Abstract
Bipolar Disorder is costly and debilitating, and many treatments have side effects. Transcranial Direct Current Stimulation (tDCS) is a well-tolerated neuromodulation technique that may be a useful treatment for Bipolar Disorder if targeted to neural regions implicated in the disorder. One potential region is the left ventrolateral prefrontal cortex (vlPFC), which shows abnormally elevated activity during reward expectancy in individuals with Bipolar Disorder. We used a counterbalanced repeated-measures design to assess the impact of cathodal (inhibitory) tDCS over the left vlPFC on reward circuitry activity, functional connectivity, and affect in adults with Bipolar Disorder, as a step toward developing novel interventions for individuals with the disorder. −1mA cathodal tDCS was administered over the left vlPFC versus a control region, left somatosensory cortex, concurrently with neuroimaging. Affect was assessed pre and post scan in remitted Bipolar Disorder(n=27) and age/gender-matched healthy (n=31) adults. Relative to cathodal tDCS over the left somatosensory cortex, cathodal tDCS over the left vlPFC lowered reward expectancy-related left ventral striatal activity (F(1,51)=9.61,p=.003), and was associated with lower negative affect post scan, controlling for pre-scan negative affect, (F(1,49)=5.57,p=.02) in all participants. Acute cathodal tDCS over the left vlPFC relative to the left somatosensory cortex reduces reward expectancy-related activity and negative affect post tDCS. Building on these findings, future studies can determine whether chronic cathodal tDCS over the left vlPFC has sustained effects on mood in individuals with Bipolar Disorder, to guide new treatment developments for the disorder.
Introduction
Bipolar Disorder is the fourth leading cause of disability worldwide1. Unfortunately, many treatments have long-term side effects. The development of new interventions for Bipolar Disorder is critical. One way forward is to identify neural biomarkers of underlying pathophysiologic processes that can act as targets for interventions. Individuals with or at-risk for Bipolar Disorder show elevated reward sensitivity2, associated with a more severe course3, impulsivity4, sensation seeking3, and high levels of reward expectancy, predisposing to hypo/mania4, a pathognomonic feature of Bipolar Disorder. Reward expectancy involves subjective evaluation of potential future rewards, with more probable rewards having greater expected value. Undue focus on such rewards in individuals with, and at-risk of developing, Bipolar Disorder during reward expectancy may predispose to hypo/mania4. Determining the neural basis of subjective evaluation of future rewards during reward expectancy is thus a promising way to elucidate neural mechanisms predisposing to hypo/mania. We reported an abnormally steep increase in left reward expectancy-related ventrolateral prefrontal cortex (vlPFC) activity with greater probability of reward in euthymic Bipolar Disorder adults5. Other studies reported greater reward expectancy-related activity in left lateral prefrontal cortex and ventral striatum (VS) in adults with high versus low impulsivity6 or reward sensitivity7. Furthermore, abnormally elevated reward-related left vlPFC activity in healthy youth at risk of Bipolar Disorder8, manic individuals9, and adults with higher levels of sensation seeking and impulsivity10, who are at higher risk of Bipolar Disorder than the general population2, have been reported.
The left vlPFC encodes relationships between stimuli and specific reward outcomes11, particularly immediate future rewards12. This lateralization of stimulus-outcome representations in vlPFC function likely reflects role of the left frontal cortex in approach behaviors13. Other regions important for reward processing are: VS, orbitofrontal cortex (OFC), dorsal and rostral anterior cingulate cortex (d/rACC), and amygdala. The VS receives inputs from the OFC, is involved in goal directed behaviors14 and processes the incentive value of expected reward/loss15. The d/rACC support responses to obtain reward15. Greater dACC activity in particular reflects preferences for higher probability reward options16. The critical interactions between VS and amygdala for reward and punishment indicate the amygdala’s important role in reward processing17. These regions are thus important for reward valuation during RE, where abnormally elevated left vlPFC activity and functional connectivity (FC) with other reward regions in individuals with Bipolar Disorder likely reflects greater valuation of potential rewards, predisposing to hypo/mania. Hence, the left vlPFC may be a viable neural target for new interventions for the disorder.
