Objectives:
Endemic and pandemic viral respiratory infections have recently emerged as a critical topic of investigation given the recent severe acute respiratory syndrome coronavirus-2 outbreak. Data from such outbreaks indicate that severe systemic comorbidities including acute neurologic illness are associated with illness and lead to significant outcome differences. Herein, we will discuss the neurologic manifestations of severe viral respiratory infections including coronavirus, influenza, respiratory syncytial virus, metapneumovirus, and enterovirus.
Data Sources:
PubMed and EMBASE were searched by two independent investigators up to March 2020.
Study Selection:
Data selection included preclinical and clinical studies detailing neurologic manifestations of viral respiratory infections.
Data Extraction and Synthesis:
Two independent investigators reviewed and extracted the data.
Conclusions:
Neurologic manifestations including seizures, status epilepticus, encephalitis, critical illness neuromyopathy, acute disseminated encephalomyelitis, acute necrotizing encephalitis, Guillan-Barré syndrome, transverse myelitis, and acute flaccid myelitis have all been associated with severe viral respiratory infections. Having an understanding of the direct neurotropism of such viruses is imperative to understanding pathogenesis, clinical presentation, and potential treatment paradigms aimed at improving morbidity and mortality.
Keywords: encephalitis, meningitis, neurology, respiratory, seizure, virus
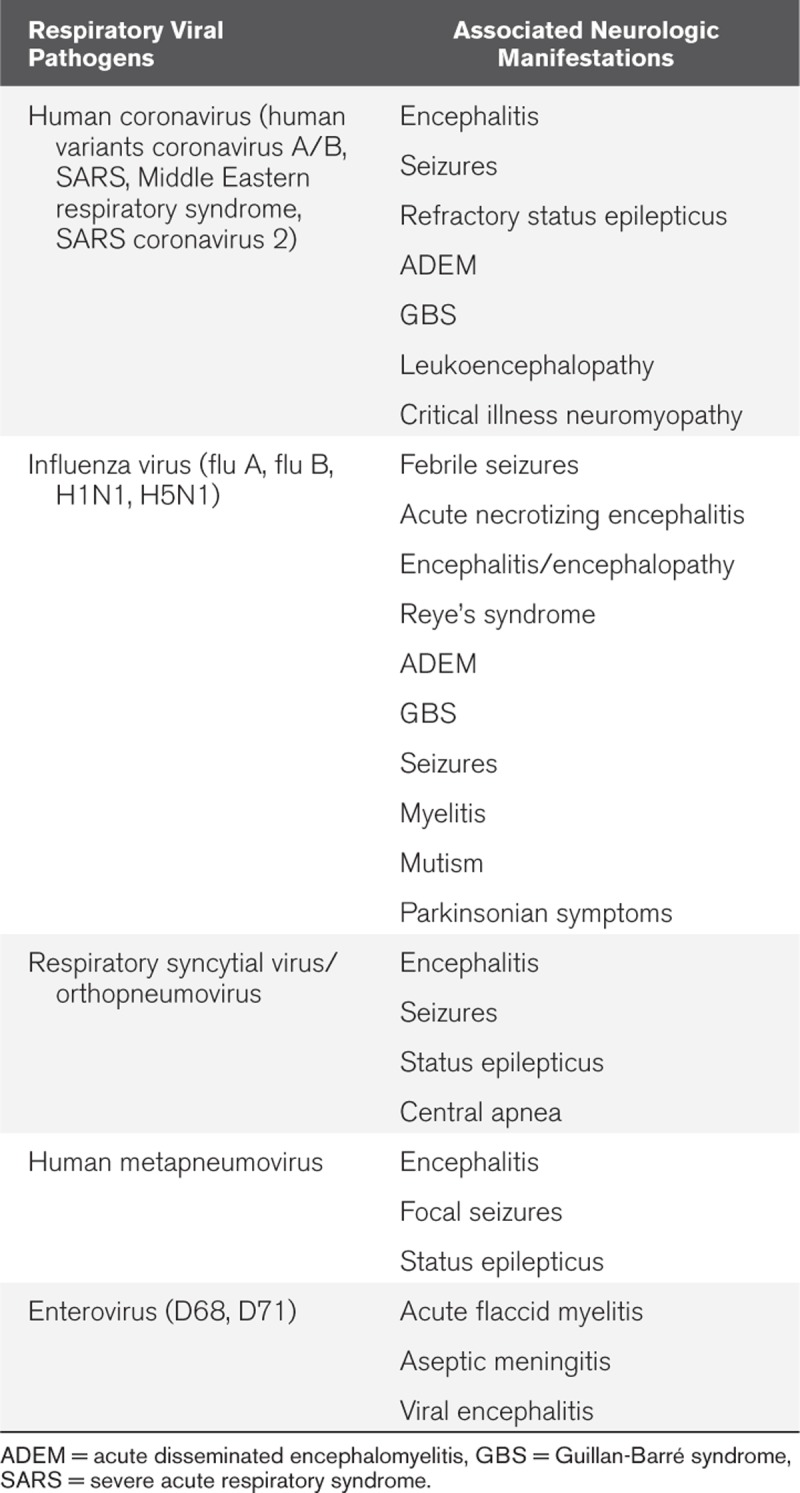
Novel viral respiratory infections are an emerging global public health emergency with high rates of morbidity and mortality. Some of the most common viral pathogens known to effect the human population with respiratory infections include coronavirus, influenza, human metapneumovirus (hMPV), respiratory syncytial virus (RSV), and enterovirus (1). Over the past 20 years, pandemics such as severe acute respiratory syndrome coronavirus (SARS Co-V), Middle Eastern respiratory syndrome (MERS), influenza (H1N1 and H5N1), and currently the coronavirus SARS Co-V2 with coronavirus disease 2019 (COVID-19) have placed a strain on the healthcare systems and societies. In addition to the costs associated with clinical care and the severe respiratory syndromes that such diseases may manifest with, severe and sometimes irreversible neurologic complications can arise. Although manifestation such as mild upper respiratory symptoms are most common, more severe manifestations such as bronchitis, pneumonia and, acute respiratory distress syndrome (ARDS) can occur as well as can neurologic manifestations varying from self-limited aseptic meningitis to fulminant status epilepticus (2–7). Severe neurologic complications can include encephalitis, acute disseminated encephalomyelitis (ADEM), cerebellitis, Guillan-Barré syndrome (GBS), acute flaccid myelitis, and necrotizing encephalopathy as well as symptomatic manifestation with status epilepticus (Table 1) (8). In this review, we will discuss the neural invasion of respiratory viruses and the typical neurologic complications of each individual respiratory infection as well as the routes by which CNS transmission occurs.
