Abstract
Background
Most guidelines for major depressive disorder recommend initial treatment with either a second-generation antidepressant (SGA) or cognitive behavioral therapy (CBT). Although most trials suggest that these treatments have similar efficacy, their health economic implications are uncertain.
Objective
To quantify the cost-effectiveness of CBT versus SGA for initial treatment of depression.
Design
Decision analytic model.
Data Sources
Relative effectiveness data from a meta-analysis of randomized controlled trials; additional clinical and economic data from other publications.
Target Population
Adults with newly diagnosed major depressive disorder in the United States.
Time Horizon
1 to 5 years.
Perspectives
Health care sector and societal.
Intervention
Initial treatment with either an SGA or group and individual CBT.
Outcome Measures
Costs in 2014 U.S. dollars, quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios.
Results of Base-Case Analysis
In model projections, CBT produced higher QALYs (3 days more at 1 year and 20 days more at 5 years) with higher costs at 1 year (health care sector, $900; societal, $1500) but lower costs at 5 years (health care sector,−$1800; societal, −$2500).
Results of Sensitivity Analysis
In probabilistic sensitivity analyses, SGA had a 64% to 77% likelihood of having an incremental cost-effectiveness ratio of $100 000 or less per QALY at 1 year; CBT had a 73% to 77% likelihood at 5 years. Uncertainty in the relative risk for relapse of depression contributed the most to overall uncertainty in the optimal treatment.
Limitation
Long-term trials comparing CBT and SGA are lacking.
Conclusion
Neither SGAs nor CBT provides consistently superior cost-effectiveness relative to the other. Given many patients’ preference for psychotherapy over pharmacotherapy, increasing patient access to CBT may be warranted.
Primary Funding Source
Department of Veterans Affairs, National Institute of Mental Health
Major depressive disorder (MDD) causes substantial morbidity worldwide, contributing 4.2% of total years lived with a disability (1). In the United States, the prevalence of MDD is 7.3% (2), with an estimated $210.5 billion annual economic cost (3). The growing burden of MDD is increasingly managed by primary care physicians (4, 5).
Per American College of Physicians guidelines, adult patients with MDD should receive either cognitive behavioral therapy (CBT) or a second-generation antidepressant (SGA), including selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors, and atypical agents (such as bupropion and mirtazapine) (6, 7). These recommendations are based on a meta-analysis showing no statistically significant differences between these treatments in initial efficacy, relapse rates, or discontinuation due to adverse events (7). Although 70% of patients with MDD prefer psychotherapy to pharmacotherapy (8), nationally representative data indicate that fewer than one quarter receive CBT or other types of psychotherapy (9).
One explanation for low psychotherapy use is its higher cost relative to pharmacotherapy (10, 11). A single CBT session costs more than $100 (12), whereas frequently used SGAs cost less than $100 per year (13, 14). Costs are an important consideration amid efforts to slow the growth of U.S. health care spending; likewise, guidelines recommend that costs should be 1 component of shared decision making between patients and providers selecting MDD treatment (6, 15).
Prior studies reached conflicting conclusions regarding the cost-effectiveness of CBT and SGA for adults with MDD. In a randomized controlled trial among U.S. patients with depression, Schoenbaum and colleagues found that, compared with SGAs, a CBT-based quality improvement program improved depression outcomes with an acceptable cost-effectiveness ratio (16, 17). In contrast, in a trial among low-income, minority women with MDD, Revicki and colleagues (17, 18) found that SGA improved depression outcomes and was cost-effective relative to CBT. Similarly in the United Kingdom, 1 trial found that Internet-based CBT improved outcomes and was cost-effective relative to usual care (19). In contrast, a U.K.-based modeling study found limited evidence and substantial uncertainty in the cost-effectiveness of CBT versus SGA (20). These studies provide reliable estimates of the cost-effectiveness of these interventions among the populations they evaluated; however, generalizability to the U.S. population with MDD is uncertain.
To address these limitations, we conducted a decision analytic modeling evaluation of the cost-effectiveness of CBT versus SGA for U.S. adults with newly diagnosed MDD. Our model integrates the best available estimates of the relative benefits and harms of CBT and SGA (7) with nationally representative cost data. Our analysis aims to estimate the 1- and 5-year cost-effectiveness of CBT versus SGA, along with overall uncertainty in the health economic consequences of each treatment, and to determine what additional evidence could reduce this uncertainty, thus better informing guidelines and policy surrounding depression treatment.
METHODS
Overview
We used a previously described decision analytic model (21) to simulate clinical and economic consequences of CBT versus SGA as initial treatment of adults with newly diagnosed MDD. We did not evaluate other treatments, such as combined treatment, other psychotherapies, or complementary and alternative medicine, because of lower quality of evidence regarding their benefits and harms (7). In describing our methods and results, we adhered to the 2013 Consolidated Health Economic Evaluation Reporting Standards (22).
At 1- and 5-year time horizons, we calculated average quality-adjusted life-years (QALYs), a measure combining survival and health-related quality of life (23), and average costs (in 2014 U.S. dollars) for each treatment from health care sector and societal perspectives (24). The 5-year time horizon allows many long-term benefits and costs to accrue without extending beyond the range of available outcomes data and most prior MDD modeling studies (25, 26).
Using the above outcomes, we then calculated the incremental cost-effectiveness ratio (ICER) of CBT as the ratio of its incremental cost relative to SGA (in 2014 U.S. dollars) to its incremental benefit (in QALYs). We designated CBT as “cost saving” if it increased QALYs and decreased costs and “dominated” if it decreased QALYs and increased costs. In the United States, medical interventions with ICERs below $50 000 to $150 000 per QALY may be considered “cost-effective” (27, 28). Hence, in our analysis, we considered treatments with ICER of $100 000 or less per QALY to be cost-effective.
We also calculated net monetary benefit (NMB), a metric that combines health and economic outcomes into a unified dollar figure (23). The NMB is calculated for a given treatment strategy (for example, CBT) as:
where QCBT is projected QALYs for CBT, CCBT is projected cost, and WTP is the willingness-to-pay threshold ($100 000 per QALY). A positive incremental NMB (for example, NMBCBT – NMBSGA) indicates an ICER below the willingness-to-pay threshold. Because NMB enables straightforward calculation and interpretation of CIs (29), we present cost-effectiveness CIs using incremental NMB rather than ICERs. In sensitivity analysis, we designate the strategy with greater NMB as “preferred.”
