Objectives:
Although the amount of information generated during this most recent coronavirus disease 2019 pandemic is enormous, much is of uncertain trustworthiness. This review summaries the many potential sources of information that clinicians turn to during pandemic illness, the challenges associated with performing methodologically sound research in this setting and potential approaching to conducting well done research during a health crisis.
Data Sources:
Not applicable.
Study Selection:
Not applicable.
Data Extraction:
Not applicable.
Data Synthesis:
Not applicable.
Conclusions:
Pandemics and healthcare crises provide extraordinary opportunities for the rapid generation of reliable scientific information but also for misinformation, especially in the early phases, which may contribute to public hysteria. The best way to combat misinformation is with trustworthy data produced by healthcare researchers. Although challenging, research can occur during pandemics and crises and is facilitated by advance planning, governmental support, targeted funding opportunities, and collaboration with industry partners. The coronavirus disease 2019 research response has highlighted both the dangers of misinformation as well as the benefits and possibilities of performing rigorous research during challenging times.
Keywords: coronavirus disease 2019, guidelines, knowledge translation, misinformation, pandemic
During times of uncertainty, it can be challenging to decipher which information is credible. Watching major news channels early during the course of an evolving and breaking story, it is usually clear that the newscasters do not have complete information; however, this does not stop the constant flow of discourse to viewers. To fill this void, it is common for broadcasters to rely upon information that is not fully vetted, much of which ends up being incorrect once the entire story becomes clear. These same themes may occur during medical crises, most clearly demonstrated during infectious pandemics that elicit a primal fear in people, bringing forth images of blockbuster films in which novel viruses wipe out large swaths of the global population. A combination of fear and a lack of credible information in the early phase of an outbreak are the largest contributors to public hysteria. Information is the best tool to combat hysteria, and as illustrated in the current infectious outbreak of severe acute respiratory syndrome coronavirus 2, in our digital media era, information is everywhere. The more important concern for clinicians and patients, similar to watching a breaking news story, in which information to believe and which to ignore.
INFORMATION EXPLOSION DURING PANDEMIC ILLNESS
The ongoing coronavirus disease 2019 (COVID-19) pandemic has demonstrated the volume of information that can be produced in a short period of time; this has been associated with both benefits (easier access for clinicians) and risks (misinformation). Media sources including newspapers, magazines, and news shows have been covering this story with fervor. Although the objectives of corporate news media include informing the public of the latest medical updates, they have an obligation to shareholders or private owners of selling more newspapers, magazines, or advertisements and the natural inclination to therefore stoke the fires of hysteria. Certainly, some sources are worse offenders when it comes to this than others who take the time to more carefully vet sources. The amount of print and news media dedicated to COVID-19 in the last few months is huge. Tangible risks of misinformation should not be ignored, as they may lead to ill-informed health decisions (1) including isolation orders, travel bans, population quarantines and even discrimination against travelers from certain countries or persons of certain ethnic origins. The use of unproven therapeutic or prophylactic interventions also introduces unnecessary risks and, unless they are used carefully in the context of an approved clinical study, increase the amount of noise thereby limiting our collective ability to discover new ways to treat patients. There are however benefits to digitalization of health media. Based on experiences with previous outbreaks, for example, influenza A(H1N1)pdm09 pandemic in 2009, the World Health Organization (WHO) and other governmental organizations are better prepared. The WHO maintains a live and up-to-date COVID-19 website which contains credible information on the outbreak (www.who.int/health-topics/coronavirus). The U.S. Centers for Disease Control and Prevention website includes updates on virus status in the United States, travel restrictions, and a world map highlighting areas with COVID-19 cases (www.cdc.gov/coronavirus). Johns Hopkins runs a website (www.gisanddata.maps.arcigis.com) that provides up-to-date and credible data describing the number of those infected broken down by severity and separated by country, as well as the number of deaths. These governmental and public health organizations sources of information should be considered most trustworthy, as they can be relied upon to avoid misinformation, and as such the public should be going here as their main source of information during the health crisis.
