Abstract
Objective
To estimate the overall risk and the temporal trend of venous thromboembolism (VTE), deep vein thrombosis (DVT), and pulmonary embolism (PE) before and after gout diagnosis in an incident gout cohort compared with the general population.
Methods
We conducted a matched cohort study using a province-wide population-based administrative health database in Canada. We calculated incidence rates (IRs) and multivariable adjusted hazard ratios (HRs) for the risk of VTE, DVT and PE before and after gout diagnosis.
Results
Among 130 708 incident individuals with gout (64% male, mean age 59 years), 2071 developed VTE, 1377 developed DVT and 1012 developed PE. IRs per 1000 person-years for gout were 2.63, 1.74 and 1.28 compared with 2.03, 1.28 and 1.06 for non-gout, respectively. The fully adjusted HRs (95% CI) for VTE, DVT and PE were 1.22 (1.13, 1.32), 1.28 (1.17, 1.41) and 1.16 (1.05, 1.29). For the pre-gout period, the fully adjusted HRs (95% CI) were 1.51 (1.38, 1.64), 1.55 (1.40, 1.72) and 1.47 (1.31, 1.66) for VTE, DVT and PE. During the third, second and first years preceding gout, the fully adjusted HRs for VTE were 1.44, 1.56 and 1.62. During the first, second, third, fourth and fifth years after gout, the fully adjusted HRs were 1.63, 1.29, 1.33, 1.28 and 1.22. Similar trends were also seen for DVT and PE.
Conclusion
Increased risks of VTE, DVT and PE were found both before and after gout diagnosis. The risk increased gradually before gout, peaking in the year prior to diagnosis, and then progressively declined. Gout-associated inflammation may contribute to venous thrombosis risk.
Keywords: gout, venous thromboembolism, deep vein thrombosis, pulmonary embolism, risk factors
Rheumatology key messages
Increased risks of VTE, DVT and PE were found both before and after gout diagnosis.
The risk increases gradually before gout diagnosis, peaks in the year prior, and then progressively declines.
Gout associated inflammation before and after gout diagnosis may be a contributing factor for VTE.
Introduction
Gout is the most common inflammatory arthritis in men [1], and is associated with increased mortality [2] and a high economic and hospitalization burden [3, 4]. Data from different countries suggest that gout is becoming more prevalent [1]. Inflammatory arthritides such as rheumatoid arthritis, systemic lupus erythematosus, and gout have been associated with an increased risk of cardiovascular diseases [5–9]. But whether the presence of hyperuricaemia as the precursor of gout or gout itself is an independent risk factor for cardiovascular disease is controversial [10, 11]. Nonetheless, the majority of studies have shown that, even after adjusting for cardiovascular risk factors, hyperuricaemia and gout remain independent risk factors for cardiovascular disease, particularly stroke and coronary heart disease (i.e. myocardial infarction, angina, ischaemic heart disease) [7–9].
Venous thromboembolism (VTE, including pulmonary embolism [PE] and deep vein thrombosis [DVT]) represents a relatively common cardiovascular event that is associated with increased mortality (i.e. one-month fatality rate of 9.7% for PE and 4.6% for DVT) [12]. An increased risk of VTE has been reported in some types of inflammatory arthritis other than gout [13, 14]. Systemic inflammation associated with those conditions might promote thrombosis by upregulating procoagulants, downregulating anticoagulants and suppressing fibrinolysis [15]. However, data on the risk of VTE in patients with hyperuricaemia or gout are scarce. Findings from three recent population-based studies are limited due to failure to adjust for medications or failure to address potential unmeasured confounders such as obesity [16, 17], use of a prevalent cohort (which is associated with survival bias) [17] and a small sample size [18].
The aims of our study, using a province-wide database of both inpatients and outpatients from the province of British Columbia (BC)’s universal healthcare system, were: (i) to estimate the overall risk of VTE, DVT and PE before and after a gout diagnosis in an incident cohort of gout; and (ii) to estimate the temporal trend of VTE, DVT and PE before and after a gout diagnosis compared with that of the general population.
Methods
Data sources
Universal healthcare coverage is available for all residents of the province of BC, Canada (population ∼ 4.7 million). Population Data BC includes all provincially funded healthcare service data since 1990, including all healthcare professional visits [19], hospitalizations [20], demographic data [21], cancer registry [22] and vital statistics [23]. Furthermore, Population Data BC includes the comprehensive prescription drug database PharmaNet [24], which captures all outpatient dispensed medications for all residents living in BC since 1996. Numerous population-based studies have been successfully conducted using this source [25–27]. All inferences, opinions and conclusions drawn from this research are those of the authors and do not reflect the opinions or policies of the data stewards. No personal identifying information was made available as part of this study. Procedures used were in compliance with British Columbia’s Freedom of Information and Privacy Protection Act. Ethical approval was obtained from the University of British Columbia’s Behavioral Research Ethics Board (H15-00887).
Study design and cohort definitions
Using Population Data BC, we conducted a 1 : 1 matched cohort study of the risk of incident VTE, DVT and PE among patients with newly diagnosed gout (gout cohort) compared with age-matched, sex-matched and index date (i.e. gout diagnosis date)-matched individuals without gout who were randomly selected from the general population (non-gout cohort).