Model Description
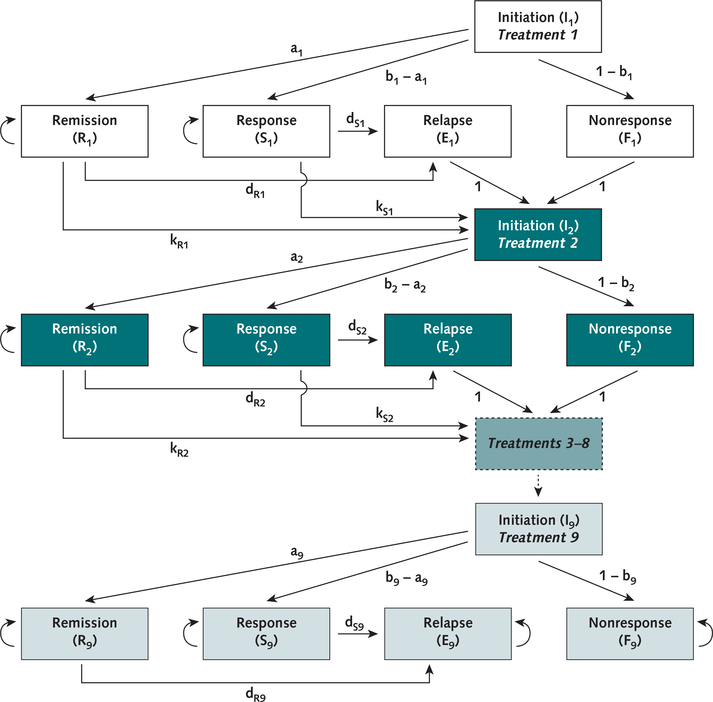
We used a deterministic, state-transition model (21) implemented in Excel 2013 (Microsoft) to simulate MDD across several treatments (Figure 1). We summarize the model’s structure here, with additional details in the Supplement (available at Annals.org).
Figure 1. Model structure.
Structure of the model used for the analysis. Health states are represented by boxes, and transition probabilities between states are represented by arrows. Each group of boxes of the same color represents 1 treatment; treatments 3 through 8 are represented by a single box with a dashed outline. For clarity, mortality probabilities are omitted from the diagram; patients in every model state are subject to a probability of mortality with each time-step. Additional information on the mathematical structure of the model is provided in the Supplement.
In the model, simulated identical patient cohorts with newly diagnosed MDD initiate treatment with either CBT or SGA; patients with nonresponse or relapse switch or augment their previously used medication or psychotherapy, up to a maximum of 9 treatments (30). After first-line CBT or SGA, the model does not explicitly specify particular medications or psychotherapies; instead, subsequent treatments reflect the aggregate costs and effectiveness of the wide variety of medications and therapies used for patients with treatment-resistant depression (30, 31).
Each treatment within the model includes 5 health states: initiation (the first month of a given treatment); remission (near-complete recovery of depression, defined by score on a validated symptom rating scale [for example, 16-item Quick Inventory of Depressive Symptomatology ≤5]) (31); response (partial recovery of depression [for example, ≥50% reduction in Hamilton Depression Rating Scale]) (32); nonresponse (initial lack of response or remission); and relapse (return of depression symptoms after initial response or remission) (31).
Patients in nonresponse and relapse states move to the subsequent treatment in the next monthly model cycle. Patients in remission and response states experience a monthly probability of discontinuation due to adverse events, which results in advancing to the next treatment.
Model Inputs
Table 1 presents base-case model input parameter values, CIs, and sources (7, 12–14, 24, 30–45).
Table 1.
Model Input Data
| Variable | Base Case (95% CI) | Distribution | References |
|---|---|---|---|
| General inputs, % | |||
| Annual discount rate | 3 | – | 24 |
| Annual mortality probability | 0.479 (0.446 to 0.515) | Normal | 31, 44, 45 |
| First-line treatment variables | |||
| Initial remission probability | |||
| SGA, % | 39.7 (32.1 to 47.8) | Logit-normal | 32 |
| CBT, relative risk vs. SGA | 1.02 (0.76 to 1.37) | Log-normal | 7 |
| Initial response probability | |||
| SGA, % | 63.1 (55.3 to 70.3) | Logit-normal | 32 |
| CBT, relative risk vs. SGA | 1.11 (0.93 to 1.32) | Log-normal | 7 |
| Annual relapse probability | |||
| SGA, % | 38.1 (34.0 to 42.2) | Beta | 33 |
| CBT, relative risk vs. SGA | 0.73 (0.26 to 2.08) | Log-normal | 7, 34, 35 |
| Annual discontinuation due to adverse event probability | |||
| SGA, % | 24.9 (15.1 to 39.1) | Logit-normal | 7, 36–38 |
| CBT, relative risk vs. SGA | 0.40 (0.06 to 2.50) | Log-normal | 7 |
| Subsequent treatment variables | |||
| Initial remission probability(relative risk vs. first-line SGA) | |||
| Treatment 2 | 0.93 (0.86 to 1.00) | Log-normal | 31 |
| Treatment 3 | 0.77 (0.70 to 0.85) | Log-normal | 31 |
| Treatment 4 | 0.35 (0.27 to 0.45) | Log-normal | 31 |
| Treatments 5–9* | 0.33 (0.21 to 0.52) | Log-normal | 31 |
| Initial response probability (relative risk vs. first-line SGA) | |||
| Treatment 2 | 0.77 (0.73 to 0.81) | Log-normal | 31 |
| Treatment 3 | 0.48 (0.44 to 0.53) | Log-normal | 31 |
| Treatment 4 | 0.27 (0.21 to 0.33) | Log-normal | 31 |
| Treatment 5–9* | 0.26 (0.17 to 0.39) | Log-normal | 31 |
| Annual relapse probability, % | 38.1 (34.0 to 42.2) | Beta | 33 |
| Annual discontinuation due to adverse event probability, % | 24.9 (15.1 to 39.1) | Logit-normal | 7, 36–38 |
| Utility with depression | |||
| Remission | 0.85 (0.83 to 0.87) | Normal | 39 |
| Response | 0.72 (0.65 to 0.79) | Normal | 39 |
| Nonresponse, relapse, initiation | 0.58 (0.50 to 0.66) | Normal | 39 |
| Costs, $ | |||
| First-line treatment, per month | |||
| SGA, months 1–3 | 76 (46 to 105) | Normal | 12–14 |
| SGA, months 4+ | 28 (17 to 39) | Normal | 12–14 |
| CBT, months 1–3 | 280 (170 to 390) | Normal | 12, 40 |
| CBT, months 4+ | 140 (85 to 195) | Normal | 12, 40 |
| Other depression, per year | |||
| Treatment 1† | 6747 (6333 to 7161) | Normal | 41 |
| Treatment 2 | 8471 (8057 to 8884) | Normal | 41 |
| Treatment 3 | 8913 (7524 to 10 286) | Normal | 30 |
| Treatment 4 | 12 862 (12 331 to 13 377) | Normal | 30 |
| Treatment 5 | 12 753 (12 159 to 13 330) | Normal | 30 |
| Treatment 6 | 14 688 (13 830 to 15 531) | Normal | 30 |
| Treatment 7 | 15 984 (14 626 to 17 326) | Normal | 30 |
| Treatment 8 | 16 998 (14 907 to 19 074) | Normal | 30 |
| Treatment 9 | 18 185 (14 501 to 21 853) | Normal | 30 |
| Indirect (productivity), per year | |||
| Remission | 2099 (1359 to 2840) | Normal | 42, 43 |
| Response | 5848 (3499 to 8197) | Normal | 42, 43 |
| Nonresponse, relapse, initiation | 11 755 (8190 to 15 321) | Normal | 42, 43 |
CBT = cognitive behavioral therapy; SGA = second-generation antidepressant.