Perhaps unique to this pandemic, compared with others, has been the response from the medical community. Although bedside practitioners are in need of data that will help them to better identify, risk-stratify, and treat affected patients, medical research often takes time. Traditionally, research is deliberate, and producing trustworthy and methodologically sound results may not be as rapid as what is required. For example, according to PubMed, although over 20,000 citations related to H1N1 influenza have been published since 2009, the large majority (14,000 of these) were published after 2011, over 2 years following the major phase of the pandemic. Major contributors to research delays include competing interests of investigators, regulatory barriers, time taken for protocol development, ethics approval, peer review and delays related to the publication process. This classic research model does not fit well with pandemic research, where there is a need for rapid information to fill gaps and address public concern. For COVID-19, some of these traditional delays have been circumvented (we will discuss how shortly), and as such, many of the major general medicine journals, including Journal of American Medical Association (JAMA), New England Journal of Medicine (NEJM), and The Lancet have prioritized publications related to COVID-19. This has been facilitated at medical journals through invited content and expedited peer review processes. JAMA, The Lancet, and NEJM, for example, maintain a Coronavirus Resource Center including research and multimedia content (www.jamanetwork.com/journals/jama/pages/coronavirus-alert), most as free online content. Providing peer-reviewed and easily accessible content has helped to overcome some of the misinformation rampant in lay media. As of March 2020, 1,801 unique citations related to COVID-19 have been indexed in PubMed, 640 in 2019, and 1,161 in 2020. This represents an enormous amount of scientific content for a disease that was first discovered in Wuhan, China in mid-December. It remains to be seen, how valid and trustworthy the data from these publications will turn out to be, given the rapidity in which they were produced and the expedited peer review and editorial decision-making required to publish so quickly. There have already been some highly visible examples of dubious and scientifically questionable reports, even some that have been published and now corrected in highly reputable journals (2). The lesson is that not everything posing as trustworthy research truly is, and it is important to both support high-quality work, but also discourage and prevent work that is not trustworthy.
Also unique to this outbreak is the role social media has played in information dissemination and at times, propagation of misinformation (3). Twitter has become entrenched as an information source for both patients and clinicians (4, 5). Although the platform is unique in allowing for engagement with experts and rapid discourse, the lack of scientific vetting and peer review (6) can contribute to hysteria, rather than alleviating it. Each day over 100,000 tweets are sent using the #COVID19 hashtag (www.symplur.com) and this is increasing exponentially. Filtering the knowledge from the misinformation in social media is extremely challenging, and probably a strategy to be avoided in times of pandemic. At the very least, if using social media, the focus must be on reliable sources presenting vetted information and avoiding conjecture and opinion. Even this rule is not absolute, as we have seen dramatic cases of prominent individuals advocating for specific unproven therapies (e.g., hydroxychloroquine and azithromycin) leading to drug shortages and increased rates of toxicity. Examples such as this reinforce the necessity of consistent and well-informed communication strategies in times when the risks of misinformation are significant.
CHALLENGES WITH PERFORMING RESEARCH DURING PANDEMICS
Research during a pandemic or health crisis presents challenges beyond the usual difficulties surrounding research in the critically ill (7). Most obviously, pandemic preparedness, planning and management requires time, resources and personnel. Clinical researchers may be diverted to the bedside caring for affected patients or working with government and public health organizations to contain the outbreak. Preliminary data from China suggests that mortality in Wuhan (the center of the COVID-19 outbreak with the largest number of cases) has been higher (> 3%) compared with other regions in China (around 0.7%) and this has been hypothesized to be at least partly due to a shortage in healthcare providers (8). Not only is researchers time diverted to clinical care but so are other resources including funding. Governments are usually the largest research funder, especially in developed nations; however, these funds may be required during an outbreak to augment capacity through infrastructure or human resources investment. The most dramatic example of this was the government of China’s investment in building a new 650,000 square foot hospital with 1,000 beds and 30 ICU beds, built in only 10 days to care strictly for COVID-19 patients.
