Abstract
Background
Acute kidney injury (AKI) significantly increases morbidity and mortality for hospitalized children, yet sociodemographic risk factors for pediatric AKI are poorly described. We examined sociodemographic differences in pediatric AKI amongst a national cohort of hospitalized children.
Methods
Secondary analysis of the most recent (2012) Kids’ Inpatient Database (KID) from the Agency for Healthcare Research and Quality. Study sample weights were used to obtain national estimates of AKI (defined by administrative data). KID is a nationally representative sample of pediatric discharges throughout the USA. Linear risk regression models were used to assess the relationship between our primary exposures (race/ethnicity, health insurance, household urbanization, gender, and age) and the diagnosis of AKI, adjusting for comorbidities.
Results
A total of 1,699,841 hospitalizations met our study criteria. In 2012, AKI occurred in approximately 12.3/1000 pediatric hospitalizations, which translates to almost 30,000 children nationally. Asian/Pacific Islander, African-American, and Hispanic children were at slightly increased risk for AKI compared to Caucasian children (adjusted risk difference (RD) 4.5 per 1000 hospitalizations, 95% confidence interval (CI) 2.9–6.0; 2.5/1000 hospitalizations, 95% CI 1.7–3.3; and 1.7/1000 hospitalizations, 95% CI 0.9–2.5, respectively). Uninsured children were more likely to suffer AKI compared to children with any health insurance (e.g., no insurance versus Medicaid: adjusted RD 14.4/1000 hospitalizations, 95% CI 12.7–16.2). Based on these national estimates, one episode of AKI might be prevented if 70 (95% CI 62–79) hospitalized children without insurance were provided with Medicaid.
Conclusions
Pediatric AKI occurs more fequently in racial minority and uninsured children, factors linked to lower socioeconomic status.
Keywords: Acute kidney injury, Child, Risk factors, Medicaid, Health services research
Introduction
Acute kidney injury (AKI) can be fatal if untreated, but, unlike chronic kidney disease (CKD), an AKI episode is usually reversible, assuming timely and accurate diagnosis and treatment. AKI, historically termed “acute renal failure,” is defined as an acute injury or sudden decrease in kidney function, which is currently diagnosed by changes in serum creatinine within 48 h to 7 days or oliguria for 6 h or more. Clinical factors associated with the development of AKI amongst hospitalized children include systemic illnesses, exposure to nephrotoxic medications, invasive procedures, and iodinated contrast [1].
Pediatric AKI is estimated to occur in approximately 5% of all hospitalized children and up to 25% of all critically ill children [2, 3]. AKI results inhighrates of short-term morbidity and mortality as well as increased hospital costs and length of stay [4, 5]. Moreover, up to 50–60% of patients may have long-term sequelae such as CKD or hypertension [6]. Knowing which subsets of patients or parameters indicate the greatest risk for AKI is paramount to instituting healthcare guidelines and policies aimed at decreasing these high rates.
Few studies have evaluated sociodemographic risk factors for AKI in pediatric populations. Those that have done so tend to focus on subsets of the pediatric population. One retrospective study in post-operative congenital cardiac surgery patients found that age less than 12 months was a risk factor for AKI [7]. Another study found that nephrotic children who were non-Caucasian were at higher risk of AKI than Caucasian children [8]. None have looked at race or other broad sociodemographic risk factors for AKI in a large, diverse pediatric population, yet we know from adult studies that those of minority populations or lower socioeconomic status have worse kidney outcomes [9–11].
We sought to determine if there are disparities in the incidence of AKI according to sociodemographic factors amongst a national pediatric cohort within the Hospital Care and Utilization Program (HCUP) Kids’ Inpatient Database (KID) [12]. HCUP KID provides detailed information on a nationally representative sample of pediatric inpatients. In high-income countries, over 99% of pediatric AKI is diagnosed in the inpatient setting [13].
Methods
Design
This study is a secondary analysis of the most recent release (2012) of KID datato assess for sociodemographic differences in the diagnosis of pediatric AKI (age 1–20 years). We used weighted sampling methods to obtain national estimates.
Data source
The KID, developed by the Agency for Healthcare Research and Quality’s HCUP, is the largest nationally representative sample of pediatric hospitalizations from > 4100 community hospitals (defined as short-term, general and specialty hospitals, excluding rehabilitation facilities) throughout the USA. The database includes discharge summary-level data only (i.e., limited demographics, ICD-9 billing codes, hospital outcome) and some limited data on hospital characteristics. Each unit of observation is a hospitalization, so it is possible for individuals to be included twice if they had two separate hospitalizations in 2012. However, AKI is also recurrent, and an individual is at risk for AKI with each hospitalization. For 2012, 44 states were included; not included were Alabama, Delaware, District of Columbia, Idaho, Maine, Mississippi, and New Hampshire.
Inclusion/exclusion criteria
All admitted children in the KID aged 1–20 years on admission were included. The database only provides age in years (not months or days). Therefore, children < 1 year of age were not included due to their potential different etiologies of AKI and higher likelihood of AKI coding errors due to the difficulty with AKI recognition. The kidneys in infants < 1 year are also still maturing and reaching their maximal functional potential. Patients were excluded if they had ICD-9 codes for renal transplants (v42.0), end-stage renal disease/chronic dialysis (585.6, 585.5, 792.5, v45.1, v45.11, v45.12, v56.0-v56.2, v56.31, v56.32, v56.8), or if they were missing data on primary diagnosis.
Outcome
AKI was a binary outcome and could occur at any point during the hospitalization. AKI was defined by ICD-9 codes (584.5–584.9, acute renal failure; 586, renal failure unspecified; 580.0, 580.4, 580.8, 580.9, acute glomerulonephritis; 593.9, acute renal disease; 866, injury to kidney with unspecified injury; 958.5, renal failure following crushing; 997.5, renal failure due to a medical procedure).
Exposures
Race/ethnicity was self-reported and categorized as Caucasian, African-American, Hispanic, Asian or Pacific Islander, Native American, and Other. Health insurance status was categorized as Private, Medicaid, Other, or No insurance. Household urbanization was defined in six categories (“central” metropolitan counties with ≥ 1 million population, “fringe” metropolitan counties with ≥ 1 million population, metropolitan counties with 250,000–999,999 population, metropolitan counties with 50,000–249,999 population, micropolitan counties (urban cluster population 10,000–49,999), or rural counties). Gender was dichotomous as either male or female. Age was continuous in years.