Remission and response probabilities from step 4 of the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) trial are assumed to apply to treatments 5 to 9 in the model.
Other depression cost for treatment 1 excludes depression-related outpatient cost and depression-related pharmaceutical cost because they are accounted for separately in the first-line treatment cost. For subsequent treatments, these cost components are included.
General
The cycle length is 1 month, ensuring patients do not discontinue a treatment because of nonresponse until after an adequate trial lasting 8 or more weeks (with 1 cycle in initiation and ≥1 cycle in remission, response, or nonresponse) (31, 40). Given this cycle length, all probabilities derived from the sources described below were converted to monthly probabilities for use in the model. All costs are presented in 2014 U.S. dollars. Cost data from earlier years were inflated using medical expenditure indices from the U.S. Bureau of Economic Analysis (46, 47), and cost data from later years were deflated using the personal consumption expenditures price index (48). Future costs and QALYs in the model are discounted at an annual rate of 3% to reflect their present value (24).
Relative Benefits and Harms of CBT Versus SGA
Data on the relative benefits and harms of CBT versus SGA were drawn from a meta-analysis by Gartlehner and colleagues (7), the primary evidence synthesis underlying recent American College of Physicians guidelines (6). Mean relative risks (RRs) for CBT versus SGA are 1.02 (95% CI, 0.76 to 1.37) for initial remission, 1.11 (CI, 0.93 to 1.32) for initial response, and 0.40 (CI, 0.06 to 2.50) for discontinuation due to adverse events (7). A pooled RR estimate is not provided for relapse, so we performed a meta-analysis of the cited studies (34, 35) using the restricted maximum likelihood method employed by Gartlehner and colleagues (7). This approach yielded a mean RR of relapse of 0.73 (CI, 0.26 to 2.08) for CBT versus SGA (Supplement Figure 1, available at Annals.org). To generate estimates of the likelihood of remission, response, or relapse with CBT within the model, these RRs are applied to the absolute estimates of SGA efficacy described below.
Treatment Efficacy
First-line SGA remission (39.7%) and response (63.1%) probabilities (with remission treated as a subset of response) were drawn from a meta-analysis of SSRI efficacy (32). Like above, we used a restricted maximum likelihood meta-analysis to estimate overall remission and response rates from the 15 studies that evaluated both remission and response (Supplement Figure 2, available at Annals.org). We derived subsequent treatment remission and response probabilities from the STAR*D (Sequenced Treatment Alternatives to Relieve Depression) trial, a pragmatic randomized trial that evaluated efficacy of several depression treatments across 4 treatment steps (31). However, remission and response rates in the first step of STAR*D were markedly lower than those seen in 2 meta-analyses of SSRI efficacy, likely reflecting prior treatment experience, so we used steps 1 to 4 of STAR*D for treatments 2 to 5 in our model (32, 49). The RRs for treatments 2 through 5 compared with first-line SGA ranged from 0.93 to 0.33 for remission and 0.77 to 0.26 for response (31).
The annual probability of relapse after initial response or remission (38.1%) was drawn from an individual patient-level meta-analysis of relapse trajectories during SGA treatment (33). We derived the annual probability of discontinuation due to adverse events (24.9%) by pooling results from the SGA groups of 3 trials (36–38) cited by Gartlehner and colleagues (7). The same probability is applied for first-line SGA and subsequent treatments.
Population Characteristics and Mortality
We simulated a cohort with 62.2% women and a mean age of 40.7 years (SD, 13.2) on the basis of the age and sex distributions in STAR*D (31). We applied age- and sex-specific mortality probabilities from the 2013 Centers for Disease Control and Prevention life tables to these distributions (44), with a relative mortality rate of 1.58 (CI, 1.47 to 1.70) for people with MDD compared with the general population (45). Aggregating across this age and sex distribution yielded an average annual mortality probability of 0.00479. We made the simplifying assumption that this mortality rate would remain constant during the 5-year analysis. On the basis of meta-analyses showing no significant change in suicide risk with antidepressants (50) or psychotherapy (51), we did not model an effect of depression treatment on suicide mortality.
Health Utility
We used utility values of 0.85, 0.72, and 0.58 for patients in remission; response; and nonresponse, relapse, or initiation states, respectively. These values were derived from a prospective study of patients treated for MDD (39) and are consistent with utility estimates from clinical trials (52, 53).
Depression Treatment Costs
To determine depression treatment costs, we combined microcosting of first-line CBT and SGA treatment (estimating the precise number of resources used by individuals) with gross costing of other components of health care cost (applying aggregate costs measured from patient cohorts) (54). First-line SGA treatment cost has 2 components: physician visits ($74 each in 2017, CPT [Current Procedural Terminology] code 99213) (12) and antidepressant medications. On the basis of 2017 National Average Drug Acquisition Costs (14) weighted by use frequency from a large insurance claims database (13), medication costs were $48 per year (Supplement Table 2, available at Annals.org). On the basis of national guidelines, we assumed patients would have monthly medication management visits during months 1 to 3 of treatment and quarterly visits thereafter (40).
First-line CBT cost has 3 components: physician visits ($74 each in 2017, CPT code 99213), individual psychotherapy ($128 per 1-hour session in 2017, CPT code 90837), and group psychotherapy ($26 per 1-hour session in 2017, CPT code 90853) (12). On the basis of national guidelines and trial protocols, we assumed patients would have 2 physician visits, 8 group CBT sessions, and 4 individual CBT sessions during months 1 to 3; thereafter, they would have individual CBT sessions every month and physician visits every 4.5 months (34, 40). As practice patterns vary regarding number of CBT sessions and individual versus group settings, we varied these assumptions widely in sensitivity analysis (40, 55).
