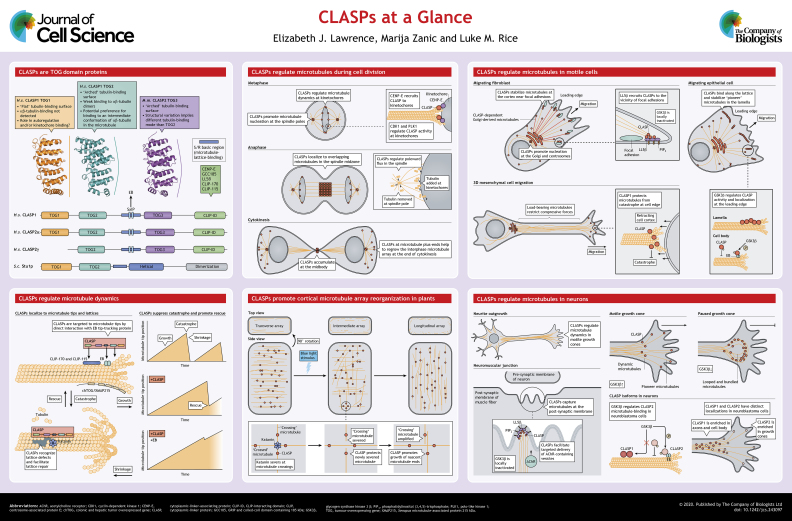
ABSTRACT
CLIP-associating proteins (CLASPs) form an evolutionarily conserved family of regulatory factors that control microtubule dynamics and the organization of microtubule networks. The importance of CLASP activity has been appreciated for some time, but until recently our understanding of the underlying molecular mechanisms remained basic. Over the past few years, studies of, for example, migrating cells, neuronal development, and microtubule reorganization in plants, along with in vitro reconstitutions, have provided new insights into the cellular roles and molecular basis of CLASP activity. In this Cell Science at a Glance article and the accompanying poster, we will summarize some of these recent advances, emphasizing how they impact our current understanding of CLASP-mediated microtubule regulation.
KEY WORDS: CLASP, Microtubule dynamics, Mitosis, Motility
Summary: An overview of recent progress deciphering the molecular mechanisms and cellular functions of CLASPs that regulate microtubule dynamics.
Introduction
The name CLASP, for CLIP-associating protein, was coined in a foundational study (Akhmanova et al., 2001) that identified proteins that interacted with cytoplasmic linker protein (CLIP)-170 and CLIP-115 (also known as CLIP1 and CLIP2, respectively), two related proteins that associate with growing microtubule ends to regulate their dynamics (see Box 1 for an overview of microtubule dynamics). By virtue of their characteristic domain organization and sequence, CLASP proteins were recognized to be orthologous to gene products that had been previously identified by genetic screening in Drosophila melanogaster (MAST/Orbit) (Inoue et al., 2000; Lemos et al., 2000) and Saccharomyces cerevisiae (Stu1) (Pasqualone and Huffaker, 1994). CLASP orthologs were also identified in Caernorhabditis elegans (Hannak and Heald, 2006) and in Arabidopsis thaliana (Ambrose et al., 2007). The first studies on human CLASP paralogs (see Box 2) reported that CLASP1 is ubiquitously expressed whereas CLASP2 is enriched in brain tissue (Akhmanova et al., 2001).
Box 1. Microtubule dynamic instability.
Microtubule dynamic instability is the hallmark behavior of microtubule polymers (Mitchison and Kirschner, 1984), in which individual microtubule ends stochastically switch between periods of growth and shrinkage. A growing microtubule end maintains a ‘cap’ of GTP-bound tubulin that protects against depolymerization. ‘Catastrophe’, the sudden transition from growth to shrinkage, occurs when this cap is lost. A shrinking microtubule can revert to growth, a transition known as ‘rescue’. CLASPs and several other microtubule-associated proteins can selectively localize to the dynamic microtubule ends, where they regulate polymerization dynamics by modulating the structure and/or biochemical properties of the microtubule end (Akhmanova and Steinmetz, 2015; Brouhard and Rice, 2018).
Box 2. Human CLASP proteins.
There are two paralogs of mammalian CLASPs, CLASP1 (also known as CLASP1α) and CLASP2, with three isoforms of CLASP2 (α, β and γ) (Akhmanova et al., 2001). CLASP1 is broadly expressed, whereas CLASP2 expression is primarily limited to the brain. CLASP1 and CLASP2α represent the longest isoforms, each containing three TOG domains, but the N-terminal TOG domain has apparently lost the ability to interact with αβ-tubulin or microtubules (Aher et al., 2018; De la Mora-Rey et al., 2013) and may instead mediate autoregulatory interactions with other regions of the protein (Aher et al., 2018). CLASP2β and CLASP2γ each lack the N-terminal TOG domain present in CLASP1 and CLASP2α. CLASP2β contains a palmitoylation motif near the N-terminus that presumably directs membrane localization, but the functional impact of membrane targeting is not known because this isoform is relatively understudied. Notably, the Clasp2-knockout mouse has a very mild phenotype (Drabek et al., 2012), whereas the double knockout (to our knowledge) is embryonic lethal. Therefore, although CLASP perturbations have revealed paralog-specific functions in cell motility (Bouchet et al., 2016), cell division (Samora et al., 2011) and neurons (Sayas et al., 2019), the CLASP paralogs may be functionally redundant in other settings. More work is needed to define the specific roles of different CLASP paralogs or isoforms and the extent to which they can or cannot compensate for each other.
Early functional observations on CLASPs indicated that CLASP activity stabilized microtubules (Akhmanova et al., 2001; Bratman and Chang, 2007; Drabek et al., 2006; Mimori-Kiyosue et al., 2005; Sousa et al., 2007), and revealed roles in cell polarization (Akhmanova et al., 2001; Efimov et al., 2007; Miller et al., 2009; Mimori-Kiyosue et al., 2005; Wittmann and Waterman-Storer, 2005) and cell division (Maiato et al., 2002, 2003, 2005; Mimori-Kiyosue et al., 2006; Ortiz et al., 2009; Pereira et al., 2006). Indeed, in multicellular organisms, CLASPs were observed to associate with growing microtubules at the leading edge of motile cells (Akhmanova et al., 2001; Mimori-Kiyosue et al., 2005; Wittmann and Waterman-Storer, 2005), and to also localize at kinetochores and the mitotic spindle during mitosis (Akhmanova et al., 2001; Hannak and Heald, 2006; Inoue et al., 2000; Lemos et al., 2000; Maiato et al., 2002, 2003; Mimori-Kiyosue et al., 2006). In yeasts, CLASPs were similarly found to bind microtubules and to have roles in spindle assembly (Bratman and Chang, 2007; Funk et al., 2014; Ortiz et al., 2009; Pasqualone and Huffaker, 1994; Yin et al., 2002). In addition to demonstrating that CLASPs could regulate microtubule dynamics, CLASPs were also shown to localize to the major microtubule-nucleating centers, including centrosomes and the Golgi, where they promote microtubule nucleation and increase microtubule density (Efimov et al., 2007; Miller et al., 2009; Mimori-Kiyosue et al., 2005). Overall, a common theme that emerged from these early studies was that CLASP activity is important for proper formation of specific networks or subpopulations of microtubules.
