Abstract
Objective
SLE is known to have an aggressive phenotype in black populations, but data from African cohorts are largely lacking. We therefore compared immunological and clinical profiles between Sudanese and Swedish patients using similar tools.
Methods
Consecutive SLE patients from Sudan (n = 115) and Sweden (n = 340) and from 106 Sudanese and 318 Swedish age- and sex-matched controls were included. All patients fulfilled the 1982 ACR classification criteria for SLE. Ten ANA-associated specificities and C1q-binding immune complexes (CICs) were measured. Cut-offs were established based on Sudanese and Swedish controls, respectively. Disease activity was measured with a modified SLEDAI and organ damage with the SLICC Damage Index. In a nested case–control design, Swedish and Sudanese patients were matched for age and disease duration.
Results
Females constituted 95.6% and 88.1% of Sudanese and Swedish patients, respectively (P = 0.02), with younger age at inclusion (33 vs 47.7 years; P < 0.0001) and shorter disease duration (5 vs 14 years; P < 0.0001) among Sudanese patients. Anti-Sm antibodies were more frequent in Sudanese patients, whereas anti-dsDNA, anti-histone and CICs were higher in Swedish patients. In the matched analyses, there was a trend for higher SLEDAI among Swedes. However, Sudanese patients had more damage, solely attributed to high frequencies of cranial/peripheral neuropathy and diabetes.
Conclusion
While anti-Sm is more common in Sudan than in Sweden, the opposite is found for anti-dsDNA. Sudanese patients had higher damage scores, mainly because of neuropathy and diabetes. Sudanese patients were younger, with a shorter SLE duration, possibly indicating a more severe disease course with impact on survival rates.
Keywords: systemic lupus erythematosus, Sudan, Africa, Sweden, autoantibodies, comparative study
Rheumatology key messages
While the anti-Sm frequency is higher, other autoantibodies are less frequent in Sudanese patients.
The higher organ damage score in matched Sudanese patients is attributed to neuropathy and diabetes.
Shorter disease duration and younger age among Sudanese patients may indicate limited survival.
Introduction
SLE is considered to be rare in West Africa but is more prevalent in other parts of the African continent. However, a number of studies have suggested that the prevalence of SLE might be underestimated in populations of African origin [1–6]. SLE severity measured by disease activity and organ damage has invariably been reported to be higher in patients of African ancestry, mainly due to more frequent occurrence and the unfavourable outcome of LN [7–9].
A number of studies have investigated the prevalence of lupus-related autoantibodies in black SLE populations. One previous study found that African American SLE patients had higher frequencies of autoantibodies against anti-dsDNA, chromatin, ribosomal P, SSA/Ro60, Sm and the Sm/U1RNP complex compared with European American patients living in the same area [10]. Other investigators have also shown that antibodies against Sm and U1RNP are more prevalent in non-Caucasian SLE patients in the USA and Europe as compared with Caucasians [11–13], while the occurrence of anti-SSB/La antibodies were similar [11]. Black South African patients had similar frequencies of anti-Sm and anti-RNP when compared with African Americans and Afro-Caribbean [14]. In all of these studies, common cut-offs as recommended by the assay manufacturer were used and no adjustment was made for the general occurrence of autoantibodies in the respective control populations. In these studies, the occurrence of autoantibodies was mostly treated as a dichotomous variable, although it is well known that not only anti-dsDNA, but also antibodies against SSA/Ro, SSB/La, Sm and U1RNP can fluctuate over time [15].
A few Sudanese reports have investigated the occurrence of autoantibodies [16, 17] and clinical phenotypes [16–19] in SLE, but none of them have compared with other ethnicities. Other limitations in previous studies are the lack of standard disease activity and/or organ damage measurement tools, as well as the use of a non-adjusted manufacturer’s cut-off to determine the prevalence of autoantibodies.
In this comparative study, we standardized investigations to compare SLE cohorts living in Sudan and Sweden with the aim of investigating differences in immunological and clinical profiles. To the best of our knowledge, there are currently no published studies comparing SLE in African and Caucasian populations using uniform clinical and laboratory analyses.
Methods
Patients and controls
This cross-sectional study included 115 and 340 consecutive SLE patients and 106 and 318 age- and sex- matched controls from Sudan and Sweden, respectively. Sudanese controls were healthy and Swedish controls were population-based non-SLE individuals. The age and gender distributions for the patients are shown in Table 1. Among the Swedish patients, 49 were of non-European ancestry, including 9 of African origin, whereas all Sudanese patients were natives. Patient history, clinical description and SLEDAI [20] and SLICC Damage Index (SLICC-DI) [21] scores in Sudan and Sweden were acquired with identical questionnaires that are used at Karolinska University Hospital, Stockholm, Sweden. All participants gave written informed consent to participate in the study, which was performed in compliance with the Declaration of Helsinki. Approval was obtained from the ethical committees of Alribat University Hospital and Omdurman Military Hospital for the Sudanese study and from the local ethics committee of the Karolinska University Hospital for the Swedish study.
Table 1.