Combining these components yielded monthly costs of $76 for SGA and $280 for CBT during months 1 to 3, and $28 for SGA and $140 for CBT thereafter. Because our microcosting analysis did not produce uncertainty estimates, we assumed an SE equal to 20% of the mean for each estimate, which we varied between 10% and 30% in sensitivity analyses. This is based on the SEs of our indirect cost estimates, which were the largest of our cost components (42).
Other health care costs were derived from 2 studies that used MarketScan insurance claims data to estimate aggregate costs for people with depression, stratified by number of prior treatments (30, 41). Because first-line SGA and CBT costs were captured by microcosting, we excluded depression-related pharmacy and outpatient costs from gross first-line treatment costs. This yielded annual health care costs ranging from $6747 to $18 185 for patients receiving treatments 1 to 9.
Indirect Costs
When evaluating cost-effectiveness from a health care sector perspective, we considered medical costs for the formal health care sector only; when taking a societal perspective, we also considered patient time and productivity costs (Supplement Table 1, available at Annals.org) (24). We valued patients’ time at $27 per hour, the average U.S. hourly earnings (43). We assumed each CBT session averages 2 hours of patient time, and each physician visit averages 1 hour (including transportation). Finally, on the basis of a nationally representative observational study, we incorporated severity-dependent productivity losses of 1.5, 4.2, and 8.4 hours per week for depression in remission, response, and nonresponse states, respectively (42).
Sensitivity and Uncertainty Analyses
We used several methods of sensitivity and uncertainty analysis to assess the robustness of our findings to alternative modeling assumptions, quantify uncertainty in our results, and establish which model parameters contributed the most to uncertainty.
In probabilistic sensitivity analysis, the value of each model parameter was drawn at random from a distribution reflecting the uncertainty in its estimated value (Table 1), and the model was run using these randomly selected parameters. We repeated this process 10 000 times to ensure an adequate sampling of parameter values. We used outcomes across these 10 000 runs to calculate CIs for model results and to estimate the likelihood that CBT or SGA was preferred (that is, produced a greater NMB) (29).
In scenario sensitivity analysis, we tested alternative modeling assumptions related to cost, treatment efficacy, and mortality. We used probabilistic sensitivity analysis to estimate the likelihood of CBT or SGA being preferred under each alternative assumption. First, we used a different source for background depression health care costs, which stratified aggregate costs by health state rather than number of treatments; annual costs ranged from $12 389 for remission to $17 551 for nonresponse (56). Second, we simulated using only group CBT sessions or only individual CBT sessions. Third, we assessed calculating efficacy of treatments after first-line SGA and CBT on the basis of a 19% reduction in odds of response and remission with each successive treatment (57), rather than using STAR*D data (31). Fourth, we increased relapse rate by a relative 15% with each successive treatment, reflecting greater relapse rates in more treatment-experienced patients seen in STAR*D (31). Fifth, we broadened our definition of treatment discontinuation to include all-cause discontinuation, rather than discontinuation specifically due to adverse events. We simulated an annual all-cause discontinuation probability of 46.0% with SGA and a mean RR (CI) for CBT versus SGA of 1.00 (CI, 0.55 to 1.81) (7). Sixth, we included vilazodone in our SGA cost analysis, increasing annual cost from $48 to $72 (Supplement Table 2).
Finally, we used value-of-information analysis to identify the parameters that contributed most to overall uncertainty in model outcomes. We estimated expected value of partial perfect information (EVPPI) for groups of parameters using the generalized additive model regression method developed by Strong and colleagues (58), implemented using the R software package (The R Foundation). Quantitatively, EVPPI represents the upper bound on the monetary value of better informing a treatment decision by eliminating uncertainty in specified model parameters (59). The EVPPI can also be interpreted as measuring the contribution of a given parameter to overall decision uncertainty; hence, EVPPI can help prioritize future research that will most efficiently reduce this uncertainty (60).
Role of the Funding Source
The Department of Veterans Affairs and the National Institute of Mental Health did not participate in the design of the study, the analysis and interpretation of the data, or the preparation and submission of the manuscript for publication.
RESULTS
Model Validation
To test the validity of our model, we compared model-predicted outcomes to results from independent observational studies. Over 2 years, the model estimates a per-patient annual cost of $9484 with first-line SGA and $9820 with first-line CBT. For comparison, recent studies of Medicaid patients (61) and primarily privately insured patients (62) receiving SGA or other treatment methods yielded annual costs of $11 263 and $9287, respectively. Over 5 years, the model projects that patients spend 47.6% (SGA) or 40.7% (CBT) of life-years depressed (that is, without remission or response), compared with 46% (CI, 34% to 58%), as reported in a meta-analysis of long-term studies of depression outcomes (25).
Base-Case Results
Table 2 presents base-case health and economic outcomes. Over a 1-year time horizon, CBT increased quality-adjusted survival relative to SGA by 3 quality-adjusted life-days or 0.008 QALYs (CI, −0.013 to 0.025). Mean costs were increased by $900 (CI, $500 to $1400) from a health care sector perspective and $1500 (CI, $500 to $2500) from a societal perspective. Using a threshold of $100 000 per QALY, CBT would not be considered cost-effective under either perspective, with ICERs of $119 000 per QALY (health care sector) and $186 000 per QALY (societal). However, the CIs for the incremental NMB of CBT were −$2400 to $1600 (health care sector) and −$3400 to $1600 (societal), indicating some likelihood that CBT could be cost-effective.
Table 2.
Health and Economic Outcomes
| Time Horizon | Base Case |
||
|---|---|---|---|
| SGA | CBT | CBT vs. SGA (95% CI) | |
| 1 y | |||
| Costs, $ | |||
| Health care sector | 8100 | 9000 | 900 (500 to 1400) |
| Societal | 14 600 | 16 100 | 1500 (500 to 2500) |
| Quality-adjusted survival, QALYs | 0.708 | 0.715 | 0.008 (−0.013 to 0.025) |
| ICER, $/QALY | |||
| Health care sector | – | – | 119 000 |
| Societal | – | – | 186 000 |
| NMB, $ | |||
| Health care sector | – | – | −200 (−2400 to 1600) |
| Societal | – | – | −700 (−3400 to 1600) |
| 5 y | |||
| Costs, $ | |||
| Health care sector | 57 200 | 55 400 | −1800 (−6800 to 3800) |
| Societal | 90 100 | 87 600 | −2500 (−10 400 to 6200) |
| Quality-adjusted survival, QALYs | 3.238 | 3.293 | 0.055 (−0.044 to 0.160) |
| ICER, $/QALY | |||
| Health care sector | – | – | Cost saving |
| Societal | – | – | Cost saving |
| NMB, $ | |||
| Health care sector | – | – | 7300 (−8100 to 21 700) |
| Societal | – | – | 8000 (−10 400 to 25 300) |
CBT = cognitive behavioral therapy; ICER = incremental cost-effectiveness ratio; NMB = net monetary benefit; QALY = quality-adjusted life-year; SGA = second-generation antidepressant.