Gout cohort
In this study, the gout cohort included all adult patients (⩾18 years of age) who received a gout diagnosis for the first time from 1 January 2000 through to 31 March 2015 in BC. We refer to the time period before the index date as the pre-gout period. A gout diagnosis was defined as follows: at least one recorded principal diagnosis of gout [International Classification of Disease ninth-version (ICD-9)-CM 274 or ICD-10-CM M10] at either a physician or hospital visit. To ensure incident gout cases, we required all newly diagnosed gout individuals to have at least ten years of prior registration in the databases (i.e. ‘run-in’ period) without a diagnosis of gout at a physician or hospital visit. The positive predictive value of a similar case definition was 86% in the US Veterans Affairs database [28].
Non-gout cohort
To establish the non-gout comparison cohort, we received data for a random sample of ∼2 000 000 BC residents registered with the provincial medical services plan during the study period, and selected individuals without any history of a gout diagnosis and matched them to gout patients (1 : 1 ratio) on age, sex and the index date. To be comparable to the gout cohort, we also excluded those non-gout individuals without at least 10 years’ run-in time before the matched index date.
Individuals were followed until they either experienced an outcome (VTE, DVT or PE), died, left BC (2.3% in the gout cohort and 4.8% in the non-gout cohort), or the follow-up period ended (31 March 2015), whichever occurred first.
Ascertainment of DVT and PE
The primary outcome was the first ever VTE event (either DVT or PE) during the follow-up. Incident DVT or PE outcomes were defined by a corresponding ICD code plus a prescription for any anticoagulant therapy (heparin, warfarin or a similar agent) between one month before and six months after the ICD code date [29]. We identified DVT (ICD-9-CM: 453; ICD-10-CM: I82.4, I82.9) from outpatient or hospitalization data, and PE (ICD-9-CM: 415.1, 673.2, 639.6; ICD-10-CM: O88.2, I26) from hospitalization data only. Deaths from DVT or PE (including out-of-hospital deaths) were identified from vital statistics. Given that VTE is a potentially fatal disease, patients may have died before they received treatment; thus, for those patients who died within 2 months after a VTE, DVT or PE diagnosis, anticoagulant therapy was not needed as part of the case definition. Similar definitions for VTE have been used in previous publications, and were found to have a positive predictive value of 94% in a general practice database [29].
Covariate assessment
To evaluate the baseline characteristics at index date, available covariates known to be potential risk factors for VTE were assessed within the 12 months prior to the index date. To evaluate baseline characteristics in patients during the pre-gout period, the same covariates were also assessed over each 12-month period prior to the year of interest. Covariates included health resource utilization (number of outpatient visits and hospitalizations), medication use (as identified through Drug Identification Numbers: NSAIDs, HRT, glucocorticoids, cyclooxygenase-2 inhibitors [cox-2], and oral contraceptives) and comorbidities (hypertension, varicose veins, sepsis, inflammatory bowel disease, chronic kidney disease and alcoholism). In addition, trauma or fracture, a history of surgery, as well as the Romano modification of Charlson Comorbidity Index for administrative data [30] were also ascertained in those periods, respectively.
Statistical analysis
We compared the baseline characteristics of gout patients with the non-gout cohort using a χ2 test for categorical variables and the Wilcoxon Rank-Sum test for continuous variables. To calculate the risk of VTE after and before the index date, we identified incident DVT and PE events during the follow-up periods, post- and pre-gout diagnosis, and calculated the incidence rates (IRs) per 1000 person-years for each outcome, individually and together (as VTE). We used Cox proportional hazard regression models [31] to calculate hazard ratios (HRs) for the risk of VTE, DVT and PE in the gout and pre-gout periods compared with the non-gout cohort, adjusting for potential confounders.
To evaluate the time trends of VTE risk before and after the index date, we also estimated HRs within 3, 2 and 1 years before the index date and 1, 2, 3, 4 and 5 years after the index date. For the analysis of VTE risk before the index date, we excluded individuals with a VTE diagnosis recorded any time before the date of interest. For the analysis of VTE risk after the index date, we excluded individuals with a VTE diagnosis recorded any time before the index date.
Sensitivity analyses
To test the robustness of our results, we performed three sensitivity analyses. First, to estimate the effect of unmeasured confounders (i.e. obesity), we calculated fully adjusted HRs by adding the simulated unmeasured confounder with a prevalence ranging from 30% to 40% in the gout cohort [32, 33] and a prevalence of 23% for the non-gout cohort (corresponding to the estimated prevalence of obesity for the BC general population aged 18 and older) [34], and odds ratios (ORs) ranging from 2.0–2.5 for the association between obesity and VTE, DVT or PE [35]. Second, we calculated the cumulative incidence of each outcome event after taking the competing risk of death into account using subdistribution models, according to Lau et al. [36]. Thirdly, we used a more restrictive gout case definition that only included gout individuals who satisfied the primary case definition and also had at least one prescription for urate-lowering therapy (ULT; allopurinol, febuxostat, probenecid or sulfinpyrazone) between 1 month before and 3 months after the index date. A similar case definition using the UK General Practice Research Database was found to have a positive predictive value of 90% [37].
SAS V.9.4 (SAS Institute, Cary, North Carolina, USA) was used for all analyses. For all HRs, we calculated 95% CIs.