CLASPs can bind directly to microtubules and to other microtubule-associated proteins including EB1 (also known as MAPRE1), as well as to other binding partners that are not associated with microtubules (Akhmanova et al., 2001; Bratman and Chang, 2007; Cheeseman et al., 2005; Efimov et al., 2007; Lansbergen et al., 2006; Maffini et al., 2009; Manning et al., 2010; Mimori-Kiyosue et al., 2005; Wittmann and Waterman-Storer, 2005). CLASP proteins were also recognized to contain a tubulin-binding TOG domain homologous to those found in otherwise unrelated microtubule polymerases of the XMAP215/Stu2/chTOG/CKAP5 family (Akhmanova et al., 2001; reviewed in Al-Bassam and Chang, 2011; Slep, 2009), providing a potential clue about their mechanism. However, deeper insights into the molecular mechanism were limited by a lack of in vitro reconstitutions. Although the first in vitro reconstitution of CLASP activity was reported in 2007 using the Schizosaccharomyces pombe CLASP (Al-Bassam et al., 2010), a second reconstitution only followed in 2016 (Moriwaki and Goshima, 2016) using CLASP from Drosophila. More recent reconstitutions are discussed below.
In this Cell Science at a Glance article and the accompanying poster, we will summarize recent developments that provide insight into the cellular function or molecular mechanism of CLASP activity, drawing on studies of migrating cells, neuronal development, microtubule reorganization in plants, in vitro reconstitution, and more.
Structural and mechanistic insights
The first structures of CLASP family TOG domains and new reconstitutions of human, insect and fungal CLASP activity have reshaped our thinking about the mechanisms by which CLASPs control microtubule dynamics.
TOG domains are helical repeat modules that bind the α-tubulin–β-tubulin heterodimers (αβ-tubulin) (Al-Bassam et al., 2006, 2007; Slep and Vale, 2007), and that can interact selectively with specific conformations of αβ-tubulin (Ayaz et al., 2012, 2014) (see poster). CLASP proteins can bind to unpolymerized αβ-tubulin, and early analyses of CLASP sequences identified one TOG domain that is homologous to those found in XMAP215 family polymerases (Akhmanova et al., 2001). Subsequent sequence analysis uncovered additional, divergent TOG domains that retained tubulin-binding elements that have been identified in studies of polymerase TOGs (Slep and Vale, 2007). Structures of CLASP-specific TOG2 domains revealed differences compared to that seen for polymerase TOGs. First, the putative tubulin-binding residues are arranged differently to those in polymerase TOGs, possibly indicating that CLASP TOG2 recognizes a different conformation of αβ-tubulin from that recognized by polymerase TOGs (Leano et al., 2013; Majumdar et al., 2018; Maki et al., 2015). Second, a portion of the primary sequence preceding TOG2 folds into an α-helix that docks onto one face of the TOG, suggesting that the linker between CLASP TOGs is less flexible than observed in polymerases (Leano et al., 2013; Majumdar et al., 2018; Maki et al., 2015). Third, a CLASP-specific conserved arginine residue likely confers a distinct character to the TOG–tubulin interface (Majumdar et al., 2018). Although their contributions to CLASP activity and/or function is not as well explored as that of the TOG2, these other CLASP TOGs also show distinctive features compared to those in polymerase TOGs, likely indicating specialized αβ-tubulin-binding properties or other functional specialization (Leano and Slep, 2019; Maki et al., 2015) (see also Box 2).
Recent reconstitutions of human CLASPs have revealed that CLASPs can autonomously recognize growing microtubule ends, suppress catastrophe and promote rescue, all without significantly affecting the rates of microtubule growing or shrinking (Aher et al., 2018; Lawrence et al., 2018; Lawrence and Zanic, 2019) (see poster). The anti-catastrophe and rescue activities of human CLASPs are increased in the presence of EB1 family plus-end-tracking proteins because direct CLASP–EB interactions increase the amount of CLASP at the growing microtubule end (Aher et al., 2018; Lawrence et al., 2018). Reconstitutions of insect CLASP have revealed that they have potent anti-catastrophe activity and suppress microtubule growth rate (Moriwaki and Goshima, 2016). Whereas the polymerase activity of XMAP215 family polymerases strictly requires two linked TOG domains (Ayaz et al., 2014; Geyer et al., 2018; Widlund et al., 2011), isolated human or fungal CLASP TOG domains by themselves are sufficient to recapitulate anti-catastrophe and rescue activity (Aher et al., 2018; Majumdar et al., 2018).
Taken together, the new structure and reconstitutions reveal that the operational principles underlying CLASP activity differ fundamentally from those that form the basis for polymerase activity (Ayaz et al., 2014; Geyer et al., 2018), and they diversify the understanding of what TOG domains can do. Indeed, the demonstration that a single CLASP TOG can recapitulate the regulatory activity of the intact protein indicates that ‘tethering’ of an unpolymerized αβ-tubulin subunit to the lattice is not strictly required for CLASP activity, unlike what is seen for polymerases where tethering is an obligate part of the mechanism (Ayaz et al., 2014; Geyer et al., 2018). Instead, CLASP TOGs must control the frequencies of microtubule catastrophe and rescue by acting directly to enhance interactions between αβ-tubulin subunits at or very near the microtubule end. The structural basis for how CLASP TOGs bind αβ-tubulin has not yet been determined, so we can only speculate that the anti-catastrophe and rescue-promoting activities occur as the result of TOG-mediated stabilization of an intermediate end-specific conformation of αβ-tubulin.
CLASPs in cell division
Early studies of CLASPs revealed that they have essential and conserved functions at the mitotic spindle (Ambrose et al., 2007; Hannak and Heald, 2006; Inoue et al., 2000; Lemos et al., 2000; Maiato et al., 2002, 2003; Pasqualone and Huffaker, 1994). In mammalian cells, loss of CLASP activity causes severe mitotic defects, including metaphase delay, disorganized, mono- or multi-polar spindles, misaligned chromosomes and cytokinesis failure (Maiato et al., 2003; Mimori-Kiyosue et al., 2006; Pereira et al., 2006) (see poster). During early mitosis, human CLASPs localize to the outer kinetochore and spindle poles (Maiato et al., 2003; Mimori-Kiyosue et al., 2006; Pereira et al., 2006). After the metaphase-anaphase transition, CLASPs re-localize to the spindle midzone where they stabilize overlapping microtubules and accumulate in the midbody during cytokinesis (Maiato et al., 2003; Mimori-Kiyosue et al., 2006; Pereira et al., 2006).