TABLE 1.
Associated Neurologic Manifestations of Severe Viral Respiratory Infections
NEURAL INVASION OF RESPIRATORY VIRUSES
Invasion of viral respiratory pathogens into the CNS requires at least a degree of neurotropism; that is, the ability to invade and survive within neural tissue. The blood-brain barrier (BBB), the essential homeostatic bridge to neural function, plays a vital role in neural protection and molecular passage of such microorganisms. Composed of endothelial cells, pericytes, astrocytes, microglia, and extracellular matrix, the BBB controls permeability and acts to control quiescence of the immune system (9). Disruption of such factors through either inflammation or vascular breakdown leads to increased BBB permeability and potentiates unwanted CNS effects. Several modes of CNS transmission by viral pathogens exist including transcellular, paracellular, and retrograde axonal transport along sensory and olfactory nerves (10). Hematogenous spread of viral pathogens, or viremia, is the common mechanism allowing for cellular migration of the virus. Transcellular migration occurs due to invasion of either host cells or phagocytic macrophages to overcome the BBB or the blood-cerebrospinal fluid (CSF) barrier, whereas paracellular migration occurs with pathogen invasion of BBB tight junctions (10). Axonal transport of viral pathogens can occur via sensory, motor, or olfactory nerves. Axonal transport occurs via adherence of motor proteins responsible for retrograde neuronal transport in the periphery as well as in the olfactory nerve which has direct communication with the nasal endothelium where respiratory viral pathogens preferably infect (11). Although each individual virus may have its own predilection for neuroinvasion, there is likely a combination or additive pathognomonic mode of transmission that occurs during viremia (12).
CORONAVIRUS
Coronaviridae are a family of RNA viruses that were first isolated in the 1960s from patients with upper respiratory symptoms (13). The virus infects both birds and mammals. Human variants of coronavirus (HCo-V) isolated as alphacoronavirus and betacoronavirus are endemic worldwide and generally produce the classical symptoms of HCo-V including rhinitis, pharyngitis, laryngitis, bronchitis, and otitis (14). In 2002, a novel coronavirus identified as SARS Co-V was isolated, propagated by zoonotic transmission from bats through and intermediate reservoir (15). Unlike typical HCo-V, SARS Co-V was extremely virulent and complicated by high rates of ARDS, multiple organ dysfunction syndrome, and mortality in up to 10% of cases (16, 17). In 2012, a second novel coronavirus, MERS Co-V evolved from zoonotic transmission causing severe respiratory symptoms with a mortality rate of 35% (18). In 2019, a third novel coronavirus, SARS Co-V2, has emerged. This virus has rapidly disseminated to become a pandemic outbreak in early 2020. It shares highly homologic sequence with SARS Co-V and causes acute, highly lethal pneumonia (COVID-19) with clinical symptoms similar to that reported for SARS Co-V and MERS Co-V (19). One difference is that upper respiratory tract signs and symptoms are rare, indicating that the target cells of SARS CoV-2 may be located in the lower airway (20).