Results
Baseline characteristics
After excluding individuals with VTE events before index date, we identified 130 708 incident gout patients (64% male, mean age 59 years), and 131 349 non-gout individuals (64% male, mean age 59 years) from 1 January 2000 to 31 March 2015. Table 1 summarizes the baseline characteristics of the cohorts. Compared with the non-gout cohort, gout patients had a higher number of outpatient visits and hospitalizations, greater Charlson Comorbidity Index, higher prevalence of hypertension, varicose veins, sepsis, inflammatory bowel disease, chronic kidney disease, alcoholism, trauma, fracture and surgery, as well as higher use of NSAIDs, cox-2 inhibitors and glucocorticoids.
Table 1.
Baseline characteristics of individuals with and without gout at the time of index date
| Variable | Gout cohort | Non-gout |
|---|---|---|
| n = 130 708 | n = 131 349 | |
| Age, mean (s.d.) | 58.98 (15.64) | 59.01 (15.65) |
| Male | 84 146 (64.38%) | 84 515 (64.34%) |
| Healthcare utilization | ||
| Number of outpatient visits, mean (s.d.) | 12.09 (13.15) | 8.92 (11.53) |
| Number with hospitalizations, n (%) | 26 322 (20.14%) | 20 861 (15.88%) |
| Comorbidities, n (%) | ||
| Charlson Comorbidity Index, mean (s.d.) | 0.53 (1.23) | 0.36 (1.04) |
| Hypertension | 49 261 (37.69%) | 33 725 (25.68%) |
| Varicose veins | 1083 (0.83%) | 954 (0.73%) |
| IBD | 893 (0.68%) | 726 (0.55%) |
| Sepsis | 919 (0.70%) | 655 (0.50%) |
| Trauma | 378 (0.29%) | 324 (0.25%) |
| Fracture | 1735 (1.33%) | 1652 (1.26%) |
| Alcoholism with liver disease | 1483 (1.13%) | 946 (0.72%) |
| Chronic kidney disease | 9276 (7.10%) | 3752 (2.86%) |
| Surgery | 439 (0.34%) | 371 (0.28%) |
| Medications, n (%) | ||
| NSAIDs | 37 661 (28.81%) | 18 986 (14.45%) |
| HRT | 3870 (2.96%) | 3632 (2.77%) |
| Glucocorticoids | 8582 (6.57%) | 5261 (4.01%) |
| Cox-2 inhibitors | 6193 (4.74%) | 4283 (3.26%) |
| Oral contraceptives | 1431 (1.09%) | 1479 (1.13%) |
Cox-2, cyclooxygenase-2.
Baseline characteristics were measured over one year prior to index date.
Risk of VTE in patients with gout
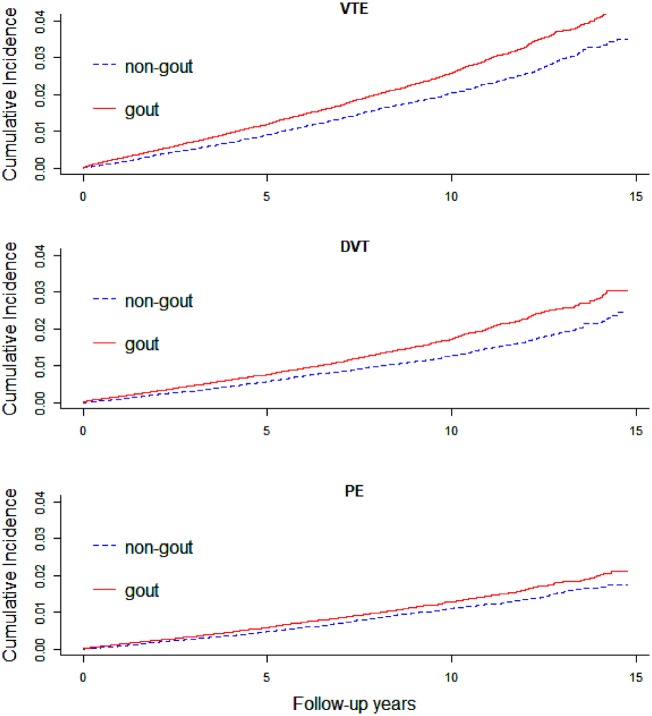
During the follow-up time after the index date, 2071 incident VTE, 1377 DVT, and 1012 PE events occurred in the gout cohort, compared with 1629, 1032 and 854 in the non-gout cohort, respectively. The cumulative incidence of VTE, DVT and PE was significantly higher in the gout cohort compared with the non-gout cohort (Fig. 1). The IRs for VTE, DVT and PE in the gout cohort were 2.63, 1.74 and 1.28 cases per 1000 person-years, respectively, while the corresponding IRs in the non-gout cohort were 2.03, 1.28 and 1.06 per 1000 person-years, respectively. After adjusting for age and sex, the HRs (95% CI) were 1.34 (1.25, 1.44), 1.40 (1.29, 1.53) and 1.26 (1.14, 1.39) for VTE, DVT and PE, respectively. The corresponding fully-adjusted HRs (95% CI) were 1.22 (1.13, 1.32), 1.28 (1.17, 1.41) and 1.16 (1.05, 1.29) (Table 2).