Organizational stress has other collateral impacts on research capacity. Research involving humans often requires regulatory or governmental support, especially if there are significant ethical, public health or safety concerns (9). More than likely, during times of institutional pressure, these regulatory pathways will be delayed, limiting the ability to get the approvals necessary to proceed. Research ethics boards may mistakenly consider the emotional pressure on patients and caregivers during a health crisis as an unsuitable environment to conduct research, thereby enacting further delays and barriers to timely investigation (10, 11). Safety concerns for research staff may keep them out of hospitals or limit their ability to enroll patients and capture study-related information. Unfortunately, the greatest impact of health crises and pandemics and the most significant challenges with outbreak tracking occur in low- or middle-income countries (LMICs), regions that are already well below capacity in terms of health and research infrastructure (12) (Fig. 1). Lack of local or regional expertise in conducting methodologically rigorous research may require external collaboration, which is challenging in the setting of travel restrictions and which runs the risk of ignoring scientific input from LMIC investigators and clinicians. This is all further complicated by a rapidly evolving landscape. Within pandemics, the clinical situation often evolves day-to-day or week-to-week, a pace uncommon in the setting of traditional epidemiologic and clinical research. A research question or medical intervention that was relevant 2 weeks ago may no longer be relevant by the time approval and funding are secured. As such, research priorities and approaches must be capable of responding nimbly and rapidly. This need for rapid information and rapid dissemination of trustworthy results is daunting and uncomfortable for most clinical researchers who are used to operating within extended timelines. The exponential increase in PubMed citations related to COVID-19 over the last 2 months is a testament to this rapid evolution in information. A we’ve learned time and time again, true salvation from pandemic times (e.g., Ebola) will come only from well-conducted research informing prevention of disease with vaccines, use of prophylaxis, improving treatments, and mitigating disease-related consequences.
Figure 1.
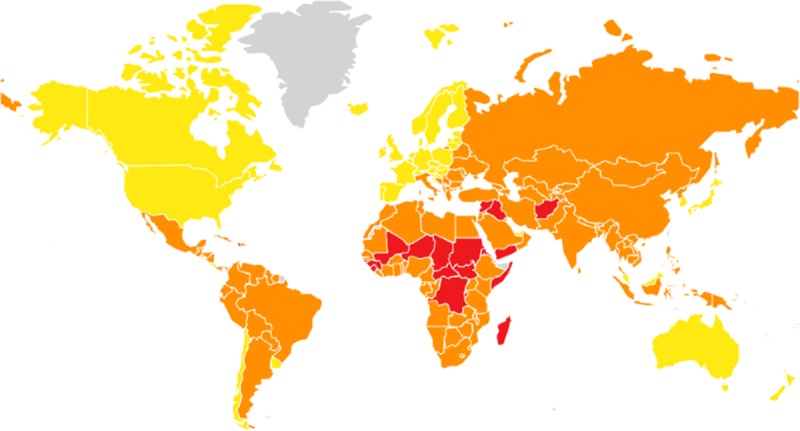
Countries judged to be most at risk for originating pandemic illness (red = high risk, orange = moderate risk, yellow = low risk). Reference: Global Health Security Index (www.ghsindex.org).
Although these barriers are significant, and clinical research during the health crisis is enormously complicated, this is the first outbreak in which rapid, potentially clinically useful research is being conducted alongside the pandemic response. At this time, approximately 75 randomized controlled trials have been registered in clinicaltrials.gov, and more than 300 in the Chinese trials registry, investigating interventions such as antivirals (multiple), IV immunoglobulin (NCT04261426), corticosteroids (NCT04273321), antibiotics (e.g., azithromycin) (multiple), Tocilizumab (NCT04317092), sildenafil (NCT04304313), thalidomide (NCT04273529), immunotherapy (NCT04268537), chloroquine (NCT04286503), recombinant angiotensin-converting enzyme (NCT04287686), thalidomide (NCT04273581), biologic agents (NCT04280588), mesenchymal stem cells (multiple), convalescent plasma (NCT04264858), nitric oxide (NCT04290858), vitamin C (NCT04264533), traditional Chinese medicine (multiple), and vaccines (NCT04283461) in the treatment of COVID-19 related illness. Research funding bodies can help by prolonging funding periods, augmenting funding envelops to help overcome the barriers mentioned above, and considering funding pandemic research even outside pandemic times.
