Abstract
Minimally invasive techniques continue to transform the field of colorectal surgery. Because traditional surgical approaches for rectal cancer are associated with significant mortality and morbidity, developing less invasive approaches to this disease is paramount. Natural orifice transluminal endoscopic surgery (NOTES), commonly known as “no incision surgery,” represents the ultimate minimally invasive approach to disease. Although transgastric and transvaginal approaches for NOTES surgery were the initially explored, a transrectal approach for colorectal disease is intuitive given that it makes use of the resected organ for transluminal access. Furthermore, the transanal approach allows for improved, precise visualization of the presacral mesorectal plane compared with an abdominal viewpoint, particularly in the narrow, male pelvis. Finally, experience with existing transanal platforms that have been used for decades for local excision of rectal disease made the development of a transanal approach to total mesorectal excision (TME) feasible. Here, we will review the evolution of minimally invasive and transanal surgical techniques that allowed for the development of transanal TME and its introduction into clinical practice.
Keywords: transanal total mesorectal excision, rectal cancer, natural orifice transluminal endoscopic surgery
The significant morbidity of open radical resection for rectal cancer has driven the evolution of novel minimally invasive approaches to this disease. This evolution has occurred in the context of growing interest in natural orifice transluminal endoscopic surgery (NOTES). Since the first report of a transgastric appendectomy by Reddy and Rao in 2004, the concept of using internal incisions to approach the peritoneal and retroperitoneal spaces has garnered considerable interest given its theoretic advantages including faster recovery, decreased postoperative pain, decreased surgical site infections, and decreased incisional hernias. 1
A transanal NOTES approach in rectal surgery is particularly appealing for several reasons. First, there is already significant experience in the colorectal field with the use of rigid transanal operating platforms for local excision of rectal lesions, including the transanal endoscopic microsurgery (TEM; Richard Wolf Company, Tubingen, Germany) and transanal endoscopic operation (TEO; Karl Storz, Tuttlingen, Germany) devices. 2 3 These rigid platforms allow for a stable operating platform with minimal distance to access the peritoneal cavity when compared with a transgastric approach. Second, the incision used for endoluminal access is resected as part of the specimen and incorporated into the surgical anastomosis, obviating the need for secure closure of an additional peritoneal access site. When compared with a transvaginal approach, the transanal approach is possible in all subjects and not limited to female patients. Finally, a transanal, “down-to-up” dissection with use of CO 2 insufflation improves visualization of the presacral dissection plane during total mesorectal excision (TME), particularly in the narrow male pelvis.
This article will outline the evolution of minimally invasive techniques in colorectal surgery that allowed for the development of transanal TME (taTME), review insights into the technique gained during preclinical trials in porcine and cadaver model, and the transition into human trials of hybrid laparoscopic-assisted taTME as well as pure NOTES taTME.
Origins of Total Mesorectal Excision
Heald et al originally described the technique of TME in 1982, referring to en bloc removal of the rectum and mesorectum along the mesorectal fascia. 4 Since that time, TME remains the gold standard for surgical management of rectal cancer. 5 Along with innovations in neoadjuvant chemoradiation, wide adoption of the TME approach since that time has dramatically reduced local recurrence rates in resectable rectal cancer, regardless of whether a sphincter-preserving low anterior resection (LAR) or abdominoperineal resection (APR) is used. 6 However, operations for rectal cancer are associated with significant postoperative mortality and morbidity. TME-related mortality ranges from 2 to 4%, while morbidity ranges from 35 to 40%. 7 8 Short-term morbidities include high rates of anastomotic leak (0–21%) and wound complications (0–47%). 9 10 11 Additionally, conventional TME is associated with high rates of long-term functional morbidities, with high rates of fecal dysfunction (0.5–37%), urinary dysfunction (5–14%), and sexual dysfunction (33–36%). 9 12 High morbidity rates and their negative impact on quality of life measures have driven the development of novel minimally invasive operative techniques for rectal cancer.
Laparoscopic and Robotic Approaches to TME
The morbidity of TME has largely been unaffected by the use of minimally invasive laparoscopic or robotic abdominal approaches. 13 Laparoscopic approaches have been shown to decrease postoperative pain, shorten the time to return of bowel function, and shorten hospital length of stay, but have had minimal impact on postoperative complications and functional outcomes. 9 12 14 The steep learning curve involved has been a significant barrier to the uptake of laparoscopic TME. Additionally, there are still lingering concerns about the oncologic safety of a laparoscopic approach, with the National Comprehensive Cancer Network guidelines still recommending its use only by experienced laparoscopic surgeons for low-risk tumors. 15 Findings of multiple large randomized control trials, including the CLASICC and COLOR II trials, demonstrated equivalent long-term oncologic outcomes between open and laparoscopic approaches. 14 16 However, more recently, two large randomized trials have failed to demonstrate noninferiority of laparoscopic TME with regards to oncologic clearance. The ACOSOG Z6051 trial compared adequacy of the circumference radial margin and distal resection margin as well as completeness of TME for laparoscopic versus open TME for patients with stage II or III rectal cancer, and found that these oncologic measures were inferior for the laparoscopic approach, without differences in length of stay, readmission, or severe complications. 17 The ALaCaRT randomized trial found similar results in patients with T1 to T3 tumors, as demonstrated in Table 1 . 18
Table 1. Comparative findings of studies of laparoscopic versus open TME.
| COLOR II 14 | ACOSOG 17 | AlaCaRT 18 | ||||
|---|---|---|---|---|---|---|
| Study period | January 2004 to May 2010 | October 2008 to September 2013 | March 2010 to November 2014 | |||
| Study location | Europe | United States | Australia/New Zealand | |||
| Total number of patients | 1103 | 486 | 475 | |||
| Laparoscopic | Open | Laparoscopic | Open | Laparoscopic | Open | |
| Patient and tumor characteristics, n | 699 | 345 | 242 | 239 | 238 | 235 |
| Male gender, n (%) | 448 (64) | 211 (61) | 156 (65) | 158 (66) | 160 (67) | 151 (64) |
| Age, mean ± SD, y | 66.8 ± 10.5 | 65.8 ± 10.9 | 57.7 ± 11.5 | 57.2 ± 12.1 | 65 (56–74) a | 65 (56–73) a |
| Body mass index, mean ± SD, kg/m 2 | 26.1 ± 4.5 | 26.5 ± 4.7 | 26.4 ± 4.0 | 26.8 ± 4.2 | 27 (24–30) a | 26 (24–30) a |
| Tumor location, n (%) | ||||||
| Lower rectum (< 5 cm from AV) | 203 (29) | 93 (27) | 124 (51) | 116 (49) | 82 (35) | 83 (35) |
| Middle rectum (5–10 cm from AV) | 273 (39) | 136 (39) | 85 (35) | 95 (40) | 103 (43) | 102 (44) |
| Upper rectum (10–15 cm from AV) | 223 (32) | 116 (34) | 33 (14) | 28 (12) | 53 (22) | 50 (21) |
| Preoperative stage, n (%) | ||||||
| I | 201 (29) | 96 (28) | 2 (1) | 3 (1) | – | – |
| II | 209 (30) | 107 (31) | 99 (41) | 92 (39) | ||
| III | 257 (37) | 126 (37) | 141 (58) | 144 (60) | ||
| Preoperative radiotherapy, n (%) | 412 (59) | 199 (58) | 235 (97) | 230 (96) | 119 (50) | 116 (49) |
| Preoperative chemotherapy | 196 (28) | 99 (29) | 231 (95) | 225 (94) | ||
| Clinical outcomes, n | 699 | 345 | 240 | 222 | ||
| Total operative time, mean ± SD, min | 200 (100–400) a | 400 (200–700) a | 266.2 ± 101.9 | 220.6 ± 92.4 | 210 (163–253) a | 190 (160–240) a |
| Conversions, n (%) | 121 (17) | – | 26 (11) | – | 21 (9) | – |
| Overall morbidity at 28 d, n (%) | 278 (40) | 128 (37) | 137 (57) | 129 (58) | – | – |
| Overall mortality at 28 d, n (%) | 8 (1) | 6 (2) | 2 (1) | 2 (1) | 1 (< 1) | 2 (1) |
| Anastomotic leak, % | 58 (8) | 25 (7) | 5 (2) | 5 (2) | 7 (3) | 8 (3) |
| Unplanned reoperation, % | 113 (16) b | 52 (15) b | 12 (5) | 5 (2) | – | – |
| Length of hospital stay, median (IQR), d | 8 (6–13) a | 9 (7–14) a | 7.3 ± 5.4 | 7.0 ± 3.4 | 8 (6–12) a | 8 (6–12) a |
| Pathological outcomes, n | 699 | 345 | 240 | 222 | ||
| Positive CRM, n (%) | 56 (8) | 30 (9) | 29 (12) | 17 (8) | 16 (7) | 7 (3) |
| Positive DRM, n (%) | – | – | 6 (3) | 4 (2) | 2 (1) | 1 (1) |
| TME quality, n (%) | ||||||
| Complete | 589 (84) | 303 (88) | 175 (73) | 181 (82) | 206 (87) | 216 (92) |
| Nearly/partially complete | 58 (8) | 19 (6) | 46 (19) | 30 (14) | 24 (10) | 17 (7) |
| Incomplete | 19 (3) | 9(3) | 19 (8) | 11 (5) | 8 (3) | 2 (1) |
| Number of lymph nodes, mean ± SD | 13 (10–18) a | 14 (10–19) a | 17.9 ± 10.1 | 16.5 ± 8.4 | – | – |
Abbreviations: AV, anal verge; CRM, circumferential resection margin; DRM, distal resection margin; IQR, interquartile range; SD, standard deviation; TME, total mesorectal excision.
Reported as median and interquartile range (IQR).
Reported reintervention.
Furthermore, these large early trials highlighted persistently high rates of conversion to an open approach (9–30%). 9 12 17 18 Alarmingly, several trials have demonstrated significantly worse overall survival in patients who were converted from a laparoscopic to an open approach (open 58.5%, laparoscopic 62.4%, and converted 49.6%, p = 0.005). 19 20 In trials with very experienced laparoscopic operators only (greater than 200 cases experience), conversion rates were lower, at 1.2%. 9
Ultimately, there has been limited adoption of the laparoscopic approach to TME, particularly when compared with growing preference of laparoscopy for colon cancer. Although use of laparoscopy for rectal cancer has been increasing recently, less than half of all rectal cancer operations in the United States are performed laparoscopically. 21
Robotic TME has the technical advantages of articulated instruments and three-dimensional visualization as well as the ability to facilitate training in minimally invasive approaches. Overall, robotic TME has shown lower conversion rates compared with laparoscopic TME (odds ratio 0.26, 95% confidence interval 0.12–0.57). 22 However, a randomized trial comparing robotic and laparoscopic techniques for rectal cancer demonstrated no differences with regards to conversion rates to open surgery, margin positivity, intraoperative or postoperative complications, or mortality. 23 Robotic TME is still associated with higher overall costs despite similar outcomes in terms of morbidity and hospital length of stay to laparoscopic TME. 24
Even in the hands of experts, there are significant technical and anatomic limitations to transabdominal minimally invasive approaches to TME, which likely contributes to ongoing high rates of conversion and positive margins. Visualization, exposure, and dissection deep in the pelvis are challenging, particularly in the narrow male pelvis or the obese patient. Transection of the distal rectum also poses a particular difficulty. Finally, regardless of whether a laparoscopic or robotic approach is utilized, a minilaparotomy is still required for specimen removal with these techniques. A transanal approach with transanal removal of the specimen could help overcome some of the shortcomings of laparoscopic and robotic TME.
