Abstract
The magnitude of the COVID-19 pandemic will result in substantial neurological disease, whether through direct infection (rare), para-infectious complications (less rare), or critical illness more generally (common). Here, we raise the importance of stringent diagnosis and data collection regarding neurological complications of COVID-19; we urge caution in the over-diagnosis of neurological disease where it does not exist, but equally strongly encourage the concerted surveillance for such conditions. Additional to the direct neurological complications of COVID-19 infection, neurological patients are at risk of harm from both structural limitations (such as number of intensive care beds), and a hesitancy to treat with certain necessary medications given risk of nosocomial COVID-19 infection. We therefore also outline the specific management of patients with neuroinflammatory diseases in the context of the pandemic. This article describes the implications of COVID-19 on neurological disease and advertises the Neurocritical Care Society’s international data collection collaborative that seeks to align data elements.
Keywords: COVID-19, Encephalitis, Guillain–Barré syndrome, Acute disseminated encephalomyelitis
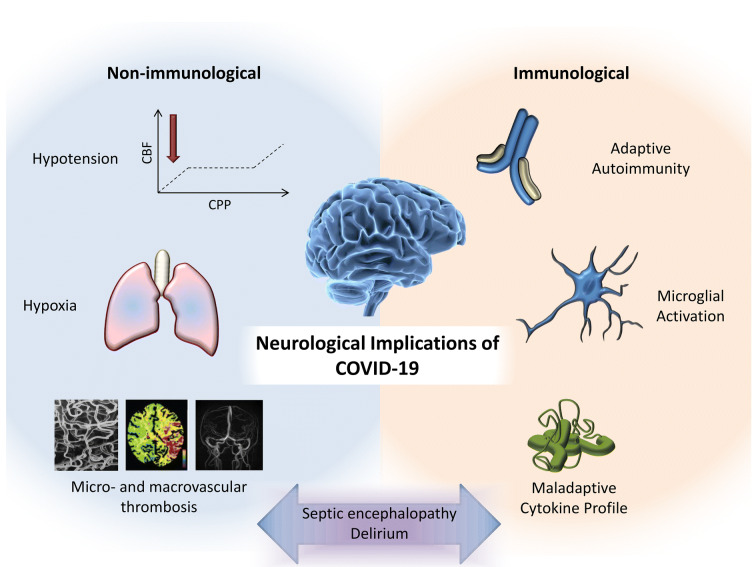
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a rampant pandemic characterized predominantly by lower respiratory tract involvement. While coronaviruses are not a common cause of neurological disease, they have been reported to cause direct central nervous system (CNS) infection, as well as presumed para-infectious disorders [1–3]. Over a million cases of confirmed COVID-19 have been reported worldwide, and while definitive evidence is sparse, emerging publications and preprints justify careful consideration of the neurological associations with COVID-19 infection (Fig. 1).
Fig. 1.
Putative mechanisms underlying neurological consequences of COVID-19
A preprint describes neurological manifestations in 36.4% of 214 patients with confirmed COVID-19 [4]. However, the symptoms described [dizziness (not further defined), headache, and impaired consciousness] are commonplace in many severe infections and represent disturbances in neurological function rather than neurological disease per se. Anosmia and ageusia have received much attention, but are ubiquitous in other common upper respiratory tract infections. While a reported increased risk of cerebrovascular disease [4] was replicated in a further preprint [5], the incidence was similar to that in critical illness more broadly [6]. A further case report [7] documents necrotizing encephalopathy in association with COVID-19, but without evidence of viral isolation from cerebrospinal fluid (CSF).
Indeed, to date, there are no definitive reports of SARS-CoV-2 detection in CSF. The only available report of CSF findings describes no abnormalities in a patient with encephalopathy during their COVID-19 illness [8]. Recent correspondence provides a secondary (Chinese language) citation of CSF positivity for SARS-CoV- 2 [9], but no clinical or laboratory details were provided, and polymerase chain reaction (PCR) techniques are at risk of sample contamination from shed airborne virus. A concerted effort by the international Human Cell Atlas community (yet to be published, but presented online at https://www.youtube.com/watch?v=gHqBoU4s63U&feature=youtu.be) has documented the relative expression of the two key co-receptors for SARS-CoV-2 entry, ACE2, and TMPRSS2, across multiple tissues, and highlights that (in health) there is minimal expression in brain tissue, suggesting that direct brain infection would not be a common phenomenon. The one brain cell type which did express both genes was the oligodendrocyte, and therefore, SARS-CoV-2 encephalitis might be expected to be a predominantly white-matter disease where it does occur.