Covariates
In addition to the above sociodemographic factors, we felt that a child’s comorbidities may be a strong confounder in assessing some of the relationships. Comorbidities were defined by HCUP through the chronic condition indicator tool [14]. The tool was developed to facilitate health services research and readily determine if a patient has a chronic condition according to administrative data. The tool uses an advanced algorithm, originally reported by Hwang et al. [15], to classify all ICD-9 codes into chronic or not chronic conditions. Examples of included chronic conditions that may influence AKI are cancer, congenital heart disease, chronic kidney disease, cystic fibrosis, diabetes mellitus, diabetes insipidus, and inflammatory bowel disease. Prematurity is not included, and we attempted to evaluate prematurity as a separate comorbidity, but we felt the variable to be too inaccurate. Only 21 hospitalizations had this as a diagnosis code amongstthe 1,699,841 eligible hospitalizations.
Analysis
Descriptive statistics were calculated for patient demographics, hospital characteristics, and hospital region stratified by racial/ethnic differences as our primary exposure of interest. HCUP provides weights to allow for national estimates based on post-stratification of hospital ownership, bed size, teaching status, urbanization of hospital location, region, and status as a freestanding children’s hospital. Further information about the KID sampling design and procedures can be found at: http://www.hcup-us.ahrq.gov/kidoverview.org [12].
Given the large sample size and method of data collection, we were able to accurately determine the risk of AKI in this population. Hence, to give a broader understanding of the public health impact of our results, we present risk differences (RD) as our contrast estimate of choice, rather than relative risks or odds ratios that can be inflated depending on the true proportion affected. Therefore, linear risk regression models with binomial distributions were used to assess the relationship between our primary sociodemographic exposures and the diagnosis of AKI.
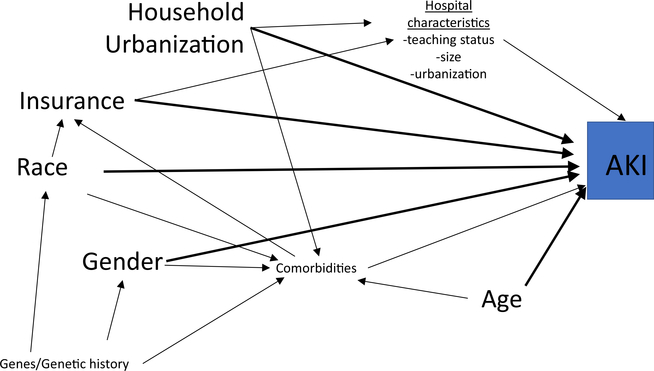
A useful tool to evaluate important confounders in a potential exposure-outcome relationship is a causal diagram, or a Directed Acyclic Graph (DAG). It allows visualization of your primary exposure (e.g., race) and outcome (e.g., AKI) in relationship to all other potential variables. It helps ensure there are not mediators or colliders included in adjustment analyses which could introduce bias. We provide simplified descriptions of key variables as DAGs can get quite complicated; for example, confounders can be hidden on indirect pathways. More in-depth descriptions and explanations of causal diagrams can be found in this overview in CJASN [16] or in the epidemiological literature [17, 18]. A confounder by definition is a variable that potentially impacts the primary exposure and the outcome, and visually is displayed on a DAG as a variable with an arrow pointing towards both the exposure and the outcome. A mediator on the other hand has an arrow coming from exposure (into mediator) and then pointing towards the outcome. Modifiers cannotbe directly visualized on DAGs. A DAG was drawn for our exposures and outcome (Fig. 1). Depending on the relationship assessed, we evaluated potential confounders of comorbidities and the other exposures based on the DAG and substantive knowledge from the literature to more explicitly evaluate each individual exposure-outcome relationship. Both traditional methods of model adjustment and stratification were used to control for potential confounders in our analyses.
Fig. 1.
A simplified directed acyclic graph of the potential relationships between sociodemographic factors and acute kidney injury. Primary exposures: race, health insurance status (insurance), household urbanization, gender, and age. Primary outcome: acute kidney injury (AKI). Confounders are variables that have an arrow going towards exposure and towards outcome (directly or indirectly). Examples would be genetic history between Race and AKI (indirectly via comorbidities). Mediators are variables along the causal pathway (arrow coming from exposure and then arrow going towards outcome). Example would be Hospital characteristics as a mediator between Insurance and AKI. Mediators are variables not typically controlled for in multivariate modeling as they can then introduce bias
All statistical analyses were conducted in SAS, version 9.4 (SAS Institute, Inc., Cary, NC). The institutional review board at the University of North Carolina at Chapel Hill reviewed this secondary data analysis of de-identified data and classified this as non-human subjects research status.
Sensitivity analyses
Roughly 8% of patients in the KID were missing data on race/ethnicity. We conducted a sensitivity analysis using multiple imputation to correct for missing race/ethnicity. The imputation model included all covariates listed in the Covariates section. Acute glomerulonephritis codes (580.x) are not typically included in AKI analyses, yet we felt given the inaccuracies with coding this may be an important group that is otherwise missed. We conducted a sensitivity analysis removing all acute glomerulonephritis codes.
Results
Patient characteristics
The KID contained 3,195,782 hospitalizations in 2012, and 1,699,841 of these met our study cohort criteria. The majority excluded were due to age < 1 year. Applying the HCUP weights to this subset translates to about 2.4 million hospitalizations for 2012 across the USA. The majority of patients were Caucasian (46.3%), followed by Hispanics and African-Americans (Table 1). Most children were female across race/ethnicities. The majority of hospitalizations occurred in large, urban teaching hospitals. While only a minority of children hospitalized did not have health insurance (4.3%), this extrapolates to over 100,000 children nationally. Lack of insurance differed by race/ethnicity, ranging from 3.7–5.5% (Table 1). Also, more Caucasian and Asian/Pacific Islander children had private insurance, while Medicaid was carried by more African-American, Hispanic, and Native American children (Table 1). These overall demographics are similar to those of US children in 2012 [19, 20]. A sensitivity analysis removing the ICD codes associated with acute glomerulonephritis revealed no differences in our results.
Table 1.