Over a 5-year time horizon, CBT increased QALYs by 0.055 (CI, −0.044 to 0.160) or 20 quality-adjusted life-days and reduced costs by approximately $2000 relative to SGA. In the base case, CBT was cost saving under both the health care sector and societal perspectives. However, CIs for the incremental NMB of CBT were −$8100 to $21 700 (health care sector) and −$10 400 to $25 300 (societal), indicating some uncertainty in CBT’s cost-effectiveness.
Sensitivity and Uncertainty Analyses
In probabilistic sensitivity analyses, SGA had a 64% to 77% chance of being preferred at 1 year, depending on perspective (Supplement Figure 3, available at Annals.org). Cognitive behavioral therapy became more likely than SGA to be preferred at time horizons of 1.5 to 2 years. At 5 years, CBT had a 73% to 77% likelihood of being preferred.
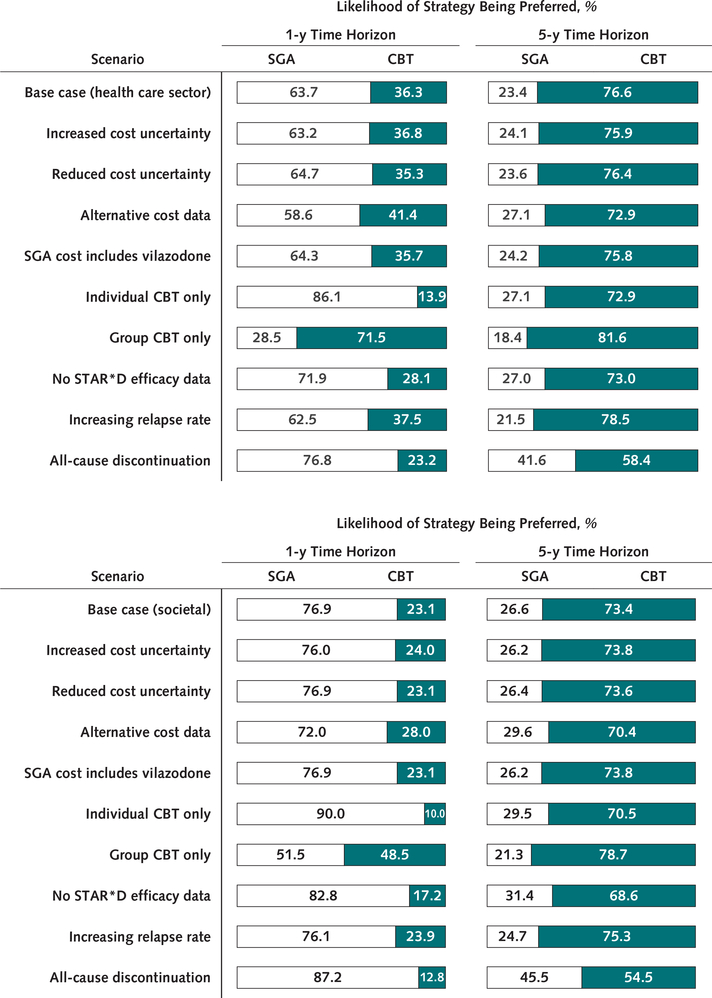
In scenario sensitivity analyses, SGA had 55% or greater likelihood of being preferred at 1 year under all scenarios except when only group CBT was used. At 5 years, CBT had 65% or greater likelihood of being preferred except when all-cause discontinuation was modeled, in which case it decreased to 55% to 58% (Figure 2).
Figure 2. Scenario sensitivity analyses.
Bars show the percentage of 10 000 probabilistic model runs in which either SGA or CBT is the preferred treatment strategy (i.e., that which produces the greatest net monetary benefit), at a willingness-to-pay threshold of $100 000 per quality-adjusted life-year. Results are shown for both 1- and 5-year time horizons. The vertical axis shows the scenario being modeled, indicating a change in either parameter values or model structure relative to the base case. CBT = cognitive behavioral therapy; SGA = second-generation antidepressant; STAR*D = Sequenced Treatment Alternatives to Relieve Depression. Top. Results from a health care sector perspective. Bottom. Results from a societal perspective. Scenarios include increased or reduced cost uncertainty (SEs of first-line SGA and CBT cost estimates are increased or reduced to 30% or 10% of the mean); alternative cost data (annual background depression costs of $12 389 for remission and $17 551 for nonremission); SGA cost includes vilazodone (vilazodone is incorporated into SGA costing analysis, increasing annual cost of SGA from $48 to $72); individual or group CBT only (exclusively individual sessions or exclusively group sessions are used to calculate CBT costs); no STAR*D efficacy data (odds of remission and response are reduced by 19% with each successive treatment rather than using STAR*D data on remission and response rates); increasing relapse rate (by a relative 15% with each successive treatment); all-cause discontinuation (all-cause discontinuation [rather than discontinuation due to adverse events] is simulated; annual probability is 46.0% for SGA, with a relative risk of 1.00 [95% CI, 0.55 to 1.81] for CBT vs. SGA).
In the value-of-information analysis (Supplement Figure 4, available at Annals.org), we found that relative initial efficacy of CBT versus SGA (EVPPI, $53 to $112) and risk for relapse with CBT versus SGA (EVPPI, $2 to $56) were the 2 most influential parameters during a 1-year time horizon. Over 5 years, risk for relapse with CBT versus SGA contributed most to overall decision uncertainty (EVPPI, $564 to $781), followed by RR for discontinuation due to adverse events (EVPPI, $324 to $457).
DISCUSSION
We used a decision analytic model to evaluate the cost-effectiveness of CBT versus SGA for initial treatment of MDD, integrating data from a recent meta-analysis of the relative benefits and harms of these treatments (7). We found that neither treatment is consistently superior from a cost-effectiveness perspective. At 1 year, there was approximately 70% likelihood that SGA was the preferred treatment, whereas at 5 years, there was approximately 75% likelihood that CBT was preferred. Of note, this uncertainty in the preferred treatment is consistent with prior U.S.-based trials, which have yielded differing conclusions about the cost-effectiveness of CBT versus SGA (16, 18).
Our findings have 3 main implications for varying stakeholders. First, for individual providers and patients, our findings lend economic support to the American College of Physicians’ conclusion that either SGA or CBT is a reasonable initial treatment of MDD (6). Given that neither treatment can be dismissed on the basis of its cost-effectiveness, shared decision making with consideration of patients’ values and preferences is essential (6, 15).