Fig. 1.
Cumulative incidence of VTE, DVT and PE among gout and non-gout
Table 2.
Overall risk of VTE, DVT and PE in gout relative to non-gout after and before index date
| Gout cohort | Non-gout | Gout cohort | Non-gout | |
|---|---|---|---|---|
| After index date | After index date | Pre-index date | Pre-index date | |
| n = 130 708 | n = 131 349 | n = 132 207 | n = 132 311 | |
| VTE | ||||
| No. of events | 2071 | 1629 | 1499 | 962 |
| IR per 1000 person-years | 2.63 | 2.03 | 1.02 | 0.65 |
| Age- and sex- adjusted HR (95% CI) | 1.34 (1.25, 1.44) | 1.00 | 1.56 (1.44, 1.69) | 1.00 |
| Fully-adjusted HR (95% CI)a | 1.22 (1.13, 1.32) | 1.00 | 1.51 (1.38, 1.64) | 1.00 |
| DVT | ||||
| No. of events | 1377 | 1032 | 1026 | 636 |
| IR per 1000 person-years | 1.74 | 1.28 | 0.70 | 0.43 |
| Age-, sex- adjusted HR (95% CI) | 1.40 (1.29, 1.53) | 1.00 | 1.62 (1.46, 1.79) | 1.00 |
| Fully adjusted HR (95% CI)a | 1.28 (1.17, 1.41) | 1.00 | 1.55 (1.40, 1.72) | 1.00 |
| PE | ||||
| No. of events | 1012 | 854 | 718 | 474 |
| IR per 1000 person-years | 1.28 | 1.06 | 0.49 | 0.32 |
| Age-, sex- adjusted HR (95% CI) | 1.26 (1.14, 1.39) | 1.00 | 1.51 (1.35, 1.70) | 1.00 |
| Fully adjusted HR (95% CI)a | 1.16 (1.05, 1.29) | 1.00 | 1.47 (1.31, 1.66) | 1.00 |
Adjusted for all variables listed in Table 1.
DVT, deep venous thrombosis; HR, hazard ratio; IR, incidence rate; PE, pulmonary embolism; VTE, venous thromboembolism.
Risk of VTE during the pre-gout period
During the pre-gout period, there were 1499 VTE, 1026 DVT and 718 PE events in the gout cohort, and 962 636 and 474 events, respectively, in the non-gout cohort. The IRs for VTE, DVT and PE during the pre-gout period in the gout cohort were 1.02, 0.70 and 0.49 cases per 1000 person-years, respectively, and 0.65, 0.43 and 0.32 per 1000 person-years in the non-gout cohort. Compared with the non-gout cohort, the age- and sex-adjusted HRs (95% CI) for VTE, DVT and PE in the gout cohort during the pre-gout period were 1.56 (1.44, 1.69), 1.62 (1.46, 1.79) and 1.51 (1.35, 1.70), respectively. The fully-adjusted HRs (95% CI) were 1.51 (1.38, 1.64), 1.55 (1.40, 1.72) and 1.47 (1.31, 1.66), respectively (Table 2).
Time trends in the risk of VTE in gout relative to non-gout before and after the index date
Before the index date, for VTE, DVT and PE, the highest HRs were observed during the first year prior to the index date (HRs (95% CI): VTE: 1.62 (1.30, 2.01), DVT: 1.75 (1.34, 2.28) and PE: 2.04 (1.46, 2.86)) (Table 3). During the third and second years preceding the index date, the fully adjusted HRs (95% CI) for VTE were 1.44 (1.26, 1.65) and 1.56 (1.33, 1.82), respectively. Similar trends were seen for DVT and PE.
Table 3.
Risk trends for VTE in gout relative to non-gout, before and after the index date
| VTE | DVT | PE | |
|---|---|---|---|
| Fully adjusted HRs (95% CI)a | Fully adjusted HRs (95% CI)a | Fully adjusted HRs (95% CI)a | |
| Before gout diagnosis | |||
| −3 years | 1.44 (1.26, 1.65) | 1.48 (1.26, 1.74) | 1.44 (1.18, 1.75) |
| −2 years | 1.56 (1.33, 1.82) | 1.61 (1.32, 1.95) | 1.61 (1.27, 2.05) |
| −1 year | 1.62 (1.30, 2.01) | 1.75 (1.34, 2.28) | 2.04 (1.46, 2.86) |
| After gout diagnosis | |||
| +1 year | 1.63 (1.32, 2.01) | 1.68 (1.29, 2.20) | 1.71 (1.25, 2.34) |
| +2 years | 1.29 (1.17, 1.49) | 1.37 (1.14, 1.65) | 1.24 (1.01, 1.53) |
| +3 years | 1.33 (1.18, 1.50) | 1.45 (1.24, 1.70) | 1.21 (1.02, 1.44) |
| +4 years | 1.28 (1.15, 1.43) | 1.34 (1.17, 1.54) | 1.18 (1.02, 1.38) |
| +5 years | 1.22 (1.10, 1.34) | 1.25 (1.10, 1.42) | 1.13 (0.99, 1.30) |
Adjusted for all variables listed in Table 1.
DVT, deep venous thrombosis; HR, hazard ratio; PE, pulmonary embolism; VTE, venous thromboembolism.