Transanal Endoscopic Surgery: TEM, TEO, and Transanal Minimally Invasive Surgery
Concurrent with the development of minimally invasive approaches to TME, colorectal surgeons have been exploring less morbid operations for rectal cancer resection in the form of local excision. In particular, transanal endoscopic surgery (TES) with local excision has been gaining popularity as an approach to early rectal cancer. The original TEM (Richard Wolf Company) platform was developed by Gerald Buess in 1982 as an endoscopic approach for local excision of low and mid-rectal lesions. 2 Although transanal excision under direct visualization had already been employed for distal rectal lesions, this approach represented a significant technical advancement, allowing for improved visualization and exposure with the ability to remove lesions in the proximal rectum. The original platform has undergone some modifications since its initial development, but still consists of a rigid and reusable 4-cm proctoscope available in two lengths. The proctoscope has an external, multiport faceplate allowing for the simultaneous use of a magnifying stereoscope and adapted dissection instruments as well as a port to provide CO 2 insufflation. Electrocautery, bipolar energy devices, and laparoscopic suturing instruments can all be used through the platform. The device is anchored to the operating table using a locking arm, which provides a stable platform for dissection and visualization.
Furthermore, the original TEM platform has been adapted for use with conventional laparoscopic instruments and a two-dimensional laparoscopic camera through the use of the TEO (Karl Storz) platform. 3 Given the high costs for TEM and TEO setups, as well as the long learning curve required for technical expertise, an alternative transanal approach using a single-incision laparoscopic disposable port was proposed in 2009, now termed transanal minimally invasive surgery. 25 26 This has allowed for more widespread adoption of TES techniques, with several different ports available currently for use (SILS Port, Covidien, Mansfield, MA; GelPOINT Path, Applied Medical, Rancho Santa Margarita, CA). Proximal rectal wall retraction and exposure is limited with these devices, particularly beyond the 2nd or 3rd Haustral valves, given their shorter length relative to the 7.5 to 20 cm rigid platforms. 27
TES platforms are currently used for resection of large rectal adenomas that cannot be removed via conventional colonoscopy or after incomplete or piecemeal resection. 28 TES has also been used in the resection of other tumors such as gastrointestinal stromal tumors, carcinoids, and presacral tumors. Finally, it has been used in benign disease including stricturoplasty, repair of complex rectourethral and rectovaginal fistulas, and repair of colorectal anastomotic complications. 25 29 30 31 32
For rectal cancer, TES has the major advantage of decreased morbidity and mortality when compared with radical excision, with major complications noted in less than 10% of patients. 33 34 35 This low morbidity is reflected in short hospital length of stay and minimal postoperative pain requirements. 36 No adverse long-term issues with continence or anorectal function have been demonstrated due to the transanal placement of the ports in TES. 37 38 39
Despite these advantages and increasing use of TES techniques for local excision of rectal cancer, its use as a curative approach is limited. Rates of local recurrence are prohibitively high except in extremely selective tumors, with early studies of unselected T1 tumors reporting rates of local recurrence as high as 26%. 40 This is likely because even in T1 tumors, the risk of occult lymph node metastasis can be high, particularly when certain histolopathologic features are present. 41 T1 tumors that are large (> 4 cm), poorly differentiated, demonstrate lymphovascular invasion or tumor budding, or involve greater than 30% of the bowel wall circumference are at high risk of local recurrence after local excision and a TES approach should not be used. 42 43 44 45 46 For T2 or more advanced lesions, a TME approach with or without neoadjuvant treatment, remains the standard of care.
The increasing use of transanal local excision has led to the development of now well-established and accessible platforms that allow for transanal access to the peritoneal cavity. This advance allowed for the leveraging of existing technology to develop a taTME approach.
Experience Gained with Peritoneal Entry and Closure during TES
In addition to increasing familiarity and experience with the general use of the TES platforms for transanal dissection, experience with local excision allowed for knowledge to be gained about the safety of transanal entry into the peritoneal cavity. Peritoneal entry is not an uncommon occurrence during TES and is no longer considered a complication of local excision procedures. Overall, the reported rate of peritoneal entry during full-thickness excision of rectal lesions ranges from 0 to 32.3%, 47 48 although the rate of complicated peritoneal entry has decreased with increasing experience with the technique to 5 to 10.7%. 49 50 Entry into the peritoneal cavity can be associated with critical loss of insufflation and difficulty maintaining adequate pneumorectum and exposure. For this reason, complicated peritoneal entry was initially associated with high rates of conversion to an open procedure in TEM cases. With additional experience, conversion rates have fallen, with rates ranging from 0 to 40% but averaging 10% or less. 51 This reflects increasing comfort with closing rectal defects endoscopically and managing the technical challenges in maintaining adequate pneumorectum. Strategies to maintain pneumorectum include complete muscle paralysis, increasing the pressure of CO 2 insufflation, and decompressing the pneumoperitoneum with a Veress needle or trocar. 25
Furthermore, there has been no additional morbidity associated with peritoneal entry during TEM cases. 48 51 52 53 54 In particular, there is no increased risk of pelvic sepsis or abscess. Additionally, to date there is no clear evidence of peritoneal seeding or increased risk of peritoneal metastatic recurrence associated with peritoneal entry during TEM. 51 These findings established the theoretical safety of peritoneal entry associated with taTME.
Natural Orifice Specimen Extraction
As previously mentioned, both laparoscopic and robotic minimally invasive approaches require a larger abdominal incision for extraction of the specimen. This requirement limits the potential for these operations to reduce wound-related complications including postoperative pain and wound infection rates as compared with an open procedure. For this reason, natural orifice specimen extraction (NOSE) has been explored as a means to eliminate the need for minilaparotomy to remove the resected specimen. Initially transvaginal and transanal approaches were performed for benign conditions, 55 56 57 58 59 but ultimately both approaches were attempted in colorectal cancer. 60 61 62 63
The initial trial of laparoscopic colectomy with transanal NOSE extraction was by Cheung et al in 2009. 60 They reported a pilot series of 10 patients with rectosigmoid or left-sided colon cancer who underwent laparoscopic resection with transanal extraction of the specimen using the TEO device. They excluded patients with tumors greater than 4 cm in size due to concerns that these large tumors could not be safely extracted via the TEO device, as well as patients with mid- or low rectal tumors. After typical laparoscopic dissection of the left colon and upper rectum, and division of the inferior mesenteric vessels, a point in the upper rectum at least 5 cm distal to the tumor was chosen as a site of distal division. The mesorectum at this level was dissected away from the bowel to create a “nude” rectal tube, and the bowel was occluded with an atraumatic laparoscopic bowel forceps so that distal rectal lavage could be performed transanally. Subsequently, the distal bowel was transected with a laparoscopic stapler. The TEO device was inserted from below, and the rectal stump was opened to allow for specimen extraction via the TEO device and subsequent stapled anastomosis using a circular stapling device. The authors reported a median operative time of 127.5 minutes, minimal blood loss, length of stay of 7 days (4–18 days), and no notable morbidity. 60
Such NOSE techniques during colorectal cancer resections have generally demonstrated decreased pain and wound complication rates, without an increase in operative time when compared with conventional laparoscopy. Park et al compared results of laparoscopic right colectomy with transabdominal versus transvaginal extraction of the specimen and found decreased pain and length of stay in the transvaginal group. 61 Leung et al randomized patients with left-sided colon cancer to undergo laparoscopic colectomy with specimen extraction either via minilaparotomy ( n = 35) or transanal ( n = 35) extraction using the TEO platform. 62 There were no differences in operating time ( p = 0.851), blood loss ( p = 0.954), or length of stay ( p = 0.990). Patients who underwent surgery with transanal extraction ( n = 35) had significantly lower pain scores and, importantly, lower rates of wound infection (4 vs. 0, p = 0.005).
The largest series of NOSE for colorectal cancer was performed by Franklin et al, who routinely incorporated transanal specimen extraction during LAR. 63 In a prospective series of 179 patients, they describe completing the TME laparoscopically followed by transanal specimen extraction using a sterilized bag and ring forceps. They reported low rates of complications overall (5.0%) with a 1.7% rate of anastomotic leak and 2.0% rate of rectal stenosis.
Fuchs et al advanced the concept of NOSE for colectomy even further, using the rectum not only as a means of specimen extraction but also as an access port for introduction of laparoscopic devices, such as the linear stapler. 59 Because all tasks requiring a port diameter greater than 5 mm were performed transanally, only a periumbilical port site for the laparoscopic camera and two 5-mm instrument port sites were needed. They studied 15 patients with benign colorectal disease and only one patient required conversion to full laparoscopy. They found low rates of complication ( n = 1, 6.7%) and improvement from baseline quality of life measures postoperatively.
Animal and Cadaver Trials
The safety and feasibility of taTME was demonstrated first in swine survival models and subsequently in fresh human cadaver trials. 64 65 66 These experiments allowed for optimization in the surgical technique prior to embarking in human trials, in particular allowing for modification to the technique to improve mobilization of the colon and achieve adequate specimen length.
Whiteford et al described the first NOTES transrectal sigmoid resection in three human cadavers in 2007 as a proof of concept, utilizing the TEM platform as the access port, and TEM instrumentation to perform full-thickness transection of the rectal wall followed by en bloc rectal and mesorectal mobilization extending to the sigmoid colon. They subsequently repeated this technique in a larger cohort of porcine and human cadaver models. 67 68
Sylla et al described the first experimental evaluation of transanal NOTES rectosigmoid resection in a nonsurvival swine model using the TEO platform ( Fig. 1 ). 64 The distal rectum was occluded with a purse-string suture to prevent fecal contamination, and subsequently a full-thickness incision of the rectal wall was made. En bloc resection of the rectum and its mesorectal envelope was then accomplished endoscopically through the platform, using conventional TEO and laparoscopic instrumentation. The peritoneal cavity was entered at the peritoneal reflection and the sigmoid colon was dissected until anatomic constraints prevented further mobilization. The colon was then exteriorized via the anus, transected, and a stapled colorectal anastomosis was performed.
Fig. 1.
