with resources to develop a personal or family safety plan including, but not limited to: alternative living arrangements to reduce transmission to family after a healthcare exposure, back-up plans for child care in case of school closure, increase in availability of clean scrubs to allow for separation of home and work clothing. If possible, arrange for access to counseling during and after the event to mitigate moral distress and post-traumatic stress disorder.
Surge Planning
Although acute surge events, by definition, occur due to the swift influx of new care demands, in a pandemic the surge conditions may be prolonged and continue to increase over time. Hospital should have or create a plan to address the “four Ss” of preparedness: space, staff, stuff, and system. This task often seems overwhelming. It may best be tackled one new care unit at a time. Ask yourself and your institution what it would take—specifically, in terms of the land, labor, and capital—to open a new, cohorted isolation ward or ICU, and where you can do it. Then what would it take to open a second one, and what would be the triggers to do so to keep ahead of demand. Determine your staffing depth and experience, and account for losses due to illness, quarantine, and opt-outs. Identify physical equipment you will need more of or that for which consumption will increase—either from use or diversion—and know that other hospitals are also thinking about ordering more.
Communication and Coordination
Frequent, accurate, and clear communication is essential in a surge event. First, you will need to have a planning and execution communication system that connects workers, local unit-based leadership, and hospital response leadership. This mechanism can provide vital “on the ground” information and questions to hospital and/or incident command leadership. Second, you will need a method to broadly communicate policies and announcements to all staff in ways that are effective at behavior change and reassurance. A searchable centralized document repository may improve compliance with the most updated policies and guidelines, but anticipate having to make frequent updates to such protocols and have a method for communicating those updates without creating confusion. Finally, you will need a public relations mechanism to communicate with patients, families, and the community. Reducing visitation—a clearly beneficial infection control practice in the midst of a surge—for example, must be must carefully messaged to minimize distress and mistrust.
Scarce Resource Allocation
In the most extreme cases, despite achieving maximal surge capacity, demand for care resources can still outpace supply. In these scenarios, the most difficult of healthcare decisions must be made: the allocation of scarce resources, such as mechanical ventilators, to some but not others. Public health emergencies require a shift from patient-focused care, the clear norm in American medicine, to society-focused care—doing the most good for the most people. Bedside clinicians who make medical decisions must be separated from any allocation decisions to protect the patient-physician relationship and absolve the clinician of that weight. Form a committee to determine allocation of scarce resources. Use guidance from state policies and documents developed by other institutions, and tailor them to your hospital, including characteristics of the disease such as load of resource utilization per patient and expected clinical course of the disease. Involve ethicists, either those within your system or via remote consultation in the development of scarce resource policies. Resources may be shared across a city or region to avoid any single hospital or health system from entering crisis standards of care while resources are available nearby or from deploying nontrivially different scare resource allocation policies. Regional coordination across institutions consistently builds public trust and attenuates staff moral distress.
CONCLUSIONS
Acute surge events are chaotic periods where a systematic but highly adaptable preparedness and response strategy is critical. Such an approach must understand where strain comes from—patient volume, patient acuity, special patient care demands, and/or resource reduction—and how to organize a continuously reassessing and evolving response effort across space, staff, stuff, and systems resources.
Footnotes
Dr. Anesi received funding from the Agency for Healthcare Research and Quality K12HS026372. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of any funders including the Agency for Healthcare Research and Quality.
REFERENCES
- 1.World Health Organization. Coronavirus Disease 2019 (COVID-19). Situation Report 64. 2020. Available at: https://www.who.int/docs/defaultsource/coronaviruse/situation-reports/20200324-sitrep-64-covid-19.pdf?sfvrsn=703b2c40_2. Accessed March 24, 2020.
- 2.World Health Organization Global Influenza Strategy 2019-2030. 2019, Geneva, Switzerland: World Health Organization; Available at: https://www.who.int/influenza/global_influenza_strategy_2019_2030/en/. Accessed April 7, 2020 [Google Scholar]
- 3.Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020; 323:707–708 [DOI] [PubMed] [Google Scholar]
- 4.Halpern SD. ICU capacity strain and the quality and allocation of critical care. Curr Opin Crit Care. 2011; 17:648–657 [DOI] [PubMed] [Google Scholar]
- 5.Anesi GL, Gabler NB, Allorto NL, et al. Intensive care unit capacity strain and outcomes of critical illness in a resource-limited setting: A 2-hospital study in South Africa. J Intensive Care Med. 2018. Dec 4. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anesi GL, Liu VX, Gabler NB, et al. Associations of intensive care unit capacity strain with disposition and outcomes of patients with sepsis presenting to the emergency department. Ann Am Thorac Soc. 2018; 15:1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hua M, Halpern SD, Gabler NB, et al. Effect of ICU strain on timing of limitations in life-sustaining therapy and on death. Intensive Care Med. 2016; 42:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerlin MP, Harhay MO, Vranas KC, et al. Objective factors associated with physicians’ and nurses’ perceptions of intensive care unit capacity strain. Ann Am Thorac Soc. 2014; 11:167–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohn R, Harhay MO, Bayes B, et al. Ward capacity strain: A novel predictor of 30-day hospital readmissions. J Gen Intern Med. 2018; 33:1851–1853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kohn R, Harhay MO, Weissman GE, et al. Ward capacity strain: A novel predictor of delays in intensive care unit survivor throughput. Ann Am Thorac Soc. 2019; 16:387–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weissman GE, Gabler NB, Brown SE, et al. Intensive care unit capacity strain and adherence to prophylaxis guidelines. J Crit Care. 2015; 30:1303–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruger JP, Lewis LM, Richter CJ. Patterns and factors associated with intensive use of ED services: Implications for allocating resources. Am J Emerg Med. 2012; 30:1884–1894 [DOI] [PubMed] [Google Scholar]
- 13.Ramgopal S, Dunnick J, Siripong N, et al. Seasonal, weather, and temporal factors in the prediction of admission to a pediatric trauma center. World J Surg. 2019; 43:2211–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christian MD, Devereaux AV, Dichter JR, et al. Introduction and executive summary: Care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014; 146:8S–34S [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelen GD, McCarthy ML, Kraus CK, et al. Creation of surge capacity by early discharge of hospitalized patients at low risk for untoward events. Disaster Med Public Health Prep. 2009; 3:S10–S16 [DOI] [PubMed] [Google Scholar]
- 16.Toerper MF, Kelen GD, Sauer LM, et al. Hospital surge capacity: A web-based simulation tool for emergency planners. Disaster Med Public Health Prep. 2018; 12:513–522 [DOI] [PubMed] [Google Scholar]
- 17.Rosenberger LH, Riccio LM, Campbell KT, et al. Quarantine, isolation, and cohorting: From cholera to Klebsiella. Surg Infect (Larchmt). 2012; 13:69–73 [DOI] [PMC free article] [PubMed] [Google Scholar]