Second, for payers and policymakers, our results highlight the potential for long-term cost savings with CBT. Although not statistically significant, our base-case analysis projected an $1800 lower health care sector cost per patient treated with CBT at 5 years. Using a conservative estimate of 2 million U.S. patients initiating depression treatment per year (63, 64), moving from current (<25%) (9) to patient-preferred (70%) (8) levels of CBT use could thus save more than $1.5 billion after 5 years. Realizing these cost savings would require overcoming barriers, including limited availability and geographic accessibility of psychotherapy providers (10, 65), reimbursement schemes favoring pharmacotherapy over psychotherapy (66, 67), and CBT’s high initial cost. With concerted effort, however, some health systems have bucked the trend of declining psychotherapy use. In the Veterans Health Administration, for example, psychotherapy use for depression increased from 20% to 26% between 2004 and 2010 (68).
However, these projected cost savings come with substantial uncertainty, which leads to the third major implication of our findings. For clinical researchers and funding agencies, certain topics of future research are critical to better inform our understanding of the health economic consequences of CBT versus SGA. We identified the relative relapse rate with CBT versus SGA as the primary driver of decision uncertainty; this reflects both the importance of long-term treatment durability to patient outcomes and the dearth of available evidence on long-term durability of CBT versus SGA (7). Our findings suggest that rectifying this evidence gap should have a high priority for those seeking to better inform initial MDD treatment.
There are several limitations to our findings. Projecting 5-year outcomes requires extrapolation beyond available data on the benefits and harms of CBT versus SGA, which primarily reflect time horizons of 1 year or less (7). In interpreting our results, one must balance the greater patient and health system relevance of longer horizons against the greater uncertainty that comes with extrapolation (28). Although our model’s input data does not extend to 5 years, validation against independent long-term results suggests the model remains accurate over this time horizon (25).
Next, it is important to recognize that CBT is practiced in both group and individual settings with varying session frequency, therapist training, and efficacy (16, 18, 34), and a physician may have little control over locally available CBT offerings. We attempted to capture practice variation in sensitivity analysis, but our results may not be applicable to every CBT intervention.
Finally, we note several limitations to our model input data. First, although we inflated them to 2014 values, much of our cost data is more than a decade old (30, 42); however, our model’s cost outcomes are well validated by more recent data (61, 62) and are robust to sensitivity analyses using alternative data sources. Second, our estimates of the relative efficacy of CBT versus SGA are based on relatively small sample sizes, with accordingly broad CIs (7). This translates into substantial uncertainty in our overall conclusions and does not permit stratification by potentially clinically important factors, such as depression severity (55).
In this decision analytic modeling study, we found that neither CBT nor SGA provides consistently superior health economic value in the initial treatment of MDD in the United States. In the absence of clear superiority of either treatment, shared decision making incorporating patient preferences is critical. Given many patients’ preference for psychotherapy over pharmacotherapy, efforts to improve patients’ access to CBT are warranted.
From University of Michigan Medical School, Ann Arbor, Michigan (E.M.M.), Harvard Medical School and Massachusetts General Hospital, Boston, and McLean Hospital, Belmont, Massachusetts (E.L.R.); Center for Clinical Management Research, VA Ann Arbor Healthcare System, and University of Michigan Medical School, Ann Arbor, Michigan (S.V.); University of Michigan Medical School and the Center for Clinical Management Research, VA Ann Arbor Healthcare System, Ann Arbor, Michigan (M.V.); and University of Michigan Medical School, Center for Clinical Management Research, VA Ann Arbor Healthcare System, University of Michigan School of Public Health, and the Institute for Social Research, University of Michigan, Ann Arbor, Michigan (K.Z.).
Supplementary Material
Acknowledgments
Grant Support: This study was supported by the U.S. Department of Veterans Affairs (Health Services Research and Development grants CD2 07-206, IIR 10-176, and IIR 14-324) and the National Institute of Mental Health (Research Training and Career Development grant R25MH094612).
Disclosures: Dr. Ross reports grants from the National Institute of Mental Health during the conduct of the study. Dr. Vijan reports grants from Department of Veterans Affairs (CD2 07-206-1) during the conduct of the study. Dr. Zivin reports a grant from Department of Veterans Affairs (CD2 07-206-1) during the conduct of the study. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-1480.
Footnotes
Reproducible Research Statement: Study protocol and data set: Not available. Statistical code: Available from Dr. Ross (ericlr@umich.edu).
Contributor Information
Eric L. Ross, Massachusetts General Hospital, Wang 812, 15 Parkman Street, Boston, MA 02144..
Sandeep Vijan, University of Michigan Internal Medicine, 375 Briarwood Circle, Building 3, Ann Arbor, MI 48108..
Erin M. Miller, University of Michigan North Campus Research Center, Building 16, 2800 Plymouth Road, Room 228W, Ann Arbor, MI 48109..
Marcia Valenstein, University of Michigan North Campus Research Center, Building 16, 2800 Plymouth Road, Room 242E, Ann Arbor, MI 48109..
Kara Zivin, University of Michigan North Campus Research Center, Building 16, 2800 Plymouth Road, Room 228W, Ann Arbor, MI 48109..
References
- 1.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–59. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weinberger AH, Gbedemah M, Martinez AM, et al. Trends in depression prevalence in the USA from 2005 to 2015: widening disparities in vulnerable groups. Psychol Med. 2018;48:1308–15. doi: 10.1017/S0033291717002781 [DOI] [PubMed] [Google Scholar]
- 3.Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76:155–62. doi: 10.4088/JCP.14m09298 [DOI] [PubMed] [Google Scholar]
- 4.Olfson M The rise of primary care physicians in the provision of US mental health care. J Health Polit Policy Law. 2016;41:559–83. doi: 10.1215/03616878-3620821 [DOI] [PubMed] [Google Scholar]
- 5.Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J Gen Intern Med. 2006;21:926–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qaseem A, Barry MJ, Kansagara D; Clinical Guidelines Committee of the American College of Physicians. Nonpharmacologic versus pharmacologic treatment of adult patients with major depressive disorder. A clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;164:350–9. doi: 10.7326/M15-2570 [DOI] [PubMed] [Google Scholar]
- 7.Gartlehner G, Gaynes BN, Amick HR, et al. Comparative benefits and harms of antidepressant, psychological, complementary, and exercise treatments for major depression. An evidence report for a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;164:331–41. doi: 10.7326/M15-1813 [DOI] [PubMed] [Google Scholar]
- 8.McHugh RK, Whitton SW, Peckham AD, et al. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74:595–602. doi: 10.4088/JCP.12r07757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olfson M, Blanco C, Marcus SC. Treatment of adult depression in the United States. JAMA Intern Med. 2016;176:1482–91. doi: 10.1001/jamainternmed.2016.5057 [DOI] [PubMed] [Google Scholar]
- 10.Pfeiffer PN, Glass J, Austin K, et al. Impact of distance and facility of initial diagnosis on depression treatment. Health Serv Res. 2011;46:768–86. doi: 10.1111/j.1475-6773.2010.01228.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei W, Sambamoorthi U, Olfson M, et al. Use of psychotherapy for depression in older adults. Am J Psychiatry. 2005;162:711–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Medicare & Medicaid Services. Physician fee schedule. Accessed at www.cms.gov/apps/physician-fee-schedule/ on 17 July 2017.