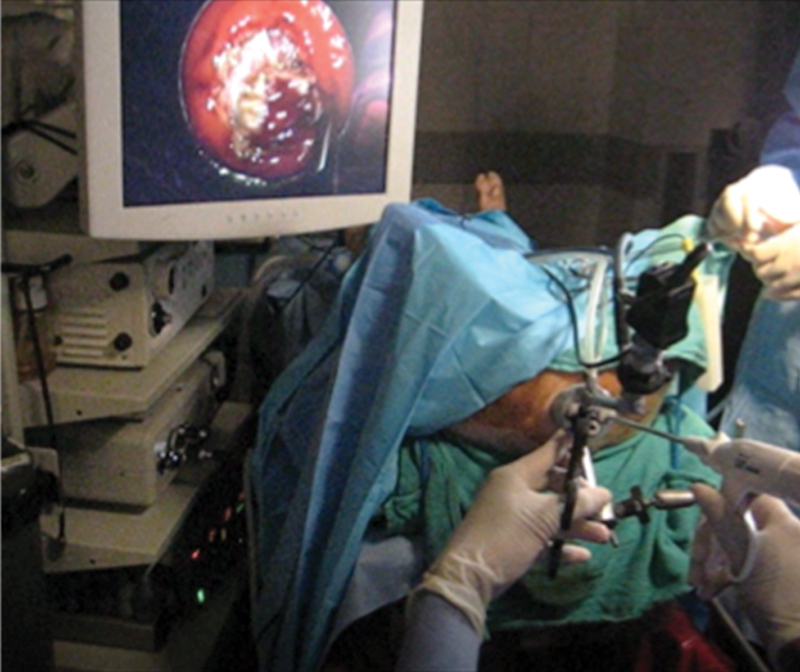
Operative setup for transanal access in a swine model using transanal endoscopic operation (TEO) instrumentation.
This model allowed for identification of several limitations in the initial taTME approach in the swine model. The sharp angle of the sacral promontory made proximal dissection difficult using the rigid platform. Based on this anatomic limitation, a combined transgastric and transanal approach was used. This dual transgastric and transanal approach increased operative time but improved retraction and mobilization of the proximal colon resulting in an average gain of 5.8 cm in colon length relative to transanal dissection alone ( Fig. 2 ). 64 Additionally, the technique of the coloanal anastomosis was optimized. In the initial nonsurvival model, the stapled anastomosis had a posterior defect in 2 out of 9 (22%) animals. 64 Placement of the purse-string suture under direct vision with retractors as opposed to undervisualization with the proctoscope, improved outcomes in subsequent trials. All resected specimens in this initial study demonstrated an intact mesorectal envelope.
Fig. 2.
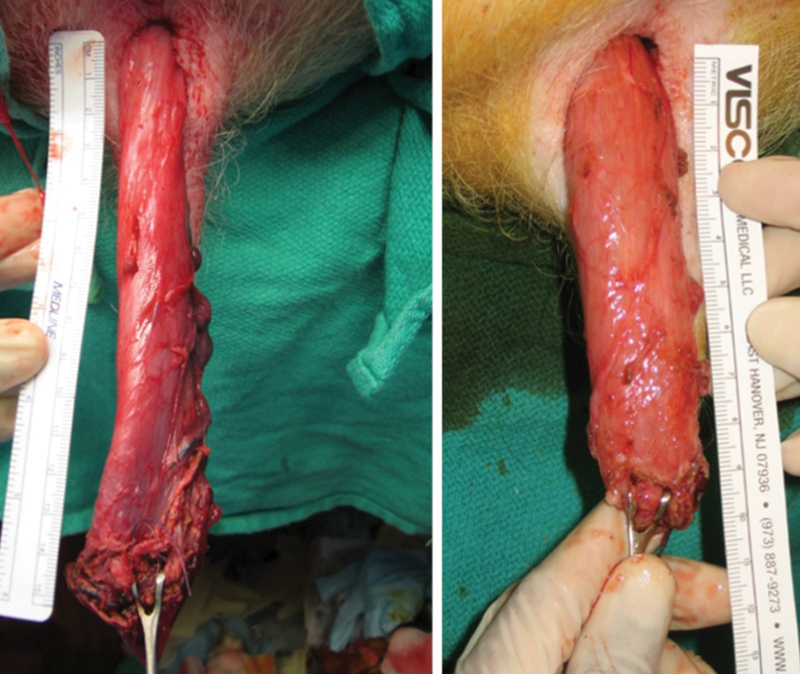
Comparison of specimen length for pure transanal approach (right) versus combined transanal and transgastric natural orifice transluminal endoscopic surgery (NOTES) approach (left) in a swine model.
Subsequently, Sylla et al proceeded with demonstrating the safety of this approach in a survival porcine model. 65 A total of 20 animals underwent either a purely transanal resection ( n = 10) or a transanal resection with transgastric assistance ( n = 10) in a 2-week survival study. Again, transgastric assistance increased procedure time but led to a significant increase in the length of the specimen, and all specimens were grossly intact. There were no mortalities and no intraoperative complications. Morbidity was also low (10%), including one intra-abdominal abscess and one abdominal wall hematoma (related to a misfire of the gastrostomy closure device). Both complications occurred in animals undergoing a combined transanal and transgastric approach.
A randomized control trial in a survival swine model, again performed by Sylla et al (unpublished data), compared transanal rectosigmoid resection using the TEM platform ( n = 15) to a transabdominal laparoscopic approach ( n = 15). 69 The operative time was shorter in the laparoscopic group (57 vs. 83 minutes, p = 0.006). However, there was no difference in overall specimen length ( p = 0.268) and all stapled anastomoses were intact. The transanal group required fewer narcotics ( p < 0.001), passed stool sooner (2 vs. 3.8 days, p = 0.004), and were less likely to develop obstructive symptoms (2 vs. 10 animals, p = 0.003). The transanal approach was also able to achieve a surgical margin closer to the anal verge, with the staple line located on average at 3.3 cm in the transanal group as compared with 14.6 cm in the laparoscopic group ( p < 0.001). One of the transanal animals did develop renal failure secondary to distal urethral obstruction resulting in death.
The same group and others subsequently returned to fresh human cadaveric models to further optimize transanal NOTES rectosigmoid resection and demonstrate both technical and theoretical oncologic feasibility prior to entering human trials. 70 71 72 73 These studies highlighted the anatomic and instrumentation constraints with transanal NOTES access to the peritoneal cavity, including the acute angle created by the sacral promontory (particularly in larger and obese subjects), limited reach of traditional TEM instrumentation, and need for extensive prior laparoscopic and TEM experience to achieve the technical challenges of the operation. Fajardo et al reported the use of the TEM platform to perform a pure transanal LAR in a cadaver model in 2010. 70 Following transanal entry into the peritoneal cavity, a hand port was transanally placed with transanal placement of two 5-mm working ports and a 10-mm camera transanally. Difficulty in maneuvering around the sacral promontory was overcome with the use of articulating instruments and a flexible-tipped laparoscopic camera ( Fig. 3 ).
Fig. 3.
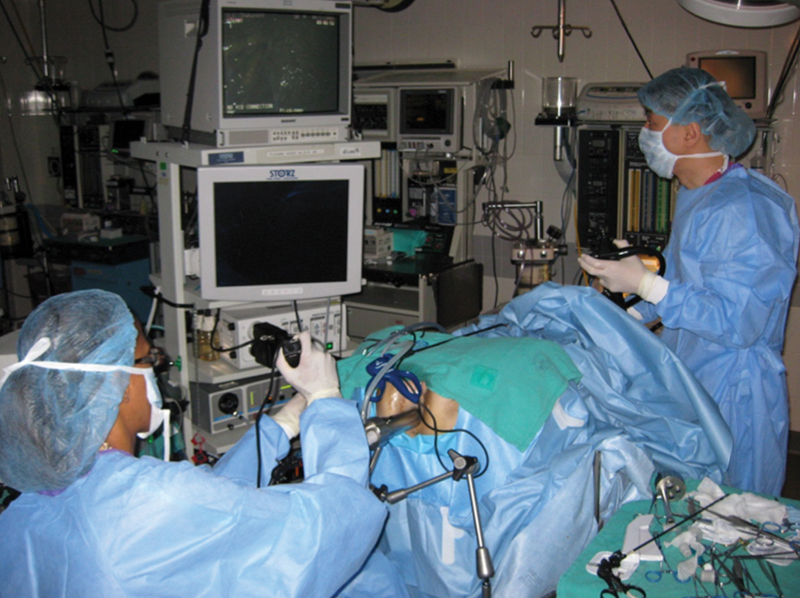
Operative setup for combined transanal and transgastric access in a human cadaver model.
Rieder et al published a small series of transanal rectosigmoid resection using TEM in human cadavers in 2011 to evaluate the oncologic adequacy of this approach. 71 Male cadavers were randomized to undergo TME by a laparoscopic ( n = 2) or a transanal ( n = 4) approach, and resection margins and lymph node yield were evaluated. They found that a pure NOTES approach using traditional TEM instrumentation did allow for good visibility of the mesorectal plane and that CO 2 insufflation assisted with the dissection, and resulted in an intact mesorectal envelope in all transanal specimens. However, the approach did not allow for adequate mobilization of the sigmoid colon and required a hybrid approach with transabdominal laparoscopic assistance in 3 out of 4 cases, and in the purely transanal case, a simulated lesion at 25 cm was not resected with the specimen. Lymph node harvest was similar between the laparoscopic and transanal cases, but failed to reach the recommended harvest and evaluation of at least 12 lymph nodes. This low rate of lymph node harvest, however, may have been a result of limitations of the cadaveric model.
Bhattacharjee et al also published a small series of pure transanal rectosigmoid resection on human cadavers using the TEM platform. 72 This setup was first tested in a bovine ex vivo model and then subsequently in human cadavers. 74 Adapted TEM instruments that were longer, more curved, and steerable compared with traditional instruments were utilized. The authors were able to achieve adequate specimen length with these novel instruments compared with previous studies, without use of transabdominal or transgastric assistance.
The largest trial of NOTES transanal rectosigmoid resection in human cadavers was conducted by Telem et al 73 and Sylla et al using the rigid TEO platform (Karl Storz) in 32 cadavers. Transanal dissection alone ( n = 19) was compared with a combined transanal and transgastric ( n = 5) approach, as well as a laparoscopic-assisted ( n = 8) approach. 73 In the pure transanal group ( n = 19), eight operations were performed using assistance with a gastroscope inserted via the TEO platform, and nine were performed with assistance from a novel multitasking rigid endoscopic platform inserted transanally (ISSA, Karl Storz).