Over the coming months, the most significant issue facing clinicians caring for COVID-19 patients will be to critically appraise the multiple research outputs and decide which to apply in clinical practice. For researchers conducting these trials, it is important to balance rapidity along with sound methodologic principles. This can be facilitated in a number of ways, which will be discussed next, including some direction on how best to incorporate new data into pandemic-based patient management.
APPROACHES TO CONDUCTING TRUSTWORTHY RESEARCH DURING HEALTH CRISIS
How might health researchers go about pursuing this in a timely manner? There are a number of strategies that have been employed to overcome some of the challenges associated with conducting research in this setting (12). Pandemics related to respiratory viruses have occurred at regular intervals throughout history (13) (Fig. 2); it is not a matter of if they will recur, but rather when. As such, rather than waiting until a pandemic occurs to build infrastructure, researchers may develop collaborative networks, initiate study protocols, and begin regulatory and ethical approval processes in anticipation of the next outbreak. Then, when the inevitable pandemic occurs, research capacity will already be in place allowing for a facilitated response. The International Severe Acute Respiratory and Emerging Infections Consortium (ISARIC), a group which was formed in collaboration with International Forum for Acute Care Trialists (InFACT) umbrella, has followed this model (14). The group, which includes 52 clinical research networks worldwide, was launched in 2011 following the H1N1 pandemic with the plan to be ready for the next viral pandemic and with the goal of ensuring timely and efficient research in the setting of health crises related to emerging infection. As introductory work has been ongoing over the last few years, with the emergence of COVID-19, ISARIC is already prepared with WHO-endorsed case report forms, clinical characterization protocols to enable harmonious clinical and biological sample data collection, and clinical trial protocols that have been collated and endorsed by the entire research network (www.isaric.tghn.org). InFACT is also guiding the WHO on supportive and adjuvant care in severe viral disease through leadership within WHO committees, a great example of intensive care physicians leading the global response and research initiatives related to this pandemic.
Figure 2.
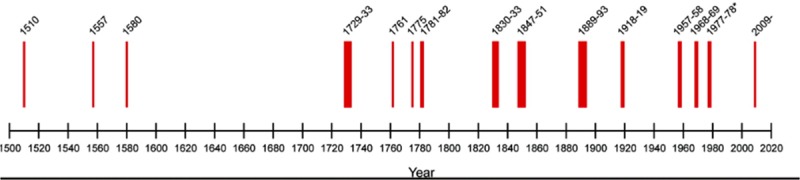
Respiratory viral pandemics since 1500. Reference: Morens, Taubenberger, Folker, and Fauci, 2010. Placed in public domain. https://contagions.wordpress.com/2010/12/31/pandemic-influenza-1510-2010/.
Randomized, Embedded, Multi-factorial, Adaptive Platform trial for Community-Acquired Pneumonia (REMAP-CAP) is another example of an InFACT-led initiative that has positioned itself well to answer timely research questions during pandemic illness such as COVID-19 (NCT02735707, www.remapcap.org). The unique study design allows for sequential investigation of a number of different interventions targeting pneumonia including specific antibiotics, antivirals, or corticosteroids, for example (15). In the setting of a pandemic, the adaptive design allows for evaluation of new interventions and multiple treatment options, even those specifically targeted to new or emerging viruses. This adaptive feature allows for trial infrastructure to be established and to even begin enrolling patients examining traditional interventions for pneumonia, while providing opportunity to change intervention mid-trial to more specific or relevant agents, targeted to specific emerging pathogens. For these reasons, the adaptive trial design is likely the optimal methodology for investigating different anti-COVID interventions within the same design. In fact, the REMAP-CAP team has already evolved their protocol to address COVID-19 and will focus on treatment domains in study centers affected by the virus, including the evaluation of prolonged macrolide therapy, corticosteroid administration strategies, antiviral use, and interferon-beta. Through central administration and wide-scale international recruitment, REMAP-CAP is well-positioned to enroll a large number of geographically diverse patients; both crucial components to study a global pandemic. Through the adaptive randomization, treatment arms that show the most promise or benefit along with the least amount of toxicity will see increased allocation of trial participants, while those with less efficacy or more toxicity will see decreased allocation (Fig. 3). Similarly, the WHO has an adaptive trial planned assessing multiple interventions which may be efficacious in the setting of COVID-19 and has developed a core outcome set to be used during pandemic research (www.who.int).