Demographics of hospitalized pediatric patients by race and ethnicity weighted for national estimates
| Totala | Caucasian | African-American | Hispanic | Asian/Pacific Islander | Native American | Other | |
|---|---|---|---|---|---|---|---|
| n = 2,392,031 | n = 1,107,114 | n = 431,738 | n = 488,040 | n = 50,553 | n = 22,232 | n = 112,779 | |
| Gender (female)b, col % | 59.1 | 58.0 | 60.9 | 61.8 | 53.9 | 62.5 | 57.3 |
| Agec, median (IQR) | 14 (6, 18) | 15 (6, 18) | 15 (6, 18) | 14 (5, 18) | 11 (3, 17) | 15 (5, 18) | 13 (4, 18) |
| Prevalence of CKD, col % | 0.3 | 0.3 | 0.4 | 0.3 | 0.4 | 0.2 | 0.3 |
| Number of chronic illnessesd, median (IQR) | 0.5 (0, 1.8) | 0.6 (0, 1.9) | 0.6 (0, 1.7) | 0.1 (0, 1.3) | 0.5 (0, 1.7) | 0.3 (0, 1.5) | 0.4 (0, 1.7) |
| Type/location of hospital, col % | |||||||
| Rural | 9.3 | 12.7 | 5.7 | 4.1 | 5.1 | 23.6 | 4.8 |
| Urban, non-teaching | 24.6 | 25.6 | 21.2 | 30.6 | 20.0 | 22.0 | 22.9 |
| Urban, teaching | 66.1 | 61.7 | 73.1 | 65.4 | 74.9 | 54.4 | 72.3 |
| Size of hospitale, col % | |||||||
| Small | 10.6 | 10.7 | 9.2 | 9.5 | 7.7 | 15.4 | 11.4 |
| Medium | 23.5 | 24.2 | 23.9 | 24.1 | 29.0 | 18.1 | 20.6 |
| Large | 65.9 | 65.1 | 66.9 | 66.3 | 63.4 | 66.5 | 68.0 |
| Location in the USA, col % | |||||||
| New England | 3.5 | 4.4 | 2.4 | 3.5 | 4.2 | 0.4 | 4.0 |
| Middle Atlantic | 14.1 | 13.9 | 17.2 | 12.4 | 16.5 | 4.0 | 29.4 |
| East North Central | 14.8 | 18.2 | 18.9 | 5.5 | 9.0 | 6.0 | 12.7 |
| West North Central | 7.4 | 7.0 | 4.1 | 1.1 | 2.2 | 10.5 | 3.2 |
| South Atlantic | 20.2 | 19.3 | 32.7 | 12.5 | 8.9 | 9.0 | 14.1 |
| East South Central | 3.8 | 6.3 | 3.4 | 0.7 | 1.4 | 0.7 | 1.0 |
| West South Central | 15.0 | 13.2 | 13.2 | 23.9 | 7.8 | 22.2 | 17.7 |
| Mountain | 6.7 | 7.1 | 2.0 | 9.4 | 6.3 | 29.5 | 5.7 |
| Pacific | 1.3 | 10.7 | 6.1 | 31.0 | 43.7 | 17.7 | 12.2 |
| Insurance statusf, col % | |||||||
| No insurance | 4.3 | 3.9 | 4.4 | 5.5 | 3.7 | 4.9 | 5.1 |
| Private insurance | 39.3 | 51.1 | 23.3 | 23.1 | 50.2 | 21.8 | 36.1 |
| Medicaid | 51.3 | 39.5 | 67.6 | 66.6 | 40.9 | 63.7 | 52.6 |
| Other | 5.1 | 5.3 | 4.4 | 4.7 | 4.9 | 8.7 | 5.8 |
| Urbanizationg, col % | |||||||
| Central metropolitan counties with ≥ 1 million population | 31.1 | 18.3 | 45.1 | 49.0 | 46.1 | 12.0 | 43.9 |
| Fringe metropolitan counties with ≥ 1 million population | 22.1 | 25.4 | 20.4 | 15.5 | 22.3 | 9.4 | 23.4 |
| Metropolitan counties with 250,000–999,999 population | 20.4 | 21.7 | 17.7 | 21.4 | 19.5 | 19.0 | 15.4 |
| Metropolitan counties with 50,000–249,999 population | 9.4 | 11.5 | 7.5 | 6.6 | 4.0 | 14.3 | 7.0 |
| Micropolitan counties | 10.3 | 13.9 | 6.1 | 5.0 | 6.5 | 20.2 | 6.1 |
| Rural counties | 6.4 | 9.0 | 3.0 | 2.0 | 1.0 | 25.0 | 3.2 |
A total of 128,742 patients missing data on race/ethnicity (unweighted)
A total of 138 patients missing data on gender (unweighted)
A total of 6,304 patients missing data on age (unweighted)
Chronic conditions defined by the chronic condition indicator of HCUP (Hospital Care and Utilization Program). Examples of included chronic conditions that may influence AKI include chronic kidney disease, diabetes mellitus, diabetes insipidus, developmental delays, inflammatory bowel disease, cystic fibrosis, congenital heart disease, and cancer
Hospital size category is determined based on a combination of location, teaching status, and region
A total of 4,274 patients missing data on health insurance status (unweighted)
A total of 6,561 patients missing data on household urbanization (unweighted)
Approximately 30,000 children aged 1–20 years were diagnosed with AKI while hospitalized (Table 1). The crude risk of an AKI episode for a pediatric hospitalization in 2012 was 1.2% or 12.3 cases per 1000 pediatric hospitalizations.