In this largest series of human cadavers, the technique of transanal TME was refined and standardized ( Figs. 4 and 5 ). A 2–0 Vicryl purse-string suture was first placed transanally under direct visualization above the sphincter complex to prevent fecal contamination. The 7.5-cm TEO proctoscope was transanally inserted followed by full-thickness and circumferential dissection of the rectum and mesorectum initiated just below the purse-string suture using electrocautery. Low pressure CO 2 insufflation was used to facilitate exposure and pneumodissection along the presacral space and the entire mesorectum was dissected sharply in a cephalad direction. The 15-cm TEO proctoscope was subsequently inserted to facilitate more proximal reach and improve visualization. The peritoneal reflection was divided to enter the peritoneal cavity. The rectosigmoid peritoneal attachments were divided using a combination of electrocautery and a bipolar device. The inferior mesenteric pedicle was transected using either the bipolar device or an endoscopic stapler (EndoGIA, Covidien). When more proximal dissection could not be transanally completed, the proctoscope was removed and the specimen was exteriorized via the anus and transected, unless additional mobilization was attempted transanally or transgastrically with a flexible endoscope, or transabdominally with laparoscopy. A Lone Star retractor (Cooper Surgical, Trumbull, CT) was placed so that a hand-sewn coloanal or stapled colorectal anastomosis was performed.
Fig. 4.
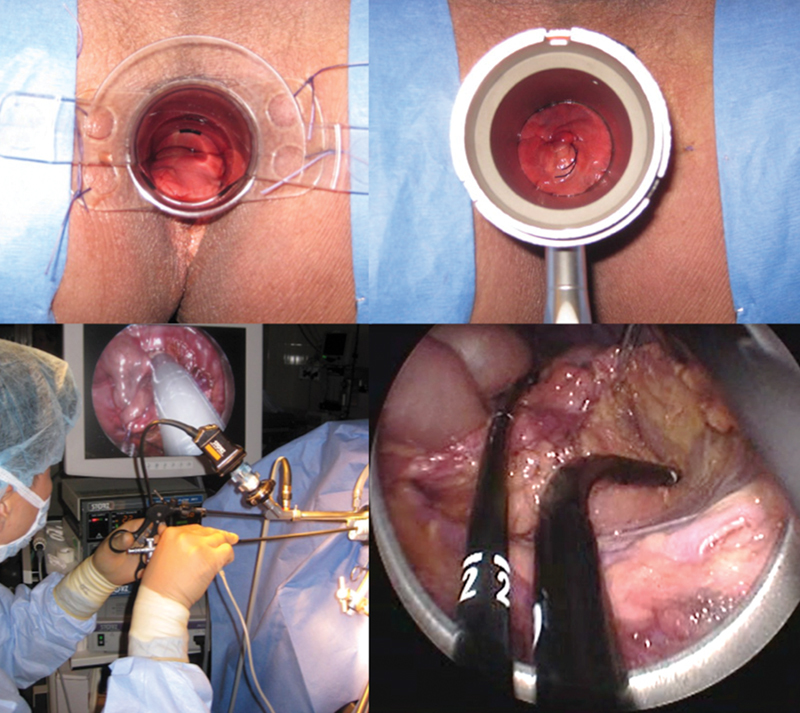
Demonstration of transanal total mesorectal excision (taTME) in a cadaver model. Top images show direct visualization and placement of the purse-string suture. Bottom images show placement of transanal endoscopic operation (TEO) platform transanally followed by circumferential dissection of the rectum and extension of the dissection into the peritoneal cavity.
Fig. 5.
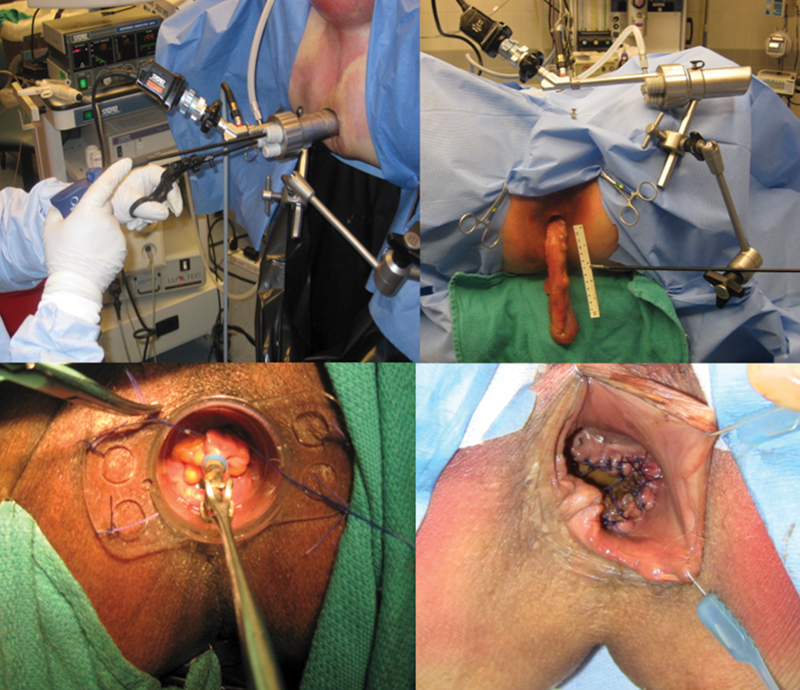
Demonstration of transanal total mesorectal excision (taTME) in a cadaver model (continued). Top figures demonstrate completion of the dissection with transanal removal of specimen. Bottom figures with hand-sewn anastomosis under direct visualization.
Oncologic feasibility was demonstrated in the cadaver model through evaluation of the mesorectum following removal of the specimen. The mesorectum was intact in 100% of specimens, proving that the taTME approach could achieve the gold standard of TME ( Fig. 6 ). Mean operative time was 5.1 hours, and specimen length was 53 cm (15–91.5 cm). As experience with the procedure improved during the trial, there was a decrease in total operative time and increase in specimen length. In particular, the laparoscopic-assisted approach allowed for the longer specimen length and shorter operative time. Furthermore, a total of 8 cases (25%) of enteric perforation occurred, all in cadavers undergoing pure NOTES rectosigmoid resection. These perforations occurred in the proximal colon ( n = 2), sigmoid colon ( n = 2), and rectum ( n = 3), and all occurred during attempts to mobilize the proximal descending colon. For this reason, a hybrid approach with laparoscopic assistance was recommended in consideration for transitioning to clinical trials.
Fig. 6.
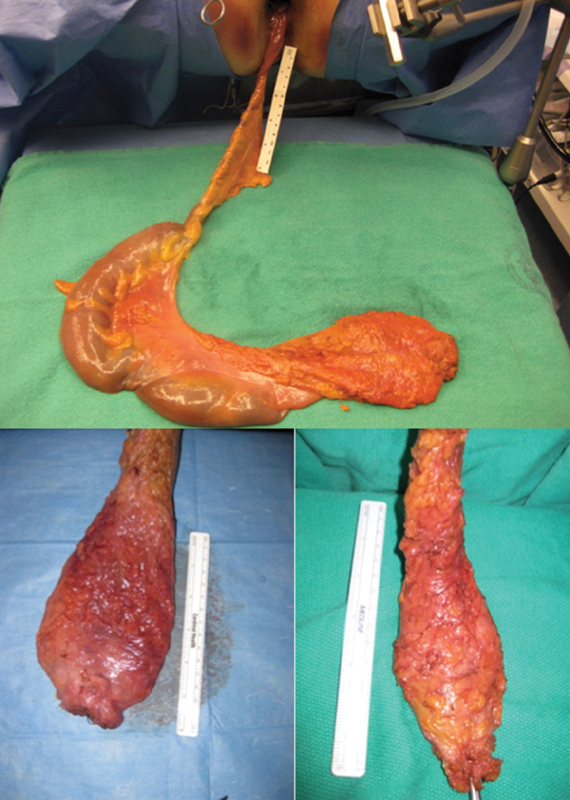
Examples of intact transanal total mesorectal excision (taTME) specimens in a human cadaver model. Top specimen demonstrating total colectomy specimen completed using transanal and transgastric access.
Transition into Early Human Trials with Hybrid NOTES
The early clinical cases of taTME were done for both malignant and benign disease using a variety of transanal platforms. Sylla et al and Lacy et al performed the first successful clinical case of hybrid taTME using the TEO platform and laparoscopic assistance in late 2009. 75 The patient was a 76-year-old female with a 3-cm clinically staged T2N1M0 rectal cancer located 6 cm from the anal verge, who had undergone neoadjuvant chemoradiation. Laparoscopic assistance was performed via one 5-mm and two 2-mm ports, with the former placed at the location of a planned stoma. Circumferential TME was performed transanally to the level of the peritoneal reflection, with the remainder of the proximal dissection performed via the TEO device using transabdominal laparoscopic visualization and assistance with retraction. A protective loop ileostomy was created as per standard practice, and the entire procedure was completed in 270 minutes and there were no postoperative complications. The specimen had negative margins and 23 sampled lymph nodes.
Chen et al also used a hybrid approach with a custom-made transanal port on a 47-year-old male with a T3 mid-rectal adenocarcinoma. Operative time was 290 minutes with negative margins, 25 sampled lymph nodes, and no postoperative complications.
Tuech et al used a combined approach in a 45-year-old female with a T1 rectal adenocarcinoma 3 cm above the dentate line. They used a single-port device (Endorec, Aspide Medical, France) for both transanal and transabdominal access (performed via the future ileostomy site). No additional abdominal incisions were used, and a hand-sewn coloanal anastomosis was performed. The case was completed in 300 minutes, with a total of 15 lymph nodes sampled.
Zorron et al 98 performed two additional cases of taTME with laparoscopic assistance. The first was in a 54-year-old male with an obstruction tumor 8 cm from the anal verge. The authors utilized a standard flexible colonoscope for transanal access. The proximal dissection was performed laparoscopically using three abdominal trocars. The second case was a 73-year-old female with an obstructing tumor 4 cm from the anal verge who had previously undergone creation of a loop colostomy and neoadjuvant therapy. Surgery was performed via a transanal disposable platform (TriPort, Olympus). Again, proximal dissection was performed using a traditional laparoscopic technique with three abdominal trocars.
Pure NOTES taTME
Leroy et al reported the first clinical case of a pure NOTES taTME in a 56-year-old female with a 5-cm mid-rectal adenoma. 76 Given the size of the lesion and preoperative radiologic workup consistent with a T1 or T2 lesion, the patient was considered appropriate for TME, and agreed to undergo a taTME approach. The patient was placed in a lithotomy position and a TEO device was used.