Figure 3.
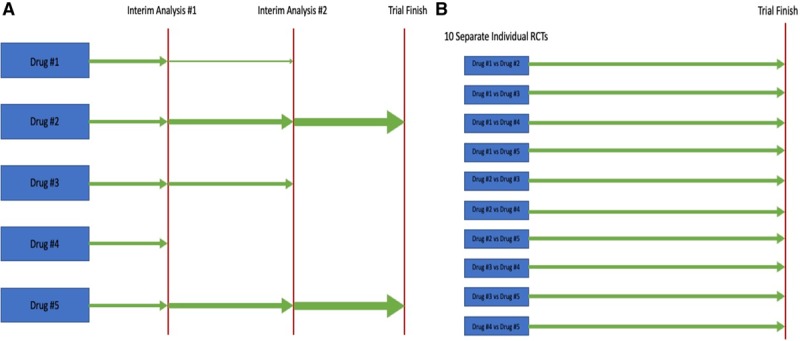
Depiction of an adaptive trial design. Width of green arrows corresponds to number of patients allocated to each trial intervention. A, Demonstrates an adaptive trial design addressing five different treatment interventions. B, Demonstrates 10 traditional randomized controlled trials (RCTs) that would be necessary to address the same five treatment interventions.
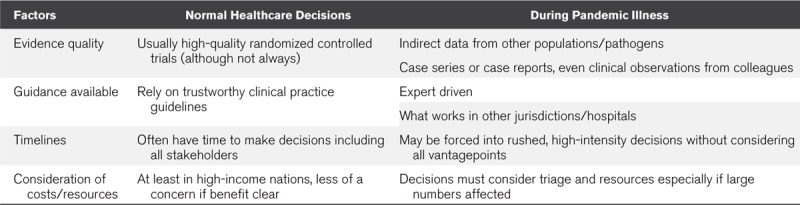
Given the rapidity of new research data associated with the COVID-19 pandemic, the next question for bedside practitioners becomes which data are of sufficient quality and trustworthiness that it should inform clinical practice (Table 1). Might we accept a lower threshold in the setting of health crises, as opposed to other settings (16)? Clinical practice guidelines (CPGs) are often considered the gold standard for informing healthcare decision-making; however, traditionally, CPGs take years to produce, limiting their ability to impact knowledge translation during pandemic illnesses. To address this, guideline developers have attempted to provide rapid guidance documents, still produced using rigorous methodology, but often addressing questions of smaller scope, using larger teams to facilitate expedited recommendations, and frequently updated (17–19). There are a number of these rapid guideline efforts, some done using Grading of Recommendations Assessment, Development and Evaluation methodology, currently underway addressing COVID-19 with a couple having just recently been published such as the Australia and New Zealand Intensive Care Society guidelines (www.anzics.com.au/coronavirusa-guidelines) and the Society of Critical Care Medicine/European Society of Intensive Care Medicine guidelines (https://www.sccm.org/SurvivingSepsisCampaign/Guidelines/COVID-19). One of the risks associated with this constantly evolving research landscape, is that evidence, and subsequently best practice, is constantly changing. As such, it is not a surprise that these guidelines are not entirely consistent with one another, and risk quickly becoming out of date. This can be overcome through frequent reassessment of recommendations based on emerging evidence (living guidelines), which although crucial in this setting, represents an added challenge to be addressed.
TABLE 1.
Healthcare Decisions During Pandemic Illness
CONCLUSIONS
Pandemics and healthcare crises provide extraordinary opportunities for the rapid generation of reliable scientific information but also for misinformation, especially in the early phases, which may contribute to public hysteria. The best way to combat misinformation is with trustworthy data produced by healthcare researchers. Although challenging, research can occur during pandemics and crises and is facilitated by advance planning, governmental support, targeted funding opportunities, and collaboration with industry partners. The COVID-19 research response has highlighted both the dangers of misinformation as well as the benefits and possibilities of performing rigorous research during challenging times.