Race/ethnicity differences
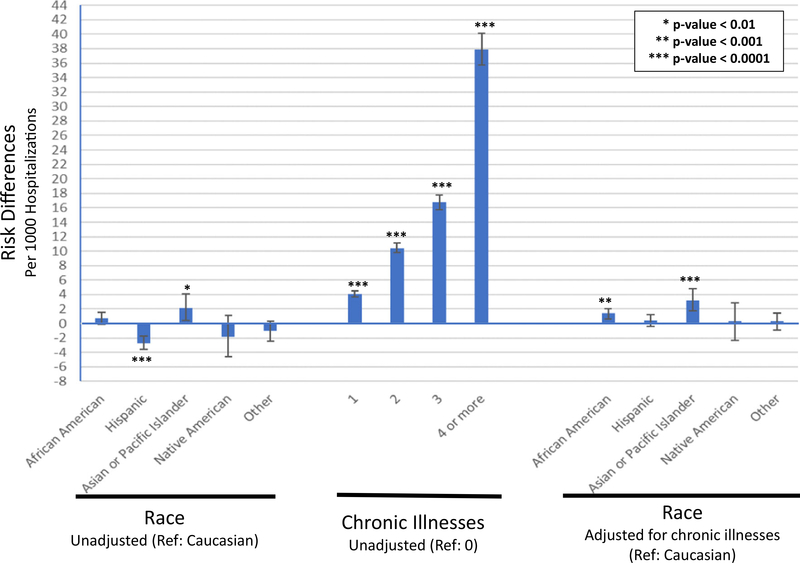
Crude risk of AKI was highest amongst Asian/Pacific Islander children (14.9 cases/1000 pediatric hospitalizations) and lowest amongst Hispanic children (10.0 cases/1000 hospitalizations) (Table 2). When adjusted for comorbidities, insurance status, gender, age, and household urbanization, disparities by race/ethnicity were attenuated (Table 3). However, AKI risks amongst African-American (RD 2.5 cases/1000 hospitalizations, 95% confidence interval (CI) 1.7–3.3), Hispanic (RD 1.7 cases/1000 hospitalizations, 95% CI 0.9–2.5), and Asian/Pacific Islander children (RD 4.5 cases/1000 hospitalizations, 95% CI 2.9–6.0) remained elevated compared to Caucasian children. Crude analyses indicated that having chronic comorbidities were a significant risk factor for AKI, and the risk increased with increasing number of chronic illnesses (Fig. 2). Adjusting for comorbidities alone greatly attenuated the racial disparities seen with AKI risks (Fig. 2). We conducted a sensitivity analysis using multiple imputation to correct for the 8% missing race/ethnicity and found no meaningful differences in our results.
Table 2.
Univariate risk estimates for sociodemographic differences in pediatric AKI episodes
| % of all hospitalizations | % of all AKI episodes | Risks | Risk differences | 95% CI | P value | |
|---|---|---|---|---|---|---|
| n = 2,392,031 | n = 29,391 | |||||
| Race/ethnicitya | ||||||
| Caucasian | 46.3 | 47.6 | 12.6 | Reference | ||
| African-American | 18.0 | 19.6 | 13.4 | 0.7 | −0.1, 1.5 | |
| Hispanic | 20.4 | 16.6 | 10.0 | −2.7 | −3.6, −1.7 | <0.0001 |
| Asian/Pacific Islander | 2.1 | 2.6 | 14.9 | 2.2 | 0.4, 4.1 | <0.01 |
| Native American | 0.9 | 0.8 | 10.9 | −1.8 | −4.6, 1.1 | |
| Other | 4.7 | 4.5 | 11.6 | −1.0 | −2.4, 0.3 | |
| Insurance statusb | ||||||
| Medicaid | 51.2 | 40.4 | 9.7 | Reference | ||
| No insurance | 4.3 | 8.1 | 23.1 | 13.4 | 11.7, 15.1 | < 0.0001 |
| Private insurance | 39.2 | 43.6 | 13.7 | 4.0 | 3.4, 4.6 | < 0.0001 |
| Other | 5.1 | 7.8 | 18.6 | 8.9 | 6.9, 11.0 | < 0.0001 |
| Genderc | ||||||
| Female | 59.1 | 43 | 8.9 | Reference | ||
| Male | 40.9 | 57 | 17.1 | 8.2 | 7.6, 8.8 | < .0001 |
| Aged | ||||||
| 1–5 years | 22.9 | 12.3 | 6.6 | Reference | ||
| 6–10 years | 12.7 | 10.7 | 10.3 | 3.7 | 3.1, 4.4 | < 0.0001 |
| 11–15 years | 17.0 | 16 | 11.6 | 5.0 | 4.2, 5.8 | < 0.0001 |
| 16–20 years | 47.0 | 59.9 | 15.6 | 9.0 | 8.4, 9.7 | < 0.0001 |
| Household urbanizatione | ||||||
| Central metropolitan counties with ≥ 1 million population | 31.2 | 30.2 | 11.9 | Reference | ||
| Fringe metropolitan counties with > 1 million population | 22.2 | 23.1 | 12.9 | 0.9 | 0, 1.9 | 0.05 |
| Metropolitan counties with 250,000–999,999 population | 20.5 | 21.3 | 12.8 | 0.9 | −0.3, 2.0 | |
| Metropolitan counties with 50,000–249,999 population | 9.5 | 9.4 | 12.3 | 0.4 | −0.9, 1.6 | |
| Micropolitan counties | 10.3 | 9.7 | 11.6 | −0.3 | −1.4, 0.7 | |
| Rural (neither metro- or micropolitan) counties | 6.4 | 5.7 | 10.9 | −1.0 | −2.2, 0.1 |
All risks and risk differences are per 1000 hospitalizations
AKI acute kidney injury, RD risk difference, CI confidence interval
A total of 128,742 patients missing data on race/ethnicity (unweighted)
A total of 4,274 patients missing data on health insurance status (unweighted)
A total of 138 patients missing data on gender (unweighted)
A total of 6,304 patients missing data on age (unweighted)
A total of 6,561 patients missing data on household urbanization (unweighted)
Table 3.
Multivariate modeling by sequential addition of potential confounders
| Race + chronic | Race + chronic + age + urban | Race + chronic + age + urban + insurance + gender | |
|---|---|---|---|
| Health insurance | |||
| Medicaid | Reference | Reference | Reference |
| No insurance | 14.4 (12.7, 16.2)**** | 12.9 (11.3, 14.6)**** | 11.8 (10.2, 13.4)**** |
| Private insurance | 3.4 (2.8, 4.0)**** | 3.4 (2.9, 4.0)**** | 2.8 (2.3, 3.4)**** |
| Other | 6.7 (4.8, 8.5)**** | 6.4 (4.5, 8.3)**** | 5.7 (3.8, 7.6)**** |
| Gender | |||
| Female | – | Reference | Reference |
| Male | – | 7.3 (6.7, 7.9)**** | 6.8 (6.2, 7.4)**** |
| Race/ethnicity | |||
| Caucasian | – | – | Reference |
| African-American | – | – | 2.5 (1.7, 3.3)**** |
| Hispanic | – | – | 1.7 (0.9, 2.5)**** |
| Asian/Pacific Islander | – | – | 4.5 (2.9, 6.0)**** |
| Native American | – | – | 0.7 (− 2.1, 3.5) |
| Other | – | – | 1.4 (0.3, 2.5)* |
Risk differences per 1000 hospitalizations presented with 95% confidence intervals in parentheses
Race=race/ethnicity; Chronic=chronic illnesses; Age=age in years; Urban=household urbanization
Italics indicates ideal set of confounders to evaluate according to DAG depicted in Fig. 1. Additional confounders are shown for comparison but may potentially introduce additional bias
p-value < 0.05; **< 0.01; ***< 0.001; ****< 0.0001
Fig. 2.