Several studies have established a role for CLASPs in regulating kinetochore microtubule dynamics (Maffini et al., 2009; Maiato et al., 2005). Notably, CLASPs are thought to be important for promoting poleward flux in spindle microtubules, thus ensuring proper spindle dynamics. CLASPs are targeted to kinetochores by direct interaction with centrosome-associated protein E (CENP-E), independently of both the microtubule-binding activity of CLASP and the motor activity of CENP-E (Maffini et al., 2009; Maiato et al., 2003). Phosphorylation of CLASP2 by CDK1 and PLK1 has been implicated in regulating the stability of kinetochore–microtubule attachments (Maia et al., 2012). However, until now, the role of CLASPs in forming the initial attachment of kinetochores to microtubules has been less well investigated.
Two new studies now add to our understanding of the role of CLASPs at kinetochores. The initial attachment of chromosomes to kinetochore microtubules occurs via a lateral interaction between the wall of the microtubule and proteins of the kinetochore (Rieder and Alexander, 1990). The lateral interaction is subsequently converted to an end-on attachment via a poorly understood mechanism. A recent in vitro study reconstituted kinetochore–microtubule attachment using surface-immobilized beads coated with purified kinetochore proteins and stabilized microtubules (Chakraborty et al., 2019). When beads were coated with CENP-E and CLASP2, microtubules formed stable end-on attachments with the protein-coated beads, with durations lasting up to 14 min. Thus, CLASP2 promotes robust, long-lived interactions with microtubule ends, a process critical for proper chromosome attachment. In dividing S. cerevisiae, the CLASP homolog Stu1 has been identified as a key component of an elegant mechanism by which unattached kinetochores promote their own capture (Kolenda et al., 2018). Here, Stu1 is sequestered at unattached kinetochores in a manner dependent on the protein kinase Mps1 and the kinetochore protein Slk19. Sequestration of Stu1 away from the spindle leads to turnover of the spindle microtubules, promoting the formation of new dynamic microtubules that search the nuclear space for unattached kinetochores. Once an attachment is formed, Stu1 stabilizes the capturing microtubule and, thus, acts to maintain the microtubule–kinetochore connection (Kolenda et al., 2018). In summary, CLASPs are involved in modulating microtubule bundling, dynamics and kinetochore attachments, all of which contribute to proper spindle assembly in dividing cells.
CLASPs in cell motility
CLASPs are essential for persistent cell motility. Indeed, a Clasp2-knockout mouse presents impaired homing of hematopoietic stem cells to the bone marrow niche and disrupted microtubule network stability and organization, indicating a role for CLASPs in regulating microtubules during stem cell migration (Drabek et al., 2012). In cultured motile cells, CLASPs locally stabilize microtubules at the leading edge, and, thus help to polarize the microtubule network towards the direction of migration (Akhmanova et al., 2001; Drabek et al., 2006; Wittmann and Waterman-Storer, 2005). In addition to acting at the cortex, CLASPs are recruited to the Golgi by GCC185 (also known as GCC2), where they stabilize nascent Golgi-derived microtubules (Efimov et al., 2007; Miller et al., 2009). These CLASP-dependent Golgi-derived microtubules preferentially orient towards the leading edge and promote the directional trafficking of vesicles to the migrating cell front, further contributing to cell polarization.
CLASPs are also implicated in the turnover of focal adhesions, large multi-protein complexes that link the intracellular cytoskeleton with the extracellular matrix. Previous work has shown that cortical CLASP is recruited near to focal adhesions by direct interaction with LL5β (also known as PHLDB2), a phosphatidylinositol (3,4,5)-trisphosphate (PIP3)-binding protein (Basu et al., 2015; Lansbergen et al., 2006). Another study in migrating human keratinocytes has shown that cortical clusters of CLASPs are essential for efficient focal adhesion disassembly (Stehbens et al., 2014). Here, CLASP-tethered microtubules provide stable tracks for the delivery, docking and fusion of exocytic vesicles in the vicinity of focal adhesions. Additionally, this work revealed that CLASPs are required for degradation of the extracellular matrix near focal adhesions (Stehbens et al., 2014), placing CLASPs in a local secretion pathway that facilitates focal adhesion turnover. Similarly, CLASP-decorated microtubules support directional trafficking in podosomes, actin-based protrusions important for degradation of extracellular matrix during cell motility and invasion (Efimova et al., 2014).
The localization and activity of CLASPs are spatially modulated in migrating cells by GSK3β-mediated phosphorylation (Akhmanova et al., 2001; Kumar et al., 2009; Watanabe et al., 2009; Wittmann and Waterman-Storer, 2005) (see poster). GSK3β activity is inhibited in the leading edge of motile cells, stimulating CLASP activity. In migrating epithelial cells, CLASPs bind to microtubule tips in the cell body and along microtubule lattices in the lamella (Kumar et al., 2009; Wittmann and Waterman-Storer, 2005). The distinct localization of CLASPs along microtubule lattices is specified by inhibition of GSK3β in the lamella, and may be important for establishing robust connections of the lamella microtubules to the cell cortex.
Interestingly, CLASP1 is implicated in regulating the mechanical properties of microtubules during 3D mesenchymal migration of cancer cells by promoting persistent microtubule growth in cell protrusions); CLASP1 knockdown completely prevented protrusion formation, 3D motility and invasiveness (Bouchet et al., 2016). In contrast, CLASP2 knockdown had no effect on motility, highlighting the potentially different functional roles of CLASP isoforms. The authors propose that CLASP1 helps microtubules to bear compressive forces during cell retraction by suppressing microtubule catastrophe, thus promoting protrusion elongation and cell migration (Bouchet et al., 2016). In 3D migrating Drosophila macrophages, the CLASP homolog Orbit (also known as MAST) promotes the formation of a microtubule bundle that protrudes into the lamella and is involved in directed migration and cell–cell contact-mediated repulsion (Stramer et al., 2010). Whether CLASPs also modulate microtubule mechanics by promoting microtubule bundling and/or increasing the rigidity of individual microtubules during cell invasion has not been investigated.