Demographics, ACR classification criteria and ongoing drug treatment in the investigated patients
| Characteristics | Sudan, all patients (n = 115) | Sweden, all patients (n = 340) | P-value | Sudan, matched patients (n = 88) | Sweden, matched patients (n = 88) | P-value |
|---|---|---|---|---|---|---|
| Gender, female/male, n/n | 110/5 | 297/40 | 0.02 | 85/3 | 79/9 | 0.07 |
| Age at inclusion, years, median/meana | 33/34.9 | 47.7/46.7 | <0.0001 | 35/36.8 | 35.4/37.3 | 0.7 |
| Age at SLE onset, years, median/mean | 28/30.0 | 26/29.1 | 0.3 | 31.5/31.6 | 28.5/31.1 | 0.7 |
| Duration of SLE, years, median/mean | 5/4.8 | 14/17.5 | <0.0001 | 5.0/5.3 | 6.0/6.1 | 0.06 |
| ACR classification criteriab, n/N (%) | ||||||
| Malar rash | 60/115 (52.2) | 175/337 (51.9) | NA | 47/86 (53.4) | 39/88 (44.3) | NA |
| Discoid rash | 7/115 (6.1) | 65/337 (19.3) | NA | 5/88 (5.7) | 13/88 (14.8) | NA |
| Photosensitivity | 61/115 (53.0) | 222/337 (65.9) | NA | 47/86 (53.4) | 49/88 (55.7) | NA |
| Oral ulcers | 73/115 (63.5) | 113/336 (33.6) | NA | 55/88 (62.5) | 28/88 (31.8) | NA |
| Arthritis | 101/115 (87.8) | 280/337 (83.1) | NA | 78/88 (88.6) | 69/88 (78.4) | NA |
| Serositis | 28/115 (24.3) | 136/337 (40.4) | NA | 21/88 (23.9) | 29/88 (32.9) | NA |
| Renal disorder | 26/114 (22.8) | 137/337 (40.6) | NA | 22/88 (25.0) | 35/88 (39.8) | NA |
| Neurologic disorder | 18/115 (15.6) | 37/337 (11.0) | NA | 14/88 (15.9) | 7/88 (7.9) | NA |
| Hematologic disorder | 20/115 (17.4) | 238/337 (70.6) | NA | 17/88 (19.3) | 69/88 (78.4) | NA |
| Immunologic disorderc | 52/64 (81.2) | 123/178 (69.1) | NA | 42/52 (80.8) | 18/32 (56.2) | NA |
| ANA by IIFd | 95/98 (96.9) | 332/336 (98.8) | NA | 74/77 (96.1) | 86/88 (97.7) | NA |
| Treatment at time of inclusion, n/N (%) | ||||||
| AZA | 69/112 (61.6) | 57/332 (17.2) | <0.0001 | 50/86 (58.1) | 19/88 (21.6) | <0.0001 |
| Prednisolone | 80/112 (71.4) | 194/337 (57.6) | 0.009 | 59/86 (68.6) | 57/88 (64.8) | 0.6 |
| Prednisolone dose, mg, median/mean | 5.0/10.0 | 2.5/5.1 | 0.007 | 5.0/9.3 | 5.0/7.3 | 0.7 |
| HCQ | 63/112 (56.3) | 116/335 (34.6) | <0.0001 | 51/86 (59.3) | 34/88 (38.6) | 0.006 |
| MMF | 14/112 (12.5) | 34/332 (10.2) | 0.5 | 13/86 (15.1) | 12/87 (13.8) | 0.8 |
| CYC | 2/112 (1.7) | 4/296 (1.3) | 0.7 | 1/86 (1.2) | 2/79 (2.5) | 0.5 |
| MTX | 10/112 (8.9) | 14/331 (4.2) | 0.06 | 9/86 (10.5) | 4/88 (4.6) | 0.1 |
| Ciclosporin | 1/112 (0.9) | 2/335 (0.6) | 0.7 | 1/86 (1.2) | 0/88 (0.0) | 0.3 |
| ASA | 9/112 (8.0) | 56/336 (16.7) | 0.02 | 9/86 (10.5) | 13/87 (14.9) | 0.4 |
| Warfarin | 4/112 (3.6) | 48/337 (15.0) | 0.002 | 3/86 (3.5) | 12/88 (13.6) | 0.02 |
Sudanese and Swedish patients are compared for the full cohorts as well as for the nested group matched for age and disease duration. Significant differences are shown in bold.
ASA, acetylsalicylic acid; NA, not applicable.
For Sudanese patients, age was reported in full years, whereas for Swedish patients, age was calculated using the date of birth and time of presentation to the clinic.
ACR fulfilled criteria were not statistically compared between the cohort since they were documented at different time points.
Including only anti-dsDNA for each cohort.
IIF was done in Sudan and Sweden at the time of diagnosis.
Sudanese SLE patients who fulfilled the 1982 revised ACR classification criteria for SLE [22] were recruited between June 2011 and December 2014 from three rheumatology clinics in Khartoum that receive patients from different parts of Sudan (Alribat University Hospital, Omdurman Military Hospital and Al Mo’alem Medical City). Healthy Sudanese controls were recruited from Neelain University in Khartoum (staff and students). Venous blood samples were collected from all subjects and immediately centrifuged and separated and the serum stored at −70°C. Blood samples were available from 93 Sudanese patients and 106 controls.
All Swedish SLE patients ⩾18 years of age receiving care at the rheumatology clinic at Karolinska University Hospital who fulfilled four or more of the 1982 ACR classification criteria for SLE during the inclusion period 2004–2010 were asked to participate. Population controls, individually matched for age, sex and region to the SLE patients, were identified through the Swedish population registry, contacted by a letter and asked to participate. SLE was the only exclusion criterion among controls. Serum samples were obtained and stored at −70°C until use.
Histories of fulfilled ACR criteria (at the time of diagnosis for Sudanese patients and at study inclusion for Swedish patients), other symptoms, comorbidities and ongoing drug treatment were obtained from patient records. As Sudanese and Swedish patients reported ACR criteria at different time points, the number of fulfilled criteria was not statistically compared in this article (Table 1). SLEDAI and SLICC-DI scores were evaluated for both cohorts at the time of inclusion. To obtain comparable data, SLEDAI was modified by excluding DNA binding and complement components for Swedish patients [23], due to lack of this information in the Sudanese cohort.
Urinary laboratory data on kidney engagement in Sudanese patients was obtained from different private laboratories in Sudan and reported considerably less kidney engagement for various measures compared with Swedish patients, which was not in agreement with other clinical and laboratory data. Because of the uncertainty concerning the comparability of urinary laboratory data, the urinary items were also excluded from the SLEDAI.
Age at disease onset was defined as the occurrence of the first symptoms of SLE. Disease duration was thus defined as the time from disease onset to inclusion in the study.
To evaluate the role of age at disease onset, patients were divided into early adult-onset SLE (>18 and <45 years) and late-onset SLE (⩾45 years) according to Feng et al. [24]. Consequently, for this analysis, eight and two young Sudanese and Swedish patients, respectively, were excluded.
Immunological testing
Quantification of autoantibodies against double-stranded DNA (dsDNA), Sm, the Sm/U1RNP complex, U1RNP, SSA/Ro52, SSA/Ro60, SSB/La, ribosomal P antigen, PCNA and histones was performed in patient and control sera using a bead-based multiplex immunoassay (FIDIS connective tissue profile, Theradiag, Marne la Vallee, France). Circulating C1q-binding immune complexes (CICs) were measured using the Quanta Lite C1q CIC ELISA (Inova Diagnostics, San Diego, CA, USA) according to the manufacturer’s instructions, except that the standard curve was extended down to 0.5 µgEq/ml. Autoantibodies and CICs were analysed at the Department of Immunology, Genetics and Pathology, Uppsala University, in parallel for the two cohorts, except for CICs, which were tested at different times. IIF ANA was tested in the Sudanese serum samples at a dilution of 1:200 using the HEp-2 cells ANA test (Immuno Concepts, Sacramento, CA, USA). Using that dilution, 5% of Uppsala controls were ANA positive [25].
Statistical analyses
For comparisons between quantitative variables, the Mann–Whitney U test was used, whereas the chi-squared test was used to test for differences between categorical variables, with the Fisher’s exact test applied when appropriate. Due to demographic differences between the Sudanese and Swedish patients, we performed matching for age at inclusion and disease duration, leaving 88 patients in each group in a nested case–control design. To create the matched groups, we conducted hierarchical clustering analysis where age at inclusion and disease duration are used as cluster variables and standardized to contribute similarly to the cluster distance matrix. Sudanese patients acted as leading labels and Swedes as joiners, so we maximized the use of the most Sudanese patients. Ward’s minimum variance method was used to calculate the distance between clusters [26]. Inclusion of new pairs to the matched groups ended when the cluster variables became statistically different.