- 13.Treviño LA, Ruble MW, Treviño K, et al. Antidepressant medication prescribing practices for treatment of major depressive disorder. Psychiatr Serv. 2017;68:199–202. doi: 10.1176/appi.ps.201600087 [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare & Medicaid Services. NADAC (National Average Drug Acquisition Cost). Accessed at https://data.medicaid.gov/Drug-Pricing-and-Payment/NADAC-National-Average-Drug-Acquisition-Cost-/a4y5-998d on 17 July 2017.
- 15.Burns RB, Smetana GW, Brady R. Should this patient receive an antidepressant? Grand rounds discussion from beth israel deaconess medical center. Ann Intern Med. 2017;167:192–9. doi: 10.7326/M17-0966 [DOI] [PubMed] [Google Scholar]
- 16.Schoenbaum M, Unützer J, Sherbourne C, et al. Cost-effectiveness of practice-initiated quality improvement for depression: results of a randomized controlled trial. JAMA. 2001;286:1325–30. [DOI] [PubMed] [Google Scholar]
- 17.Brettschneider C, Djadran H, Härter M, et al. Cost-utility analyses of cognitive-behavioural therapy of depression: a systematic review. Psychother Psychosom. 2015;84:6–21. doi: 10.1159/000365150 [DOI] [PubMed] [Google Scholar]
- 18.Revicki DA, Siddique J, Frank L, et al. Cost-effectiveness of evidence-based pharmacotherapy or cognitive behavior therapy compared with community referral for major depression in predominantly low-income minority women. Arch Gen Psychiatry. 2005;62:868–75. [DOI] [PubMed] [Google Scholar]
- 19.Hollinghurst S, Peters TJ, Kaur S, et al. Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry. 2010;197:297–304. doi: 10.1192/bjp.bp.109.073080 [DOI] [PubMed] [Google Scholar]
- 20.Koeser L, Donisi V, Goldberg DP, et al. Modelling the cost-effectiveness of pharmacotherapy compared with cognitive-behavioural therapy and combination therapy for the treatment of moderate to severe depression in the UK. Psychol Med. 2015;45:3019–31. doi: 10.1017/S0033291715000951 [DOI] [PubMed] [Google Scholar]
- 21.Ross EL, Zivin K, Maixner DF. Cost-effectiveness of electroconvulsive therapy vs pharmacotherapy/psychotherapy for treatment-resistant depression in the United States. JAMA Psychiatry. 2018;75:713–22. doi: 10.1001/jamapsychiatry.2018.0768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Husereau D, Drummond M, Petrou S, et al. ; ISPOR Health Economic Evaluation Publication Guidelines-CHEERS Good Reporting Practices Task Force. Consolidated health economic evaluation reporting standards (CHEERS)—explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16:231–50. doi: 10.1016/j.jval.2013.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York: Oxford Univ Pr; 2006. [Google Scholar]
- 24.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316:1093–103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 25.Forte A, Baldessarini RJ, Tondo L, et al. Long-term morbidity in bipolar-I, bipolar-II, and unipolar major depressive disorders. J Affect Disord. 2015;178:71–8. doi: 10.1016/j.jad.2015.02.011 [DOI] [PubMed] [Google Scholar]
- 26.Zimovetz EA, Wolowacz SE, Classi PM, et al. Methodologies used in cost-effectiveness models for evaluating treatments in major depressive disorder: a systematic review. Cost Eff Resour Alloc. 2012;10:1. doi: 10.1186/1478-7547-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–7. doi: 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- 28.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–22. doi: 10.1016/j.jacc.2014.03.016 [DOI] [PubMed] [Google Scholar]
- 29.Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ. 1998;7:723–40. [DOI] [PubMed] [Google Scholar]
- 30.Russell JM, Hawkins K, Ozminkowski RJ, et al. The cost consequences of treatment-resistant depression. J Clin Psychiatry. 2004; 65:341–7. [DOI] [PubMed] [Google Scholar]
- 31.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163:1905–17. [DOI] [PubMed] [Google Scholar]
- 32.Weinmann S, Becker T, Koesters M. Re-evaluation of the efficacy and tolerability of venlafaxine vs SSRI: meta-analysis. Psychopharmacology (Berl). 2008;196:511–20; discussion 521–2. [DOI] [PubMed] [Google Scholar]
- 33.Gueorguieva R, Chekroud AM, Krystal JH. Trajectories of relapse in randomised, placebo-controlled trials of treatment discontinuation in major depressive disorder: an individual patient-level data meta-analysis. Lancet Psychiatry. 2017;4:230–7. doi: 10.1016/S2215-0366(17)30038-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.David D, Szentagotai A, Lupu V, et al. Rational emotive behavior therapy, cognitive therapy, and medication in the treatment of major depressive disorder: a randomized clinical trial, posttreatment outcomes, and six-month follow-up. J Clin Psychol. 2008;64:728–46. doi: 10.1002/jclp.20487 [DOI] [PubMed] [Google Scholar]
- 35.Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008;76:468–77. doi: 10.1037/0022-006X.76.3.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeRubeis RJ, Hollon SD, Amsterdam JD, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry. 2005;62:409–16. [DOI] [PubMed] [Google Scholar]
- 37.Dimidjian S, Hollon SD, Dobson KS, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol. 2006;74:658–70. [DOI] [PubMed] [Google Scholar]
- 38.Mynors-Wallis LM, Gath DH, Day A, et al. Randomised controlled trial of problem solving treatment, antidepressant medication, and combined treatment for major depression in primary care. BMJ. 2000;320:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sapin C, Fantino B, Nowicki ML, et al. Usefulness of EQ-5D in assessing health status in primary care patients with major depressive disorder. Health Qual Life Outcomes. 2004;2:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Third edition. Accessed at http://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf on 27 August 2017. [PubMed] [Google Scholar]
- 41.Crown WH, Finkelstein S, Berndt ER, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry. 2002;63:963–71. [DOI] [PubMed] [Google Scholar]
- 42.Stewart WF, Ricci JA, Chee E, et al. Cost of lost productive work time among US workers with depression. JAMA. 2003;289:3135–44. [DOI] [PubMed] [Google Scholar]
- 43.Bureau of Labor Statistics. Current and real (constant 1982–1984 dollars) earnings for all employees on private nonfarm payrolls, seasonally adjusted. Accessed at www.bls.gov/news.release/realer.t01.htm on 22 April 2018.