The case was performed using a retroperitoneal approach to sigmoid mobilization, using what the authors termed “Perirectal Oncologic Gateway for Retroperitoneal Endoscopic Single-Site Surgery” or PROGRESS, which was previously described by the authors in a porcine model. 77 A purse-string closure of the rectum was performed distal to the lesion and a full-thickness incision was made posteriorly from approximately the 2-O'clock to the 10-O'clock positions. The dissection was then advanced cephalad in the posterior presacral space, until the TEO device could be inserted through the rectotomy and dissection continued in this plane to the level of the sacral promontory. Subsequently, the lateral dissection was performed, and the previous full-thickness rectotomy was continued anteriorly to complete a circumferential, full-thickness rectal incision. The anterior portion of the dissection was completed within the rectovaginal plane to the level of the peritoneal reflection. Then, the peritoneum was incised and the entire rectum mobilized into the peritoneal cavity with exposure of the sigmoid mesocolon and retroperitoneum to the level of the left colic artery. When the medial and lateral attachments of the sigmoid colon were adequately mobilized, the specimen was removed transanally. The TEO device was reinserted parallel to the bowel and dissection continued, allowing for even further mobilization of the descending colon. The superior hemorrhoidal artery was ligated distal to the left colic artery with a “low-tie” technique, all through the transanal platform. The bowel was transected proximally using a linear stapling device, and the mesentery was ligated and divided. A hand-sewn, side-to-end coloanal anastomosis was performed. The procedure time was190 minutes, specimen length was 20 cm, and 16 lymph nodes were harvested. Final pathology demonstrated a large tubulovillous adenoma with low-grade dysplasia. The procedure was complicated by a pelvic hematoma that required computed tomography-guided drain placement.
Early Case Series
Since these initial case reports, there has been an explosion of smaller case series of taTME, beginning with smaller series by Lacy et al and Sylla et al. 78 79 These case reports have used a variety of transanal platforms including the TEO platform, the Endorec Trocar, the GelPOINT Path Transanal Access Platform (Applied Medical), and the SILS port (Covidien). Many of these early institutional series were performed under institutional review board approval by groups with prior experience with this approach in the experimental setting. Results of these mid-sized series with at least 20 taTME cases for rectal cancer are summarized in Table 2 . These series consistently demonstrated the procedural feasibility and safety of taTME primary performed using laparoscopic assistance, with acceptable morbidity and preliminary oncologic outcomes.
Table 2. Comparative findings of outcomes for series describing over 20 taTME cases.
| Lacy et al 90 | Veltcamp Helbach et al 93 | Tuech et al 94 | Burke et al 95 | Chen et al 96 | Buchs et al 97 | |
|---|---|---|---|---|---|---|
| Study period | 10/2011–11/2014 | 6/2012–9/2014 | 2/2010–9/2014 | 3/2010–7/2015 | 12/2013–4/2015 | 5/2013–5/2015 |
| Study location | Barcelona, Spain | Amsterdam, the Netherlands | Rouen, France | Orlando, United States | Taipei, Taiwan | Oxford, United Kingdom |
| Number of institutions | 1 | 2 | 3 | 1 | 2 | 2 |
| Transanal device used | GelPOINT | GelPOINT or SILS | GelPOINT or endorec or SILS | GelPOINT | GelPOINT | GelPOINT |
| Total number of patients | 140 | 80 | 56 | 50 | 50 | 40 |
| Patient and tumor characteristics | ||||||
| Male gender, n (%) | 89 (64) | 48 (60) | 41 (73) | 30 (60) | 38 (76) | 32 (80) |
| Age, mean ± SD or median (range), y | 65.5 ± 12.7 | 65.3 ± 2.7 | 63 ± 2.7 | 57 ± 1.6 | 57.3 ± 11.9 | 64.4 ± 10.2 |
| Body mass index, mean ± SD or median (range), kg/m 2 | 25.2 ± 3.9 | 28.6 ± 1.8 | 29.0 ± 1.9 | 26.5 ± 1.2 | 24.2 ± 3.7 | 27.4 ± 4.9 |
| Tumor height, mean ± SD or median (range), cm | From AV 7.6 ± 3.6 |
From ARJ 5.3 (1–10) |
From AV 4 (0–5) |
From AV 4.4 (3.0–5.5) |
From AV 5.8 ± 2.1 |
From ARJ 3 (0–10) |
| Preoperative stage, n (%) | ||||||
| T1–2 | 29 (21) | All T2–T3 | 10 (18) | 7 (14) | – | Reported as ≥T3 |
| T3 | 90 (64) | 44 (79) | 35 (70) | 20 (40) | 23 (58) | |
| T4 | 11 (8) | 2 (4) | 8 (16) | 30 (60) | ||
| Neoadjuvant therapy, n (%) | 94 (67) | 65 (81) | 47 (84) | 43 (86) | 50 (100) | 12 (30) |
| Clinical outcomes | ||||||
| Total operative time, mean ± SD or median (range), min | 166 (60–360) | 204 (91–447) | 270 (150–495) | 267 (227–331) | 182.1 ± 55.4 | 368 ± 101.7 |
| Conversions, n (%) | 0 | – | 3 (7) | 1 (2) | 1 (2) | 3 (8) |
| Overall morbidity at 28 d, n (%) | 48 (34) | 31 (39) | 15 (26) | 18 (36) | 13 (26) | 16 (40) |
| Overall mortality at 28 d, n (%) | 0 | 1 (1) | 0 | 0 | 0 | 0 |
| Anastomotic leak, % | 12 (9) | – | 3 (5) | 3 (6) | 3 (6) | 1 (3) |
| Unplanned reoperation, % | 12 (9) | 9 (11) | – | 6 (12) | 2 (4) | 3 (8) |
| Length of hospital stay, mean ± SD or median (range), d | 6 (3–39) | 8 (3–41) | 10 (6–21) | 4.5 (4–8) | 7.4 ± 2.5 | 7 (3–92) |
| Pathological outcomes | ||||||
| Positive CRM, n (%) | 9 (6) | 2 (3) a | 3 (5) | 2 (4) | 2 (4) | 2 (5) |
| Positive DRM, n (%) | 0 | 0 | 0 | 1 (2) | 0 | 0 |
| TME quality, n (%) | ||||||
| Complete | 136 (97) | 71 (89) | 47 (84) | 36 (72) | – | 37 (93) |
| Moderate/near-complete | 3 (2) | 7 (9) | 9 (16) | 13 (26) | – | 2 (5) |
| Incomplete | 1 (1) | 2 (3) | 0 | 1 (2) | – | 1 (3) |
| Number of lymph nodes, mean ± SD or median (range) | 14.7 ± 6.8 | 16 ± 2 | 15 ± 1.9 | 18 ± 1.4 | 16.7 ± 7.8 | 20 ± 9.7 |
| Median follow-up, mo | 15 | 21 | 29 | 15 | – | 11 |
| Recurrence rate, n (%) | ||||||
| Local | 1 (1) | 2 (3) | 1 (2) | 2 (4) | – | – |
| Distant | 8 (6) | – | – | 7 (15) | – | 6 (15.0) |
| Both local and distant | 2 (2) | – | – | – | – | – |
| Serra-Aracil et al 80 | de'Angelis et al 81 | Rouanet et al 82 | Muratore et al 83 | Perdawood et al 84 | Rink et al 85 | Rasulov et al 86 | |
|---|---|---|---|---|---|---|---|
| Study period | 9/2012–9/2014 | 1/2011–12/2014 | 1/2009–6/2011 | 1/2012–12/2013 | 12/2013–4/2015 | 5/2013–3/2015 | 10/2013–1/2015 |
| Study location | Barcelona, Spain | Creteil, France | Montpelier, France | Candiolo, Italy | Slagelse, Denmark | Leverkusen and Mainz, Germany | Moscow, Russia |
| Number of institutions | 1 | 1 | 1 | 1 | 1 | 2 | 1 |
| Transanal device used | TEO | GelPOINT | TEO | SILS | GelPOINT | GelPOINT or SILS | TEO |
| Total number of patients | 32 | 32 | 30 | 26 | 25 | 24 | 22 |
| Patient and tumor characteristics | |||||||
| Male gender, n (%) | 24 (75) | 21 (66) | 30 (100) | 16 (62) | 19 (76) | 18 (75) | 11 (50) |
| Age, mean ± SD or median (range), y | 68 (39–88) | 64.9 ± 10.0 | 65 (43–82) | 65.8 (38–84) | 70 (54–76) | 57 (35–77) | 56 (30–69) |
| Body mass index, mean ± SD or median (range), kg/m 2 | 25 (20–35) | 25.2 ± 3.5 | 26.0 (21.0–32.4) | 26.2 (16.9–38.2) | 28 (18–46) | 25 (19–38) | 26 (20–32) |
| Tumor height, mean ± SD or median (range), cm | From AV 8 (5–10) |
From AV 4 (2.5–5) |
From LA 1 (0–7) |
From AV 4.4 (3–6) |
From AV 8 (4–10) |
From AV 5 (3–6) |
From AV 6.5 (0–10) |
| Preoperative stage, n (%) | |||||||
| T1–2 | All T1–T3 | 13 (41) | 2 (6) | 2 (8) | 4 (16) | 7 (29) | 5 (23) |
| T3 | 17 (53) | 21 (70) | 6 (23) | 19 (76) | 16 (67) | 14 (64) | |
| T4 | 2 (6) | 7 (23) | 18 (69) | 2 (8) | 1 (4) | 3 (13) | |
| Neoadjuvant therapy, n (%) | 16 (50) | 27 (84) | 26 (87) | 19 (73) | 7 (28) | 19 (79) | 19 (87) |
| Clinical outcomes | |||||||
| Total operative time, mean ± SD or median (range), min | 240 (165–360) | 195 ± 43.6 | 304 (120–500) | 241 (150–360) | 300 (235–420) | 339 (183–458) | 320 (265–495) |
| Conversions, n (%) | 0 | 1 (3) | 2 (7) | 0 | 0 | – | 1 (5) |
| Overall morbidity at 28 d, n (%) | 10 (31) | 8 (25) | 9 (30) | 7 (27) | 13 (52) | 7 (29) | 8 (36) |
| Overall mortality at 28 d, n (%) | 0 | 0 | 0 | 1 (4) | 0 | 0 | 0 |
| Anastomotic leak, % | 3 (9) | 2 (6) | 1 (3) | 2 (8) | 2 (8) | 1 (4) | 0 |
| Unplanned reoperation, % | 0 | – | 2 (7) | – | – | – | 0 |
| Length of hospital stay, mean ± SD or median (range), d | 8 (4–20) | 7.8 ± 2.1 | 14 (9–25) | 7 (3–25) | 5 (2–43) | – | 8 (6–18) |
| Pathological outcomes | |||||||
| Positive CRM, n (%) | 0 | 1 (3) | 4 (13) | 0 | 1 (4) | 2 (8) | 1 (5) |
| Positive DRM, n (%) | 0 | 2 (6.2) | – | 0 | – | 0 | 0 |
| TME quality, n (%) | |||||||
| Complete | 30 (93) | 27 (84) | 30 | 23 (86) | 20 (80) | 22 (92) | 15 (68) |
| Moderate/near-complete | 2 (6) | 3 (9) | 0 | 3 (12) | 5 (20) | 2 (8) | 3 (14) |
| Incomplete | 0 | 2 (6) | 0 | 0 | 0 | 0 | 4 (18) |
| Number of lymph nodes, mean ± SD or median (range) | 15 | 17.1 ± 7.1 | 13 (8–32) | 10 | 21 (9–42) | 14 (4–32) | 17 (0–54) |
| Median follow-up, mo | – | – | 21 | 21 | – | – | 11 |
| Recurrence rate, n (%) | |||||||
| Local | – | – | 4 (13) | 0 | – | – | – |
| Distant | – | – | 8 (27) | 2 (8) | – | – | – |
| Both local and distant | – | – | – | 0 | – | – | – |
Abbreviations: ARJ, anorectal junction; AV, anal verge; CRM, circumferential resection margin; DRM, distal resection margin; LA, levator ani muscles; SD, standard deviation; taTME, transanal total mesorectal excision; TEO, transanal endoscopic operation; TME, transanal endoscopic microsurgery.