The innate virility of Co-V has tropism for both the respiratory tract and the CNS. The neuroinvasive and neurotropic potential of the virus has been well described in studies isolating viral RNA in brain parenchyma outside the blood vessels and is accepted as a vector leading to acute or chronic neurologic disease (5, 21). Viral particles are almost exclusively discovered in neuronal cells, and hematologic or lymphatic routes of viral dissemination are postulated in addition to direct invasion of peripheral nerve terminals and subsequent trans-synaptic transfer (21–23). For acute neurologic disease, the neuropathogenicity of Co-V has been described with associated cases of encephalitis, refractory status epilepticus, GBS, and ADEM during active infection with confirmatory CSF results (24–28). In addition to acute virulence, chronic and persistent infection of human oligodendrocytes and glial cells by Co-V has been demonstrated and proposed to be involved in neuropathologic changes (29, 30). Specifically, an association with development of multiple sclerosis or other demyelinating diseases has been posed (5). Similarly, several acute neurologic manifestations have been reported for SARS Co-V and MERS Co-V, including critical illness neuromyopathy, considered secondary to direct nerve invasion, ADEM, and leukoencephalopathy (31, 32). To date, no rigorous evidence exists describing neurologic involvement of the novel SARS Co-V2. Extrapolating knowledge of neuroinvasion from Co-V in general, it has been proposed that SARS Co-V’s neurotropism may play a role in the severe respiratory symptoms, by invasion of medullary neurons and interference with functions of nucleus ambiguous and nucleus tractus solitarus, potentially causing central apnea, and lack of feedback from lung and respiratory tract receptors (19). Furthermore, headaches were reported by 8% in one of the published series from China (19). In a retrospective case series (not yet peer reviewed) of patients hospitalized with COVID-19 in Wuhan, China, the authors describe that of 214 cases, 36% had neurologic manifestations, with higher prevalence in more severe cases (33). CNS symptoms were present in 25%, with dizziness and headache being the most common, whereas peripheral neuromuscular symptoms were present in 19%. Severe manifestations with impaired consciousness or cerebrovascular disease was also reported, but much less common (33). Acute management strategies for such presentations should be individualized for each underlying manifestation, but overall remains largely supportive and symptomatic. Several drugs have been proposed to be repurposed for the new coronavirus (34). Effectiveness of corticosteroids remains unclear and likely dependent on the phase of illness during which steroids are given (35). Currently, several medications are being investigated for their efficacy and safety for COVID-19 pneumonia, including complement mediators and nucleotide analogues (36), but no specific treatment or prevention of neurologic manifestations exist, other than primary prevention of exposure and infection.
INFLUENZA
The influenza viruses, formally classified as orthomyxoviridae, are a family of RNA viruses commonly implicated in severe upper respiratory syndromes. There are three genera: influenza virus A, influenza virus B, and influenza virus C, of which, type A is the most virulent human pathogen and causes the most severe form of the disease (37). Diverse phenotypic symptoms are common, from pyrexia, headache, pharyngitis/laryngitis, to ARDS. Influenza infects upwards of 10% of the population annually and has a mortality rate of 4–8.8 deaths per 100,000 persons (38, 39). Individual subtypes of the influenza virus are determined by the structural proteins hemagglutinin (H) and neuraminidase (N). Antigenic shifts that occur within these proteins during zoonotic transmission have led to several pandemics including H1N1 and H5N1 (40, 41). Phenotypic expression within these subtypes tends to be more severe with higher rates of severe lower respiratory infection, ARDS, and death. In addition to the classical respiratory symptoms associated with influenza, a variety of extrapulmonary complications including neurologic, cardiovascular, ocular, hematologic, renal, and hepatic can occur (42).
The neurologic manifestations of influenza have been extensively described (43), vary widely, and have important clinical and prognostic implications. Although generally more prevalent in children, encephalitis, GBS, ADEM, Reye’s syndrome, seizures, myelitis, mutism, and Parkinsonian symptoms have been reported in both adult and pediatric populations (42). Historically, febrile seizure is the most frequently encountered influenza-associated CNS complication, with one in five children hospitalized with influenza affected (44). Thirty percent of pediatric deaths related to H1N1 in the United Kingdom presented with encephalopathy and seizures, and neurologic sequelae are among the most severe consequences of the pandemic influenza A H1N1 in survivors (45). The epidemic influenza A (H3N2) strain was associated with a surge in cases of encephalitis/encephalopathy in Japan, Europe, and the United States (43). One of the best characterized and severe neurologic complications of influenza viremia is acute necrotizing encephalopathy (ANE). Although originally described in the pediatric population, ANE also can affect adults and presents with rapid deterioration in consciousness, symmetric bithalamic, cortical, or infratentorial lesions on MRI and often absence of CSF pleocytosis (see case example in Fig. 1) (46–50). Presence of hemorrhage and tissue loss on imaging in conjunction with CSF are poor prognostic indicators (51). Secondary complications include seizures and status epilepticus. The mortality rate of ANE is upwards of 30% with a significant proportion of patients having long-term neurologic sequelae following recovery (52). Progression to brain death is also a documented complication of ANE (53). In a study of influenza A and B strains in Japan, elevated serum aspartate aminotransferase and creatine phosphokinase and thrombocytopenia (<50,000 platelets/μL) appeared to correlate with unfavorable outcome in influenza-associated encephalopathy (54). Another commonly recognized complication of influenza is GBS, a para- or postinfectious demyelinating polyneuropathy resulting in flaccid quadriparesis and cranial nerve deficits (55, 56). In addition to active infection, the influenza vaccine has also been associated with GBS, although a recent large case-series refutes this evidence (57). Reye’s syndrome has also been highly implicated as a parainfectious complication of the influenza virus, especially in children (58). Defined as acute encephalopathy and fatty liver infiltration, Reye’s patients generally have a poor prognosis with high mortality. Additional but rare neurologic complications resulting from viral infiltration of specific neuronal structures (basal ganglia, hypothalamus, myelinated axons) include Klein-Levin syndrome, Parkinsonism, transverse myelitis, and ADEM (44).
Figure 1.