Transcranial direct current stimulation (tDCS) involves passing a weak current through the brain to modulate endogenous electric fields produced by transmembrane currents18. Anodal (excitatory) tDCS results in subthreshold depolarization of cortical pyramidal cells, increasing neuronal excitability and leading to increased neuronal-neuronal connectivity19. Cathodal (inhibitory) tDCS results in hyperpolarization, decreasing neuronal excitability and leading to reduced connectivity19. As the magnitude of de/hyperpolarization imposed by tDCS is quite small, the effects of stimulation are dependent on endogenous currents as well. Thus, tDCS is thought to preferentially modulate neural networks with heightened activity during task performance- the “activity-selectivity” hypothesis20.
Prior studies have used tDCS to treat psychiatric disorders, including Bipolar Disorder, and report that it is well tolerated21. Importantly, in an early tDCS trial applying anodal tDCS over the left lateral prefrontal cortex in depressed individuals with Major Depressive Disorder or Bipolar II Disorder 22, one Bipolar II Disorder individual developed hypomania23. Notably, the electrode montage used provided relatively high-amperage anodal (.08-.15V/m) tDCS to the left vlPFC24. Another case report showed that tDCS over the left lateral prefrontal cortex was associated with the development of hypomania in individuals with Bipolar II Disorder25. Thus, anodal tDCS over the left vlPFC may be associated with elevated activity in, and/or functional connectivity with, this region, and development of hypomania in individuals with Bipolar Disorder. Non-clinical studies reported altered lateral prefrontal cortex activity and functional connectivity in reward circuitry during task performance in healthy adults after tDCS over lateral prefrontal cortical regions26. Additionally, anodal tDCS over the right vlPFC reduces negative affect in healthy individuals27. Together, these findings indicate that tDCS can modulate activity in reward circuitry regions, and affect, and may be a potential intervention for Bipolar Disorder.
Given our findings of abnormally elevated reward expectancy-related left vlPFC activity in euthymic individuals with Bipolar Disorder5, we aimed to determine whether acute cathodal tDCS over the left vlPFC would impact affect and reward expectancy-related left vlPFC activity and functional connectivity with other reward regions in remitted Bipolar Disorder and healthy individuals. We recruited Bipolar Disorder individuals in remission in order to focus on predisposition to, rather than present, hypo/mania, and avoid any potential confounds of higher severity affective symptoms and related higher levels of psychotropic medication on the impact of tDCS in individuals with Bipolar Disorder in this first-stage, proof of concept study. Given the activity-selectivity hypothesis, we administered tDCS during the reward task, while measuring BOLD fMRI. We hypothesized that acute cathodal tDCS over the left vlPFC versus over a control neural region (control condition tDCS) would: 1. significantly reduce activity and functional connectivity in reward circuitry; and 2. significantly lower positive and hypo/mania-related negative affect. We further hypothesized that these effects would be of greater magnitude in Bipolar Disorder versus healthy participants, given the likely higher levels of these measures in Bipolar Disorder than healthy participants during control condition tDCS.
Methods
Participants
We recruited adults with Bipolar Disorder type-I (remitted:≥2 months euthymic and not psychotic). 27 adults with Bipolar Disorder and 31 age and gender ratio-matched healthy adults were included (mean age= (28.5), SD= (7.16), 36 female; Table1; Supplement Figure1 for recruitment stream, Supplement for clinical measures, exclusion criteria, and numbers of included and excluded participants). 18 Bipolar Disorder participants were taking one or more mood stabilizers, 3 were taking antipsychotic medication, 5 an antidepressant, 5 benzodiazepines, and 1 was taking propranolol.
Table 1.
