Background:
Pediatric oncology patients with sepsis are at higher risk of morbidity and mortality compared with pediatric patients without malignancy. Historically, patients with relapsed and/or refractory disease were not considered candidates for aggressive life support strategies including extracorporeal membrane oxygenation support.
Case Summary:
We report a 4-year-old female with relapsed refractory pre-B cell acute lymphoblastic leukemia preparing for chimeric antigen receptor T cell therapy with tisagenlecleucel who was admitted with fever and neutropenia. She progressed to refractory septic shock secondary to Escherichia coli bacteremia and required escalation of hemodynamic support to venoarterial extracorporeal membrane oxygenation cannulation. She cleared her E. coli bacteremia, was decannulated, subsequently received her chimeric antigen receptor T-cell therapy, and was declared disease free 1 month from her initial presentation.
Conclusion:
The ability to provide chimeric antigen receptor T-cell therapy at designated institutions can augment extracorporeal membrane oxygenation candidacy discussions in oncology patients with relapsed disease and may make extracorporeal membrane oxygenation candidacy for oncology patients with refractory sepsis more favorable.
Keywords: chimeric antigen receptor, extracorporeal membrane oxygenation, leukemia, pediatrics, sepsis
Pediatric oncology patients with sepsis are at higher risk of morbidity and mortality as compared to pediatric patients without malignancy. The increased risk of severe infection and sepsis in the setting of neutropenia has been well documented, especially in the presence of an indwelling central catheter (1–5). Historically, oncologic patients, especially those with active disease, have not been considered optimal candidates for extracorporeal membrane oxygenation (ECMO) support, although sepsis itself is an accepted indication for ECMO (6). However, new treatment modalities have become available to patients with relapsed and refractory leukemia, including chimeric antigen receptor T-cell (CAR T-cell) therapy, which has transformed the disease course and survival rates of a once incurable disease (7).
We present the following case report as an example of ECMO for refractory pediatric septic shock and multiple organ failure in a patient with relapsed refractory acute lymphoblastic leukemia (ALL) undergoing lymphodepletion for CAR T-cell therapy.
PRESENTING CONCERNS AND CLINICAL FINDINGS
The patient was a 4-year-old Mexican female with a history of relapsed refractory pre-B cell ALL, who transferred her care to our facility to facilitate CAR T-cell therapy.
Past medical history was pertinent for the diagnosis of standard risk pre-B ALL with blasts that coexpressed CD19, CD22, CD58, CD10, CD38, and cytogenetics that revealed p53 deletion, t(1;19) with a complex karyotype. She had no CNS disease. Patient was treated with the National Mexican Protocol (based off Berlin-Frankfurt-Münster and St. Jude Total XIII). There was no evidence of morphologic or minimal residual disease via flow cytometry at the end of induction chemotherapy. She relapsed 199 days after diagnosis then failed reinduction chemotherapy as well as two cycles of blinatumomab. She was then treated with reinduction therapy per ALL R3 and did not achieve remission. She experienced a high burden of extramedullary disease in the liver, spleen, kidneys, lymph nodes, and a chloroma to left orbit causing a significant cranial nerve II palsy. She experienced renal insufficiency requiring long-term electrolyte replacement. Due to poor response to therapy, plans were made for transfer of care for CAR T-cell therapy. She received bridging oral chemotherapy with 6-mercaptopurine and methotrexate.
Approximately 2 weeks prior to presentation to the ICU, she was treated for a Staphylococcal epidermidis central catheter infection and septic shock with a 10-day course of vancomycin. In preparation for CAR T-cell infusion, she received lymphoid depleting chemotherapy that consisted of fludarabine and cyclophosphamide. She presented to the emergency department on day 2 of lymphoid depleting therapy (1 wk prior to planned CAR T-cell treatment) with fever, nausea, headache, and fatigue. After initial resuscitation, work-up, and initiation of broad-spectrum antibiotics for fever with neutropenia, she was admitted to the hematology/oncology team.