MRI of a 56-yr-old female who presented with 2 d of shortness of breath and upper respiratory congestion, followed by onset of rapidly progressive confusion culminating in seizures and then obtundation. She tested positive for influenza A antigen. Cerebrospinal fluid analysis showed a mild pleocytosis of 13 (82% neutrophils), and polymerase chain reaction testing for other viral encephalitides remained negative. She was treated with oseltamivir immediately upon confirmation of positive influenza testing. MRI at admission showed restricted diffusion and susceptibility along with Fluid-attenuated inversion recovery (FLAIR) hyperintensity, without enhancement, in bilateral thalami (A–C) and bilateral mesial temporal lobes (D–F) (A+D, FLAIR, B+E, diffusion weighted imaging, C+F, susceptibility weighted imaging sequences).
The exact pathogenesis of the neurologic manifestations of influenza are not entirely clear. Data showing ANE but absence of the influenza virus in the CNS have led to conclude that the inflammatory insult outside the CNS is the trigger for ANE (59), whereas others have hypothesized that disruption of the BBB in the presence of systemic hypercytokinemia could be responsible for inducing necrotic brain lesions (44). Historically, occurrence of Parkinsonian symptoms and “encephalitis lethargica” was reported after the 1918 influenza pandemic (60). However, although there was documented presence of influenza A antigens in patients with “encephalitis lethargica” (61), viral RNA could not be detected in brains of postencephalitic Parkinsonian patients, questioning the exact role of the influenza virus in the genesis of Parkinsonism (62), and calling for long-term follow-up studies among survivors of H5N1-infected individuals to further elucidate the possible direct destructive effect of influenza to the neurons of the substantia nigra.
The mainstay of treatment for influenza infection remains supportive. Patients in high-risk groups are recommended to receive antiviral therapy to shorten illness duration and decrease the occurrence of secondary complications (63), but not all data are affirming strong efficacy for complication prevention (64, 65). For ANE, steroids given within 24 hours after the onset were associated with better outcome of children without brainstem lesions (51) in one series. The use of salicylates should be avoided in children and adolescents due to the association with Reye syndrome.
RESPIRATORY SYNCYTIAL VIRUS
Human RSV is an RNA virus of the pneumoviridae family. Accordingly, the virus has been renamed orthopneumovirus, but for the clinical context of this article, we will use “RSV” (66). Bronchiolitis, pneumonia, and bronchopneumonia from RSV largely infects the pediatric population and is responsible for a majority of PICU admissions related to pulmonary disease (67). Immunocompromised individuals are also susceptible to infection, and emerging literature suggests a large infection burden within adult populations (68). Extrapulmonary manifestations of RSV are well reported and organ systems typically involved include neurologic, cardiovascular, and hepatic (69).
Neurologic manifestations of acute RSV infection include encephalitis, seizures, status epilepticus, and central apnea and are reported in up to 39% of patients (70, 71). Application of very stringent criteria for diagnosis of neurologic disease in the largest available series yielded, however, only a prevalence of 1.2% (71). Seizure frequency among pediatric patients with RSV was 6.6% in one of the pediatric series (70) but only made for 0.7% of all patients and represented the majority of neurologic manifestations in other large series (6, 71). Another important neurologic complication following RSV infection is central apnea, which is found in 16%–21% of patients admitted to the ICU (72). Central apnea at admission is a strong predictor for recurrent apneic events and clinical predictor for mechanical ventilation (72). Studies have shown an abnormal laryngeal chemoreflex in patients infected with RSV, implicating CNS pathology as a culprit (73). The exact mechanism of CNS penetration and pathologic response of RSV are unknown; however, CSF samples were positive for the virus in 50% of patients with seizures in the above series (70). With reference to the adult population, scant evidence exists in the literature implicating RSV as a neuropathogen. Treatment for the neurologic sequelae of RSV is generally supportive and symptomatic.
HUMAN METAPNEUMOVIRUS
Human metapneumovirus (hMPV) is an RNA virus of the paramyxovirus family responsible for upper respiratory infection in all age groups, with more severe infections such as bronchiolitis and pneumonia affecting the pediatric and immunocompromised populations (74). Since its discovery in 2001, extrapulmonary neurologic manifestations of hMNV have been reported including encephalitis, focal seizures, and status epilepticus or even severe fatal encephalitis mostly in the pediatric but also in the adult population (75–77). The overall prevalence of neurologic complications seems rare, with exact prevalence unknown and most data coming from small case series or single case reports (75). Among 205 pediatric patients referred to the California encephalitis project between 2004 and 2006, hMPV was detected in nasopharyngeal swabs of five patients (6). Compared with infection with RSV, the frequency of seizures is reported to be higher (6.3% vs 0.7%) (6). In a literature review of pediatric cases of hMPV infection complicated by encephalitis, seizures occurred in 10 of 13 cases (75). Infection likely occurs due to direct CNS invasion, as hMPV has been found in brain tissue after autopsy (78). MRI findings can range from normal parenchymal structures to having various degrees of white matter involvement. CSF analysis can range from normal to showing varying degrees of pleocytosis and inconsistent polymerase chain reaction positivity (6). Treatment for the secondary neurologic complications of hMNV remains largely supportive.