In consideration of national demographic differences between cohorts, cut-offs for all analyses were defined as the 98th percentiles for the respective national controls and clinical associations to immunological tests were evaluated accordingly. Due to the presence of outliers for anti-SSA/Ro52, SSA/Ro60 and SSB/La among healthy Sudanese controls, the 98th percentile was calculated after retracting these values, with the corresponding Swedish cut-offs treated similarly. Data are also presented using the general cut-offs suggested by the assay manufacturers. In some comparisons (mentioned below) between Sudanese and Swedish patients, statistical significances were recalculated after exclusion of the non-European patients from the Swedish cohort. All statistical analyses were conducted using JMP statistical software (SAS Institute, Cary, NC, USA). P-values <0.05 were considered significant.
Results
Demographics
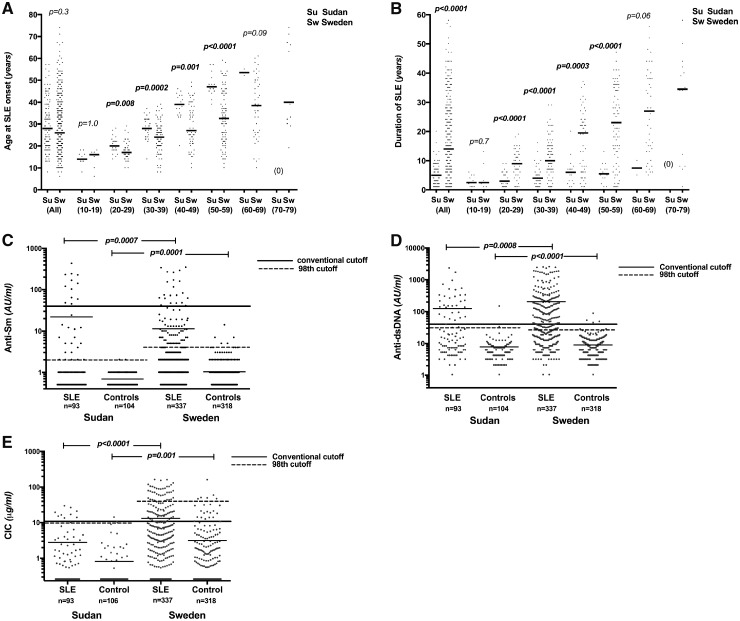
The female preponderance was stronger among Sudanese than among Swedish patients 95.6% vs 88.1% (P = 0.02). The median age at study inclusion was considerably lower among the Sudanese patients (33.0 vs 47.7 years; P < 0.0001; Table 1). A total of 82.3% of Sudanese and 83.2% of Swedish patients had early adult-onset SLE (>18 and <45 years; P = 0.8). There was also no difference in age at disease onset between the two full cohorts (28.0 vs 26.0 years; P = 0.3). However, when patients were stratified to subgroups according to age at study inclusion, Sudanese patients were older at disease onset in all groups between 20 and 60 years of age (Fig. 1A).
Fig. 1.
Age at disease onset, disease duration and immunological profile among Sudanese and Swedish SLE subjects
Comparison of (A) age at SLE onset and (B) disease duration between Sudanese and Swedish patients in the full cohorts and after stratification according to age at study inclusion. Levels of (C) anti-Sm, (D) anti-dsDNA and (E) CICs among patients and controls in each cohort. Cut-offs recommended by the manufacturer and cut-offs according to the 98th percentile among national controls are indicated.
Disease duration was significantly lower among Sudanese patients, both in the full cohort and after stratification (Fig. 1B), and was not associated with SLEDAI or SLICC scores but was associated with worse SLICC-DI scores in Swedes (P < 0.0001; data not shown). Among the Sudanese patients, 78.0%, 15.0% and 6.0% reported Arab, Afro-Arab and African tribal origin, respectively.
Immune profile
At study inclusion, IIF ANA performed in Uppsala was positive in 58% of Sudanese patients and 9% of Sudanese controls. Comparing the full cohorts, levels of anti-Sm, anti-dsDNA and anti-ribosomal P protein as well as CICs were lower among Sudanese patients (Table 2, Fig. 1C–E). When comparing the nine Swedish patients of African origin with Sudanese patients, we found no difference in autoantibody levels but higher CIC levels among Swedes (P = 0.048; data not shown). Using conventional cut-offs recommended by the manufacturer, the occurrence of anti-Sm was increased and anti-dsDNA decreased among Sudanese patients. Autoantibody levels also differed between controls in the two populations, with higher levels of antibodies against SSA/Ro52, U1RNP and histone among Sudanese patients and against SSA/Ro60, Sm, dsDNA and ribosomal P antigens among Swedes (Table 2). When we instead used separate cut-offs based on the national controls, the occurrence of anti-SSA/Ro52 and anti-histone antibodies was also increased among the Swedish patients. Using the nation-based cut-off for the Sudanese cohort, a mean of nine patients turned positive for the tested autoantibodies except for anti-SSA/Ro52 in which five patients lost positivity and for anti-histone in which no change in occurrence was observed. Data for all antibodies are shown in Table 2 and graphically for anti-Sm, anti-dsDNA and CICs in Fig. 1C–E.
Table 2.