Demographic and clinical information. Anxiety scale = Hamilton Rating Scale for Anxiety, Depression scale = Hamilton Rating Scale for Depression, Mania Scale = Young Mania Rating Scale, BPRS = Brief Psychiatric Rating Scale. Mean (SD) or frequency (percentage) are reported.
| Healthy Control |
Bipolar Disorder I Remitted |
|||
|---|---|---|---|---|
| n=31 | n=27 | statistic | p value |
|
| age | 27.7(5.7) | 29.4(8.5) | t(44.6)=−.893 | 0.377 |
| sex (F) | 19 (61.3%) | 17 (62.9%) | x2(1)=.000 | 1 |
| IQ | 111(6.6) | 110.8(7.6) | t(55)=.132 | 0.895 |
| Anxiety Scan 1 | 1.58(1.57) | 3.48(2.83) | t(39.2)=−3.1 | 0.004 |
| Anxiety Scan 2 | 1.16(1.7) | 3.33(3.5) | t(37.1)=−2.96 | 0.005 |
| Depression Scan 1 | 1.29(1.32) | 2.93(1.49) | t(56)=−4.42 | <.001 |
| Depression Scan 2 | 1.39(1.7) | 3.30(3.3) | t(37.3)=−2.7 | 0.01 |
| Mania Scan 1 | .16(.52) | .74(1.3) | t(33.7)=−2.23 | 0.032 |
| Mania Scan 2 | .07(.36) | .70(1.86) | t(27.7)=−1.76 | 0.089 |
| BPRS Scan 1 | 18.19(.48) | 19.56(1.34) | t(31.7)=−5.01 | <.001 |
| BPRS Scan 2 | 18.42(1.1) | 19.33(2.5) | t(34.4)=−1.75 | 0.089 |
| On medications | ||||
| Benzodiazepines | n/a | 5 (18.5%) | ||
| Mood Stabilizers | n/a | 18 (66.7%) | ||
| Antipsychotic | n/a | 3 (11.1%) | ||
| Antidepressant | n/a | 5 (18.5%) |
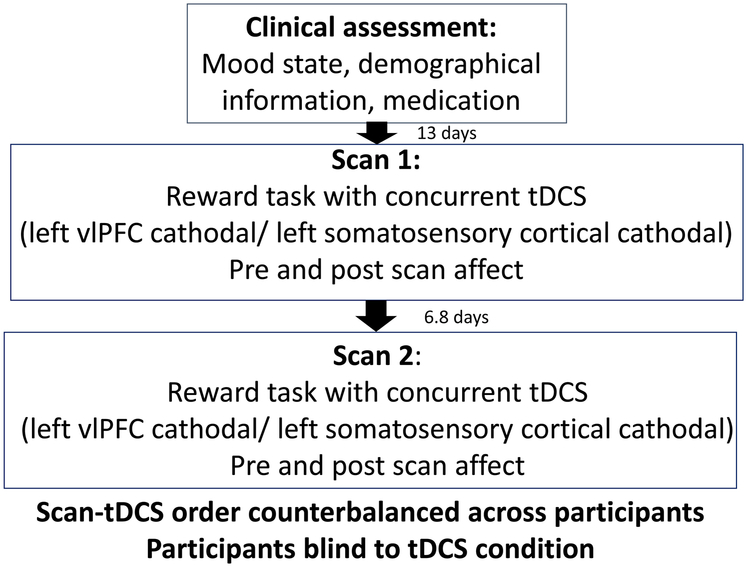
The University of Pittsburgh Institutional Review Board approved this study, and participant consent was acquired. See Figure 1 for the study design.
Figure 1.
Study design
Reward task
Participants completed two, 8-minute blocks of an event-related card-guessing game examining neural activity during expectancy and receipt of reward/loss, comprising 96 trials, including 12 possible win, 12 possible loss, 12 possible win/loss trials (either win or loss), or 12 neutral condition in each of the two blocks. All 4 trial types were used to compute the reward expectancy regressor. Trials were presented in pseudorandom order. Participants believed that their performances determined outcome, with $1 for winning and 75¢ deducted for losing. The outcome of each trial was however, predetermined, with $6 won (Supplement).
tDCS procedure
Concurrent with the reward task during fMRI a constant −1mA current was administered using the Neuroelectrics Starstim tCS system via saline-soaked sponge electrodes and non-ferromagnetic wires (www.neuroelectrics.com Barcelona, Spain). −1mA current was used, as this is sufficient to produce neural inhibition beneath the cathode28 while avoiding paradoxical excitation observed with higher current29. The cathode was positioned at the F7 EEG electrode location (10-10 system, over left vlPFC). The anode was extracephalic (EC), placed on the contralateral shoulder, as in previous tDCS studies22. Electrodes and sponges were circular (5.8cm diameter). tDCS was administered during the reward task (duration 16.5 minutes), with 30 seconds ramp up at the start, and 30 seconds ramp down at the end of the task: total time 17.5 minutes.