Note: In instances with multiple publications on a single cohort, data from most recent publication is shown.
Margins < 2 mm defined as positive, all other studies used 1 mm margin.
Current State of taTME in Clinical Practice
A large number of small and mid-size case series have been published demonstrating promising results with respect to the safety and efficacy of taTME. Recently, the results from the international taTME registry have highlighted short-term outcomes in the first 720 cases reported worldwide between July 2014 and December 2015 for both benign and malignant disease. 87 These included cases performed at 66 individual sites in 23 different countries, demonstrating the remarkable spread of this technique since its inception. The majority of cases were performed for rectal cancer (88.1%), particularly low rectal cancer and predominantly in male subjects. Most cases were performed with minimally invasive transabdominal assistance, although 3.1% of cases used open assistance. Most patients underwent creation of a diverting stoma (91%) if they were undergoing LAR. A wide variety of rigid and flexible transanal access platforms were used. Both stapled and hand-sewn anastomoses were performed. Conversion from laparoscopic to open abdominal approach occurred in 6.3% of cases and conversion from a transanal to open approach occurred in 2.8% of cases. Rates of positive circumferential radial margins (CRMs) were comparable to those for laparoscopic, robotic, and open resections in prior randomized trials. This was similar to results from a randomized trial comparing laparoscopic TME to hybrid taTME, which found lower rates of CRM positivity in the taTME arm (18% vs. 4%, p = 0.025). 88
Overall postoperative mortality and morbidity results from the international registry were comparable to prior trials of open and laparoscopic TME, but with a lower overall rate of anastomotic leak (6.3%). 87 Of note, however, there is a unique risk of iatrogenic injury to the male urethra during taTME. The inherent risk of this complication with taTME is due to the fact that during the anterior dissection in the rectoprostatic plane, the dissection can be carried too far anterior along the superior border of the prostate, ultimately leading to injury to the preprostatic urethra. 89 Although the rates of injury reported in larger case series (0–6.7%) and the international registry (0.7%) are generally low, this complication is particularly concerning given the fact that this is not a described complication of sphincter-preserving operations with an open or laparoscopic approach and is only a rare complication of APR. 82 87 90 91 Furthermore, current case series likely underreport the true incidence of urethral injury, which is more likely to occur in the hands of less experienced surgeons. 92 Risks of other intraoperative visceral organ injuries reported from the registry were low, including bladder (0.3%), vaginal perforation (0.1%), and rectal perforation (0.3%). 87 For laparoscopic and open procedures, the previously mentioned large randomized trials have noted complications of bladder injury (0–0.4% and none, respectively), splenic injury (none and 0–1.4%, respectively), ureteral injury (0.4–1% and less than 1%, respectively), colon perforation (0–1.3% and 0–2.0%, respectively), and rectal perforation (0–4.2% and 1–1.4%, respectively). 14 17 Thus, while different visceral organs appear to be at risk with the taTME approach as compared with open surgery or laparoscopy, with the potential exception of urethral injury, overall rates are very low.
Conclusion
TaTME continues to develop as a promising minimally invasive approach to treat rectal cancer. Preliminary results from small and mid-sized series as well as a large registry suggest that the technique facilitates completion of good quality TME, particularly in the most challenging cases of distal rectal tumors in obese male patients. While this approach results in similar morbidity as conventional TME, the ability to extract the TME specimen transanally, when safe to do so, may minimize postoperative pain and wound-related complications. Although taTME is most typically employed with a hybrid approach using laparoscopic transabdominal assistance, pure NOTES taTME has been performed in small case series. Initial results suggest that taTME is safe and effective, and several phase II and III trials are currently underway to evaluate the long-term oncologic safety of this approach in rectal cancer, and compare results to laparoscopic TME. The need for structured training and standardization of the procedure is also paramount for safe adoption and implementation of this novel approach at centers with preexisting expertise in TES and minimally invasive TME.
Abbreviations
- APR
abdominoperineal resection
- CI
confidence interval
- CRM
circumferential resection margins
- LAR
low anterior resection
- NOSE
natural orifice specimen extraction
- NOTES
natural orifice transluminal endoscopic surgery
- OR
odds ratio
- taTME
transanal total mesorectal excision
- TAMIS
transanal minimally invasive surgery
- TEM
transanal endoscopic microsurgery
- TEO
transanal endoscopic operation
- TES
transanal endoscopic surgery
- TME
total mesorectal excision
Footnotes
Conflict of Interest Dr. Sylla reports personal fees from Ethicon, personal fees from Medtronic, personal fees from Olympus, personal fees from Karl Storz, outside the submitted work.
References
- 1.Fuchs K H, Breithaupt W, Varga G, Schulz T, Reinisch A, Josipovic N. Transanal hybrid colon resection: from laparoscopy to NOTES. Surg Endosc. 2013;27(03):746–752. doi: 10.1007/s00464-012-2534-7. [DOI] [PubMed] [Google Scholar]
- 2.Buess G, Theiss R, Hutterer F et al. Transanal endoscopic surgery of the rectum - testing a new method in animal experiments [in German] Leber Magen Darm. 1983;13(02):73–77. [PubMed] [Google Scholar]
- 3.Nieuwenhuis D H, Draaisma W A, Verberne G HM, van Overbeeke A J, Consten E CJ. Transanal endoscopic operation for rectal lesions using two-dimensional visualization and standard endoscopic instruments: a prospective cohort study and comparison with the literature. Surg Endosc. 2009;23(01):80–86. doi: 10.1007/s00464-008-9918-8. [DOI] [PubMed] [Google Scholar]
- 4.Heald R J, Husband E M, Ryall R D. The mesorectum in rectal cancer surgery--the clue to pelvic recurrence? Br J Surg. 1982;69(10):613–616. doi: 10.1002/bjs.1800691019. [DOI] [PubMed] [Google Scholar]
- 5.Ridgway P F, Darzi A W. The role of total mesorectal excision in the management of rectal cancer. Cancer Contr. 2003;10(03):205–211. doi: 10.1177/107327480301000303. [DOI] [PubMed] [Google Scholar]
- 6.MacFarlane J K, Ryall R D, Heald R J.Mesorectal excision for rectal cancer Lancet 1993341(8843):457–460. [DOI] [PubMed] [Google Scholar]
- 7.Marijnen C AM, Kapiteijn E, van de Velde C JH et al. Acute side effects and complications after short-term preoperative radiotherapy combined with total mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20(03):817–825. doi: 10.1200/JCO.2002.20.3.817. [DOI] [PubMed] [Google Scholar]
- 8.Snijders H S, Wouters M WJM, van Leersum N J et al. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38(11):1013–1019. doi: 10.1016/j.ejso.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 9.Kang S-B, Park J W, Jeong S-Y et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11(07):637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 10.Jeong S-Y, Park J W, Nam B H et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol. 2014;15(07):767–774. doi: 10.1016/S1470-2045(14)70205-0. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani H, Tamamori Y, Azuma T et al. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and conventional open surgery for rectal cancer. J Gastrointest Surg. 2011;15(08):1375–1385. doi: 10.1007/s11605-011-1547-1. [DOI] [PubMed] [Google Scholar]
- 12.Guillou P J, Quirke P, Thorpe Het al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial Lancet 2005365(9472):1718–1726. [DOI] [PubMed] [Google Scholar]
- 13.Bentrem D J, Okabe S, Wong W Det al. T1 adenocarcinoma of the rectum: transanal excision or radical surgery? Ann Surg 200524204472–477., discussion 477–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Pas M H, Haglind E, Cuesta M A et al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14(03):210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 15.NCCN.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Rectal CancerVersion 3. 2017. Available at:https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed October 1, 2019
- 16.Green B L, Marshall H C, Collinson F et al. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100(01):75–82. doi: 10.1002/bjs.8945. [DOI] [PubMed] [Google Scholar]
- 17.Fleshman J, Branda M, Sargent D J et al. Effect of laparoscopic-assisted resection vs open resection of stage II or III rectal cancer on pathologic outcomes the ACOSOG Z6051 randomized clinical trial. JAMA. 2015;314(13):1346–1355. doi: 10.1001/jama.2015.10529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson A RL, Solomon M J, Lumley J W et al. Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA. 2015;314(13):1356–1363. doi: 10.1001/jama.2015.12009. [DOI] [PubMed] [Google Scholar]
- 19.Jayne D G, Thorpe H C, Copeland J, Quirke P, Brown J M, Guillou P J. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97(11):1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 20.Mroczkowski P, Hac S, Smith B, Schmidt U, Lippert H, Kube R. Laparoscopy in the surgical treatment of rectal cancer in Germany 2000-2009. Colorectal Dis. 2012;14(12):1473–1478. doi: 10.1111/j.1463-1318.2012.03058.x. [DOI] [PubMed] [Google Scholar]