Risk of AKI by race and chronic illnesses. Error bars represent 95% confidence intervals
Health insurance status
Lack of health insurance was the sociodemographic risk factor most strongly associated with AKI in hospitalized children (Table 2). The uninsured population had nearly double the crude risk of AKI compared to all pediatric inpatients (23.1 cases/1000 hospitalizations amongst the uninsured versus 12.3 cases/1000 hospitalizations overall). The absolute risk difference for those without insurance versus Medicaid was 14.4/1000 hospitalizations.
After adjusting for chronic illnesses, Medicaid remained a protective factor against AKI even when stratified further by race and gender (Fig. 2). Model including chronic illnesses and race yielded similar results (Table 3). We conducted a sensitivity analysis excluding 19- and 20-year-old patients as these patients may have different access to health insurance (i.e., typically not able to access Medicaid as easily as those ≤18 years old) and in our population almost half of those without insurance were 19–20 years of age (47.1%) (Supplementary Table 1). Interestingly, the risk of AKI remained higher for those without insurance versus Medicaid but it was greatly attenuated (RD 4.4, 95% CI 2.8–5.9) in this sensitivity analysis, controlling for race/ethnicity and comorbidities.
Household urbanization
Our one neighborhood-level factor (household urbanization) was not a significant risk factor for AKI. Only fringe vs central metropolitan counties with ≥ 1 million population had a risk difference of 0.9 per 1000 hospitalizations (95% CI 0–1.9) with p value 0.05 (Table 2).
Gender
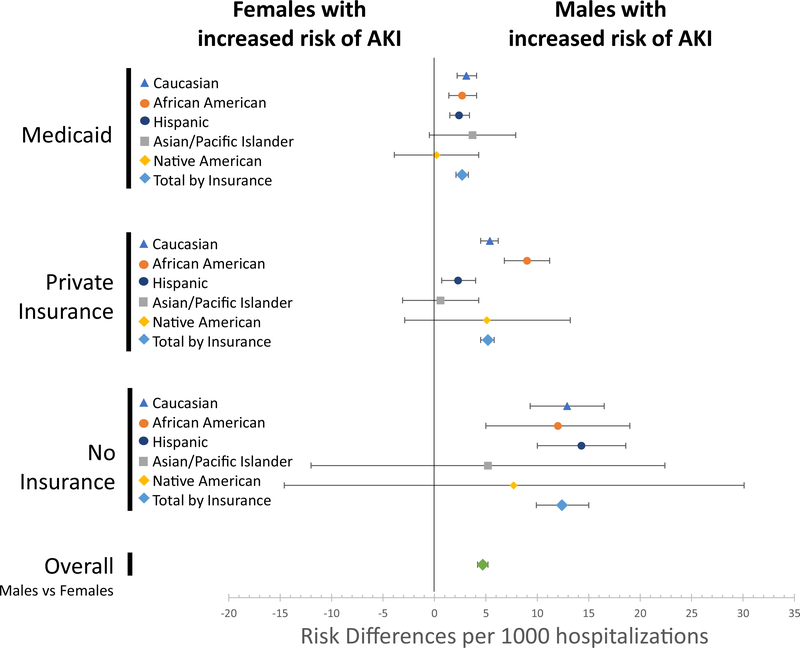
Compared to female patients, males were associated with a higher risk of AKI (RD 8.2/1000 hospitalizations, 95% CI 7.6–8.8) (Table 2), and this difference persisted after adjustment for chronic conditions (Fig. 3). This increased risk was present for all Caucasian, African-American, and Hispanic boys, regardless of insurance strafa (Fig. 3). The risk remained after modeling controlled for comorbidities, race/ethnicity, age, and household urbanization (RD 7.3, 95% CI 6.7–7.9) but was again attenuated with sensitivity analysis removing 19- and 20-year-olds (RD 3.1, 95% CI 2.6–3.6; Supplementary Table 1).
Fig. 3.
Risk differences of AKI for those with no health Insurance versus those with Medicaid, adjusted for chronic illnesses and stratified by race and gender. Error bars represent 95% confidence intervals
Age
Older age was associated with a higher risk of AKI diagnosis (Table 2). To evaluate the trend further, we evaluated smaller subsets of children and compared risk differences in similar combinations of multivariate analyses as presented in Table 3. Those aged 15–16 years of age had similar risk difference associations to those presented in Supplementary Table 1 (all children excluding those 19- and 20-year-olds). The risk differences continue to diverge as we evaluated 17–18-year-olds and then 19–20-year-olds; the 19–20-year-olds who are uninsured have a risk of 20 more episodes of AKI per 1000 hospitalizations (RD 20.1, 95% CI 17.7–22.6) compared to 19–20-year-olds with Medicaid, adjusting for race and chronic illnesses.
Chronic kidney disease sub-group
If we limited our analyses to only the sub-group of children diagnosed with CKD (n = 7280, 0.3% of our cohort, weighted), then one-third of those hospitalizations had an episode of AKI (n = 2401 weighted). This means that AKI amongst kids with CKD made up 8.2% of the total AKI episodes in this cohort. Also, the AKI risks amongst males and those without insurance greatly increased amongst children with CKD (Supplementary Table 2). For example, amongst children with CKD, those without insurance have almost 300 more episodes of AKI per 1000 hospitalizations compared to those with Medicaid (RD 298.3/1000 hospitalizations, 95% CI 227.1–369.4, p value < 0.0001). However, the racial disparities disappeared, and there were no significant differences in AKI risk between any racial minority and Caucasian children with CKD.
Discussion
This is the first large, nationally representative pediatrie kidney study to show that lack of health insurance is a risk factor for poor kidney outcomes. Only slight racial differences existed for AKI. Chronic illnesses, particularly CKD, also were associated with pediatric AKI, and they attenuated the racial disparities. Male gender was a risk factor for increased risk of pediatric AKI, and the increased risk in males was highest amongst racial minorities and uninsured children.