Approximately 36 hours after admission, she was transferred to the PICU due to progressive septic shock with hypotension. On examination, she was afebrile, blood pressure was 69/31, heart rate 165 beats per minute, respiratory rate 56 breaths per minute, and oxygen saturation was 100% on room air. Poor perfusion and a capillary refill time of 5 seconds were noted. Pulses were noted to be 3+ in bilateral radial arteries. Despite tachycardia, no murmur or gallop was appreciated. She remained hypotensive despite aggressive fluid resuscitation (100 mL/kg normal saline over 24 hr), high doses of inotropes, and broad-spectrum antibiotics. She underwent intubation for persistent shock within an hour of PICU transfer. At this time, author (J.O.M.) contacted the patient’s oncology team to discuss expected prognosis with her upcoming CAR T-cell therapy and gain initial impressions of her ECMO candidacy.
DIAGNOSTIC FOCUS AND ASSESSMENT
Her blood culture was found to be positive for Escherichia coli, which was suspected to be multidrug resistant based on preliminary sensitivity profile and history of prolonged care in Mexico, which complicated her antibiotic management. She was maintained on minimal respiratory support with mechanical ventilation with good oxygenation and ventilation. An initial echocardiogram obtained following PICU transfer revealed severe global hypokinesis of the left ventricle with an ejection fraction (EF) of 31% while on significant vasoactive support including epinephrine, norepinephrine, and vasopressin with maximal Vasoactive-Inotropic Score of 98. With an ongoing shock state and acute kidney injury including severe oliguria and total body fluid overload (18%), in addition to lack of response to escalation of vasoactive support, stress dose steroids, bicarbonate infusion, and continuous renal replacement therapy (CRRT) were initiated. At 8 hours after CRRT initiation, there was evidence of progression to multiple organ dysfunction with ongoing shock (peak lactate of 24 mmol/L), myocardial ischemia (troponin 2.03 nanogram/mL), renal dysfunction (three-fold rise in baseline serum creatinine 0.2–0.6), coagulopathy (prothrombin time 28.6 s, partial thromboplastin time 111.2 s, international normalized ratio 2.50), and hepatobiliary dysfunction (aspartate aminotransferase 224 unit/L, total bilirubin 4.3 mg/dL, direct bilirubin 3.7 mg/dL) (Fig. 1).
Figure 1.
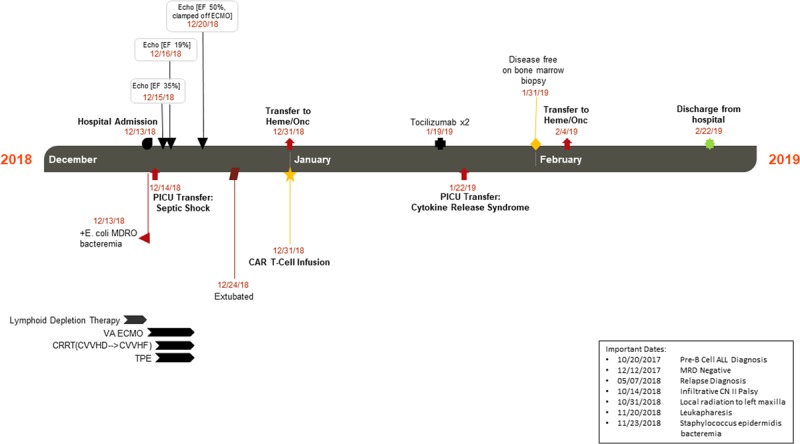
Patient transferred to the PICU on hospital day 2 and subsequently initiated on venoarterial extracorporeal membrane oxygenation (VA ECMO) on PICU day 2, decannulated on PICU day 6, and extubated on PICU day 10. To support her multiple organ dysfunction, she was initiated on continuous venovenous hemodialysis (CVVHD) the evening prior to VA ECMO cannulation and remained on continuous renal replacement therapy (CRRT) through decannulation. In addition, she received therapeutic plasma exchange (TPE) for a total of five treatments. Her cardiac function improved, and she was able to receive her chimeric antigen receptor T-cell (CAR T-cell) infusion. She remained in the hospital for ongoing monitoring related to her treatment with a short return to the PICU for cytokine release syndrome. Repeat bone marrow biopsy deemed her disease free, and she was discharged 10 wk after admission. ALL = acute lymphoblastic leukemia, CN II = cranial nerve two, CVVHF = continuous venovenous hemo filtration, E. coli MDRO = Escherichia coli multidrug-resistant organism, EF = ejection fraction, MRD = minimal residual disease.