Given high rates of COVID-19 infection in the general population, coincidental occurrence of neurological diseases is likely, and we must be cautious about inferring causal linkages. However, we must also recognize that in a pandemic, neurological manifestations of COVID-19 may be overlooked. This dilemma predicates a low threshold for imaging and CSF analysis in COVID-19 patients displaying unexpected neurological symptoms (recognizing that magnetic resonance imaging may be challenging in this context).
A greater concern than direct viral invasion of the CNS may be para-infectious neurological diseases such as Guillain–Barré syndrome, transverse myelitis, or acute disseminated encephalomyelitis, such as seen in the 2015–2016 Zika virus epidemic, but on a much greater scale given the numbers of people infected. It is reassuring that, despite the peak onset of para-infectious conditions typically occurring within 4 weeks, there has been no clear signal from countries affected early in the pandemic course. However, such associations may emerge over time and have clear clinical relevance. Patients with neurological complications may require protracted intensive care stays and represent an additional strain on already overstretched facilities.
Further considerations relate to patients with neurological conditions requiring treatments that could worsen outcome from COVID-19, such as immunosuppressant medication for autoimmune neurological diseases. Although recent reports suggest some benefit in the most severe cases of COVID-19-related ARDS [10], evidence from previous coronavirus outbreaks suggests that viral shedding may be prolonged in patients treated with corticosteroids [11], and their routine use is currently avoided. Typical second-line treatments for neuroinflammatory crises, intravenous immunoglobulins (IVIg) or plasma exchange (PLEX), are less likely to delay viral clearance in COVID-19, and given some reports of benefit in sepsis [12, 13], they may even be of potential benefit. However, IVIg is associated with an increased risk of thromboembolism [14], a particular issue given the reports of increased D-dimer levels [10] and (as yet unquantified) concerns regarding the risk of microthrombosis in COVID-19 infections.
Third-line therapies for neuroinflammatory conditions such as cyclophosphamide or rituximab are likely to represent the highest risk treatments with regard to subsequent COVID-19 infection, but are already only used when less toxic medications have failed. We would advocate that those not currently infected with SARS-CoV-2 should receive any treatment required to treat their neurological condition, but in a positive-pressure room where possible (in order to provide against acquiring nosocomial COVID-19 infection). For those who develop contemporaneous COVID-19 infection, we would suggest temporizing with second-line treatments (IVIg/PLEX) until the active infection has cleared, and ensuring meticulous monitoring and prophylaxis for thromboembolism if IVIg is used.
Our last concern is around the potential chronic neurological consequences of this pandemic. The sheer volume of those suffering critical illness is likely to result in an increased burden of long-term cognitive impairment [15]. In addition, there also remains the as yet unquantified risk of both “common” para-infectious processes such as acute disseminated encephalomyelitis, as well as atypical disorders akin to encephalitis lethargica, which continues to be linked to the 1918 H1N1 pandemic [16], though this link remains unproven.
Given that the scale of this pandemic is likely to result in a significant burden of neurological disease, we advocate a unified approach to reporting COVID-19 patients who develop neurological complications, and where possible compiling a repository of biological samples. The Neurocritical Care Society (margin note: https://www.neurocriticalcare.org/home) is leading a multicenter, international collaborative effort to develop guidelines and tools to align data and sample collection protocols so that individual centers can undertake standardized data collection which can eventually be integrated into a harmonized larger dataset that is widely available for analysis.
Such an effort must cater to different granularities of data collection, using the Common Data Elements (CDEs) approach espoused by the National Institute for Neurological Diseases and Stroke (Table 1) [17]. At the most basic level, Core CDEs may be eligible for expedited ethical board review and waived consent. The collection of such pragmatic data should be feasible in a global pandemic, with incorporation into wider pandemic research efforts, which currently collect some limited neurological data [18]. They could also provide a basis for retrospective clinical record review in cohorts of patients affected earlier in the pandemic, looking for neurological associations with COVID-19 infection. More specialized units could seek to collect more detailed basic CDEs, which require more effort and pose a greater regulatory burden. Finally, supplemental CDEs (e.g., neuroimaging, biospecimens, and postmortem brain examination) would only be collected by centers that had appropriate resources. One key (but organizationally demanding) supplemental CDE would be the long-term follow-up of survivors of the COVID-19 pandemic, to allow effective monitoring of late neurological sequelae.
Table 1.