Health insurance status
Medicaid was the sociodemographic factor most protective against AKI. Compared to uninsured children, if a hospitalized child had Medicaid, his/her absolute risk of being diagnosed with AKI decreased by 14 per 1000 hospitalizations, controlling for comorbidities and race. Translating this to a number needed to treat approach, this would require 70 uninsured, hospitalized children (95% CI 62–79) to receive Medicaid to potentially prevent one AKI episode. These results should be interpreted with caution. We cannot account for all unmeasured confounding in this observational study, and we are limited by administrative data. Therefore, we cannot conclude a causal relationship between insurance and AKI. However, our results are consistent with other studies that have found poor health outcomes amongst uninsured pediatric patients [21].
In hospitalized children at risk for AKI, health insurance status may very well be a proxy for further upstream factors related to access to healthcare. We were limited in our ability to assess additional indicators for social determinants of health at the individual level (e.g., income, parents’ level of education) or neighborhood level (e.g., percent in poverty, employment rates, percent with high school diploma). We found that household urbanization did not affect one’s AKI risk yet one’s health insurance status did. So perhaps, we are seeing that our medical system is able to reach the poorest or most remote neighborhoods, yet the more important sociodemographic factor to prevent AKI is one’s individual ability and ease to gain access to our medical system, both preventative and emergency services because of one’s individual healthcare insurance status. Out-of-pocket expenses can be a driving force for healthcare seeking behaviors. Therefore, this also seems congruent with our findings that both uninsured and those with private insurance had higher risks of AKI than those with Medicaid. Patients with private insurance may have high co-pays, different levels of coverage depending on their insurer, and may fluctuate in and out of insurance depending on parental job stability, while those with Medicaid have little to no co-pays and more stability on the insurance plan.
Health insurance status is unique in the pediatric population and is considered a social determinant of health by the Healthy People 2020 initiative [22]. In all states, many children who are not eligible for private insurance are eligible for some form of government-assisted insurance, such as Medicaid [23–25]. Pediatric patients who have no insurance are a unique group of patients, typically due to health literacy barriers, eligibility issues such as citizenship status, or family preference to decline coverage. If our results are confirmed in future research studies that include granular data beyond the limited administrative data in this database, then perhaps interventions should evaluate ways to improve access to healthcare insurance to all children at risk of AKI.
The only other pediatric kidney study to look at social determinants of health focused on parental, self-reported income level in children with chronic kidney disease, and they found no difference in the rate of kidney disease based on income [26]. However, that study was limited by its smaller size (n = 572) and inclusion only of children actively involved in medical care, creating a selection bias against those with poor access to care and without insurance. Future pediatric nephrology studies are needed to assess disparities in social determinants of health, not just in those at risk for AKI.
A unique feature of AKI risk amongst patients uninsured in our cohort is that it seems to be worst amongst the 19- and 20-year-olds. This may be a true risk or a glimpse at the underdiagnosis of AKI in younger children. Nineteen- and twenty-year-old patients may be a high-risk population that are no longer eligible for most state Medicaid programs (47% of uninsured patients are 19–20 years of age). However, older pediatric patients have greater muscle mass and hence higher baseline creatinine levels making them more akin to adult levels, which may allow easier recognition and subsequently improved coding of AKI amongst older children. This was also seen as gender AKI risks were attenuated when we removed 19- and 20-year-olds from the analysis.
Race/ethnicity
Disparities amongst populations with lower socioeconomic status (SES) and racial minorities often are intertwined due to their co-existence. Just as lower SES groups are more likely to experience poorer health outcomes, minority groups have been more likely to report poorer health status compared to Caucasians [9, 10, 27]. As an example, a recent study on appendiceal ruptures saw improvements in all racial groups after insurance coverage increased, but more pronounced improvements amongst minorities [28].
Though the differences are small, our finding of increased AKI risk in minority groups is of significance given the large number of hospitalized pediatric patients at risk of AKI annually in the USA. This analysis of a large, nationally representative cohort allowed us to see these small differences that may be missed in smaller studies. These risks are not completely attenuated after stratifying by insurance status and controlling for comorbidities. Asian/Pacific Islander, African-American, and Hispanic children have a slightly higher risk of AKI compared to Caucasian children.
Part of the racial differences seen in this study may be due to differing levels of medical care in hospitals predominantly caring for minority groups or other SES factors we were unable to capture in this analysis. Chronic conditions alone are also a major risk factor for AKI, and they attenuated much of the racial disparities seen in this study, suggesting the possibility of a genetic component or other uncaptured confounder of the association between race and AKI. Risk variants of the apolipoprotein L1 (APOL1) gene have been consistently cited to explain much of the racial burden of chronic kidney disease as APOL1 is more prevalent in African-Americans, but this has not been consistently shown in AKI literature [29–33]. A large adult cohort study did not find evidence that APOL1 risk variants had a higher risk of AKI compared to those without these variants; though this evaluation assessed APOL1 risk variants found in chronic renal conditions [34]. A large genome-wide association study in AKI found two distinct genetic loci (single-nucleotide polymorphisms) on chromosome 4 nearby yet distinct from the APOL1-regulator, IRF2, which has been associated with the high risk of chronic kidney failure in African-Americans [35].
Further research is needed to evaluate the higher AKI incidence in Asian/Pacific Islander children as this has not been seen in other studies. However, other studies often focus only on Caucasian, African-American, and/or Hispanic sub-groups, excluding Asian and Pacific Islander children due to sample size issues. It is known in adults that people from the Pacific Islands have higher rates of chronic renal failure than any other racial or ethnic group [36]. This cohort did not allow for further refinement as children were a priori grouped together as “Asian/Pacific Islander.”
Gender
Our study also found a higher AKI risk amongst male patients when compared with females, after controlling for comorbidities, race/ethnicity, age, and household urbanization (Fig. 4). While adult AKI studies also show males to be at increased risk [37], this is often explained by the gender differences in undiagnosed comorbid conditions (e.g., hypertension, hyperglycemia), which are less common in the pediatric population. The gender differences in pediatric AKI risk may be related to underlying disease rate differences in congenital conditions, such as congenital anomalies of the kidney and urinary tract, which are more common in boys. There also may be a differential bias in detection of AKI, perhaps due to differing muscle mass in the adolescent population.
Fig. 4.