Footnotes
Dr. Rochwerg is supported by the Hamilton Health Sciences Early Career Research Award. Dr. Lamontagne is supported by a Fonds de recherche du Québec - Santé Award. Dr. Sevransky’s institution has received funding from the Marcus Foundation for a sepsis clinical trial. The remaining authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Lo B, Katz MH. Clinical decision making during public health emergencies: Ethical considerations. Ann Intern Med. 2005; 143:493–498 [DOI] [PubMed] [Google Scholar]
- 2.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020; 382:970–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Merchant RM, Lurie N. Social media and emergency preparedness in response to novel coronavirus. JAMA. 2020. Mar 23. [online ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Lampos V, Cristianini N.Tracking the flu pandemic by monitoring the social web.. Paper presented at: 2010 2nd International Workshop on Cognitive Information Processing; June 14–16; Elba Island, Italy: 2010. [Google Scholar]
- 5.Xu WW, Chiu IH, Chen Y, et al. Twitter hashtags for health: Applying network and content analyses to understand the health knowledge sharing in a Twitter-based community of practice. Qual Quant. 2015; 49:1361–1380 [Google Scholar]
- 6.Choo EK, Ranney ML, Chan TM, et al. Twitter as a tool for communication and knowledge exchange in academic medicine: A guide for skeptics and novices. Med Teach. 2015; 37:411–416 [DOI] [PubMed] [Google Scholar]
- 7.Ortiz JR, Rudd KE, Clark DV, et al. Clinical research during a public health emergency: A systematic review of severe pandemic influenza management. Crit Care Med. 2013; 41:1345–1352 [DOI] [PubMed] [Google Scholar]
- 8.Ji Y, Ma Z, Peppelenbosch MP, et al. Potential association between COVID-19 mortality and health-care resource availability. Lancet Glob Health. 2020; 8:e480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook D, Burns K, Finfer S, et al. Clinical research ethics for critically ill patients: A pandemic proposal. Crit Care Med. 2010; 38:e138–e142 [DOI] [PubMed] [Google Scholar]
- 10.London AJ. Social value, clinical equipoise, and research in a public health emergency. Bioethics. 2019; 33:326–334 [DOI] [PubMed] [Google Scholar]
- 11.Petrini C. Ethics of clinical science in a public health emergency: Reflections on the role of research ethics boards. Am J Bioeth. 2013; 13:27–29 [DOI] [PubMed] [Google Scholar]
- 12.Oshitani H, Kamigaki T, Suzuki A. Major issues and challenges of influenza pandemic preparedness in developing countries. Emerg Infect Dis. 2008; 14:875–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tognotti E. Influenza pandemics: A historical retrospect. J Infect Dev Ctries. 2009; 3:331–334 [DOI] [PubMed] [Google Scholar]
- 14.Dunning JW, Merson L, Rohde GGU, et al. ; ISARIC Working Group 3, ISARIC Council. Open source clinical science for emerging infections. Lancet Infect Dis. 2014; 14:8–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med. 2016; 375:65–74 [DOI] [PubMed] [Google Scholar]
- 16.Bhadelia N, Sauer L, Cieslak TJ, et al. ; National Ebola Training and Education Center’s Special Pathogens Research Network (SPRN)’s Medical Countermeasures Working Group. Evaluating promising investigational medical countermeasures: Recommendations in the absence of guidelines. Health Secur. 2019; 17:46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garritty CM, Norris SL, Moher D. Developing WHO rapid advice guidelines in the setting of a public health emergency. J Clin Epidemiol. 2017; 82:47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morgan RL, Florez I, Falavigna M, et al. Development of rapid guidelines: 3. GIN-McMaster Guideline Development Checklist extension for rapid recommendations. Health Res Policy Syst. 2018; 16:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siemieniuk RA, Agoritsas T, Macdonald H, et al. Introduction to BMJ rapid recommendations. BMJ. 2016; 354:i5191. [DOI] [PubMed] [Google Scholar]