CLASPs in neurons
CLASPs are crucial regulators of neuronal development and regeneration. CLASPs are specifically enriched in neuronal growth cones, where they play a pivotal role in axonal growth (Beffert et al., 2012; Hur et al., 2011; Lee et al., 2004; Marx et al., 2013). Furthermore, CLASP2 protein levels steadily rise throughout neuronal development, suggesting a continued requirement for CLASP2 activity (Beffert et al., 2012). Paradoxically, while CLASP2 is required for axon growth, too much CLASP activity inhibits axon growth (Hur et al., 2011; Lee et al., 2004). The contradictory effects of CLASP2 can be attributed to GSK3β-mediated modulation of CLASP localization (Hur et al., 2011). When CLASP2 activity is elevated, such as when GSK3β is inhibited, CLASP2 shifts from the microtubule tips to lattices, resulting in microtubule bundling and looping, and preventing axon extension (Hur et al., 2011; Lee et al., 2004). Therefore, CLASP2 can differentially regulate growth cone motility depending on its microtubule-binding activity (see poster).
Recent studies have revealed unexpected differences in the function of the two CLASP paralogs, as well as GSK3β-dependent modulation of CLASP activity. Specifically, it has been reported that the two paralogs have distinct localization in neuroblastoma cells, with CLASP2 decorating growing microtubule ends in the growth cone, and CLASP1 being enriched towards the cell body (Sayas et al., 2019). CLASP1 and CLASP2 were also found to have differential sensitivity to GSk3β-mediated phosphorylation in that study. Overall, the authors concluded that CLASP1 stimulates, while CLASP2 inhibits, neurite extension, suggesting that the activities of the two proteins are finely balanced to achieve proper neuronal differentiation. CLASP2 has also been identified as a cytoskeletal effector in the reelin signaling pathway, which is central to neocortical development (Dillon et al., 2017). Knockdown of CLASP2 in vivo led to the mislocalization of neurons and impaired lamination of the developing cortex. These effects are thought to be mediated by GSK3β-dependent phosphorylation of CLASP2, which modulates its interaction with the reelin pathway component DAB1 (Dillon et al., 2017). Since GSK3β-mediated phosphorylation of CLASP2 modulates the CLASP–microtubule interaction (Akhmanova et al., 2001; Kumar et al., 2009; Watanabe et al., 2009; Wittmann and Waterman-Storer, 2005), it is possible that GKS3β in the reelin pathway also regulates CLASP2 localization on microtubules.
In addition to the role of CLASPs in growth cone motility, CLASPs also regulate synaptic morphology and protein composition. Indeed, CLASP2 overexpression in hippocampal neurons resulted in an increase in the number and area of synapses and disrupted synaptic protein levels (Beffert et al., 2012). Several recent studies have shed light on the function of CLASPs at neuromuscular junctions. Here, CLASPs capture microtubules at the post synaptic membrane, which then serve as stable tracks for the delivery of vesicles containing acetylcholine receptors (Schmidt et al., 2012). CLASP2 is recruited to the post-synaptic membrane by LL5β and is regulated by GSK3β-mediated phosphorylation (Basu et al., 2014, 2015). Thus, CLASPs facilitate directed vesicle transport by tethering and stabilizing microtubules to the post-synaptic membrane, in a manner that is reminiscent of CLASP activity near focal adhesions in motile cells (see poster).
Plant CLASP in microtubule cytoskeleton remodeling
Plants have a single isoform of CLASP that plays important roles in cell and tissue morphogenesis and in cell division (Ambrose et al., 2007; Kirik et al., 2007; Pietra et al., 2013). In Arabidopsis, cortical microtubules undergo dramatic rearrangements both during growth and upon acute blue-light stimulus (see poster). A recent study demonstrated that CLASP activity is central to the brassinosteroid hormone pathway, regulating sustained cell division in Arabidopsis root apical meristems (Ruan et al., 2018). The authors found that CLASP mediates cortical microtubule re-organization in response to brassinosteroid signaling. Interestingly, CLASP functions in a negative feedback loop in which CLASP-stabilized microtubules facilitate the delivery of factors that suppress CLASP transcript levels, thereby downregulating CLASP expression. In addition, CLASP mutants have significant defects in cortical microtubule organization, exhibiting a delayed timing of array reorganization during growth (Thoms et al., 2018) and complete failure of microtubule reorientation in response to blue light (Lindeboom et al., 2019). Earlier studies implicated CLASP in directly linking microtubules to the cortex and locally preventing microtubule catastrophe, thereby preferentially stabilizing cortical microtubules (Ambrose et al., 2007, 2011; Ambrose and Wasteneys, 2008). Now, new work provides novel insight into the interplay between CLASP and the microtubule-severing enzyme katanin during the process of microtubule array reorganization.
Microtubule severing is a major mechanism for microtubule network remodeling in plants (Lindeboom et al., 2013). Severing a single microtubule gives rise to two polymers, which, if stabilized, can serve as templates for new polymer growth and overall network amplification (Kuo et al., 2019; Vemu et al., 2018). Two recent studies in plants have shown that CLASP is required to maintain the population of nascent microtubule plus ends during the array organization. CLASP mutants had ∼30% fewer growing microtubules and a lower density of longitudinally oriented microtubules during cortical array pattern formation (Thoms et al., 2018). CLASP mutants also displayed a significantly smaller probability of microtubule regrowth immediately after severing (Lindeboom et al., 2019).
A possible mechanism for the protective action of CLASP is promoting microtubule rescue at discrete CLASP puncta localized along the lattice of longitudinally oriented microtubules. Surprisingly, however, the effects of CLASP mutations on the parameters of microtubule dynamics, including rescue frequency, were mild (Lindeboom et al., 2019; Thoms et al., 2018). Furthermore, CLASP puncta were also found on transverse microtubules, a population that is not stabilized and is eventually lost (Lindeboom et al., 2019). Alternatively, CLASP could act through the recognition of a lattice ‘defect’. A microtubule crossover event could introduce defects in the lattice of the newer ‘crossing’ microtubule (Aumeier et al., 2016; de Forges et al., 2016); such defects may be recognized by both CLASP and katanin (Díaz-Valencia et al., 2011). Indeed, katanin specifically targets microtubule crossovers and preferentially severs the crossing, as opposed to the crossed microtubule (Lindeboom et al., 2013; Zhang et al., 2013). However, recruitment of CLASP to lattice defect sites may facilitate incorporation of GTP-bound tubulin for lattice repair, and enhance the resistance of the microtubule to mechanical force (Aher et al., 2019 preprint). Thus, CLASP may compete with katanin for binding to sites of microtubule defects, as well as target intermediate lattice structures that are generated during severing (Vemu et al., 2018) (see poster). Although enhanced accumulation of CLASP at microtubule crossovers has not been observed (Lindeboom et al., 2019), the localized action of even a small number of CLASP molecules may be sufficient to stabilize the growing microtubule end (Aher et al., 2018, 2019 preprint). Therefore, CLASP at severing sites may allow immediate continuous growth of nascent microtubule plus ends, while the minus ends are simultaneously protected by SPIRAL2 (Nakamura et al., 2018). In this way, the resulting balance between microtubule severing and stabilization facilitates overall network amplification and reorganization.