- 21.Keller D S, Qiu J, Senagore A J. Predicting opportunities to increase utilization of laparoscopy for rectal cancer. Surg Endoscopy Other Intervention Techniques. 2017:1–8. doi: 10.1007/s00464-017-5844-y. [DOI] [PubMed] [Google Scholar]
- 22.Liao G, Zhao Z, Lin S et al. Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol. 2014;12(01):122. doi: 10.1186/1477-7819-12-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jayne D, Pigassi A, Tsang C et al. ROLARR: robotic versus laparoscopic resection for rectal cancer. Color Dis. 2010;12:28–29. [Google Scholar]
- 24.Tyler J A, Fox J P, Desai M M, Perry W B, Glasgow S C. Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum. 2013;56(04):458–466. doi: 10.1097/DCR.0b013e31827085ec. [DOI] [PubMed] [Google Scholar]
- 25.Albert M R, Atallah S B, deBeche-Adams T C, Izfar S, Larach S W. Transanal minimally invasive surgery (TAMIS) for local excision of benign neoplasms and early-stage rectal cancer: efficacy and outcomes in the first 50 patients. Dis Colon Rectum. 2013;56(03):301–307. doi: 10.1097/DCR.0b013e31827ca313. [DOI] [PubMed] [Google Scholar]
- 26.Atallah S, Albert M, Larach S. Transanal minimally invasive surgery: a giant leap forward. Surg Endosc. 2010;24(09):2200–2205. doi: 10.1007/s00464-010-0927-z. [DOI] [PubMed] [Google Scholar]
- 27.McLemore E C, Weston L A, Coker A M et al. Transanal minimally invasive surgery for benign and malignant rectal neoplasia. Am J Surg. 2014;208(03):372–381. doi: 10.1016/j.amjsurg.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Arolfo S, Allaix M E, Migliore M, Cravero F, Arezzo A, Morino M. Transanal endoscopic microsurgery after endoscopic resection of malignant rectal polyps: a useful technique for indication to radical treatment. Surg Endosc. 2014;28(04):1136–1140. doi: 10.1007/s00464-013-3290-z. [DOI] [PubMed] [Google Scholar]
- 29.Kanehira E, Tanida T, Kamei A, Nakagi M, Iwasaki M, Shimizu H. Transanal endoscopic microsurgery for surgical repair of rectovesical fistula following radical prostatectomy. Surg Endosc. 2015;29(04):851–855. doi: 10.1007/s00464-014-3737-x. [DOI] [PubMed] [Google Scholar]
- 30.Tielen R, Bremers A JA, van der Graaf W TA, Flucke U E, de Wilt J HW. Transanal endoscopic microsurgery following treatment with imatinib : a case report of a patient with a rectal gastrointestinal stromal tumor. Acta Chir Belg. 2015;115(02):166–169. doi: 10.1080/00015458.2015.11681089. [DOI] [PubMed] [Google Scholar]
- 31.Duek S D, Gilshtein H, Khoury W. Transanal endoscopic microsurgery: also for the treatment of retrorectal tumors. Minim Invasive Ther Allied Technol. 2014;23(01):28–31. doi: 10.3109/13645706.2013.872663. [DOI] [PubMed] [Google Scholar]
- 32.Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Corredera-Cantarin C, Gomez-Diaz C, Navarro-Soto S. Atypical indications for transanal endoscopic microsurgery to avoid major surgery. Tech Coloproctol. 2014;18(02):157–164. doi: 10.1007/s10151-013-1040-9. [DOI] [PubMed] [Google Scholar]
- 33.Dias A R, Nahas C SR, Marques C FS, Nahas S C, Cecconello I. Transanal endoscopic microsurgery: indications, results and controversies. Tech Coloproctol. 2009;13(02):105–111. doi: 10.1007/s10151-009-0466-6. [DOI] [PubMed] [Google Scholar]
- 34.Kunitake H, Abbas M A. Transanal endoscopic microsurgery for rectal tumors: a review. Perm J. 2012;16(02):45–50. doi: 10.7812/tpp/11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moore J S, Cataldo P A, Osler T, Hyman N H.Transanal endoscopic microsurgery is more effective than traditional transanal excision for resection of rectal masses Dis Colon Rectum 200851071026–1030., discussion 1030–1031 [DOI] [PubMed] [Google Scholar]
- 36.Ford S J, Wheeler J MD, Borley N R. Factors influencing selection for a day-case or 23-h stay procedure in transanal endoscopic microsurgery. Br J Surg. 2010;97(03):410–414. doi: 10.1002/bjs.6857. [DOI] [PubMed] [Google Scholar]
- 37.Allaix M E, Rebecchi F, Giaccone C, Mistrangelo M, Morino M. Long-term functional results and quality of life after transanal endoscopic microsurgery. Br J Surg. 2011;98(11):1635–1643. doi: 10.1002/bjs.7584. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy M L, Lubowski D Z, King D W. Transanal endoscopic microsurgery excision: is anorectal function compromised? Dis Colon Rectum. 2002;45(05):601–604. doi: 10.1007/s10350-004-6252-7. [DOI] [PubMed] [Google Scholar]
- 39.Kreis M E, Jehle E C, Ohlemann M, Becker H D, Starlinger M J. Functional results after transanal rectal advancement flap repair of trans-sphincteric fistula. Br J Surg. 1998;85(02):240–242. doi: 10.1046/j.1365-2168.1998.00557.x. [DOI] [PubMed] [Google Scholar]
- 40.Whitehouse P A, Armitage J N, Tilney H S, Simson J NL. Transanal endoscopic microsurgery: local recurrence rate following resection of rectal cancer. Colorectal Dis. 2008;10(02):187–193. doi: 10.1111/j.1463-1318.2007.01291.x. [DOI] [PubMed] [Google Scholar]
- 41.Sengupta S, Tjandra J J. Local excision of rectal cancer: what is the evidence? Dis Colon Rectum. 2001;44(09):1345–1361. doi: 10.1007/BF02234796. [DOI] [PubMed] [Google Scholar]
- 42.Heidary B, Phang T P, Raval M J, Brown C J. Transanal endoscopic microsurgery: a review. Can J Surg. 2014;57(02):127–138. doi: 10.1503/cjs.022412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morino M, Arezzo A, Allaix M E. Transanal endoscopic microsurgery. Tech Coloproctol. 2013;17 01:S55–S61. doi: 10.1007/s10151-012-0936-0. [DOI] [PubMed] [Google Scholar]
- 44.Serra-Aracil X, Mora-Lopez L, Alcantara-Moral M, Caro-Tarrago A, Gomez-Diaz C J, Navarro-Soto S. Transanal endoscopic surgery in rectal cancer. World J Gastroenterol. 2014;20(33):11538–11545. doi: 10.3748/wjg.v20.i33.11538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.You Y N. Local excision: is it an adequate substitute for radical resection in T1/T2 patients? Semin Radiat Oncol. 2011;21(03):178–184. doi: 10.1016/j.semradonc.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 46.Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of rectal cancer by transanal endoscopic microsurgery: experience with 425 patients. World J Gastroenterol. 2014;20(28):9556–9563. doi: 10.3748/wjg.v20.i28.9556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bignell M B, Ramwell A, Evans J R, Dastur N, Simson J NL.Complications of transanal endoscopic microsurgery (TEMS): a prospective audit Colorectal Dis 201012(7 Online):e99–e103. [DOI] [PubMed] [Google Scholar]
- 48.Gavagan J A, Whiteford M H, Swanstrom L L. Full-thickness intraperitoneal excision by transanal endoscopic microsurgery does not increase short-term complications. Am J Surg. 2004;187(05):630–634. doi: 10.1016/j.amjsurg.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 49.Allaix M E, Arezzo A, Caldart M, Festa F, Morino M. Transanal endoscopic microsurgery for rectal neoplasms: experience of 300 consecutive cases. Dis Colon Rectum. 2009;52(11):1831–1836. doi: 10.1007/DCR.0b013e3181b14d2d. [DOI] [PubMed] [Google Scholar]
- 50.Barendse R M, Dijkgraaf M G, Rolf U R et al. Colorectal surgeons' learning curve of transanal endoscopic microsurgery. Surg Endosc. 2013;27(10):3591–3602. doi: 10.1007/s00464-013-2931-6. [DOI] [PubMed] [Google Scholar]
- 51.Morino M, Allaix M E, Famiglietti F, Caldart M, Arezzo A. Does peritoneal perforation affect short- and long-term outcomes after transanal endoscopic microsurgery? Surg Endosc. 2013;27(01):181–188. doi: 10.1007/s00464-012-2418-x. [DOI] [PubMed] [Google Scholar]
- 52.Baatrup G, Borschitz T, Cunningham C, Qvist N. Perforation into the peritoneal cavity during transanal endoscopic microsurgery for rectal cancer is not associated with major complications or oncological compromise. Surg Endosc. 2009;23(12):2680–2683. doi: 10.1007/s00464-008-0281-6. [DOI] [PubMed] [Google Scholar]
- 53.Marks J H, Frenkel J L, Greenleaf C E, D'Andrea A P. Transanal endoscopic microsurgery with entrance into the peritoneal cavity: is it safe? Dis Colon Rectum. 2014;57(10):1176–1182. doi: 10.1097/DCR.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 54.Ramwell A, Evans J, Bignell M, Mathias J, Simson J. The creation of a peritoneal defect in transanal endoscopic microsurgery does not increase complications. Colorectal Dis. 2009;11(09):964–966. doi: 10.1111/j.1463-1318.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 55.Lacy A M, Delgado S, Rojas O A, Almenara R, Blasi A, Llach J. MA-NOS radical sigmoidectomy: report of a transvaginal resection in the human. Surg Endosc. 2008;22(07):1717–1723. doi: 10.1007/s00464-008-9956-2. [DOI] [PubMed] [Google Scholar]
- 56.Awad Z T, Qureshi I, Seibel B, Sharma S, Dobbertien M A. Laparoscopic right hemicolectomy with transvaginal colon extraction using a laparoscopic posterior colpotomy: a 2-year series from a single institution. Surg Laparosc Endosc Percutan Tech. 2011;21(06):403–408. doi: 10.1097/SLE.0b013e31823945ac. [DOI] [PubMed] [Google Scholar]
- 57.Torres R A, Orban R D, Tocaimaza L, Vallejos Pereira G, Arévalo J R. Transvaginal specimen extraction after laparoscopic colectomy. World J Surg. 2012;36(07):1699–1702. doi: 10.1007/s00268-012-1528-x. [DOI] [PubMed] [Google Scholar]
- 58.Franklin M E, Jr, Liang S, Russek K. Natural orifice specimen extraction in laparoscopic colorectal surgery: transanal and transvaginal approaches. Tech Coloproctol. 2013;17 01:S63–S67. doi: 10.1007/s10151-012-0938-y. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs K H, Breithaupt W, Varga G, Schulz T, Reinisch A, Josipovic N. Transanal hybrid colon resection: from laparoscopy to NOTES. Surg Endosc. 2013;27(03):746–752. doi: 10.1007/s00464-012-2534-7. [DOI] [PubMed] [Google Scholar]
- 60.Cheung H YS, Leung A LH, Chung C C, Ng D CK, Li M KW. Endo-laparoscopic colectomy without mini-laparotomy for left-sided colonic tumors. World J Surg. 2009;33(06):1287–1291. doi: 10.1007/s00268-009-0006-6. [DOI] [PubMed] [Google Scholar]