During the first, second, third, fourth and fifth year after the index date, the HRs for VTE decreased over time, but remained significant, with fully adjusted HRs (95% CI) of 1.63 (1.32, 2.01), 1.29 (1.17, 1.49), 1.33 (1.18, 1.50), 1.28 (1.15, 1.43) and 1.22 (1.10, 1.34). Similar trends were also seen in DVT and PE (Table 3).
Sensitivity analyses
We performed three sensitivity analyses. Firstly, when assessing the robustness of our results to the potential effect of the unmeasured confounder, obesity, HRs remained significant, but attenuated at values of 40% prevalence in the gout cohort and OR of 2.0 for the association between the unmeasured confounder and all outcomes except PE (Table 4). Secondly, after accounting for the competing risk of death using subdistribution models, the results also remained significant, but the effect sizes were attenuated (Table 4). Thirdly, overall, fully adjusted HRs remained significant except DVT when using the stricter gout case definition (i.e. gout diagnosis and ULT) when assessing the risk before and after gout diagnosis (Table 5).
Table 4.
Sensitivity analyses (unmeasured confounder and competing risk of death) for the risk of VTE in gout
| Analyses | VTE | DVT | PE |
|---|---|---|---|
| HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | |
| Primary analyses | 1.22 (1.13, 1.32) | 1.28 (1.17, 1.41) | 1.16 (1.05, 1.29) |
| Sensitivity analyses modeling obesity with prevalence = 30% and OR = 2.0 | 1.19 (1.10, 1.28) | 1.27 (1.15, 1.39) | 1.14 (1.03, 1.27) |
| Sensitivity analyses modeling obesity with prevalence = 40% and OR = 2.0 | 1.11 (1.02, 1.20) | 1.16 (1.05, 1.28) | 1.02 (0.91, 1.14) |
| Sensitivity analyses modeling obesity with prevalence = 30% and OR = 2.5 | 1.07 (0.99, 1.16) | 1.13 (1.03, 1.25) | 0.99 (0.89, 1.11) |
| Sensitivity analyses modeling obesity with prevalence = 40% and OR = 2.5 | 0.97 (0.89, 1.05) | 1.04 (0.93, 1.15) | 0.89 (0.79, 1.00) |
| Sensitivity analyses accounting for competing risk of death | 1.20 (1.14, 1.26) | 1.27 (1.20, 1.36) | 1.13 (1.06, 1.22) |
Adjusted for all variables listed in Table 1.
DVT, deep venous thrombosis; HR, hazard ratio; OR, odds ratio; PE, pulmonary embolism; VTE, venous thromboembolism; PE, pulmonary embolism.
Table 5.
Sensitivity analyses evaluating the risk of VTE using the alternative case definition
| Gout cohort | Non-gout | Pre-gout | Non-gout | |
|---|---|---|---|---|
| After index date | After index date | Pre-index date | Pre-index date | |
| n = 41 962 | n = 42 302 | n = 42 669 | n = 42 735 | |
| VTE | ||||
| No. of events | 717 | 545 | 707 | 433 |
| IR per 1000 person-years | 3.17 | 2.33 | 1.43 | 0.87 |
| Age- and sex- adjusted HR (95% CI) | 1.41 (1.25, 1.59) | 1.00 | 1.64 (1.46, 1.85) | 1.00 |
| Fully-adjusted HR (95% CI)a | 1.18 (1.03, 1.35) | 1.00 | 1.65 (1.45, 1.87) | 1.00 |
| DVT | ||||
| No. of events | 461 | 365 | 478 | 302 |
| IR per 1000 person-years | 2.03 | 1.56 | 0.96 | 0.61 |
| Age- and sex- adjusted HR (95% CI) | 1.30 (1.12, 1.51) | 1.00 | 1.60 (1.38, 1.85) | 1.00 |
| Fully-adjusted HR (95% CI)a | 1.06 (0.89, 1.26) | 1.00 | 1.62 (1.38, 1.90) | 1.00 |
| PE | ||||
| No. of events | 363 | 275 | 343 | 201 |
| IR per 1000 person-years | 1.60 | 1.17 | 0.69 | 0.40 |
| Age- and sex-adjusted HR (95% CI) | 1.49 (1.26, 1.77) | 1.00 | 1.69 (1.42, 2.01) | 1.00 |
| Fully-adjusted HR (95% CI)a | 1.29 (1.07, 1.57) | 1.00 | 1.67 (1.38, 2.01) | 1.00 |
Adjusted for all variables listed in Table 1.
DVT, deep venous thrombosis; HR, hazard ratio; IR, incidence rate; PE, pulmonary embolism; VTE, venous thromboembolism.
Discussion
In this large population-based study of an incident gout cohort, we demonstrated that the overall risks of VTE, DVT and PE were significantly increased both before and after gout diagnosis when compared with the general population. Furthermore, we observed that the risk increased gradually before gout diagnosis, and peaked during the one year prior to diagnosis and one year following. Though the risks decreased progressively thereafter, with the exception of PE, they remained statistically significant even five years after the index date when compared with the general population.