Antinuclear-associated autoantibody specificities in Sudanese and Swedish SLE patients
| Autoantibody | Levels in all Sudanese patients, median/ mean | Levels in all Swedish patients, median/ mean | P-value | Occurrence in all Sudanese patients, 98th cut-off, n (%) | Occurrence in all Swedish patients, 98th cut-off, n (%) | P-value | Occurrence in all Sudanese patients, manufacturer’s cut-off, n (%) | Occurrence in all Swedish patients, manufacturers’ cut-off, n (%) | P-value | Levels in Sudanese controls, median/ mean | Levels in Swedish controls, median/ mean | P-value |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-SSA/Ro52 (AU/ml) | 16/52.1 | 17/52.6 | 0.8 | 25 (26.9) | 130 (38.6) | 0.04 | 30 (32.3) | 107 (31.8) | 0.9 | 11/16.9 | 9/10.3 | <0.0001 |
| Anti-SSA/Ro60 (AU/ml) | 2/47.2 | 3/48.8 | 0.2 | 42 (45.2) | 144 (42.7) | 0.7 | 34 (36.6) | 124 (36.8) | 1 | 0/4.1 | 1/2.5 | <0.0001 |
| Anti-SSB/La (AU/ml) | 2/19.0 | 2/22.1 | 0.7 | 22 (23.7) | 73 (21.7) | 0.7 | 15 (16.1) | 66 (19.6) | 0.5 | 1/4.0 | 1/3.8 | 0.4 |
| Anti-Sm (AU/ml) | 1/21.7 | 1/11.3 | 0.0007 | 30 (32.3) | 75 (22.3) | 0.046 | 12 (12.9) | 21 (6.2) | 0.03 | 0/0.4 | 1/0.8 | 0.0001 |
| Anti-Sm/U1RNP (AU/ml) | 1/20.5 | 1/15.8 | 0.2 | 28 (30.1) | 133 (39.5) | 0.09 | 15 (16.1) | 42 (12.5) | 0.4 | 0/0.6 | 0/0.5 | 0.9 |
| Anti-U1RNP (AU/ml) | 6/43.8 | 7/39.6 | 0.9 | 23 (24.7) | 94 (27.9) | 0.5 | 20 (21.5) | 69 (20.5) | 0.8 | 3/5.3 | 2/4.3 | 0.02 |
| Anti-dsDNA (IU/ml) | 14/124.6 | 29/205.9 | 0.0008 | 33 (35.5) | 178 (52.8) | 0.003 | 31 (33.3) | 152 (45.1) | 0.04 | 5/7.8 | 7/9.0 | <0.0001 |
| Anti-ribosomal P (AU/ml) | 1/9.3 | 2/11.6 | 0.0005 | 16 (17.2) | 80 (23.7) | 0.2 | 4 (4.3) | 20 (5.9) | 0.5 | 1/1.2 | 1/1.7 | <0.0001 |
| Anti-histone (AU/ml) | 5/20.5 | 6/20.0 | 0.2 | 10 (10.8) | 80 (23.7) | 0.006 | 10 (10.8) | 37 (11.0) | 1 | 3/4.4 | 2/3.7 | 0.02 |
| Anti-PCNA (AU/ml) | 5/8.4 | 5/9.1 | 0.6 | 11 (11.8) | 23 (6.8) | 0.1 | 1 (1.1) | 11 (3.3) | 0.3 | 4/4.9 | 4/4.9 | 0.7 |
| CIC (μgEq/ml) | 0.25/2.8 | 2.0/13.1 | <0.0001 | 9 (9.7) | 38 (11.2) | 0.7 | 9 (9.7) | 81 (23.9) | 0.003 | 0.2/0.8 | 0.2/3.1 | 0.001 |
| Levels in matched Sudanese patients, median/ mean | Levels in matched Swedish patientsa, median/ mean | P-value | Occurrence in matched Sudanese patientsa, 98th cut-off, n (%) | Occurrence in matched Swedish patientsa, 98th cut-off, n (%) | P-value | Occurrence in matched Sudanese patientsa, manufacturer’s cut-off, n (%) | Occurrence in matched Swedish patientsa, manufacturer’s cut-off, n (%) | P-value | ||||
| Anti-SSA/Ro52 (AU/ml) | 15/49.9 | 18/68.4 | 0.3 | 20 (26.7) | 31 (41.3) | 0.06 | 23 (30.7) | 27 (36.0) | 0.5 | |||
| Anti-SSA/Ro60 (AU/ml) | 1/46.6 | 22/65.0 | 0.02 | 33 (44.0) | 40 (53.3) | 0.2 | 27 (36.0) | 35 (46.7) | 0.2 | |||
| Anti-SSB/La (AU/ml) | 2/19.5 | 4/29.0 | 0.08 | 19 (25.3) | 19 (25.3) | 1 | 13 (17.3) | 19 (25.3) | 0.2 | |||
| Anti-Sm (AU/ml) | 1/24.4 | 2/17.1 | 0.001 | 26 (34.7) | 27 (36.0) | 0.9 | 10 (13.3) | 6 (8.0) | 0.3 | |||
| Anti-Sm/U1RNP (AU/ml) | 1/19.5 | 4/25.3 | 0.02 | 24 (32.0) | 39 (52.0) | 0.01 | 12 (16.0) | 16 (21.3) | 0.4 | |||
| Anti-U1RNP (AU/ml) | 6/40.3 | 9/62.0 | 0.03 | 18 (24.0) | 27 (36.0) | 0.1 | 16 (21.3) | 21 (28.0) | 0.3 | |||
| Anti-dsDNA (IU/ml) | 15/105.1 | 79.0/435.1 | <0.0001 | 28 (37.3) | 51 (68.0) | 0.0002 | 26 (34.7) | 41 (54.7) | 0.01 | |||
| Anti-ribosomal P (AU/ml) | 1/10.7 | 3/15.9 | <0.0001 | 14 (18.7) | 23 (30.7) | 0.09 | 4 (5.3) | 7 (9.3) | 0.3 | |||
| Anti-histone (AU/ml) | 5/15.2 | 10/35.9 | 0.001 | 6 (8.0) | 32 (42.7) | <0.0001 | 6 (8.0) | 14 (18.7) | 0.05 | |||
| Anti-PCNA (AU/ml) | 5/8.6 | 8/11.9 | 0.008 | 10 (13.3) | 9 (12.0) | 0.8 | 1 (1.3) | 2 (2.7) | 0.6 | |||
| CIC (µgEq/ml) | 0.5/2.6 | 12.2/24.1 | <0.0001 | 6 (8.0) | 16 (21.3) | 0.02 | 6 (8.0) | 38 (50.7) | <0.0001 |
The upper part shows a comparison between the full Sudanese (n = 93) and Swedish (n = 337) cohorts, whereas the lower part is the matched Sudanese (n = 75) and Swedish (n = 75) groups. The left columns show comparisons between levels, the middle columns show occurrence based on cut-off determined as the 98th percentile and common cut-offs recommended by the manufacturers. Comparisons between serum levels of Sudanese and Swedish controls are shown to the right. Control subjects with laboratory measurements are n = 106 for Sudanese and n = 318 for Swedes. Significant differences are shown in bold.
13 of 88 matched Sudanese patients did not deliver serum samples and their corresponding Swedish pairs were excluded in the matched study for the laboratory comparisons.
In the smaller nested case–control study, matched for age and disease duration, all autoantibody specificities except SSA/Ro52 and SSB were present at higher levels among Swedish patients; also CIC levels remained highest among Swedish patients (Table 2).
After exclusion of Swedish patients with non-European ancestry, there was no difference in the occurrence of anti-dsDNA between the cohorts using the manufacturer-defined cut-off, whereas more Swedes expressed higher anti-SSA/Ro60 and anti-SSB using the national cut-off (P < 0.0001 and P = 0.003, respectively). All other immunological comparisons yielded the same results.
Medication, disease activity and damage
AZA was the most commonly used immunosuppressive treatment by both Sudanese and Swedish SLE patients. When comparing the full cohorts, more Sudanese patients received AZA, HCQ and prednisolone as well as higher daily dosages of prednisolone than Swedes. The opposite was found for acetylsalicylic acid and warfarin. In the matched nested case–control study, these differences remained for AZA, HCQ and warfarin (Table 1). The use of CYC, MTX, ciclosporin and MMF treatments did not differ between Sudan and Sweden in both the full and matched groups.
Disease activity measured with a modified SLEDAI did not differ between the full cohorts, whereas Swedish patients showed marginally higher activity in the nested cohorts (P = 0.0505). Myositis and oral ulcers were more common in Sudanese compared with Swedish patients in the full cohorts. After correction for differences in age and disease duration, Swedish patients instead had an increased occurrence of alopecia and leucopenia (Table 3).