Risk differences of AKI for males versus females, adjusted for chronic illnesses and stratified by health Insurance status and race/ethnicity. Error bars represent 95% confidence intervals
Age
The higher AKI risk found in older children in this study should be viewed with caution as the outcomes of AKI are from administrative data only. There may be a true increasing risk of AKI with increasing age, but our study may also be highlighting the lack of recognition of AKI in younger children and not a true increased risk. Younger children with low baseline creatinine levels (e.g., 0.2–0.4 mg/dL) may have a significant AKI with doubling of their creatinine and still remain with creatinine levels ≤ 1 mg/dL (normal for an adult), an easy miss for providers less familiar with pediatric variations in creatinine levels.
Limitations
This study is a secondary analysis ofvoluntary, administrative data submitted by hospitals to a national database. Hospitals can choose whether to participate, which may potentially introduce bias to the available data. The unit of measurement is a hospitalization, so recurrent AKI episodes in one individual in separate hospitalizations is possible. We are unable to link individuals and consequently cannot account for potential repeat outcomes. Our primary outcome (AKI) relies solely on appropriate recognition and ICD coding, which has been shown to be flawed with significant underreporting, in particular for AKI [38–41 ]. Hence, it is likely that our AKI estimates under-report the true incidence of disease and perhaps the disparities that exist. This also likely explains the discrepancy between our national incidence of AKI (1.2%) being much below other reports of about 5%.
We attempted to evaluate both an individual social determinant of health and a neighborhood-level factor. In assessing these variables, we consider both the individual’s access to medical care as well as geographic barriers to care. This analysis was limited by the available indicators for social determinants of health in the KID. Evaluating other factors, such as citizenship status, census tract income level, and parental education level, may provide a clearer understanding of the higher risk of AKI amongst lower-resourced or lower SES populations as seen in this study.
However, this study also has important strengths. By analyzing such a large national database with a diverse patient population that represents almost every state in the country, we can provide an overview of the AKI burden and associated disparities at a national level that would not otherwise be apparent. Specifically, we included a large proportion of minorities who are often not represented in kidney research (Hispanics, Asian/Pacific Islanders, and uninsured populations). Unlike other research on insurance disparities, we are able to distinguish Medicaid from no insurance, highlighting a distinct risk difference associated with AKI risk in these two different populations. This study was not designed to evaluate AKI risks amongst children with CKD specifically, but it certainly deserves further exploration as there was quite a high incidence of AKI amongst children with CKD (33%).
Conclusions
Pediatric AKI occurs in 12 of every 1000 pediatric hospitalizations. In the largest, nationally representative cohort, the biggest protective factor against developing AKI is a child having health insurance. Our research should be confirmed with data sources other than administrative data as we are limited by what is accurately coded. Potential improved data sources would be prospective cohorts or network collaborations amongst hospitals that provide laboratory data to more accurately capture all AKI episodes. However, our data suggest that we could potentially prevent 1 episode of AKI for every 70 uninsured hospitalized children provided with Medicaid. Our study showed that racial disparities exist amongst those with pediatric AKI. Though these risks are greatly attenuated by chronic conditions, racial minorities had higher risks compared to Caucasians. Also, hospitalized boys were at an increased risk of AKI compared to girls, controlling for comorbidities, race, age, and household urbanization. More granular data is needed to explore the sociodemographic disparities seen in this large cohort. We need to evaluate if these disparities persist when we can evaluate all episodes of AKI and not just those recognized and subsequently coded by clinicians as these are just the tip of the iceberg.
If these disparities are confirmed, this is concerning as this may be contributing to disparities seen later in life with adult disparities in CKD. Expanding health insurance coverage to children currently uninsured should be explored further as a potential preventative approach for pediatric AKI, particularly amongst potentially high-risk populations 19- and 20-year-olds and those with CKD.
Supplementary Material
Acknowledgments
We would like to acknowledge all the HCUP Data Partners that contribute to HCUP because without their contributions, our work would not have been possible. The full list of state organizations can be found at: https://www.hcup-us.ahrq.gov/db/hcupdatapartners.jsp.
Funding information ECB was supported by NIH/NIDDK T32-DK00775 Training Grant.
Footnotes
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
The institutional review board at the University of North Carolina at Chapel Hill reviewed this secondary data analysis of de-identified data and classified this as non-human subjects research status.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s00467-020-04470-1) contains supplementary material, which is available to authorized users.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sutherland SM, Ji J, Sheikhi FH et al. (2013) AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 10.2215/CJN.00270113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL (2017) Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 10.1056/NEJMoa1611391 [DOI] [PubMed] [Google Scholar]
- 3.McGregor TL, Jones DP, Wang L et al. (2016) Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: aretrospective observational study. Am J Kidney Dis. 10.1053/j.ajkd.2015.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alkandari O, Eddington KA, Hyder A et al. (2011) Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care. 10.1186/cc10269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hessey E, Morissette G, Lacroix J et al. (2018) Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol 13(5):685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg JH, Coca S, Parikh CR(2014) Long-termriskofchronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol. 10.1186/1471-2369-15-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S-K, Hur M, Kim E et al. (2016) Risk factors for acute kidney injury after congenital cardiac surgery in infants and children: a retrospective observational study. PLoS One. 10.1371/joumal.pone.0166328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rheault MN, Zhang L, Selewski DT et al. (2015) AKI in children hospitalized with nephrotic syndrome. Clin J Am Soc Nephrol. 10.2215/CJN.06620615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicholas SB, Kalantar-Zadeh K, Norris KC (2015) Socioeconomic disparities in chronic kidney disease. Adv Chronic Kidney Dis. https://doi.org/10.1053Zj.ackd.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoham DA, Vupputuri S, Kaufman JS et al. (2008) Kidney disease and the cumulative burden of life course socioeconomic conditions: the Atherosclerosis Risk in Communities (ARIC) study. Soc Sci Med. https://doi.org/10.1016Zj.socscimed.2008.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vart P, Gansevoort RT, Joosten MM, Bültmann U, Reijneveld SA (2015) Socioeconomic disparities in chronic kidney disease: a systematic review and meta-analysis. Am J Prev Med. 10.1016/j.amepre.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 12.HCUP Kids’ Inpatient Database (KID). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD: http://www.hcup-us.ahrq.gov/kidoverview.org. Published 2012 Accessed August 1, 2018 [Google Scholar]