Perspectives
CLASP proteins have been subject of significant interest in the last few years, with studies ranging from structures of individual CLASP TOG domains to the roles of CLASPs in 3D cell migration and neuronal development. Connecting the findings from a range of scales, from molecular to organismal, and from different contexts presents an exciting challenge for the immediate future. At the molecular level, how do distinct features of CLASP TOG domains ‘encode’ selective association of CLASPs with dynamic lattice and microtubule ends to mediate microtubule rescue and repair? Answering questions such as these will provide a deeper understanding of the molecular mechanisms underlying CLASP function and also provide new insights into the ways that microtubule end configurations and lattice defects contribute to microtubule dynamics. At the cellular level, how does CLASP function in concert with a multitude of other regulatory factors, and how do concerted actions of these protein ensembles simultaneously control nucleation, growth and stability of microtubule networks built from a shared pool of tubulin in space and time? Given its central role in microtubule network reorganization in a vast array of cellular contexts, CLASP promises to remain at the center stage for years to come.
Acknowledgements
We thank Jelmer Lindeboom (Carnegie Institution) and Irina Kaverina (Vanderbilt University) for providing feedback on the manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
E.J.L. acknowledges the support of the National Institutes of Health IBSTO training grant T32CA119925. M.Z. acknowledges support from the National Institutes of Health grant R35GM119552, and the Searle Scholars Program. Work on TOG domain proteins in L.M.R.’s lab has been supported by grants from the National Institutes of Health (R01-GM098543) and from the Robert A. Welch Foundation (I-1908). Deposited in PMC for release after 12 months.
Cell Science at a Glance
A high-resolution version of the poster and individual poster panels are available for downloading at http://jcs.biologists.org/lookup/doi/10.1242/jcs.243097.supplemental
References
- Aher A., Kok M., Sharma A., Rai A., Olieric N., Rodriguez-Garcia R., Katrukha E. A., Weinert T., Olieric V., Kapitein L. C. et al. (2018). CLASP suppresses microtubule catastrophes through a single TOG domain. Dev. Cell 46, 40-58.e8. 10.1016/j.devcel.2018.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aher A., Rai D., Schaedel L., Gaillard J., John K., Blanchoin L., Thery M. and Akhmanova A. (2019). CLASP mediates microtubule repair by promoting tubulin incorporation into damaged lattices. bioRxiv 15 10.1101/809251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhmanova A. and Steinmetz M. O. (2015). Control of microtubule organization and dynamics: two ends in the limelight. Nat. Rev. Mol. Cell Biol. 16, 711-726. 10.1038/nrm4084 [DOI] [PubMed] [Google Scholar]
- Akhmanova A., Hoogenraad C. C., Drabek K., Stepanova T., Dortland B., Verkerk T., Vermeulen W., Burgering B. M., De Zeeuw C. I., Grosveld F. et al. (2001). Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104, 923-935. 10.1016/S0092-8674(01)00288-4 [DOI] [PubMed] [Google Scholar]
- Al-Bassam J. and Chang F. (2011). Regulation of microtubule dynamics by TOG-domain proteins XMAP215/Dis1 and CLASP. Trends Cell Biol. 21, 604-614. 10.1016/j.tcb.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam J., van Breugel M., Harrison S. C. and Hyman A. (2006). Stu2p binds tubulin and undergoes an open-to-closed conformational change. J. Cell Biol. 172, 1009-1022. 10.1083/jcb.200511010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Bassam J., Larsen N. A., Hyman A. A. and Harrison S. C. (2007). Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure 15, 355-362. 10.1016/j.str.2007.01.012 [DOI] [PubMed] [Google Scholar]
- Al-Bassam J., Kim H., Brouhard G., van Oijen A., Harrison S. C. and Chang F. (2010). CLASP promotes microtubule rescue by recruiting tubulin dimers to the microtubule. Dev. Cell 19, 245-258. 10.1016/j.devcel.2010.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose J. C. and Wasteneys G. O. (2008). CLASP modulates microtubule-cortex interaction during self-organization of acentrosomal microtubules. Mol. Biol. Cell 19, 4730-4737. 10.1091/mbc.e08-06-0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose J. C., Shoji T., Kotzer A. M., Pighin J. A. and Wasteneys G. O. (2007). The Arabidopsis CLASP gene encodes a microtubule-associated protein involved in cell expansion and division. Plant Cell 19, 2763-2775. 10.1105/tpc.107.053777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrose C., Allard J. F., Cytrynbaum E. N. and Wasteneys G. O. (2011). A CLASP-modulated cell edge barrier mechanism drives cell-wide cortical microtubule organization in Arabidopsis. Nat. Commun. 2, 430 10.1038/ncomms1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumeier C., Schaedel L., Gaillard J., John K., Blanchoin L. and Théry M. (2016). Self-repair promotes microtubule rescue. Nat. Cell Biol. 18, 1054-1064. 10.1038/ncb3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz P., Ye X., Huddleston P., Brautigam C. A. and Rice L. M. (2012). A TOG:alphabeta-tubulin complex structure reveals conformation-based mechanisms for a microtubule polymerase. Science 337, 857-860. 10.1126/science.1221698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayaz P., Munyoki S., Geyer E. A., Piedra F.-A., Vu E. S., Bromberg R., Otwinowski Z., Grishin N. V., Brautigam C. A. and Rice L. M. (2014). A tethered delivery mechanism explains the catalytic action of a microtubule polymerase. eLife 3, e03069 10.7554/eLife.03069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Sladecek S., Pemble H., Wittmann T., Slotman J. A., van Cappellen W., Brenner H.-R. and Galjart N. (2014). Acetylcholine receptor (AChR) clustering is regulated both by glycogen synthase kinase 3beta (GSK3beta)-dependent phosphorylation and the level of CLIP-associated protein 2 (CLASP2) mediating the capture of microtubule plus-ends. J. Biol. Chem. 289, 30857-30867. 10.1074/jbc.M114.589457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Sladecek S., Martinez de la Peña y Valenzuela I., Akaaboune M., Smal I., Martin K., Galjart N. and Brenner H. R. (2015). CLASP2-dependent microtubule capture at the neuromuscular junction membrane requires LL5beta and actin for focal delivery of acetylcholine receptor vesicles. Mol. Biol. Cell 26, 938-951. 