Table 3.
SLICC-DI, modified SLEDAI scores and occurrence of individual components at the time of inclusion
| Sudan, all patients (n = 115) | Sweden, all patients (n = 337) | P-value | Sudan, matched patients (n = 88) | Sweden, matched patients (n = 88) | P-value | |
|---|---|---|---|---|---|---|
| SLICC-DI score, median/mean | 1/0.8 | 1/1.4 | 0.001 | 1/0.9 | 0/0.6 | 0.04 |
| SLICC, ocular | 3 (2.6) | 41 (12.2) | 0.003 | 3 (3.4) | 4 (4.6) | 0.7 |
| SLICC, neuropsychiatric | 43 (37.4) | 72 (21.4) | 0.0007 | 31 (35.2) | 11 (12.5) | 0.0004 |
| SLICC, renal | 5 (4.4) | 30 (9.0) | 0.1 | 5 (5.7) | 2 (2.3) | 0.2 |
| SLICC, pulmonary | 4 (3.5) | 17 (5.0) | 0.5 | 3 (3.4) | 3 (3.4) | 1 |
| SLICC, cardiovascular | 7 (6.1) | 33 (9.8) | 0.2 | 5 (5.7) | 1 (1.1) | 0.09 |
| SLICC, peripheral vascular | 6 (5.2) | 29 (8.6) | 0.2 | 6 (6.8) | 5 (5.7) | 0.8 |
| SLICC, gastrointestinal | 1 (0.9) | 6 (1.8) | 0.5 | 1 (1.1) | 3 (3.4) | 0.3 |
| SLICC, musculoskeletal | 5 (4.3) | 54 (16.2) | 0.001 | 4 (4.6) | 7 (8.0) | 0.3 |
| SLICC, malignancy | 1 (0.9) | 9 (2.7) | 0.3 | 1 (1.1) | 0 (0) | 0.3 |
| SLICC, skin | 4 (3.5) | 60 (17.8) | 0.0001 | 4 (4.6) | 11(12.5) | 0.06 |
| SLICC, premature gonadal failure | 2 (1.7) | 27 (8.0) | 0.02 | 2 (2.3) | 2 (2.3) | 1 |
| SLICC, diabetes | 8 (7.0) | 3 (0.9) | 0.0003 | 7 (8.0) | 0 (0) | 0.007 |
| SLEDAI score, median/mean | 0/1.8 | 0/1.8 | 0.2 | 0/1.6 | 1/2.3 | 0.0505 |
| SLEDAI, seizures | 0 (0) | 1 (0.3) | 0.5 | 0 (0) | 0 (0) | NA |
| SLEDAI, psychosis | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| SLEDAI, organic brain syndrome | 1 (0.9) | 7 (2.1) | 0.4 | 0 (0) | 3 (3.4) | 0.08 |
| SLEDAI, visual disturbance | 1 (0.9) | 0 (0) | 0.09 | 1 (1.1) | 0 (0) | 0.3 |
| SLEDAI, cranial nerve disease | 0 (0) | 0 (0) | NA | 0 (0) | 0 (0) | NA |
| SLEDAI, lupus headache | 4 (3.5) | 12 (3.6) | 0.9 | 4 (4.6) | 5 (5.7) | 0.7 |
| SLEDAI, cerebrovascular accidents | 0 (0) | 1 (0.3) | 0.6 | 0 (0) | 0 (0) | NA |
| SLEADI, vasculitis | 1 (0.9) | 3 (0.9) | 1 | 0 (0) | 0 (0) | NA |
| SLEDAI, arthritis | 15 (13.0) | 44 (13.1) | 1 | 10 (11.4) | 15 (17.1) | 0.3 |
| SLEDAI, myositis | 4 (3.5) | 0 (0) | 0.0006 | 2 (2.3) | 0 (0) | 0.2 |
| SLEDAI, alopecia | 4 (3.5) | 36 (10.7) | 0.02 | 3 (3.4) | 10 (11.4) | 0.04 |
| SLEDAI, mucosal ulcers | 12 (10.4) | 12 (3.6) | 0.004 | 8 (9.1) | 4 (4.6) | 0.2 |
| SLEDAI, pleurisy | 2 (1.7) | 8 (2.4) | 0.7 | 1 (1.1) | 4 (4.6) | 0.2 |
| SLEDAI, pericarditis | 1 (0.9) | 2 (0.6) | 0.8 | 0 (0) | 1 (1.1) | 0.3 |
| SLEDAI, fever | 1 (0.9) | 16 (4.8) | 0.06 | 1 (1.1) | 6 (6.8) | 0.05 |
| SLEDAI, thrombocytopenia | 2 (1.7) | 8 (2.4) | 0.7 | 2 (2.3) | 2 (2.3) | 0.7 |
| SLEDAI, leucopenia | 6 (5.2) | 36 (10.7) | 0.08 | 5 (5.7) | 14 (15.9) | 0.03 |
Values presented as n (%) unless stated otherwise.
Sudanese and Swedish patients are compared concerning the full cohorts as well as the matched groups. Groups are compared with the Mann–Whitney U test for SLICC-DI and SLEDAI and with the chi-squared test for the occurrence of individual components. Significant differences are shown in bold.
NA: not applicable.
At the time of inclusion, Swedish patients had more organ damage when the full cohorts were compared (P = 0.001). In the nested cohort where the effect of the discrepant age and disease duration had been corrected for, Sudanese patients exhibited more damage measured with the SLICC-DI (P = 0.04; Table 3). This difference remained after excluding the Swedes with non-European ancestry (data not shown).
For the total SLICC-DI score, Sudanese patients were more often affected by neuropsychiatric damage and diabetes mellitus (P = 0.0007 and P = 0.0003, respectively) in the full cohort, and this difference remained in the nested case–control analysis (Table 3). When the neuropsychiatric damage item was divided into its components, cranial/peripheral neuropathy was more common in Sudanese patients in the full cohort (34/115 vs 12/337; P < 0.0001) as well as in the nested cohort (26/88 vs 1/88; P = 0.004). When adding cranial/peripheral neuropathy and diabetes as well as their interaction factor into a logistic regression model for the full cohorts, both associations remained (P = 0.001 and P = 0.01, respectively), but without interaction (P = 0.4). None of the Swedish patients in the matched cohort had diabetes, thus a similar regression could not be performed in this group. We found no statistical difference between the other neurological components (Table 4). Whereas the SLICC-DI score in the full cohort was higher among Sudanese patients only in the 20–29 years group, neuropsychiatric manifestations were also more common in the 30–39 years group and cranial/peripheral engagement likewise in the 40–49 and 50–59 years groups (Table 5).
Table 4.