With this ongoing decline despite escalation of support, conversations continued between the pediatric intensivist (J.S.W.) and her primary oncologist (T.F.) as to her ECMO candidacy and prognosis in the setting of her relapsed refractory ALL. With our institution’s experience and success with CAR T-cell therapy, it was felt the patient’s survival was estimated to be greater than 50% with respect to her underlying disease, and the family was consented for venoarterial ECMO with the aid of a Spanish interpreter.
THERAPEUTIC FOCUS AND ASSESSMENT
The patient was cannulated in the PICU for venoarterial ECMO with a 16F arterial cannula in the right common carotid and 21F venous cannula in the right internal jugular vein with confirmation of positioning on echocardiography, using flows of 140 mL/kg/min. During cannulation, her indwelling central catheter was removed for infectious source control. Anticoagulation was managed with systemic heparin initially with lower targets due to catheter removal and subsequently escalated to goal activated clotting time 160–180 seconds and anti-Xa level 0.5–0.7 IU/mL without bleeding or thrombotic complications. CRRT via Prismaflex and therapeutic plasma exchange (TPE, 1.5× volume day 1 followed by 1× volume for a total of 5 d) were immediately used in tandem with the ECMO circuit for refractory sepsis with multiple organ dysfunctions. Broad-spectrum antimicrobial therapy to cover her confirmed extended spectrum beta-lactamase producing E. coli included meropenem, ceftazidime/avibactam, gentamicin, and micafungin.
FOLLOW-UP AND OUTCOMES
Following ECMO cannulation, she was rapidly weaned off the previously mentioned vasoactive and inotropic support. She was initiated on milrinone for afterload reduction and improvement in systemic vascular resistance. As she maintained good antegrade flow across the aortic valve, she did not require left atrial decompression. Her bacteremia cleared, and the cardiac dysfunction quickly improved with repeat echo demonstrating low normal biventricular systolic function with an EF of 51% during an ECMO clamp trial after 120 hours of ECMO. She was decannulated following a total of 124 hours on venoarterial ECMO (PICU day 6) and extubated on PICU day 10. With recovery of organ dysfunction, she received her CAR T-cell infusion (tisagenlecleucel) on PICU day 17 and was then transferred to the hematology/oncology service. She developed cytokine release syndrome requiring tocilizumab and brief PICU readmission. She was identified to be in remission 1 month later and was discharged from the hospital shortly thereafter.
DISCUSSION
Patients with active relapsed and/or refractory ALL have previously not been considered optimal candidates for ECMO, and interpretation of the ELSO Red Book would at best consider this situation as a relative contraindication due to the presence of a preexisting chronic illness with low survival rate and at worst an absolute contraindication due to incurable malignancy (8). Yet, this very population is at notably higher risk for increased morbidity and mortality in the face of sepsis, often requiring a higher intensity of therapy and interventions (9–13). Additionally, ECMO has been successfully used in patients with active curable malignancy for a variety of indications (14–17).
New cancer treatment modalities have become available for relapsed refractory pre-B ALL, including CAR T-cell therapy, that have transformed the disease course and survival rates of what was once an incurable disease. Pediatric CAR T-cell clinical trials have reported 81%–93% complete remission and 52%–79% overall 12-month survival (7). Additionally, experience with ECMO in refractory sepsis is increasing, and survival rates for this rescue modality are approaching those for refractory respiratory failure at 50%–60% in recent years (18–25).
Although our patient had maximized the supports as outlined in the American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock, she continued to have progressive refractory shock with concern for impending cardiac arrest (6). She had already undergone harvest of T-cells and was undergoing lymphoid-depleting therapy in preparation for CAR T-cell therapy; thus, the time course to her definitive therapy was short, and her estimated prognosis with this therapy was significantly higher and above the threshold set by the Extracorporeal Life Support Organization as a contraindication for ECMO. Additionally, she was at a quaternary care center with the resources and experience for both ECMO and CAR T-cell therapy.