- 61.Park J S, Choi G S, Kim H J, Park S Y, Jun S H. Natural orifice specimen extraction versus conventional laparoscopically assisted right hemicolectomy. Br J Surg. 2011;98(05):710–715. doi: 10.1002/bjs.7419. [DOI] [PubMed] [Google Scholar]
- 62.Leung A LH, Cheung H YS, Fok B KL, Chung C CC, Li M KW, Tang C N. Prospective randomized trial of hybrid NOTES colectomy versus conventional laparoscopic colectomy for left-sided colonic tumors. World J Surg. 2013;37(11):2678–2682. doi: 10.1007/s00268-013-2163-x. [DOI] [PubMed] [Google Scholar]
- 63.Franklin M E, Jr, Liang S, Russek K. Integration of transanal specimen extraction into laparoscopic anterior resection with total mesorectal excision for rectal cancer: a consecutive series of 179 patients. Surg Endosc. 2013;27(01):127–132. doi: 10.1007/s00464-012-2440-z. [DOI] [PubMed] [Google Scholar]
- 64.Sylla P, Willingham F F, Sohn D K, Gee D, Brugge W R, Rattner D W. NOTES rectosigmoid resection using transanal endoscopic microsurgery (TEM) with transgastric endoscopic assistance: a pilot study in swine. J Gastrointest Surg. 2008;12(10):1717–1723. doi: 10.1007/s11605-008-0637-1. [DOI] [PubMed] [Google Scholar]
- 65.Sylla P, Sohn D K, Cizginer S et al. Survival study of natural orifice translumenal endoscopic surgery for rectosigmoid resection using transanal endoscopic microsurgery with or without transgastric endoscopic assistance in a swine model. Surg Endosc. 2010;24(08):2022–2030. doi: 10.1007/s00464-010-0898-0. [DOI] [PubMed] [Google Scholar]
- 66.Telem D A, Berger D L, Bordeianou L G, Rattner D W, Sylla P. Update on transanal NOTES for rectal cancer: Transitioning to human trials. Minim Invasive Surg. 2012;2012:287613. doi: 10.1155/2012/287613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Whiteford M H, Denk P M, Swanström L L. Feasibility of radical sigmoid colectomy performed as natural orifice translumenal endoscopic surgery (NOTES) using transanal endoscopic microsurgery. Surg Endosc. 2007;21(10):1870–1874. doi: 10.1007/s00464-007-9552-x. [DOI] [PubMed] [Google Scholar]
- 68.Denk P M, Swanström L L, Whiteford M H. Transanal endoscopic microsurgical platform for natural orifice surgery. Gastrointest Endosc. 2008;68(05):954–959. doi: 10.1016/j.gie.2008.03.1115. [DOI] [PubMed] [Google Scholar]
- 69.Sylla P, Kim M-C, Dursun A . San Antonio, TX: Society of American Gastrointestinal and Endoscopic Surgeons; 2011. Laparoscopic Versus NOTES Rectosigmoid Resection Using Transanal Endoscopic Microsurgery (TEM) in a Swine Survival Model. [Google Scholar]
- 70.Fajardo A D, Hunt S R, Fleshman J W, Mutch M G. Video. Transanal single-port low anterior resection in a cadaver model. Surg Endosc. 2010;24(07):1765. doi: 10.1007/s00464-009-0838-z. [DOI] [PubMed] [Google Scholar]
- 71.Rieder E, Spaun G O, Khajanchee Y S et al. A natural orifice transrectal approach for oncologic resection of the rectosigmoid: an experimental study and comparison with conventional laparoscopy. Surg Endosc. 2011;25(10):3357–3363. doi: 10.1007/s00464-011-1726-x. [DOI] [PubMed] [Google Scholar]
- 72.Bhattacharjee H K, Kirschniak A, Storz P, Wilhelm P, Kunert W. Transanal endoscopic microsurgery-based transanal access for colorectal surgery: experience on human cadavers. J Laparoendosc Adv Surg Tech A. 2011;21(09):835–840. doi: 10.1089/lap.2011.0045. [DOI] [PubMed] [Google Scholar]
- 73.Telem D A, Han K S, Kim M C et al. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013;27(01):74–80. doi: 10.1007/s00464-012-2409-y. [DOI] [PubMed] [Google Scholar]
- 74.Bhattacharjee H K, Buess G F, Becerra Garcia F C et al. A novel single-port technique for transanal rectosigmoid resection and colorectal anastomosis on an ex vivo experimental model. Surg Endosc. 2011;25(06):1844–1857. doi: 10.1007/s00464-010-1476-1. [DOI] [PubMed] [Google Scholar]
- 75.Sylla P, Rattner D W, Delgado S, Lacy A M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24(05):1205–1210. doi: 10.1007/s00464-010-0965-6. [DOI] [PubMed] [Google Scholar]
- 76.Leroy J, Barry B D, Melani A, Mutter D, Marescaux J.No-scar transanal total mesorectal excision: the last step to pure NOTES for colorectal surgery JAMA Surg 201314803226–230., discussion 231 [DOI] [PubMed] [Google Scholar]
- 77.Leroy J, Diana M, Barry B et al. Perirectal Oncologic Gateway to Retroperitoneal Endoscopic Single-Site Surgery (PROGRESSS): a feasibility study for a new NOTES approach in a swine model. Surg Innov. 2012;19(04):345–352. doi: 10.1177/1553350612452346. [DOI] [PubMed] [Google Scholar]
- 78.Lacy A M, Adelsdorfer C, Delgado S, Sylla P, Rattner D W. Minilaparoscopy-assisted transrectal low anterior resection (LAR): a preliminary study. Surg Endosc. 2013;27(01):339–346. doi: 10.1007/s00464-012-2443-9. [DOI] [PubMed] [Google Scholar]
- 79.Sylla P, Bordeianou L G, Berger D et al. A pilot study of natural orifice transanal endoscopic total mesorectal excision with laparoscopic assistance for rectal cancer. Surg Endosc. 2013;27(09):3396–3405. doi: 10.1007/s00464-013-2922-7. [DOI] [PubMed] [Google Scholar]
- 80.Serra-Aracil X, Mora-López L, Casalots A, Pericay C, Guerrero R, Navarro-Soto S. Hybrid NOTES: TEO for transanal total mesorectal excision: intracorporeal resection and anastomosis. Surg Endosc. 2016;30(01):346–354. doi: 10.1007/s00464-015-4170-5. [DOI] [PubMed] [Google Scholar]
- 81.de'Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg. 2015;400(08):945–959. doi: 10.1007/s00423-015-1350-7. [DOI] [PubMed] [Google Scholar]
- 82.Rouanet P, Mourregot A, Azar C C et al. Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum. 2013;56(04):408–415. doi: 10.1097/DCR.0b013e3182756fa0. [DOI] [PubMed] [Google Scholar]
- 83.Muratore A, Mellano A, Marsanic P, De Simone M. Transanal total mesorectal excision (taTME) for cancer located in the lower rectum: short- and mid-term results. Eur J Surg Oncol. 2015;41(04):478–483. doi: 10.1016/j.ejso.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 84.Perdawood S K, Al Khefagie G AA. Transanal vs laparoscopic total mesorectal excision for rectal cancer: initial experience from Denmark. Colorectal Dis. 2016;18(01):51–58. doi: 10.1111/codi.13225. [DOI] [PubMed] [Google Scholar]
- 85.Rink A D, Kauff D W, Paschold M, Vestweber K-H, Lang H, Kneist W. Hybrid-TAMIS totale mesorektale Exzision. Der Chiryo. 2016;87(03):225–232. doi: 10.1007/s00104-015-0043-7. [DOI] [PubMed] [Google Scholar]
- 86.Rasulov A O, Mamedli Z Z, Gordeyev S S, Kozlov N A, Dzhumabaev H E. Short-term outcomes after transanal and laparoscopic total mesorectal excision for rectal cancer. Tech Coloproctol. 2016;20(04):227–234. doi: 10.1007/s10151-015-1421-3. [DOI] [PubMed] [Google Scholar]
- 87.Penna M, Hompes R, Arnold S et al. Transanal total mesorectal excision: international registry results of the first 720 cases. Ann Surg. 2017;266(01):111–117. doi: 10.1097/SLA.0000000000001948. [DOI] [PubMed] [Google Scholar]
- 88.Denost Q, Adam J P, Rullier A, Buscail E, Laurent C, Rullier E. Perineal transanal approach: a new standard for laparoscopic sphincter-saving resection in low rectal cancer, a randomized trial. Ann Surg. 2014;260(06):993–999. doi: 10.1097/SLA.0000000000000766. [DOI] [PubMed] [Google Scholar]
- 89.Atallah S, Albert M, Monson J RT. Critical concepts and important anatomic landmarks encountered during transanal total mesorectal excision (taTME): toward the mastery of a new operation for rectal cancer surgery. Tech Coloproctol. 2016;20(07):483–494. doi: 10.1007/s10151-016-1475-x. [DOI] [PubMed] [Google Scholar]
- 90.Lacy A M, Tasende M M, Delgado S et al. Transanal total mesorectal excision for rectal cancer: outcomes after 140 patients. J Am Coll Surg. 2015;221(02):415–423. doi: 10.1016/j.jamcollsurg.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 91.Sawkar H P, Kim D Y, Thum D J et al. Frequency of lower urinary tract injury after gastrointestinal surgery in the nationwide inpatient sample database. Am Surg. 2014;80(12):1216–1221. [PubMed] [Google Scholar]
- 92.Atallah S B, DuBose A C, Burke J P et al. Uptake of transanal total mesorectal excision in North America: initial assessment of a structured training program and the experience of delegate surgeons. Dis Colon Rectum. 2017;60(10):1023–1031. doi: 10.1097/DCR.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 93.Veltcamp Helbach M, Deijen C L, Velthuis S, Bonjer H J, Tuynman J B, Sietses C. Transanal total mesorectal excision for rectal carcinoma: short-term outcomes and experience after 80 cases. Surg Endosc. 2016;30(02):464–470. doi: 10.1007/s00464-015-4221-y. [DOI] [PubMed] [Google Scholar]
- 94.Tuech J-J, Karoui M, Lelong B et al. A step toward NOTES total mesorectal excision for rectal cancer: endoscopic transanal proctectomy. Ann Surg. 2015;261(02):228–233. doi: 10.1097/SLA.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 95.Burke J P, Martin-Perez B, Khan A et al. Transanal total mesorectal excision for rectal cancer: early outcomes in 50 consecutive patients. Color Dis. 2016;18(06):570–577. doi: 10.1111/codi.13263. [DOI] [PubMed] [Google Scholar]
- 96.Chen C-C, Lai Y-L, Jiang J-K et al. Transanal total mesorectal excision versus laparoscopic surgery for rectal cancer receiving neoadjuvant chemoradiation: a matched case-control study. Ann Surg Oncol. 2016;23(04):1169–1176. doi: 10.1245/s10434-015-4997-y. [DOI] [PubMed] [Google Scholar]
- 97.Buchs N C, Wynn G, Austin R et al. A two-centre experience of transanal total mesorectal excision. Colorectal Dis. 2016;18(12):1154–1161. doi: 10.1111/codi.13394. [DOI] [PubMed] [Google Scholar]
- 98.Zorron R, Phillips H N, Coelho D, Flach L, Lemos F B, Vassallo R C. Perirectal NOTES Access. Surg Innov. 2012;19(01):11–19. doi: 10.1177/1553350611409956. [DOI] [PubMed] [Google Scholar]