Neuropsychiatric domains of the SLICC-DI in Sudanese and Swedish SLE patients
| Clinical manifestations | Sudan (n = 115) | Sweden (n = 337) | P-value | Sudan (n = 88) | Sweden (n = 88) | P-value |
|---|---|---|---|---|---|---|
| Cognitive impairment, n (%) | 6 (5.2) | 37 (11.0) | 0.07 | 2 (2.3) | 8 (9.1) | 0.1 |
| Seizures, n (%) | 2 (1.7) | 18 (5.3) | 0.1 | 2 (2.3) | 2 (2.3) | 1.0 |
| CVA, n | 3(2.6) | 22(6.5) | 0.3 | 3 (3.4) | 2 (2.3) | 0.6 |
| Cranial or peripheral neuropathy, n (%) | 34 (29.6) | 12 (3.6) | <0.0001 | 26 (29.6) | 1 (1.1) | <0.0001 |
| Transverse myelitis, n (%) | 0 (0.0) | 1 (0.3) | 0.5 | 0 (0.0) | 0 (0.0) | NA |
To the left the full cohorts are compared and to the right, the group matched for age at inclusion and disease duration. Significant differences are shown in bold.
CVA: cerebrovascular accident.
Table 5.
SLICC-DI, neuropsychiatric and cranial/peripheral neuropathy components in Sudanese and Swedish patients stratified for inclusion age
| Age group (years) | 10–19 | 20–29 | 30–39 | 40–49 | 50–59 | 60–69 |
|---|---|---|---|---|---|---|
| SLICC-DI | ||||||
| Sudan/Sweden, median | 0/0 | 1/0 | 0/0 | 1/1 | 1/2 | 0/2 |
| P-value | 0.5 | 0.004 | 0.9 | 0.07 | 0.03 | (0.0501) |
| Neuropsychiatric | ||||||
| Sudan/Sweden, % | 27.3/16.7 | 51.8/11.9 | 33.3/8.4 | 34.8/28.6 | 37.5/29.9 | 0/26.1 |
| P-value | 1.0 | 0.0003 | 0.0007 | 0.6 | 0.5 | 0.4 |
| Cranial or peripheral neuropathy | ||||||
| Sudan/Sweden, % | 18.1/0.0 | 40.7/2.4 | 27.8/0 | 21.7/1.8 | 37.5/6.9 | 0/6.5 |
| P-value | 0.5 | <0.0001 | <0.0001 | 0.007 | 0.0005 | 0.7 |
Groups are compared with the Mann–Whitney U test for SLICC-DI and with the chi-squared test for the occurrence of individual components. Significant differences are shown in bold.
Swedish patients suffered more ocular, musculoskeletal and skin manifestations and more premature ovarian failure in the full cohort; however, these differences disappeared in the nested case–control design (Table 3). The musculoskeletal engagement was more common in Swedes in the 40–49 and 50–59 years groups; age stratification yielded no significant differences concerning ocular and skin manifestations or premature ovarian failure (data not shown).
Clinical associations to individual autoantibodies
Comparing adult and late-onset SLE, we found a higher prevalence of anti-dsDNA (37.3% vs 7.7%; P = 0.04) among Sudanese adult-onset patients and a higher prevalence of anti-Sm/U1RNP and anti-U1RNP (41.5% vs 20.5%; P = 0.009 and 32.7% vs.13.6%; P = 0.01, respectively) in Swedes. Other autoantibodies did not show such an association.
Sudanese SLE patients with antibodies against Sm, Sm/U1RNP, U1RNP or SSA/Ro60 had significantly higher disease activity (P = 0.006, 0.006, 0.002 and 0.03, respectively) compared with seronegative patients. In contrast, among Swedish patients, disease activity was associated with the presence of antibodies against all ANAs tested [SSA/Ro52 (P = 0.04), SSA/Ro60 (P = 0.03), SSB (P = 0.02), Sm (P = 0.002), Sm/U1RNP (P = 0.003), U1RNP (P = 0.03), dsDNA (P = 0.01), ribosomal P (P < 0.0001), histone (P = 0.0009) and PCNA proteins (P = 0.04)] as well as CICs (P = 0.004).
Discussion
To our knowledge, this is the first study to consistently compare immunological and clinical aspects of well-defined Sudanese lupus cohort living in Africa and Swedish patients living in Europe.
The levels of autoantibodies differed between the two full cohorts. Swedish patients exhibited higher levels of anti-dsDNA, anti-ribosomal P and anti-Sm. Interestingly, there were also considerable differences in autoantibody levels between healthy controls from Sudan and Sweden, albeit in the low range, a finding that has to be taken into account when comparing autoantibody findings in different SLE populations. Mostly the Swedish controls had higher levels of autoantibodies as compared with the Sudanese controls, which is in contrast to previous studies reporting higher ANA levels in African American as compared with Caucasian healthy individuals [27, 28].
There is no consensus concerning whether reference intervals should be adjusted to different geographical regions [29]. Normal levels of biochemical analytes can differ between racial groups [30], and a number of investigators and previous guidelines have recommended national/regional adjustments [30–32]. In contrast to most previous studies, we have therefore used autoantibody cut-offs corresponding to the 98th percentile of national controls. Using such adjustments in Sudanese and Swedish controls, we found more significant differences between Sudanese and Swedish SLE patients, and clinical associations to the occurrence of autoantibodies reached a stronger significance than when using conventional common cut-offs recommended by the assay providers (data not shown). We believe that other autoimmune serology tests would benefit from such national adjustments when performed in African populations.
Disease activity, measured as a modified SLEDAI, where anti-dsDNA, complement and the urinary items have been excluded, showed minimal differences between Sudanese and Swedish patients. Although an issue of concern, no certain conclusion can be drawn about the occurrence of active LN in Sudan from the SLEDAI data available.
Although we found more damage among Swedish patients, this was associated with considerably older age and longer disease duration and was only present in the large non-adjusted cohorts. In the nested case–control study, matched for inclusion age and disease duration, Sudanese SLE patients showed higher SLICC-DI scores compared with Swedish patients. This finding is in agreement with most previous publications demonstrating more unfavourable SLE outcomes in black patients either living in Africa [1, 4, 33, 34] or patients of African ancestry elsewhere [3, 9, 35–38]. Higher SLICC-DI scores among Sudanese patients were associated with two factors: the neuropsychiatric domain and diabetes. Subanalysis revealed the neuropsychiatric difference to be totally attributed to more cranial/peripheral neuropathy in Sudanese patients, without an increase in any other neuronal component. Stratification for inclusion year also showed the higher SLICC-DI scores attributed to more neuropsychiatric manifestations to be especially prominent in the 20–29 year stratum. These findings have, to our knowledge, never been described before and might draw attention to the importance of neurological involvement in early SLE in Sudan. Furthermore, diabetes was more common among the Sudanese patients. A possible reason could be steroid treatment, since more Sudanese than Swedish patients were treated with higher prednisolone doses in the full cohort. Although the increased occurrence of diabetes in the Sudanese population is a plausible reason for neuropathy, both factors were independently associated with Sudanese origin. Although access to SLE treatment in Sudan and Sweden varies, the use of some drugs was comparable in the current cohorts. In Sudan, ciclosporin has been provided by the Sudanese Kidney Association, whereas CYC is subsided by the Sudanese government. MMF is often imported from Egypt and India.