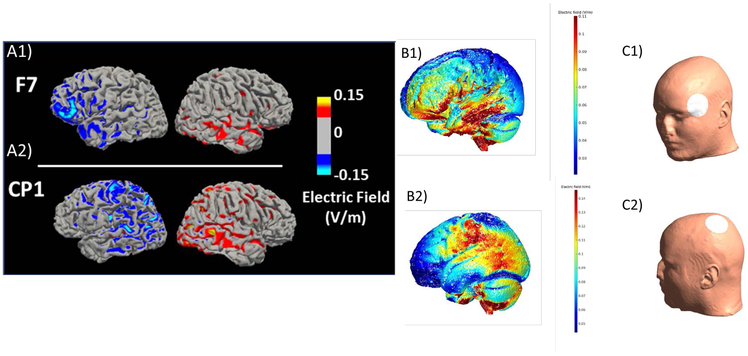
Participants completed two scans in counterbalanced order approximately one week (interscan interval =6.8 days 1.1 SD) apart: one scan was with F7-EC montage targeting the left vlPFC as described above; and the other scan was with CP1-EC montage targeting the left somatosensory cortex. Participants were blind to montage order. (See Supplement for montage order assignment methods). The left somatosensory cortex was chosen as the control region, as somatosensory cortex tDCS has minimal influence on vlPFC and subcortical regions30. Neurotargeting (SIMnIBS:simnibs.org and ROAST, version 2.7, http://www.parralab.org/roast/; Supplement) confirmed that cathodal tDCS over the left vlPFC resulted in a more focal and higher magnitude electric field at (and current flow to) the left vlPFC than did cathodal tDCS over the left somatosensory cortex (Figures 2A, B and Supplement Figures 2-8)31. The order of the two scans was counterbalanced across participants in each group to avoid conflation of montage type with any potential practice effects over the two scans on neural and behavioral measures of interest.
Figure 2.
Electric (E)field modeling using two software packages, (A) Simulation of Non-Invasive Brain Stimulation (SimNIBS) http://www.simnibs.org/ and (B) Realistic vOlumetric Approach to Stimulate Transcranial Electric Stimulation (ROAST, version 2.7, http://www.parralab.org/roast/). A1) Electric field magnitude modeling with a F7 cathode-right EC anode montage on a representative Bipolar Disorder participant from the study. A2) Electric field magnitude modeling with a CP1 cathode - right EC anode montage on a representative Bipolar Disorder participant from the study. B1) F7 e-field modeling using ROAST on the MNI Head. B2) CP1 e-field modeling using ROAST on the MNI Head. C1) The white circle on the human head shows the F7 electrode position in the 10-10 EEG system .C2) The white circle on the human head shows the CP1 electrode position in the 10-10 EEG system. All models used 5.8 cm diameter electrodes and −1mA current; color bar: v/m (Supplement for simulation details, subcortical slices, and additional head models)
Affect
Affect was assessed using the Positive and Negative Affect Schedule (PANAS)32 immediately before and after each scan.
Data processing
Please see Supplement for data preprocessing and processing. Three primary neural regions were: left vlPFC (mni:−45 26 −8, k=344), constructed using activation likelihood estimation (ALE) (Supplement), and left and right VS (8mm sphere ±9, 9, −8)33, constructed using the WFU PickAtlas (Wake Forest University, USA)34. Secondary neural regions were: Pickatlas-Brodmann Area-defined right and left amygdala, d/rACC (BA32,24), and OFC (BA11). Left vlPFC functional connectivity was measured using Generalized Psychophysiological Interaction (gPPI)35, with primary targets: left and right VS, and secondary targets: left and right amygdala, d/rACC, and OFC. At the second level whole region eigenvariate parameter estimates were extracted using SPM12 (Table 2).