- 13.Mehta RL, Burdmann EA, Cerdá J et al. (2016) Recognition and management of acute kidney injury in the International Society of Nephrology 0by25 global snapshot: a multinational cross-sectional study. Lancet. 10.1016/S0140-6736(16)30240-9 [DOI] [PubMed] [Google Scholar]
- 14.HCUP Chronic Condition Indicator (CCI). Healthcare Cost and Utilization Project (HCUP). 2012. Agency for Healthcare Research and Quality, Rockville, MD: http://www.hcup-us.ahrq.gov/toolssoftware/chronic/chronic.jsp. Published 2012 Accessed May 1, 2018 [Google Scholar]
- 15.Hwang W, Weller W, Ireys H, Anderson G (2001) Out-of-pocket medical spending for care of chronic conditions. Health Aff. 10.1377/hlthaff.20.6.267 [DOI] [PubMed] [Google Scholar]
- 16.Staplin N, Herrington WG, Judge PK et al. (2017) Use of causal diagrams to inform the design and interpretation of observational studies: an example from the study of heart and renal protection (SHARP). Clin J Am Soc Nephrol. 10.2215/CJN.02430316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenland S, Pearl J, Robins JM (1999) Causal diagrams for epidemiologic research. Epidemiology 10.1097/00001648-199901000-00008 [DOI] [PubMed] [Google Scholar]
- 18.Hernán MA, Hernández-Díaz S, Robins JM (2004) A structural approach to selection bias. Epidemiology 10.1097/01.ede.0000135174.63482.43 [DOI] [PubMed] [Google Scholar]
- 19.United States Census Bureau. Quick Facts United States. https://census.gov/quickfacts/fact/table/US/pst045218. Accessed December 2, 2019
- 20.National Center for Education Statistics. Estimates of resident population, by race/ethnicity and age group: selected years, 1980 through 2016. https://nces.ed.gov/programs/digest/d16/tables/dt16_101.20.asp?referer=raceindicators. Accessed December 2, 2019
- 21.Abdullah F, Zhang Y, Lardaro T et al. (2010) Analysis of 23 million US hospitalizations: uninsured children have higher all-cause inhospital mortality. J Public Health (Bangkok) 32(2):236–244. 10.1093/pubmed/fdp099 [DOI] [PubMed] [Google Scholar]
- 22.Office of Disease Prevention and Health Promotion. Social Determinants of Health | Healthy People 2020. Healthy People 2019 Topics and Objectives; https://www.healthypeople.gov. Published 2015 [Google Scholar]
- 23.Centers for Medicare & Medicaid Services. Medicaid.gov https://www.medicaid.gov. Published 2018 Accessed August 7, 2018
- 24.Centers for Medicare & Medicaid Services. Healthcare.gov https://www.healthcare.gov/medicaid-chip/. Published 2018 Accessed August 7, 2018
- 25.Benefits.gov State Children’s Health Insurance Program. https://benefits.gov/benefits/benefit-details/607. Published 2018 Accessed August 8, 2018 [Google Scholar]
- 26.Hidalgo G, Ng DK, Moxey-Mims M et al. (2013) Association of income level with kidney disease severity and progression among children and adolescents with CKD: a report from the chronic kidney disease in children (CKiD) study. Am J Kidney Dis. 10.1053/j.ajkd.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shone LP, Dick AW, Brach C et al. (2003) The role of race and ethnicity in the state Children’s health insurance program (SCHIP) in four states: are there baseline disparities, and what do they mean for SCHIP? Pediatrics 10.1542/peds.112.6.SE1.521 [DOI] [PubMed] [Google Scholar]
- 28.Scott JW, Rose JA, Tsai TC et al. (2016) Impact of ACA insurance coverage expansion on perforated appendix rates among young adults. Med Care. 10.1097/MLR.0000000000000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olabisi OA, Heneghan JF (2017) APOL1 nephrotoxicity: what does ion transport have to do with it? Semin Nephrol. 10.1016/j.semnephrol.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ng DK, Robertson CC, Woroniecki RP et al. (2017) APOL1-associated glomerular disease among African-American children: a collaboration of the chronic kidney disease in children (CKiD) and nephrotic syndrome study network (NEPTUNE) cohorts. Nephrol Dial Transplant. 10.1093/ndt/gfw061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Woroniecki RP, Ng DK, Limou S et al. (2016) Renal and cardiovascular morbidities associated with APOL1 status among African-American and non-African-American children with focal segmental glomerulosclerosis. Front Pediatr 10.3389/fped.2016.00122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsa A, Kao WHL, Xie D et al. (2013) APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 10.1056/NEJMoa1310345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman DJ (2017) A brief history of APOL1: a gene evolving. Semin Nephrol. 10.1016/j.semnephrol.2017.07.003 [DOI] [PubMed] [Google Scholar]
- 34.Grams ME, Matsushita K, Sang Y et al. (2014) Explaining the racial difference in AKI incidence. J Am Soc Nephrol. 10.1681/ASN.2013080867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao B, Lu Q, Cheng Y et al. (2017) A genome-wide association study to identify single-nucleotide polymorphisms for acute kidney injury. Am J Respir Crit Care Med. 10.1164/rccm.201603-0518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Renal Data System (2017) USRDS annual data report: epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda: 10.1053/j.ajkd.2017.01.020 [DOI] [Google Scholar]
- 37.Neugarten J, Golestaneh L (2018) Female sex reduces the risk of hospital-associated acute kidney injury: a meta-analysis. BMC Nephrol 10.1186/s12882-018-1122-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J (2014) Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 10.2215/CJN.07650713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko S, Venkatesan S, Nand K, Levidiotis V, Nelson C, Janus E (2018) International statistical classification of diseases and related health problems coding underestimates the incidence and prevalence of acute kidney injury and chronic kidney disease in general medical patients. Intern Med J. 10.1111/imj.13729 [DOI] [PubMed] [Google Scholar]
- 40.Borzecki AM, Cevasco M, Chen Q, Shin M, Itani KMF, Rosen AK (2011) How valid is the AHRQ patient safety indicator postoperative physiologic and metabolic derangement? J Am Coll Surg. 10.1016/j.jamcollsurg.2011.01.001 [DOI] [PubMed] [Google Scholar]
- 41.Waikar SS, Wald R, Chertow GM et al. (2006) Validity of international classification of diseases, ninth revision, clinical modification codes for acute renal failure. J Am Soc Nephrol 17(6):1688–1694. 10.1681/ASN.2006010073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.