Tiered approach to facilitate parallel development and rapid deployment of investigations of neurologic manifestations of COVID-19. Centers can elect to participate in Tier 1, Tiers 1 + 2 or 1 + 3, or Tiers 1 + 2 + 3
| Design considerations | Common data elements | Ethical board considerations | Participating centers | Implementation considerations | |
|---|---|---|---|---|---|
| Tier 1 |
Prospective Registry Simple inclusion and exclusion criteria Small # of core data elements => Low burden to research team Low data granularity, capture basic groups Outcomes: acute phase outcome, e.g., mortality |
Core |
Qualifies for expedited review Qualifies for waiver of consent |
All centers All centers able to participate regardless of resource levels Many centers Large sample size |
Practical in COVID-19 pandemic and compatible with infection containment: No direct contact with study subjects All data can be collected remotely from electronic health records or via telecommunication with clinical team Highly pragmatic (lean) workflow |
| Tier 2 |
More detailed clinical and neurodiagnostic data collection. Examples: Detailed neuroexam Clinical laboratory data Clinical imaging/neurophysiologic data Outcome: global Functional outcome assessment beyond mortality. Acute + subacute phase outcomes |
Basic |
Likely require full board review Likely require informed consent |
Able/willing centers participate Smaller # of sites compared to Tier 1 Smaller overall sample size but more granular outcome |
May require contact with study subjects—possibly utilize telecommunication tools to reduce exposure risk More onerous and granular data collection Standardization considerations in clinical laboratory, imaging, and electrodiagnostic data Missing data considerations |
| Tier 3 |
Advanced, nonstandard neurodiagnostics (e.g., advanced MR imaging) Prospective biospecimens collection (CSF, blood, other) for experimental biomarkers investigation Possible postmortem tissue study Longitudinal study to capture subacute and long-term events |
Supplemental |
Requires full board review Requires written informed consent |
Small # of centers with necessary resources participate Smaller # of sites Smaller overall sample size but with longitudinal data and biomarker data |
Requires direct contact with subject or specimen, higher risk for exposure Biospecimens will need biocontainment facilities for banking/storage Advanced neurodiagnostics resources available at participating centers Standardization considerations in experimental biomarkers (molecular and imaging) |
Source of Support
No financial support was received to support this article.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375:497–498. doi: 10.1056/NEJMc1509458. [DOI] [PubMed] [Google Scholar]
- 2.Turgay C, Emine T, Ozlem K, Muhammet SP, Haydar AT. A rare cause of acute flaccid paralysis: human corona viruses. J Pediatr Neurosci. 2015;10(3):280–281. doi: 10.4103/1817-1745.165716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arabi YM, Harthi A, Hussein J, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43(4):495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mao L, Wang M, Chen S et al. 2020. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv. 10.1101/2020.02.22.20026500.
- 5.Li Y, Wang M, Zhou Y et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study (3/3/2020). Available at SSRN: https://ssrn.com/abstract=3550025 or 10.2139/ssrn.3550025. [DOI] [PMC free article] [PubMed]
- 6.Boehme AK, Ranawat P, Luna J, Kamel H, Elkind MS. Risk of acute stroke after hospitalization for sepsis: a case-crossover study. Stroke. 2017;48(3):574–580. doi: 10.1161/STROKEAHA.116.016162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID-19–associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology ePub ahead of print. Accessed 31 March 2020 (10.1148/radiol.2020201187). [DOI] [PMC free article] [PubMed]
- 8.Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong S, Guan J. Novel coronavirus and central nervous system. Eur J Neurol. 2020. Epub 26th March. [DOI] [PubMed]
- 10.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russel CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rimmer E, Houston BL, Kumar A, et al. The efficacy and safety of plasma exchange in patients with sepsis and septic shock: a systematic review and meta-analysis. Crit Care. 2014;18(6):699. doi: 10.1186/s13054-014-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Busani S, Damiani E, Cavazzuti I, et al. Intravenous immunoglobulin in septic shock: review of the mechanisms of action and meta-analysis of the clinical effectiveness. Minerva Anestesiol. 2016;82(5):559–572. [PubMed] [Google Scholar]
- 14.Stiehm ER. Adverse effects of human immunoglobulin therapy. Transfus Med Rev. 2013;27(3):171–178. doi: 10.1016/j.tmrv.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Pandharipande PP, Girard TD, Jackson JC, et al. Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306–1316. doi: 10.1056/NEJMoa1301372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maurizi CP. Influenza caused epidemic encephalitis (encephalitis lethargica): the circumstantial evidence and a challenge to the nonbelievers. Med Hypotheses. 2010;74(5):798–801. doi: 10.1016/j.mehy.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 17.https://www.commondataelements.ninds.nih.gov/ Accessed 31 March 2020.
- 18.https://isaric.tghn.org/covid-19-clinical-research-resources/ Accessed 31 March 2020.