ENTEROVIRUS D68 AND 71
Enteroviruses (EV) D68 and 71 (EV68, EV71) are RNA viruses belonging to the picornaviridae family. Initially discovered in 1968, EV6868 was described as a nonpolio enterovirus that infected the upper respiratory tract (79). Isolated in 1969, EV71 was a novel enterovirus found causes hand foot and mouth syndrome (80). A majority of the enterovirus family cause mild symptoms; however, certain strains with specific neurotropism such as EV68 and EV71 have been implicated in severe secondary neurologic sequelae of infection (80–82). EV71 specifically has been demonstrated to cause both aseptic meningitis and viral encephalitis (83, 84). More notably, however, are the recent spikes in cases of acute flaccid paralysis or acute flaccid myelitis (AFM) affecting the pediatric population worldwide (85). Both EV68 and EV71 can result in nonpolio enterovirus paralysis, with EV68 being causative during the most recent outbreak (82, 86, 87). A diagnostic classification for AFM has been proposed for epidemiologic purposes and describes the condition as the acute onset of flaccid paralysis with neuroimaging evidence of spinal cord grey matter lesions (85, 88). High levels of CSF EV-specific antibodies are frequently identified in AFM cases, providing evidence for a causal role of nonpolio EV in AFM (89). Treatment is usually supportive and rehabilitative with outcomes varying from complete clinical recovery to those having persistent residual neurologic deficits (85) and slow recovery (90).
OTHER COMMON RESPIRATORY VIRUSES
Several other well-known respiratory viruses including rhinovirus, adenovirus, and parainfluenza virus commonly infect the general population. The respiratory symptoms related to infection are generally mild and include upper respiratory manifestations including rhinitis, pharyngitis, and tonsillitis. although usually self-limited, mild neurologic sequelae including aseptic meningitis, encephalopathy, and febrile seizures have been associated in up to 3% of patients with such infections (91–95). Aseptic or viral meningitis specifically can have varying clinical presentations ranging from fatigue or headache to alterations in consciousness and seizures (96). CSF analysis typically shows a lymphocytic pleocytosis with hyperproteinemia, although patients can present with normal CSF findings (96, 97). Neurologic symptoms with such viruses tend to be mild, improve with supportive care, and have low-cause morbidity and mortality (95, 98).
CONCLUSIONS
The neurologic manifestations of severe respiratory viruses including Co-V, influenza, RSV, hMPV, and enterovirus D68 and D71 are a diagnostic and therapeutic challenge for many physicians. Clinical syndromes including status epilepticus, encephalitis, critical illness neuromyopathy, ADEM, ANE, GBS, transverse myelitis, and flaccid paralysis have all been associated with these viral respiratory infections. Having an understanding of the direct neurotropism of such viruses is imperative to understanding pathogenesis, clinical presentation and potential treatment paradigms aimed at improving morbidity and mortality.
Footnotes
Dr. Robinson has received honoraria for serving as an expert legal witness for traumatic brain injury. Dr. Busl disclosed that she does not have potential conflicts of interest.
REFERENCES
- 1.Shi T, Arnott A, Semogas I, et al. The etiological role of common respiratory viruses in acute respiratory infections in older adults: A systematic review and meta-analysis. J Infect Dis. 2019; pii:jiy662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akins PT, Belko J, Uyeki TM, et al. H1N1 encephalitis with malignant edema and review of neurologic complications from influenza. Neurocrit Care. 2010; 13:396–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Algahtani H, Subahi A, Shirah B. Neurological complications of middle east respiratory syndrome coronavirus: A report of two cases and review of the literature. Case Rep Neurol Med. 2016; 2016:3502683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antonucci R, Chiappe S, Porcella A, et al. Bronchiolitis-associated encephalopathy in critically-ill infants: An underestimated complication? J Matern Fetal Neonatal Med. 2010; 23:431–436 [DOI] [PubMed] [Google Scholar]
- 5.Arbour N, Day R, Newcombe J, et al. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000; 74:8913–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arnold JC, Singh KK, Milder E, et al. Human metapneumovirus associated with central nervous system infection in children. Pediatr Infect Dis J. 2009; 28:1057–1060 [DOI] [PubMed] [Google Scholar]
- 7.Mariotti P, Iorio R, Frisullo G, et al. Acute necrotizing encephalopathy during novel influenza A (H1N1) virus infection. Ann Neurol. 2010; 68:111–114 [DOI] [PubMed] [Google Scholar]
- 8.Bohmwald K, Gálvez NMS, Ríos M, et al. Neurologic alterations due to respiratory virus infections. Front Cell Neurosci. 2018; 12:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liebner S, Dijkhuizen RM, Reiss Y, et al. Functional morphology of the blood-brain barrier in health and disease. Acta Neuropathol. 2018; 135:311–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahm T, Rudolph H, Schwerk C, et al. Neuroinvasion and inflammation in viral central nervous system infections. Mediators Inflamm. 2016; 2016:8562805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson PA, McGavern DB. Dorovini-Zis K. Portals of viral entry into the central nervous system. The Blood-Brain Barrier in Health and Disease, Volume Two: Pathophysiology and Pathology. 2015, Cleveland, OH: CRC Press, 23 [Google Scholar]