10.1091/mbc.E14-06-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beffert U., Dillon G. M., Sullivan J. M., Stuart C. E., Gilbert J. P., Kambouris J. A. and Ho A. (2012). Microtubule plus-end tracking protein CLASP2 regulates neuronal polarity and synaptic function. J. Neurosci. 32, 13906-13916. 10.1523/JNEUROSCI.2108-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchet B. P., Noordstra I., van Amersfoort M., Katrukha E. A., Ammon Y.-C., Ter Hoeve N. D., Hodgson L., Dogterom M., Derksen P. W. B. and Akhmanova A. (2016). Mesenchymal cell invasion requires cooperative regulation of persistent microtubule growth by SLAIN2 and CLASP1. Dev. Cell 39, 708-723. 10.1016/j.devcel.2016.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratman S. V. and Chang F. (2007). Stabilization of overlapping microtubules by fission yeast CLASP. Dev. Cell 13, 812-827. 10.1016/j.devcel.2007.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouhard G. J. and Rice L. M. (2018). Microtubule dynamics: an interplay of biochemistry and mechanics. Nat. Rev. Mol. Cell Biol. 19, 451-463. 10.1038/s41580-018-0009-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty M., Tarasovetc E. V., Zaytsev A. V., Godzi M., Figueiredo A. C., Ataullakhanov F. I. and Grishchuk E. L. (2019). Microtubule end conversion mediated by motors and diffusing proteins with no intrinsic microtubule end-binding activity. Nat. Commun. 10, 1673 10.1038/s41467-019-09411-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman I. M., MacLeod I., Yates J. R. III, Oegema K. and Desai A. (2005). The CENP-F-like proteins HCP-1 and HCP-2 target CLASP to kinetochores to mediate chromosome segregation. Curr. Biol. 15, 771-777. 10.1016/j.cub.2005.03.018 [DOI] [PubMed] [Google Scholar]
- de Forges H., Pilon A., Cantaloube I., Pallandre A., Haghiri-Gosnet A.-M., Perez F. and Poüs C. (2016). Localized mechanical stress promotes microtubule rescue. Curr. Biol. 26, 3399-3406. 10.1016/j.cub.2016.10.048 [DOI] [PubMed] [Google Scholar]
- De la Mora-Rey T., Guenther B. D. and Finzel B. C. (2013). The structure of the TOG-like domain of Drosophila melanogaster Mast/Orbit. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 69, 723-729. 10.1107/S1744309113015182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Valencia J. D., Morelli M. M., Bailey M., Zhang D., Sharp D. J. and Ross J. L. (2011). Drosophila Katanin-60 depolymerizes and severs at microtubule defects. Biophys. J. 100, 2440-2449. 10.1016/j.bpj.2011.03.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon G. M., Tyler W. A., Omuro K. C., Kambouris J., Tyminski C., Henry S., Haydar T. F., Beffert U. and Ho A. (2017). CLASP2 links reelin to the cytoskeleton during neocortical development. Neuron 93, 1344-1358.e5. 10.1016/j.neuron.2017.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabek K., van Ham M., Stepanova T., Draegestein K., van Horssen R., Sayas C. L., Akhmanova A., Ten Hagen T., Smits R., Fodde R. et al. (2006). Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr. Biol. 16, 2259-2264. 10.1016/j.cub.2006.09.065 [DOI] [PubMed] [Google Scholar]
- Drabek K., Gutiérrez L., Vermeij M., Clapes T., Patel S. R., Boisset J.-C., van Haren J., Pereira A. L., Liu Z., Akinci U. et al. (2012). The microtubule plus-end tracking protein CLASP2 is required for hematopoiesis and hematopoietic stem cell maintenance. Cell Rep. 2, 781-788. 10.1016/j.celrep.2012.08.040 [DOI] [PubMed] [Google Scholar]
- Efimov A., Kharitonov A., Efimova N., Loncarek J., Miller P. M., Andreyeva N., Gleeson P., Galjart N., Maia A. R., McLeod I. X. et al. (2007). Asymmetric CLASP-dependent nucleation of noncentrosomal microtubules at the trans-Golgi network. Dev. Cell 12, 917-930. 10.1016/j.devcel.2007.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efimova N., Grimaldi A., Bachmann A., Frye K., Zhu X., Feoktistov A., Straube A. and Kaverina I. (2014). Podosome-regulating kinesin KIF1C translocates to the cell periphery in a CLASP-dependent manner. J. Cell Sci. 127, 5179-5188. 10.1242/jcs.149633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk C., Schmeiser V., Ortiz J. and Lechner J. (2014). A TOGL domain specifically targets yeast CLASP to kinetochores to stabilize kinetochore microtubules. J. Cell Biol. 205, 555-571. 10.1083/jcb.201310018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer E. A., Miller M. P., Brautigam C. A., Biggins S. and Rice L. M. (2018). Design principles of a microtubule polymerase. eLife 7, e34574 10.7554/eLife.34574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannak E. and Heald R. (2006). Xorbit/CLASP links dynamic microtubules to chromosomes in the Xenopus meiotic spindle. J. Cell Biol. 172, 19-25. 10.1083/jcb.200508180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur E.-M., Saijilafu, Lee B. D., Kim S. J., Xu W. L. and Zhou F. Q. (2011). GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev. 25, 1968-1981. 10.1101/gad.17015911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y. H., do Carmo Avides M., Shiraki M., Deak P., Yamaguchi M., Nishimoto Y., Matsukage A. and Glover D. M. (2000). Orbit, a novel microtubule-associated protein essential for mitosis in Drosophila melanogaster. J. Cell Biol. 149, 153-166. 10.1083/jcb.149.1.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V., Herrmann U., Parupalli C., Sedbrook J. C., Ehrhardt D. W. and Hülskamp M. (2007). CLASP localizes in two discrete patterns on cortical microtubules and is required for cell morphogenesis and cell division in Arabidopsis. J. Cell Sci. 120, 4416-4425. 10.1242/jcs.024950 [DOI] [PubMed] [Google Scholar]
- Kolenda C., Ortiz J., Pelzl M., Norell S., Schmeiser V. and Lechner J. (2018). Unattached kinetochores drive their own capturing by sequestering a CLASP. Nat. Commun. 9, 886 10.1038/s41467-018-03108-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Lyle K. S., Gierke S., Matov A., Danuser G. and Wittmann T. (2009). GSK3beta phosphorylation modulates CLASP–microtubule association and lamella microtubule attachment. J. Cell Biol. 184, 895-908. 10.1083/jcb.200901042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo Y.-W., Trottier O., Mahamdeh M. and Howard J. (2019). Spastin is a dual-function enzyme that severs microtubules and promotes their regrowth to increase the number and mass of microtubules. Proc. Natl Acad. Sci. USA 116, 5533-5541. 10.1073/pnas.1818824116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen G., Grigoriev I., Mimori-Kiyosue Y., Ohtsuka T., Higa S., Kitajima I., Demmers J., Galjart N., Houtsmuller A. B., Grosveld F. et al. (2006). CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev. Cell 11, 21-32. 10.1016/j.devcel.2006.05.012 [DOI] [PubMed] [Google Scholar]