Three main precursors to VTE were proposed by Virchow: damage to the vessel wall, increasing blood coagulability and venous stasis [38]. Inflammation during the hyperuricaemia phase and gout attacks might be a plausible key factor responsible for damaging the vascular endothelium and increasing blood coagulability. Deposition of uric acid can activate the formation of the nucleotide-binding domain, leucine-rich-containing family, pyrin domain-containing-3 (NLRP3) inflammasome, which increases the production of interleukin-1ß and can amplify the inflammatory response on vascular cells [39]. At the same time, hyperuricaemia can also activate the production of reactive oxygen species, which can increase the formation of NLRP3 [40] and inhibit the secretion of nitric oxide, a suppressant of NLRP3 [41]. This inflammatory process can damage the vascular endothelium in both arteries and veins through endothelial disturbance by leading to vascular smooth muscle cells proliferation and endothelial cells inhibition [18, 42]. In addition, von Willebrand factor, tissue factor and plasminogen activator inhibitor V are also produced, which upregulates blood coagulation [18, 42].
As proposed by previous studies[18, 43], inflammation may increase gradually in the pre-diagnosis phase of gout as untreated hyperuricaemia worsens, until the onset of symptoms causes patients to seek medical care, leading to the diagnosis of gout. Thus, hyperuricaemia and untreated inflammation would peak in the first year before the index date, with a subsequent decline in risk after the index date resulting from reduced inflammation following treatment with anti-inflammatory drugs, as well as ULTs [44].
Our findings of increased risk of VTE after gout diagnosis are consistent with recent studies. A cohort study from Taiwan reported adjusted HRs for DVT and PE of 1.66 and 1.53, respectively [16]. However, only one ICD-9 CM or ICD-10 CM code was used to define DVT or PE, and they did not adjust for any prescription use that are known as risk factors for VTE. Another study, using the same dataset, also reported an adjusted HR for DVT of 1.38 compared with the general population [17], which is also consistent with our findings. However, their study design used a prevalent cohort that has an inherent survival bias, as the cohort only includes surviving individuals, who may be less susceptible to cardiovascular complications such as VTE.
Our finding that the risk of VTE is increased before the index date suggests that serum uric acid (SUA) level may play a role in thrombosis even before the diagnosis of gout. This finding is consistent with evidence that SUA itself has a pro-inflammatory effect on vascular cells and with evidence that SUA level is positively associated with atherothrombotic cardiovascular diseases [45]. Using the cohort component of the Atherosclerosis Risk in Communities Study, Kubota et al. [18] recruited 14 126 participants without history of VTE at baseline and divided SUA levels into six categories to assess the risk of VTE. The authors found a positive association between SUA levels and VTE risk ranging from 1.44 (95% CI, 1.06, 1.96) to 1.89 (95% CI, 1.15, 3.12) compared with the lowest SUA level group [18]. A similar trend was observed for VTE subtypes. The authors concluded that elevated SUA may increase the risk of VTE through inflammation and proposed SUA as a low-cost biomarker that could be studied for adding to VTE risk prediction.
We acknowledge the potential limitations inherent to our observational study using administrative data. The uncertainty for the accuracy of the gout case definition cannot be completely ruled out, especially as we used one billing code from outpatient or inpatient visit for the gout case definition; however, similar results were also found using our alternative case definition (validity of 90%) in our sensitivity analyses, which restricted the definition to those gout patients who had at least one prescription of ULT [37]. The measurement of disease onset using administrative data may also be imprecise; for example, there would be no record if patients did not have any healthcare utilization during the first gout flare [46, 47]. The time difference between gout onset and the first clinical billing code might lead to the higher risk of VTE in pre-gout period than the general population. Finally, we did not have access to laboratory data to assess the SUA levels before and after the index date, nor other parameters such the C-reactive protein.
While our multivariable Cox models adjusted for many known VTE risk factors, certain confounding variables were unavailable in our dataset, such as body mass index to assess obesity. However, in our sensitivity analyses adjusting for unmeasured confounders, the results remained statistically significant for VTE and DVT even when using values of 40% prevalence of obesity in the gout cohort and an odds ratio of 2.0 for the association between obesity and VTE or DVT. Even so, as with other retrospective observational studies, our results could have been influenced by other unknown confounders.
Despite these limitations, there are several notable strengths in our study. We used a large Canadian administrative database based on the entire BC population, which makes our findings more generalizable. We were also able to use the largest gout cohort assembled to date to study the relationship between gout and VTE in a general population-based setting. The sample size provided sufficient statistical power to find an association between gout and VTE. Besides, this is also the first study assessing the yearly trends of VTE risk before and after the index date. Furthermore, we required at least 10 years of run-in time before the index date to only include the purely incident patients as our exposure. Finally, we used the strictest VTE case definition to capture true events.
If our results are confirmed by others, the critical question is how to maximize the clinical utility of these results. As is known, VTE is the most common preventable cause of inpatient death (PE occupies 5% to 10% of death in hospitalized patients) [48] and prevention should be the ultimate goal. However, not all patients with gout carry the same risk and therefore further research to identify high-risk patients should be the next step, so prevention or prophylaxis can be implemented. In the meantime, adequate vigilance and awareness for VTE risk should be targeted in patients with gout or conceivable hyperuricaemia. This could be an additional incentive for patients and clinicians to adhere to treatment guidelines under the assumption that this will decrease the risk of gout flares and, thus, likely VTE.