- 12.Suen WW, Prow NA, Hall RA, et al. Mechanism of West Nile virus neuroinvasion: A critical appraisal Viruses. 2014; 6:2796–2825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyrrell DA, Bynoe ML. Cultivation of a novel type of common-cold virus in organ cultures. Br Med J. 1965; 1:1467–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vabret A, Dina J, Brison E, et al. [Human coronaviruses]. Pathol Biol (Paris). 2009; 57:149–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan Y, Zheng BJ, He YQ, et al. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science. 2003; 302:276–278 [DOI] [PubMed] [Google Scholar]
- 16.Chan-Yeung M, Xu RH. SARS: Epidemiology. Respirology. 2003; 8SupplS9–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med. 2005; 202:415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nassar MS, Bakhrebah MA, Meo SA, et al. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: Epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci. 2018; 22:4956–4961 [DOI] [PubMed] [Google Scholar]
- 19.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may be at least partially responsible for the respiratory failure of COVID-19 patients. J Med Virol. 2020. Feb 27. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005; 41:1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding Y, He L, Zhang Q, et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol. 2004; 203:622–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YC, Bai WZ, Hirano N, et al. Coronavirus infection of rat dorsal root ganglia: Ultrastructural characterization of viral replication, transfer, and the early response of satellite cells. Virus Res. 2012; 163:628–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016; 375:497–498 [DOI] [PubMed] [Google Scholar]
- 25.Yeh EA, Collins A, Cohen ME, et al. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics. 2004; 113:e73–e76 [DOI] [PubMed] [Google Scholar]
- 26.Turgay C, Emine T, Ozlem K, et al. A rare cause of acute flaccid paralysis: Human coronaviruses. J Pediatr Neurosci. 2015; 10:280–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma K, Tengsupakul S, Sanchez O, et al. Guillain-Barré syndrome with unilateral peripheral facial and bulbar palsy in a child: A case report. SAGE Open Med Case Rep. 2019; 7:2050313X19838750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau KK, Yu WC, Chu CM. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004; 10:342–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbour N, Ekandé S, Côté G, et al. Persistent infection of human oligodendrocytic and neuroglial cell lines by human coronavirus 229E. J Virol. 1999; 73:3326–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazzini E, Fleming J, Fahn S. Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Mov Disord. 1992; 7:153–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 2004; 61:1669–1673 [DOI] [PubMed] [Google Scholar]
- 32.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV). Infection. 2015; 43:495–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mao LWM, Chen S, He Q, et al. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: A retrospective case series study. mdRxiv. 2020. Feb 25. [online ahead of print] [Google Scholar]
- 34.Fan HH, Wang LQ, Liu WL, et al. Repurposing of clinically approved drugs for treatment of coronavirus disease 2019 in a 2019-novel coronavirus (2019-nCoV) related coronavirus model. Chin Med J (Engl). 2020. Mar 6. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou YH, Qin YY, Lu YQ, et al. Effectiveness of glucocorticoid therapy in patients with severe novel coronavirus pneumonia: protocol of a randomized controlled trial. Chin Med J (Engl). 2020. Mar 5. [online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends. 2020; 14:72–73 [DOI] [PubMed] [Google Scholar]
- 37.Dolin R. Kasper DLBE, Fauci AS, Hauser SL. Influenza. Harrisson’s Principles of Internal Medicine. 2005. Sixteenth Edition, New York, NY: McGraw Hill, 1066–1070 [Google Scholar]
- 38.Uyeki TM, Bernstein HH, Bradley JS, et al. Clinical practice guidelines by the infectious diseases Society of America: 2018 update on diagnosis, treatment, chemoprophylaxis, and institutional outbreak management of seasonal influenzaa. Clin Infect Dis. 2019; 68:e1–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iuliano AD, Roguski KM, Chang HH, et al. ; Global Seasonal Influenza-associated Mortality Collaborator Network. Estimates of global seasonal influenza-associated respiratory mortality: A modelling study. Lancet. 2018; 391:1285–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jang H, Boltz D, Sturm-Ramirez K, et al. Highly pathogenic H5N1 influenza virus can enter the central nervous system and induce neuroinflammation and neurodegeneration. Proc Natl Acad Sci U S A. 2009; 106:14063–14068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fineberg HV. Pandemic preparedness and response–lessons from the H1N1 influenza of 2009. N Engl J Med. 2014; 370:1335–1342 [DOI] [PubMed] [Google Scholar]