Certainly, there are ongoing patient-centric and center-centric limitations to this consideration and approach. One serious initial consideration for our patient was the possibility of anthracycline-induced cardiac dysfunction which has been associated with higher intensity treatment and mortality (26). As this would have altered the expected recovery and survival, it is an important consideration; our patient was ultimately deemed to have a lower likelihood of this complication with normal recent preadmission echo reports. Additionally, as sepsis tends to be a more rapidly reversible etiology with shorter ECMO runs, this rationale may not be applicable to other indications for ECMO cannulation and support. Finally, had our patient not had plans for and/or begun preparatory stages of CAR T-cell therapy, the expected survival would likely have precluded the use of ECMO.
We propose that as more novel and targeted therapies such as CAR T-cell therapy become available, that the contraindications for aggressive life support strategies in our oncology patients may need to be modified or re-examined on a case-by-case basis.
CONCLUSIONS
The ability to provide CAR T-cell therapy at designated institutions can augment ECMO candidacy discussions in oncology patients with relapsed disease. CAR T-cell therapy can improve survival from underlying relapsed disease and may make ECMO candidacy for oncology patients with refractory sepsis more favorable.
ACKNOWLEDGMENTS
We would like to thank Drs. Doug Myers and Erin Hall for their expertise in chimeric antigen receptor T-cell therapy.
Footnotes
Institutional informed consent was obtained to facilitate the production of this case report.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Hord JD, Lawlor J, Werner E; Children’s Hospital Association Childhood Cancer and Blood Disorders Network. Central line associated blood stream infections in pediatric hematology/oncology patients with different types of central lines. Pediatr Blood Cancer. 2016; 63:1603–1607 [DOI] [PubMed] [Google Scholar]
- 2.Miliaraki M, Katzilakis N, Chranioti I, et al. Central line-associated bloodstream infection in childhood malignancy: Single-center experience. Pediatr Int. 2017; 59:769–775 [DOI] [PubMed] [Google Scholar]
- 3.Moskalewicz RL, Isenalumhe LL, Luu C, et al. Bacteremia in nonneutropenic pediatric oncology patients with central venous catheters in the ED. Am J Emerg Med. 2017; 35:20–24 [DOI] [PubMed] [Google Scholar]
- 4.Thurman CB, Abbott M, Liu J, et al. Risk for health care-associated bloodstream infections in pediatric oncology patients with various malignancies. J Pediatr Oncol Nurs. 2017; 34:196–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Viana Taveira MR, Lima LS, de Araújo CC, et al. Risk factors for central line-associated bloodstream infection in pediatric oncology patients with a totally implantable venous access port: A cohort study. Pediatr Blood Cancer. 2017; 64:336–342 [DOI] [PubMed] [Google Scholar]
- 6.Davis AL, Carcillo JA, Aneja RK, et al. American college of critical care medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017; 45:1061–1093 [DOI] [PubMed] [Google Scholar]
- 7.Forsberg MH, Das A, Saha K, et al. The potential of CAR T therapy for relapsed or refractory pediatric and young adult B-cell ALL. Ther Clin Risk Manag. 2018; 14:1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brogan TV, Lequier L, Lorusso R, et al. Extracorporeal Life Support: The Elso Red Book. 2017Fifth Edition Ann Arbor, MI: Extracorporeal Life Support Organization [Google Scholar]
- 9.Ali AM, Sayed HA, Mohammed MM. The outcome of critically ill pediatric cancer patients admitted to the pediatric intensive care unit in a tertiary university oncology center in a developing country: A 5-year experience. J Pediatr Hematol Oncol. 2016; 38:355–359 [DOI] [PubMed] [Google Scholar]