In summary, this is the largest population-based study to demonstrate that patients with gout have an increased risk of VTE, DVT and PE (∼20%, 30% and 20%, respectively) after a gout diagnosis compared with the general population. This risk is independent of traditional VTE risk factors. Moreover, we also demonstrated that the risk is higher in the years prior to diagnosis, peaking in the first year before diagnosis (∼60–100%) and decreasing with time thereafter, but remaining elevated when compared with the general population. Certainly, this risk should also be confirmed in future prospective studies, especially among people who have hyperuricaemia.
Acknowledgements
The authors thank Hamid Tavakoli for his assistance in SAS code checking and Dami Ojo for her editorial assistance in the preparation of this manuscript. We would like to thank the Ministry of Health of British Columbia and Population Data BC for providing access to the administrative data. N.M. is supported by a Fellowship Award from the Canadian Institutes of Health Research. J.A.A.-Z. is the Michael Smith Foundation for Health Research Scholar and the BC Lupus Society research scholar.
Funding: This work was supported by the Canadian Institutes of Health Research [CIHR Grants MOP 125960, THC 135235].
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Roddy E, Choi HK.. Epidemiology of gout. Rheum Dis Clin North Am 2014;40:155–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fisher MC, Rai SK, Lu N, Zhang Y, Choi HK.. The unclosing premature mortality gap in gout: a general population-based study. Ann Rheum Dis 2017;76:1289–94. [DOI] [PubMed] [Google Scholar]
- 3. Rai SK, Burns LC, De Vera MA. et al. The economic burden of gout: a systematic review. Semin Arthritis Rheum 2015;45:75–80. [DOI] [PubMed] [Google Scholar]
- 4. Rai SK, Aviña-Zubieta JA, McCormick N. et al. Trends in gout and rheumatoid arthritis hospitalizations in Canada from 2000 to 2011. Arthritis Care Res 2017;69:758–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maradit-Kremers H, Crowson CS, Nicola PJ. et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum 2005;52:402–11. [DOI] [PubMed] [Google Scholar]
- 6. Aviña-zubieta JA, To F, Vostretsova K. et al. Risk of myocardial infarction and stroke in newly diagnosed systemic lupus erythematosus: a general population-based study. Arthritis Care Res 2017;69:849–56. [DOI] [PubMed] [Google Scholar]
- 7. Choi HK, Curhan G.. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007;116:894–900. [DOI] [PubMed] [Google Scholar]
- 8. Seminog OO, Goldacre MJ.. Gout as a risk factor for myocardial infarction and stroke in England: evidence from record linkage studies. Rheumatology 2013;52:2251–9. [DOI] [PubMed] [Google Scholar]
- 9. Bos MJ, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM.. Uric acid is a risk factor for myocardial infarction and stroke: the Rotterdam study. Stroke 2006;37:1503–7. [DOI] [PubMed] [Google Scholar]
- 10. Culleton BF, Larson MG, Kannel WB, Levy D.. Serum uric acid and risk for cardiovascular disease and death: the Framingham Heart Study. Ann Intern Med 1999;131:7–13. [DOI] [PubMed] [Google Scholar]
- 11. Wannamethe SG, Shaper AG, Whincup PH.. Serum urate and the risk of major coronary heart disease events. Heart 1997;78:147–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naess IA, Christiansen SC, Romundstad P. et al. Incidence and mortality of venous thrombosis: a population-based study. J Thromb Haemost 2007;5:692–9. [DOI] [PubMed] [Google Scholar]
- 13. Aviña-zubieta JA, Jansz M, Sayre EC, Choi HK.. The risk of deep venous thrombosis and pulmonary embolism in primary sjögren syndrome: a general population-based study. J Rheumatol 2017;44:1184–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi HK, Rho YH, Zhu Y. et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis 2013;72:1182–7. [DOI] [PubMed] [Google Scholar]
- 15. Xu J, Lupu F, Esmon CT.. Inflammation, innate immunity and blood coagulation. Hamostaseologie 2010;30:5–9. [PubMed] [Google Scholar]
- 16. Huang CC, Huang PH, Chen JH. et al. An independent risk of gout on the development of deep vein thrombosis and pulmonary embolism: a nationwide, population-based cohort study. Medicine 2015;94:e2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chiu CC, Chen YT, Hsu CY. et al. Association between previous history of gout attack and risk of deep vein thrombosis - a nationwide population-based cohort study. Sci Rep 2016;6:26541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubota Y, Mcadams-demarco M, Folsom AR.. Serum uric acid, gout, and venous thromboembolism: the atherosclerosis risk in communities study. Thromb Res 2016;144:144–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.British Columbia Ministry of Health [creator] (2017): Medical Services Plan (MSP) Payment Information File. Population Data BC [publisher]. Data Extract. MOH (2017). https://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 20.Canadian Institute for Health Information [creator] (2017): Discharge Abstract Database (Hospital Separations). Population Data BC [publisher]. Data Extract. MOH (2017). http://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 21.British Columbia Ministry of Health [creator] (2017): Consolidation File (MSP Registration & Premium Billing). Population Data BC [publisher]. Data Extract. MOH (2017). http://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 22.BC Cancer Agency Registry Data (2017). Population Data BC [publisher]. Data Extract. BC Cancer Agency (2017). http://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 23.BC Vital Statistics Agency [creator] (2017): Vital statistics Deaths. Population Data BC [publisher].Data Extract BC Vital Statistics Agency (2017).http://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 24.BC Ministry of Health [creator] (2018): PharmaNet. BC Ministry of Health [publisher]. Data Extract. Data Stewardship Committee (2018). http://www.popdata.bc.ca/data (15 August 2019, date last accessed).