- Lawrence E. J. and Zanic M. (2019). Rescuing microtubules from the brink of catastrophe: CLASPs lead the way. Curr. Opin. Cell Biol. 56, 94-101. 10.1016/j.ceb.2018.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence E. J., Arpag G., Norris S. R. and Zanic M. (2018). Human CLASP2 specifically regulates microtubule catastrophe and rescue. Mol. Biol. Cell 29, 1168-1177. 10.1091/mbc.E18-01-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leano J. B. and Slep K. C. (2019). Structures of TOG1 and TOG2 from the human microtubule dynamics regulator CLASP1. PLoS ONE 14, e0219823 10.1371/journal.pone.0219823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leano J. B., Rogers S. L. and Slep K. C. (2013). A cryptic TOG domain with a distinct architecture underlies CLASP-dependent bipolar spindle formation. Structure 21, 939-950. 10.1016/j.str.2013.04.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Engel U., Rusch J., Scherrer S., Sheard K. and Van Vactor D. (2004). The microtubule plus end tracking protein Orbit/MAST/CLASP acts downstream of the tyrosine kinase Abl in mediating axon guidance. Neuron 42, 913-926. 10.1016/j.neuron.2004.05.020 [DOI] [PubMed] [Google Scholar]
- Lemos C. L., Sampaio P., Maiato H., Costa M., Omel'yanchuk L. V., Liberal V. and Sunkel C. E. (2000). Mast, a conserved microtubule-associated protein required for bipolar mitotic spindle organization. EMBO J. 19, 3668-3682. 10.1093/emboj/19.14.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindeboom J. J., Nakamura M., Hibbel A., Shundyak K., Gutierrez R., Ketelaar T., Emons A. M. C., Mulder B. M., Kirik V. and Ehrhardt D. W. (2013). A mechanism for reorientation of cortical microtubule arrays driven by microtubule severing. Science 342, 1245533 10.1126/science.1245533 [DOI] [PubMed] [Google Scholar]
- Lindeboom J. J., Nakamura M., Saltini M., Hibbel A., Walia A., Ketelaar T., Emons A. M. C., Sedbrook J. C., Kirik V., Mulder B. M. et al. (2019). CLASP stabilization of plus ends created by severing promotes microtubule creation and reorientation. J. Cell Biol. 218, 190-205. 10.1083/jcb.201805047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffini S., Maia A. R. R., Manning A. L., Maliga Z., Pereira A. L., Junqueira M., Shevchenko A., Hyman A., Yates J. R. III, Galjart N. et al. (2009). Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr. Biol. 19, 1566-1572. 10.1016/j.cub.2009.07.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia A. R. R., Garcia Z., Kabeche L., Barisic M., Maffini S., Macedo-Ribeiro S., Cheeseman I. M., Compton D. A., Kaverina I. and Maiato H. (2012). Cdk1 and Plk1 mediate a CLASP2 phospho-switch that stabilizes kinetochore-microtubule attachments. J. Cell Biol. 199, 285-301. 10.1083/jcb.201203091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., Sampaio P., Lemos C. L., Findlay J., Carmena M., Earnshaw W. C. and Sunkel C. E. (2002). MAST/Orbit has a role in microtubule-kinetochore attachment and is essential for chromosome alignment and maintenance of spindle bipolarity. J. Cell Biol. 157, 749-760. 10.1083/jcb.200201101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiato H., Fairley E. A. L., Rieder C. L., Swedlow J. R., Sunkel C. E. and Earnshaw W. C. (2003). Human CLASP1 is an outer kinetochore component that regulates spindle microtubule dynamics. Cell 113, 891-904. 10.1016/S0092-8674(03)00465-3 [DOI] [PubMed] [Google Scholar]
- Maiato H., Khodjakov A. and Rieder C. L. (2005). Drosophila CLASP is required for the incorporation of microtubule subunits into fluxing kinetochore fibres. Nat. Cell Biol. 7, 42-47. 10.1038/ncb1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S., Kim T., Chen Z., Munyoki S., Tso S. C., Brautigam C. A. and Rice L. M. (2018). An isolated CLASP TOG domain suppresses microtubule catastrophe and promotes rescue. Mol. Biol. Cell 29, 1359-1375. 10.1091/mbc.E17-12-0748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki T., Grimaldi A. D., Fuchigami S., Kaverina I. and Hayashi I. (2015). CLASP2 has two distinct TOG domains that contribute differently to microtubule dynamics. J. Mol. Biol. 427, 2379-2395. 10.1016/j.jmb.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning A. L., Bakhoum S. F., Maffini S., Correia-Melo C., Maiato H. and Compton D. A. (2010). CLASP1, astrin and Kif2b form a molecular switch that regulates kinetochore-microtubule dynamics to promote mitotic progression and fidelity. EMBO J. 29, 3531-3543. 10.1038/emboj.2010.230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A., Godinez W. J., Tsimashchuk V., Bankhead P., Rohr K. and Engel U. (2013). Xenopus cytoplasmic linker-associated protein 1 (XCLASP1) promotes axon elongation and advance of pioneer microtubules. Mol. Biol. Cell 24, 1544-1558. 10.1091/mbc.e12-08-0573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P. M., Folkmann A. W., Maia A. R. R., Efimova N., Efimov A. and Kaverina I. (2009). Golgi-derived CLASP-dependent microtubules control Golgi organization and polarized trafficking in motile cells. Nat. Cell Biol. 11, 1069-1080. 10.1038/ncb1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Grigoriev I., Lansbergen G., Sasaki H., Matsui C., Severin F., Galjart N., Grosveld F., Vorobjev I., Tsukita S. et al. (2005). CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J. Cell Biol. 168, 141-153. 10.1083/jcb.200405094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y., Grigoriev I., Sasaki H., Matsui C., Akhmanova A., Tsukita S. and Vorobjev I. (2006). Mammalian CLASPs are required for mitotic spindle organization and kinetochore alignment. Genes Cells 11, 845-857. 10.1111/j.1365-2443.2006.00990.x [DOI] [PubMed] [Google Scholar]
- Mitchison T. and Kirschner M. (1984). Dynamic instability of microtubule growth. Nature 312, 237-242. 10.1038/312237a0 [DOI] [PubMed] [Google Scholar]