The estimation of autoimmunity risk in Africa is complex and challenging, both because of diverse environmental influences and genetic admixtures and also due to the difficulties in tracing individuals until identification of a clinically overt disease. Since health resources and knowledge about demography are limited in many African countries, including Sudan, it is difficult to conduct epidemiological studies. It is more feasible to explore clinical characteristics and standardized laboratory measures in defined patient populations.
We found a higher percentage of females among Sudanese than among Swedish SLE patients. This is in agreement with what we previously have reported in a comparative Sudanese–Swedish RA study [39]. In South Africa and Nigeria, female SLE preponderance was very similar to what we observed in Sudan [4, 34]. Also, in the PROFILE multi-ethnic lupus cohort [40], the percentage of females was higher among African American as compared with Caucasian patients (91.7% and 86%, respectively). Collectively these studies imply that the female dominance may be higher in non-Caucasian SLE cohorts.
Earlier studies have mostly connected younger age at SLE onset to higher disease activity and nephropathy [41–44], higher prevalence of anti-dsDNA [43, 44] and Sm autoantibodies [24, 44], but less organ damage [43] and better survival [42]. However, in the current study, disease onset associated mainly with immunological variables in both cohorts and we were unable to draw conclusions regarding clinical phenotypes in early onset SLE.
Sudanese SLE patients were generally younger at inclusion. However, there is a considerable difference in life expectancy between Sudan and Sweden: 66.0 vs 84.0 years for females and 62.0 vs 80.3 years for males [45]. When we stratified for age at study inclusion, Sudanese patients demonstrated later SLE onset in younger as well as older age groups. Previous reports on age at onset have shown varying results. Budhoo et al. [34] reported no ethnic differences with regard to disease onset among South African SLE patients. African American patients are likely to be affected at a younger age compared with whites [41, 46], whereas Johnson et al. [38] found no difference in early and late-onset SLE between African Canadians and Caucasian Canadians. Our current finding of older age at disease onset after stratification thus differs from all the above studies, but coincides with what we have previously described concerning RA in Sudan compared with Sweden [39]. The majority of the Sudanese cohort is of Arabian ancestry, and George et al. [47] reported data on SLE onset and duration among Arab SLE patients from Israel, Lebanon, Kuwait, United Arab Emirates and Saudi Arabia that are comparable to our findings. Younger age and shorter duration of SLE in our cross-sectional Sudanese cohort could also be attributed to early mortality in Sudanese patients with severe disease. This might be due to the aggressive natural history of SLE and/or difficulty in accessing health care and treatment. Further longitudinal studies are needed to address such speculation.
Our objective was to compare incipient hospital-based SLE cohorts in Sudan and Sweden as accurately and commensurable as possible. Our findings of a higher prevalence of anti-Sm and a lower prevalence of anti-dsDNA, considerably shorter disease duration and more damage, especially concerning cranial/peripheral neuropathy and diabetes, will hopefully add to the general understanding of SLE in Sudan and demonstrate the importance of investigating this heterogeneous disease in larger cohorts and longitudinal designs from different parts of Africa.
Acknowledgement
The company Theradiag provided reagents for the analysis of specific ANA-associated autoantibodies.
Funding: This work was supported by the Swedish Rheumatism Association, King Gustav Vth 80-year Foundation, Agnes and Mac Rudberg Foundation, Signe and Reinhold Sund Foundation for Rheumatological Research, Ingegerd Johansson Foundation (SLS-713911), Swedish Research Council, Uppsala County Council (ALF), Stockholm County Council (ALF) and Swedish Heart–Lung Foundation.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Symmons DP. Frequency of lupus in people of African origin. Lupus 1995;4:176–8. [DOI] [PubMed] [Google Scholar]
- 2. Westlake SL, Edwards CJ.. Anti-malarials and lupus in West Africa use and lupus in Africans. Lupus 2009;18:193–5. [DOI] [PubMed] [Google Scholar]
- 3. Bae SC, Fraser P, Liang MH.. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the “prevalence gradient hypothesis”. Arthritis Rheum 1998;41:2091–9. [DOI] [PubMed] [Google Scholar]
- 4. Adelowo OO, Oguntona SA.. Pattern of systemic lupus erythematosus among Nigerians. Clin Rheumatol 2009;28:699–703. [DOI] [PubMed] [Google Scholar]
- 5. Dzifa D, Boima V, Yorke E. et al. Predictors and outcome of systemic lupus erythematosus (SLE) admission rates in a large teaching hospital in sub-Saharan Africa. Lupus 2018;27:336–42. [DOI] [PubMed] [Google Scholar]
- 6. Molokhia M, McKeigue PM, Cuadrado M, Hughes G.. Systemic lupus erythematosus in migrants from West Africa compared with Afro-Caribbean people in the UK. Lancet 2001;357:1414–5. [DOI] [PubMed] [Google Scholar]
- 7. Urowitz MB, Gladman DD, Ibanez D. et al. Evolution of disease burden over five years in a multicenter inception systemic lupus erythematosus cohort. Arthritis Care Res (Hoboken) 2012;64:132–7. [DOI] [PubMed] [Google Scholar]
- 8. Alarcon GS, Calvo-Alen J, McGwin G Jr. et al. Systemic lupus erythematosus in a multiethnic cohort: LUMINA XXXV. Predictive factors of high disease activity over time. Ann Rheum Dis 2006;65:1168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bruce IN, O’Keeffe AG, Farewell V. et al. Factors associated with damage accrual in patients with systemic lupus erythematosus: results from the Systemic Lupus International Collaborating Clinics (SLICC) Inception Cohort. Ann Rheum Dis 2015;74:1706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bruner BF, Guthridge JM, Lu R. et al. Comparison of autoantibody specificities between traditional and bead-based assays in a large, diverse collection of patients with systemic lupus erythematosus and family members. Arthritis Rheum 2012;64:3677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnett FC, Hamilton RG, Roebber MG, Harley JB, Reichlin M.. Increased frequencies of Sm and nRNP autoantibodies in American blacks compared to whites with systemic lupus erythematosus. J Rheumatol 1988;15:1773–6. [PubMed] [Google Scholar]
- 12. Jurencak R, Fritzler M, Tyrrell P. et al. Autoantibodies in pediatric systemic lupus erythematosus: ethnic grouping, cluster analysis, and clinical correlations. J Rheumatol 2009;36:416–21. [DOI] [PubMed] [Google Scholar]