- 25. Lacaille D, Avina-Zubieta JA, Sayre EC, Abrahamowicz M.. Improvement in 5-year mortality in incident rheumatoid arthritis compared with the general population-closing the mortality gap. Ann Rheum Dis 2017;76:1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Etminan M, Forooghian F, Brophy JM, Bird ST, Maberley D.. Oral fluoroquinolones and the risk of retinal detachment. JAMA 2012;307:1414–9. [DOI] [PubMed] [Google Scholar]
- 27. Solomon DH, Massarotti E, Garg R. et al. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA 2011;305:2525–31. [DOI] [PubMed] [Google Scholar]
- 28. Singh JA. Veterans Affairs databases are accurate for gout-related health care utilization: a validation study. Arthritis Res Ther 2013;15:R224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Huerta C, Johansson S, Wallander MA, García Rodríguez LA.. Risk factors and short-term mortality of venous thromboembolism diagnosed in the primary care setting in the United Kingdom. Arch Intern Med 2007;167:935–43. [DOI] [PubMed] [Google Scholar]
- 30. Romano PS, Roos LL, Jollis JG.. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol 1993;46:1075–9. [DOI] [PubMed] [Google Scholar]
- 31. Cox DR. Regression models and life-tables. J R Stat Soc Ser B 1972;34:187–220. [Google Scholar]
- 32. Zhu Y, Pandya BJ, Choi HK.. Comorbidities of gout and hyperuricemia in the US general population: NHANES 2007-2008. Am J Med 2012;125:679–87. [DOI] [PubMed] [Google Scholar]
- 33. Cea Soriano L, Rothenbacher D, Choi HK, García Rodríguez LA.. Contemporary epidemiology of gout in the UK general population. Arthritis Res Ther 2011;13:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Statistics Canada. Adjusting the scales: Obesity in the Canadian population after correcting for respondent bias, 82–624-X. 2014. https://www150.statcan.gc.ca/n1/pub/82-624-x/2014001/article/11922-eng.htm (1 June 2019, date last accessed)
- 35. Yang G, De Staercke C, Hooper WC.. The effects of obesity on venous thromboembolism: a review. Open J Prev Med 2012;2:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lau B, Cole SR, Gange SJ.. Practice of epidemiology competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meier CR, Jick H.. Omeprazole, other antiulcer drugs and newly diagnosed gout. Br J Clin Pharmacol 1997;44:175–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Previtali E, Bucciarelli P, Passamonti SM, Martinelli I.. Risk factors for venous and arterial thrombosis. Blood Transfus 2011;9:120–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dalbeth N, Merriman TR, Stamp LK.. Gout. Lancet 2016;388:2039–52. [DOI] [PubMed] [Google Scholar]
- 40. Zheng Q, Ren Y, Reinach PS. et al. Reactive oxygen species activated NLRP3 inflammasomes initiate inflammation in hyperosmolarity stressed human corneal epithelial cells and environment-induced dry eye patients. Exp Eye Res 2015;134:133–40. [DOI] [PubMed] [Google Scholar]
- 41. Mao K, Chen S, Chen M. et al. Nitric oxide suppresses NLRP3 inflammasome activation and protects against LPS-induced septic shock. Cell Res 2013;23:201–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wakefield TW, Myers DD, Henke PK.. Mechanisms of venous thrombosis and resolution. Arterioscler Thromb Vasc Biol 2008;28:387–91. [DOI] [PubMed] [Google Scholar]
- 43. Krishnan E, Baker JF, Furst DE, Schumacher HR.. Gout and the risk of acute myocardial infarction. Arthritis Rheum 2006;54:2688–96. [DOI] [PubMed] [Google Scholar]
- 44. Volterrani M, Iellamo F, Sposato B, Romeo F.. Uric acid lowering therapy in cardiovascular diseases. Int J Cardiol 2016;213:20–2. [DOI] [PubMed] [Google Scholar]
- 45. Mazzali M, Hughes J, Kim YG. et al. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 2001;38:1101–6. [DOI] [PubMed] [Google Scholar]
- 46. Miller DR, Rogers WH, Kazis LE. et al. Patients’ self-report of diseases in the Medicare Health Outcomes Survey based on comparisons with linked survey and medical data from the Veterans Health Administration. J Ambul Care Manage 2008;31:161–77. [DOI] [PubMed] [Google Scholar]
- 47. Wijnands JM, Boonen A, Arts IC. et al. Large epidemiologic studies of gout: challenges in diagnosis and diagnostic criteria. Curr Rheumatol Rep 2011;13:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohen AT, Tapson VF, Bergmann J. et al. Venous thromboembolism risk and prophylaxis in the acute hospital care setting (ENDORSE study): a multinational cross-sectional study. Lancet 2008;371:387–94. [DOI] [PubMed] [Google Scholar]