- 44.Arias E, Heron M, Xu J. United States life tables, 2013. Natl Vital Stat Rep. 2017;66:1–64. [PubMed] [Google Scholar]
- 45.Cuijpers P, Vogelzangs N, Twisk J, et al. Comprehensive meta-analysis of excess mortality in depression in the general community versus patients with specific illnesses. Am J Psychiatry. 2014;171:453–62. doi: 10.1176/appi.ajp.2013.13030325 [DOI] [PubMed] [Google Scholar]
- 46.Bureau of Economic Analysis. Health care. Accessed at www.bea.gov/national/health_care_satellite_account.htm on 22 April 2018.
- 47.Dunn A, Grosse SD, Zuvekas SH. Adjusting health expenditures for inflation: a review of measures for health services research in the United States. Health Serv Res. 2018;53:175–96. doi: 10.1111/1475-6773.12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Federal Reserve Bank of St. Louis. Personal consumption expenditures. Accessed at https://fred.stlouisfed.org/series/DPCERG3A086NBEA on 20 October 2018.
- 49.Papakostas GI, Homberger CH, Fava M. A meta-analysis of clinical trials comparing mirtazapine with selective serotonin reuptake inhibitors for the treatment of major depressive disorder. J Psychopharmacol. 2008;22:843–8. doi: 10.1177/0269881107083808 [DOI] [PubMed] [Google Scholar]
- 50.Braun C, Bschor T, Franklin J, et al. Suicides and suicide attempts during long-term treatment with antidepressants: a meta-analysis of 29 placebo-controlled studies including 6,934 patients with major depressive disorder. Psychother Psychosom. 2016;85:171–9. doi: 10.1159/000442293 [DOI] [PubMed] [Google Scholar]
- 51.Cuijpers P, de Beurs DP, van Spijker BA, et al. The effects of psychotherapy for adult depression on suicidality and hopelessness: a systematic review and meta-analysis. J Affect Disord. 2013;144:183–90. doi: 10.1016/j.jad.2012.06.025 [DOI] [PubMed] [Google Scholar]
- 52.Benedict A, Arellano J, De Cock E, et al. Economic evaluation of duloxetine versus serotonin selective reuptake inhibitors and venlafaxine XR in treating major depressive disorder in Scotland. J Affect Disord. 2010;120:94–104. doi: 10.1016/j.jad.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 53.Mrazek DA, Hornberger JC, Altar CA, et al. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatr Serv. 2014;65:977–87. doi: 10.1176/appi.ps.201300059 [DOI] [PubMed] [Google Scholar]
- 54.Fishman PA, Hornbrook MC. Assigning resources to health care use for health services research: options and consequences. Med Care. 2009;47:S70–5. doi: 10.1097/MLR.0b013e3181a75a7f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parikh SV, Quilty LC, Ravitz P, et al. ; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 2. Psychological treatments. Can J Psychiatry. 2016;61:524–39. doi: 10.1177/0706743716659418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dennehy EB, Robinson RL, Stephenson JJ, et al. Impact of non-remission of depression on costs and resource utilization: from the COmorbidities and symptoms of DEpression (CODE) study. Curr Med Res Opin. 2015;31:1165–77. doi: 10.1185/03007995.2015.1029893 [DOI] [PubMed] [Google Scholar]
- 57.Amsterdam JD, Williams D, Michelson D, et al. Tachyphylaxis after repeated antidepressant drug exposure in patients with recurrent major depressive disorder. Neuropsychobiology. 2009;59:227–33. doi: 10.1159/000226611 [DOI] [PubMed] [Google Scholar]
- 58.Strong M, Oakley JE, Brennan A. Estimating multiparameter partial expected value of perfect information from a probabilistic sensitivity analysis sample: a nonparametric regression approach. Med Decis Making. 2014;34:311–26. doi: 10.1177/0272989X13505910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Briggs AH, Weinstein MC, Fenwick EA, et al. ; ISPOR-SMDM Modeling Good Research Practices Task Force. Model parameter estimation and uncertainty analysis: a report of the ISPOR-SMDM modeling good research practices task force working group-6. Med Decis Making. 2012;32:722–32. [DOI] [PubMed] [Google Scholar]
- 60.Campbell JD, McQueen RB, Libby AM, et al. Cost-effectiveness uncertainty analysis methods: a comparison of one-way sensitivity, analysis of covariance, and expected value of partial perfect information. Med Decis Making. 2015;35:596–607. doi: 10.1177/0272989X14556510 [DOI] [PubMed] [Google Scholar]
- 61.Olfson M, Marcus S, Benson C, et al. Health care costs of treatment-resistant depression in a Medicaid population (M22) In: International Society for Pharmacoeconomics and Outcomes Research 22nd Annual Meeting, Boston, Massaschusetts, 20–24 May 2017. [Google Scholar]
- 62.Gauthier G, Guérin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry. 2017;17:222. doi: 10.1186/s12888-017-1385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kessler RC, Berglund P, Demler O, et al. ; National Comorbidity Survey Replication. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R). JAMA. 2003;289:3095–105. [DOI] [PubMed] [Google Scholar]
- 64.Pence BW, O’Donnell JK, Gaynes BN. The depression treatment cascade in primary care: a public health perspective. Curr Psychiatry Rep. 2012;14:328–35. doi: 10.1007/s11920-012-0274-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hundt NE, Barrera TL, Mott JM, et al. Predisposing, enabling, and need factors as predictors of low and high psychotherapy utilization in veterans. Psychol Serv. 2014;11:281–9. doi: 10.1037/a0036907 [DOI] [PubMed] [Google Scholar]
- 66.Mojtabai R, Olfson M. National trends in psychotherapy by office-based psychiatrists. Arch Gen Psychiatry. 2008;65:962–70. doi: 10.1001/archpsyc.65.8.962 [DOI] [PubMed] [Google Scholar]
- 67.Wilk JE, West JC, Rae DS, et al. Patterns of adult psychotherapy in psychiatric practice. Psychiatr Serv. 2006;57:472–6. [DOI] [PubMed] [Google Scholar]
- 68.Mott JM, Hundt NE, Sansgiry S, et al. Changes in psychotherapy utilization among veterans with depression, anxiety, and PTSD. Psychiatr Serv. 2014;65:106–12. doi: 10.1176/appi.ps.201300056 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.