- Moriwaki T. and Goshima G. (2016). Five factors can reconstitute all three phases of microtubule polymerization dynamics. J. Cell Biol. 215, 357-368. 10.1083/jcb.201604118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Lindeboom J. J., Saltini M., Mulder B. M. and Ehrhardt D. W. (2018). SPR2 protects minus ends to promote severing and reorientation of plant cortical microtubule arrays. J. Cell Biol. 217, 915-927. 10.1083/jcb.201708130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J., Funk C., Schafer A. and Lechner J. (2009). Stu1 inversely regulates kinetochore capture and spindle stability. Genes Dev. 23, 2778-2791. 10.1101/gad.541309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasqualone D. and Huffaker T. C. (1994). STU1, a suppressor of a beta-tubulin mutation, encodes a novel and essential component of the yeast mitotic spindle. J. Cell Biol. 127, 1973-1984. 10.1083/jcb.127.6.1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietra S., Gustavsson A., Kiefer C., Kalmbach L., Hörstedt P., Ikeda Y., Stepanova A. N., Alonso J. M. and Grebe M. (2013). Arabidopsis SABRE and CLASP interact to stabilize cell division plane orientation and planar polarity. Nat. Commun. 4, 2779 10.1038/ncomms3779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A. L., Pereira A. J., Maia A. R., Drabek K., Sayas C. L., Hergert P. J., Lince-Faria M., Matos I., Duque C., Stepanova T. et al. (2006). Mammalian CLASP1 and CLASP2 cooperate to ensure mitotic fidelity by regulating spindle and kinetochore function. Mol. Biol. Cell 17, 4526-4542. 10.1091/mbc.e06-07-0579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L. and Alexander S. P. (1990). Kinetochores are transported poleward along a single astral microtubule during chromosome attachment to the spindle in newt lung cells. J. Cell Biol. 110, 81-95. 10.1083/jcb.110.1.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruan Y., Halat L. S., Khan D., Jancowski S., Ambrose C., Belmonte M. F. and Wasteneys G. O. (2018). The microtubule-associated protein CLASP sustains cell proliferation through a brassinosteroid signaling negative feedback loop. Curr. Biol. 28, 2718-2729.e5. 10.1016/j.cub.2018.06.048 [DOI] [PubMed] [Google Scholar]
- Samora C. P., Mogessie B., Conway L., Ross J. L., Straube A. and McAinsh A. D. (2011). MAP4 and CLASP1 operate as a safety mechanism to maintain a stable spindle position in mitosis. Nat. Cell Biol. 13, 1040-1050. 10.1038/ncb2297 [DOI] [PubMed] [Google Scholar]
- Sayas C. L., Basu S., van der Reijden M., Bustos-Morán E., Liz M., Sousa M., van IJcken W. F. J., Avila J. and Galjart N. (2019). Distinct functions for mammalian CLASP1 and −2 during neurite and axon elongation. Front. Cell. Neurosci. 13, 5 10.3389/fncel.2019.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N., Basu S., Sladecek S., Gatti S., van Haren J., Treves S., Pielage J., Galjart N. and Brenner H. R. (2012). Agrin regulates CLASP2-mediated capture of microtubules at the neuromuscular junction synaptic membrane. J. Cell Biol. 198, 421-437. 10.1083/jcb.201111130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slep K. C. (2009). The role of TOG domains in microtubule plus end dynamics. Biochem. Soc. Trans. 37, 1002-1006. 10.1042/BST0371002 [DOI] [PubMed] [Google Scholar]
- Slep K. C. and Vale R. D. (2007). Structural basis of microtubule plus end tracking by XMAP215, CLIP-170, and EB1. Mol. Cell 27, 976-991. 10.1016/j.molcel.2007.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa A., Reis R., Sampaio P. and Sunkel C. E. (2007). The Drosophila CLASP homologue, Mast/Orbit regulates the dynamic behaviour of interphase microtubules by promoting the pause state. Cell Motil. Cytoskeleton 64, 605-620. 10.1002/cm.20208 [DOI] [PubMed] [Google Scholar]
- Stehbens S. J., Paszek M., Pemble H., Ettinger A., Gierke S. and Wittmann T. (2014). CLASPs link focal-adhesion-associated microtubule capture to localized exocytosis and adhesion site turnover. Nat. Cell Biol. 16, 561-573. 10.1038/ncb2975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B., Moreira S., Millard T., Evans I., Huang C. Y., Sabet O., Milner M., Dunn G., Martin P. and Wood W. (2010). Clasp-mediated microtubule bundling regulates persistent motility and contact repulsion in Drosophila macrophages in vivo. J. Cell Biol. 189, 681-689. 10.1083/jcb.200912134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms D., Vineyard L., Elliott A. and Shaw S. L. (2018). CLASP facilitates transitions between cortical microtubule array patterns. Plant Physiol. 178, 1551-1567. 10.1104/pp.18.00961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemu A., Szczesna E., Zehr E. A., Spector J. O., Grigorieff N., Deaconescu A. M. and Roll-Mecak A. (2018). Severing enzymes amplify microtubule arrays through lattice GTP-tubulin incorporation. Science 361, eaau1504-eaau1514. 10.1126/science.aau1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Noritake J., Kakeno M., Matsui T., Harada T., Wang S., Itoh N., Sato K., Matsuzawa K., Iwamatsu A. et al. (2009). Phosphorylation of CLASP2 by GSK-3beta regulates its interaction with IQGAP1, EB1 and microtubules. J. Cell Sci. 122, 2969-2979. 10.1242/jcs.046649 [DOI] [PubMed] [Google Scholar]
- Widlund P. O., Stear J. H., Pozniakovsky A., Zanic M., Reber S., Brouhard G. J., Hyman A. A. and Howard J. (2011). XMAP215 polymerase activity is built by combining multiple tubulin-binding TOG domains and a basic lattice-binding region. Proc. Natl. Acad. Sci. USA 108, 2741-2746. 10.1073/pnas.1016498108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann T. and Waterman-Storer C. M. (2005). Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J. Cell Biol. 169, 929-939. 10.1083/jcb.200412114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H., You L., Pasqualone D., Kopski K. M. and Huffaker T. C. (2002). Stu1p is physically associated with beta-tubulin and is required for structural integrity of the mitotic spindle. Mol. Biol. Cell 13, 1881-1892. 10.1091/mbc.01-09-0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Fishel E., Bertroche T. and Dixit R. (2013). Microtubule severing at crossover sites by Katanin generates ordered cortical microtubule arrays in Arabidopsis. Curr. Biol. 23, 2191-2195. 10.1016/j.cub.2013.09.018 [DOI] [PubMed] [Google Scholar]