- 42.Sellers SA, Hagan RS, Hayden FG, et al. The hidden burden of influenza: A review of the extra-pulmonary complications of influenza infection. Influenza Other Respir Viruses. 2017; 11:372–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Surana P, Tang S, McDougall M, et al. Neurological complications of pandemic influenza A H1N1 2009 infection: European case series and review. Eur J Pediatr. 2011; 170:1007–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Toovey S. Influenza-associated central nervous system dysfunction: A literature review. Travel Med Infect Dis. 2008; 6:114–124 [DOI] [PubMed] [Google Scholar]
- 45.Sachedina N, Donaldson LJ. Paediatric mortality related to pandemic influenza A H1N1 infection in England: An observational population-based study. Lancet. 2010; 376:1846–1852 [DOI] [PubMed] [Google Scholar]
- 46.Mizuguchi M. Acute necrotizing encephalopathy of childhood: A novel form of acute encephalopathy prevalent in Japan and Taiwan. Brain Dev. 1997; 19:81–92 [DOI] [PubMed] [Google Scholar]
- 47.Abdelrahman HS, Safwat AM, Alsagheir MM. Acute necrotizing encephalopathy in an adult as a complication of H1N1 infection. BJR Case Rep. 2019; 5:20190028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ochi N, Takahashi K, Yamane H, et al. Acute necrotizing encephalopathy in an adult with influenza A infection. Ther Clin Risk Manag. 2018; 14:753–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mizuguchi M, Abe J, Mikkaichi K, et al. Acute necrotising encephalopathy of childhood: A new syndrome presenting with multifocal, symmetric brain lesions. J Neurol Neurosurg Psychiatry. 1995; 58:555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee YJ, Smith DS, Rao VA, et al. Fatal H1N1-related acute necrotizing encephalopathy in an adult. Case Rep Crit Care. 2011; 2011:562516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Okumura A, Mizuguchi M, Kidokoro H, et al. Outcome of acute necrotizing encephalopathy in relation to treatment with corticosteroids and gammaglobulin. Brain Dev. 2009; 31:221–227 [DOI] [PubMed] [Google Scholar]
- 52.Togashi T, Matsuzono Y, Narita M. Epidemiology of influenza-associated encephalitis-encephalopathy in Hokkaido, the northernmost island of Japan. Pediatr Int. 2000; 42:192–196 [DOI] [PubMed] [Google Scholar]
- 53.Martin A, Reade EP. Acute necrotizing encephalopathy progressing to brain death in a pediatric patient with novel influenza A (H1N1) infection. Clin Infect Dis. 2010; 50:e50–e52 [DOI] [PubMed] [Google Scholar]
- 54.Morishima T, Togashi T, Yokota S, et al. ; Collaborative Study Group on Influenza-Associated Encephalopathy in Japan. Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin Infect Dis. 2002; 35:512–517 [DOI] [PubMed] [Google Scholar]
- 55.Tam CC, O’Brien SJ, Rodrigues LC. Influenza, campylobacter and mycoplasma infections, and hospital admissions for Guillain-Barre syndrome, England. Emerg Infect Dis. 2006; 12:1880–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tam CC, O’Brien SJ, Petersen I, et al. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS One. 2007; 2:e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grave C, Boucheron P, Rudant J, et al. Seasonal influenza vaccine and Guillain-Barre syndrome: A self-controlled case series study. Neurology. 2020. Feb 25. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Hurwitz ES, Nelson DB, Davis C, et al. National surveillance for Reye syndrome: A five-year review. Pediatrics. 1982; 70:895–900 [PubMed] [Google Scholar]
- 59.Nakai Y, Itoh M, Mizuguchi M, et al. Apoptosis and microglial activation in influenza encephalopathy. Acta Neuropathol. 2003; 105:233–239 [DOI] [PubMed] [Google Scholar]
- 60.Taubenberger JK. The origin and virulence of the 1918 “Spanish” influenza virus. Proc Am Philos Soc. 2006; 150:86–112 [PMC free article] [PubMed] [Google Scholar]
- 61.Gamboa ET, Wolf A, Yahr MD, et al. Influenza virus antigen in postencephalitic parkinsonism brain. Detection by immunofluorescence. Arch Neurol. 1974; 31:228–232 [DOI] [PubMed] [Google Scholar]
- 62.McCall S, Henry JM, Reid AH, et al. Influenza RNA not detected in archival brain tissues from acute encephalitis lethargica cases or in postencephalitic Parkinson cases. J Neuropathol Exp Neurol. 2001; 60:696–704 [DOI] [PubMed] [Google Scholar]
- 63.Treanor JJ, Hayden FG, Vrooman PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. US Oral Neuraminidase Study Group. JAMA. 2000; 283:1016–1024 [DOI] [PubMed] [Google Scholar]
- 64.Jefferson T, Jones MA, Doshi P, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults and children. Cochrane Database Syst Rev. 2014; (4):CD008965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Del Mar C, Doshi P, Hama R, et al. Neuraminidase inhibitors for influenza complications. Lancet. 2014; 384:1260–1261 [DOI] [PubMed] [Google Scholar]
- 66.King AMQ, Lefkowitz EJ, Mushegian AR, et al. Changes to taxonomy and the international code of virus classification and nomenclature ratified by the international committee on taxonomy of viruses (2018). Arch Virol. 2018; 163:2601–2631 [DOI] [PubMed] [Google Scholar]
- 67.Pilar Orive FJ, Casado Flores J, García Teresa MA, et al. [Acute respiratory infections in pediatric intensive care units. A multicenter prospective study]. An Esp Pediatr. 1998; 48:138–142 [PubMed] [Google Scholar]
- 68.Binder W, Thorsen J, Borczuk P. RSV in adult ED patients: Do emergency providers consider RSV as an admission diagnosis? Am J Emerg Med. 2017; 35:1162–1165 [DOI] [PubMed] [Google Scholar]
- 69.Eisenhut M. Extrapulmonary manifestations of severe respiratory syncytial virus infection–a systematic review. Crit Care. 2006; 10:R107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kho N, Kerrigan JF, Tong T, et al. Respiratory syncytial virus infection and neurologic abnormalities: retrospective cohort study. J Child Neurol. 2004; 19:859–864 [DOI] [PubMed] [Google Scholar]