- 13. Gulko PS, Reveille JD, Koopman WJ. et al. Survival impact of autoantibodies in systemic lupus erythematosus. J Rheumatol 1994;21:224–8. [PubMed] [Google Scholar]
- 14. Tikly M, Burgin S, Mohanlal P, Bellingan A, George J.. Autoantibodies in black South Africans with systemic lupus erythematosus: spectrum and clinical associations. Clin Rheumatol 1996;15:143–7. [DOI] [PubMed] [Google Scholar]
- 15. Faria AC, Barcellos KS, Andrade LE.. Longitudinal fluctuation of antibodies to extractable nuclear antigens in systemic lupus erythematosus. J Rheumatol 2005;32:1267–72. [PubMed] [Google Scholar]
- 16. Ahmed N, Shigidi M, Al Agib AN, Abdelrahman H, Taha E.. Clinical features and antinuclear antibodies profile among adults with systemic lupus erythematosus and lupus nephritis: a cross-sectional study. Pan Afr Med J 2017;27:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eltom HMH, Ali TH, A N.. Clinical implications of the presence of anti-Ro antibodies in systemic lupus erythematosus in Sudan. Sudan JMS 2015;10:93–8. [Google Scholar]
- 18. Kaballo BG, Ahmed AE, Nur MM, Khalid IO, Abu-Aisha H.. Mycophenolate mofetil versus azathioprine for maintenance treatment of lupus nephritis. Saudi J Kidney Dis Transpl 2016;27:717–25. [DOI] [PubMed] [Google Scholar]
- 19. Kaballo BG AWA, Nur MM, Modawi MA. et al. Ethnic distribution and clinical features of systemic lupus erythematosus in the Sudan. Sudan Med J 2009;45:49–57. [Google Scholar]
- 20. Bombardier C, Gladman DD, Urowitz MB. et al. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum 1992;35:630–40. [DOI] [PubMed] [Google Scholar]
- 21. Gladman D, Ginzler E, Goldsmith C. et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996;39:363–9. [DOI] [PubMed] [Google Scholar]
- 22. Tan EM, Cohen AS, Fries JF. et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- 23. Uribe AG, Vila LM, McGwin G Jr. et al. The Systemic Lupus Activity Measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol 2004;31:1934–40. [PubMed] [Google Scholar]
- 24. Feng X, Zou Y, Pan W. et al. Associations of clinical features and prognosis with age at disease onset in patients with systemic lupus erythematosus. Lupus 2014;23:327–34. [DOI] [PubMed] [Google Scholar]
- 25. Agmon-Levin N, Damoiseaux J, Kallenberg C. et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann Rheum Dis 2014;73:17–23. [DOI] [PubMed] [Google Scholar]
- 26. Hair JF., Black WC, Babin BJ, Anderson RE.. Multivariate data analysis. 7th edn Upper Saddle River, NJ: Prentice Hall, 2010. [Google Scholar]
- 27. Satoh M, Chan EK, Ho LA. et al. Prevalence and sociodemographic correlates of antinuclear antibodies in the United States. Arthritis Rheum 2012;64:2319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wandstrat AE, Carr-Johnson F, Branch V. et al. Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun 2006;27:153–60. [DOI] [PubMed] [Google Scholar]
- 29. Siest G, Henny J, Grasbeck R. et al. The theory of reference values: an unfinished symphony. Clin Chem Lab Med 2013;51:47–64. [DOI] [PubMed] [Google Scholar]
- 30. Malati T. Whether western normative laboratory values used for clinical diagnosis are applicable to Indian population? An overview on reference interval. Indian J Clin Biochem 2009;24:111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horn PS, Pesce AJ.. Effect of ethnicity on reference intervals. Clin Chem 2002;48:1802–4. [PubMed] [Google Scholar]
- 32. Kost GJ, Hale KN.. Global trends in critical values practices and their harmonization. Clin Chem Lab Med 2011;49:167–76. [DOI] [PubMed] [Google Scholar]
- 33. Khanfir MS, Houman MH, Cherif E. et al. TULUP (TUnisian LUPus): a multicentric study of systemic lupus erythematosus in Tunisia. Int J Rheum Dis 2013;16:539–46. [DOI] [PubMed] [Google Scholar]
- 34. Budhoo A, Mody GM, Dubula T, Patel N, Mody PG.. Comparison of ethnicity, gender, age of onset and outcome in South Africans with systemic lupus erythematosus. Lupus 2017;26:438–46. [DOI] [PubMed] [Google Scholar]
- 35. Hopkinson ND, Jenkinson C, Muir KR, Doherty M, Powell RJ.. Racial group, socioeconomic status, and the development of persistent proteinuria in systemic lupus erythematosus. Ann Rheum Dis 2000;59:116–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pons-Estel BA, Catoggio LJ, Cardiel MH. et al. The GLADEL multinational Latin American prospective inception cohort of 1, 214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004;83:1–17. [DOI] [PubMed] [Google Scholar]
- 37. Alarcon GS, McGwin G Jr., Petri M. et al. Time to renal disease and end-stage renal disease in PROFILE: a multiethnic lupus cohort. PLoS Med 2006;3:e396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Johnson SR, Urowitz MB, Ibanez D, Gladman DD.. Ethnic variation in disease patterns and health outcomes in systemic lupus erythematosus. J Rheumatol 2006;33:1990–5. [PubMed] [Google Scholar]
- 39. Elshafie AI, Elkhalifa AD, Elbagir S. et al. Active rheumatoid arthritis in Central Africa: a comparative study between Sudan and Sweden. J Rheumatol 2016;43:1777–86. [DOI] [PubMed] [Google Scholar]
- 40. Alarcon GS, McGwin G Jr, Petri M. et al. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus 2002;11:95–101. [DOI] [PubMed] [Google Scholar]
- 41. Tucker LB, Uribe AG, Fernandez M. et al. Adolescent onset of lupus results in more aggressive disease and worse outcomes: results of a nested matched case-control study within LUMINA, a multiethnic US cohort (LUMINA LVII ). Lupus 2008;17:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Merola JF, Bermas B, Lu B. et al. Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus 2014;23:778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Aljohani R, Gladman DD, Su J, Urowitz MB.. Disease evolution in late-onset and early-onset systemic lupus erythematosus. Lupus 2017;26:1190–1196. [DOI] [PubMed] [Google Scholar]
- 44. Webb R, Kelly JA, Somers EC. et al. Early disease onset is predicted by a higher genetic risk for lupus and is associated with a more severe phenotype in lupus patients. Ann Rheum Dis 2011;70:151–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Countries. Sudan. Statistics. http://www.who.int/countries/sdn/en/ (28 September 2017, date last accessed).
- 46. Reveille JD, Moulds JM, Ahn C. et al. Systemic lupus erythematosus in three ethnic groups: I. The effects of HLA class II, C4, and CR1 alleles, socioeconomic factors, and ethnicity at disease onset. LUMINA Study Group. Lupus in minority populations, nature versus nurture. Arthritis Rheum 1998;41:1161–72. [DOI] [PubMed] [Google Scholar]
- 47. Habib GS, Saliba WR.. Systemic lupus erythematosus among Arabs. Isr Med Assoc J 2002;4:690–3. [PubMed] [Google Scholar]