- 10.Dagher GA, Safa R, Hajjar K, et al. Characteristics and outcomes of pediatric septic patients with cancer: A retrospective cohort study. J Emerg Med. 2019; 57:216–226 [DOI] [PubMed] [Google Scholar]
- 11.Faraci M, Bagnasco F, Giardino S, et al. Intensive care unit admission in children with malignant or nonmalignant disease: Incidence, outcome, and prognostic factors: A single-center experience. J Pediatr Hematol Oncol. 2014; 36:e403–e409 [DOI] [PubMed] [Google Scholar]
- 12.Pillon M, Sperotto F, Zattarin E, et al. Predictors of mortality after admission to pediatric intensive care unit in oncohematologic patients without history of hematopoietic stem cell transplantation: A single-center experience. Pediatr Blood Cancer. 2019; 66:e27892. [DOI] [PubMed] [Google Scholar]
- 13.Zinter MS, DuBois SG, Spicer A, et al. Pediatric cancer type predicts infection rate, need for critical care intervention, and mortality in the pediatric intensive care unit. Intensive Care Med. 2014; 40:1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorjup V, Fister M, Noc M, et al. Treatment of sepsis and ARDS with extracorporeal membrane oxygenation and interventional lung assist membrane ventilator in a patient with acute lymphoblastic leukemia. Respir Care. 2012; 57:1178–1181 [DOI] [PubMed] [Google Scholar]
- 15.Gow KW, Heiss KF, Wulkan ML, et al. Extracorporeal life support for support of children with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Crit Care Med. 2009; 37:1308–1316 [DOI] [PubMed] [Google Scholar]
- 16.Gow KW, Lao OB, Leong T, et al. Extracorporeal life support for adults with malignancy and respiratory or cardiac failure: The extracorporeal life support experience. Am J Surg. 2010; 199:669–675 [DOI] [PubMed] [Google Scholar]
- 17.Huprikar NA, Peterson MR, DellaVolpe JD, et al. Salvage extracorporeal membrane oxygenation in induction-associated acute respiratory distress syndrome in acute leukemia patients: A case series. Int J Artif Organs. 2019; 42:49–54 [DOI] [PubMed] [Google Scholar]
- 18.Falk L, Hultman J, Broman LM. Extracorporeal membrane oxygenation for septic shock. Crit Care Med. 2019; 47:1097–1105 [DOI] [PubMed] [Google Scholar]
- 19.Grasso C, Annich GM. Venoarterial extracorporeal membrane oxygenation in severe pediatric septic shock. Pediatr Crit Care Med. 2018; 19:1000–1999 [DOI] [PubMed] [Google Scholar]
- 20.Oberender F, Ganeshalingham A, Fortenberry JD, et al. Venoarterial extracorporeal membrane oxygenation versus conventional therapy in severe pediatric septic shock. Pediatr Crit Care Med. 2018; 19:965–972 [DOI] [PubMed] [Google Scholar]
- 21.Park TK, Yang JH, Jeon K, et al. Extracorporeal membrane oxygenation for refractory septic shock in adults. Eur J Cardiothorac Surg. 2015; 47:e68–e74 [DOI] [PubMed] [Google Scholar]
- 22.Ro SK, Kim WK, Lim JY, et al. Extracorporeal life support for adults with refractory septic shock. J Thorac Cardiovasc Surg. 2018; 156:1104–1109.e1 [DOI] [PubMed] [Google Scholar]
- 23.Robb K, Badheka A, Wang T, et al. Use of extracorporeal membrane oxygenation and associated outcomes in children hospitalized for sepsis in the united states: A large population-based study. PLoS One. 2019; 14:e0215730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Solé A, Jordan I, Bobillo S, et al. Venoarterial extracorporeal membrane oxygenation support for neonatal and pediatric refractory septic shock: More than 15 years of learning. Eur J Pediatr. 2018; 177:1191–1200 [DOI] [PubMed] [Google Scholar]
- 25.Vogel DJ, Murray J, Czapran AZ, et al. Veno-arterio-venous ECMO for septic cardiomyopathy: A single-centre experience. Perfusion. 2018; 33:57–64 [DOI] [PubMed] [Google Scholar]
- 26.Wolfe KK, Reichek J, Marsillio LE. Critical illness and cardiac dysfunction in anthracycline-exposed pediatric oncology patients. Pediatr Crit Care Med. 2019; 20:595–602 [DOI] [PubMed] [Google Scholar]