- 71.Sweetman LL, Ng YT, Butler IJ, et al. Neurologic complications associated with respiratory syncytial virus. Pediatr Neurol. 2005; 32:307–310 [DOI] [PubMed] [Google Scholar]
- 72.Kneyber MC, Brandenburg AH, de Groot R, et al. Risk factors for respiratory syncytial virus associated apnoea. Eur J Pediatr. 1998; 157:331–335 [DOI] [PubMed] [Google Scholar]
- 73.Lindgren C, Grögaard J. Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr. 1996; 85:798–803 [DOI] [PubMed] [Google Scholar]
- 74.Edwards KM, Zhu Y, Griffin MR, et al. ; New Vaccine Surveillance Network. Burden of human metapneumovirus infection in young children. N Engl J Med. 2013; 368:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vehapoglu A, Turel O, Uygur Sahin T, et al. Clinical significance of human metapneumovirus in refractory status epilepticus and encephalitis: Case report and review of the literature. Case Rep Neurol Med. 2015; 2015:131780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mergeay M, Coeckelbergh E, De Cauwer H, et al. An adult case of metapneumovirus-induced acute encephalitis. Acta Neurol Belg. 2019; 119:645–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jeannet N, van den Hoogen BG, Schefold JC, et al. Cerebrospinal fluid findings in an adult with human metapneumovirus-associated encephalitis. Emerg Infect Dis. 2017; 23:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schildgen O, Glatzel T, Geikowski T, et al. Human metapneumovirus RNA in encephalitis patient. Emerg Infect Dis. 2005; 11:467–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schieble JH, Fox VL, Lennette EH. A probable new human picornavirus associated with respiratory diseases. Am J Epidemiol. 1967; 85:297–310 [DOI] [PubMed] [Google Scholar]
- 80.Schmidt NJ, Lennette EH, Ho HH. An apparently new enterovirus isolated from patients with disease of the central nervous system. J Infect Dis. 1974; 129:304–309 [DOI] [PubMed] [Google Scholar]
- 81.Gear JH. Nonpolio causes of polio-like paralytic syndromes. Rev Infect Dis. 1984; 6Suppl 2S379–S384 [DOI] [PubMed] [Google Scholar]
- 82.Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis. 2018; 18:e239–e247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berlin LE, Rorabaugh ML, Heldrich F, et al. Aseptic meningitis in infants < 2 years of age: Diagnosis and etiology. J Infect Dis. 1993; 168:888–892 [DOI] [PubMed] [Google Scholar]
- 84.Wang Y, Zou G, Xia A, et al. Enterovirus 71 infection in children with hand, foot, and mouth disease in Shanghai, China: Epidemiology, clinical feature and diagnosis. Virol J. 2015; 12:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: A clinical review of US cases 2012-2015. Ann Neurol. 2016; 80:326–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greninger AL, Naccache SN, Messacar K, et al. A novel outbreak enterovirus D68 strain associated with acute flaccid myelitis cases in the USA (2012-14): A retrospective cohort study. Lancet Infect Dis. 2015; 15:671–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen CY, Chang YC, Huang CC, et al. Acute flaccid paralysis in infants and young children with enterovirus 71 infection: MR imaging findings and clinical correlates. AJNR Am J Neuroradiol. 2001; 22:200–205 [PMC free article] [PubMed] [Google Scholar]
- 88.Maloney JA, Mirsky DM, Messacar K, et al. MRI findings in children with acute flaccid paralysis and cranial nerve dysfunction occurring during the 2014 enterovirus D68 outbreak. AJNR Am J Neuroradiol. 2015; 36:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schubert RD, Hawes IA, Ramachandran PS, et al. Pan-viral serology implicates enteroviruses in acute flaccid myelitis. Nat Med. 2019; 25:1748–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bove R. Social media in the age of the “New Polio”. N Engl J Med. 2019; 380:1195–1197 [DOI] [PubMed] [Google Scholar]
- 91.Lema CL, Cisterna DM, Freire MC. [Neurologic disease due to adenovirus infection]. Medicina (B Aires). 2005; 65:196–200 [PubMed] [Google Scholar]
- 92.Soeur M, Wouters A, de Saint-Georges A, et al. Meningoencephalitis and meningitis due to an adenovirus type 5 in two immunocompetent adults. Acta Neurol Belg. 1991; 91:141–150 [PubMed] [Google Scholar]
- 93.Landry ML, Hsiung GD. Adenovirus-associated meningoencephalitis in a healthy adult. Ann Neurol. 1988; 23:627–628 [DOI] [PubMed] [Google Scholar]
- 94.Lee KS, Lee BL, Heo YJ. Acute encephalopathy with biphasic seizures and late reduced diffusion associated with adenoviral pneumonia. Child Neurol Open. 2019; 6:2329048X19826288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang YC, Huang SL, Chen SP, et al. Adenovirus infection associated with central nervous system dysfunction in children. J Clin Virol. 2013; 57:300–304 [DOI] [PubMed] [Google Scholar]
- 96.Wright WF, Pinto CN, Palisoc K, et al. Viral (aseptic) meningitis: A review. J Neurol Sci. 2019; 398:176–183 [DOI] [PubMed] [Google Scholar]
- 97.Dawood N, Desjobert E, Lumley J, et al. Confirmed viral meningitis with normal CSF findings. BMJ Case Rep. 2014; 2014:bcr2014203733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCarthy VP, Zimmerman AW, Miller CA. Central nervous system manifestations of parainfluenza virus type 3 infections in childhood. Pediatr Neurol. 1990; 6:197–201 [DOI] [PubMed] [Google Scholar]