Table 2.
Results of repeated measures ANOVA comparing tDCS (left vlPFC vs left SS) in Bipolar Disorder and healthy participants on primary and secondary neural measures. Abbreviations: ventrolateral prefrontal cortex (vlPFC), ventral striatum (VS), orbitofrontal cortex (OFC), d/rACC (dorsal/rostral anterior cingulate cortex (BA 24 and BA 32), somatosensory cortex (SS), transcranial direct current stimulation (tDCS).
| Effect of montage | Effect of group | montage × group interaction |
||||
|---|---|---|---|---|---|---|
| Primary neural measures | F(1,51) = | p= | F(1,51) = | p= | F(1,51) = | p= |
| Activity | ||||||
| left VS | 9.61 | .003* | .20 | .656 | .34 | .563 |
| right VS | 5.00 | .030 | .10 | .759 | .10 | .751 |
| left vlPFC | .31 | .583 | 1.47 | .213 | .50 | .481 |
| Functional connectivity | ||||||
| left vlPFC-left VS FC | 4.99 | .030 | .06 | .806 | .50 | .482 |
| left vlPFC-right VS FC | 3.56 | .065 | .39 | .536 | .81 | .372 |
| Secondary neural measures |
||||||
| Activity | ||||||
| left amygdala | 5.29 | .026 | .89 | .349 | 2.20 | .145 |
| right amygdala | 4.66 | .036 | 1.16 | .286 | 8.86 | .004* |
| left BA 24 | 7.15 | .010* | .01 | .920 | 1.67 | .202 |
| right BA 24 | 8.24 | .006* | .01 | .932 | 1.53 | .222 |
| left BA 32 | 8.58 | .005* | .45 | .507 | 2.39 | .128 |
| right BA 32 | 6.01 | .018 | .00 | .966 | .42 | .522 |
| left OFC | 1.27 | .265 | .55 | .464 | .36 | .554 |
| Functional connectivity | ||||||
| left vlPFC seed | ||||||
| left vlPFC-left amygdala | .00 | .949 | .13 | .722 | .37 | .546 |
| left vlPFC-right amygdala | .02 | .892 | 1.26 | .268 | 2.04 | .159 |
| left vlPFC-left BA24 | .38 | .542 | 5.02 | .029 | .02 | .877 |
| left vlPFC-right BA24 | 1.62 | .209 | 5.48 | .023 | .17 | .678 |
| left vlPFC-left BA32 | .15 | .703 | .60 | .443 | 1.37 | .247 |
| left vlPFC-right BA32 | 1.14 | .290 | 5.09 | .028 | .19 | .666 |
| left vlPFC-left OFC | .44 | .512 | .14 | .714 | .19 | .664 |
FDR corrected p-value = .01
Statistical analysis methods
Analysis of the impact of tDCS on neural measures
Please see Supplement for power calculation and assumption tests. Given that recruitment of the matched participants in this study was from the community and participants were not hierarchically related, repeated measures ANOVAs were used. We tested the effect of tDCS montage (tDCS over left vlPFC versus left somatosensory cortex), group (Bipolar Disorder versus healthy), and tDCS montage×group interaction, on primary and secondary neural regional reward expectancy-related activity and functional connectivity (left vlPFC seed), accounting for age, gender, IQ, and counterbalance order. Repeated measures ANOVA is similar to one-way ANOVA with the ability to test non-independent within subjects’ effects. False Discovery Rate (FDR) was used to correct for parallel ANOVAs (p-value=0.01) 36. We used IBM Statistics SPSS 24 37 and report degrees of freedom, test statistics, and p-values for each repeated measures ANOVA.
Analysis of the impact of tDCS on post-scan affect
Two repeated measures ANOVAs, accounting for pre-scan affect, age, gender, IQ, and counterbalanced order, examined the effect of tDCS montage, group, and tDCS montageXgroup interaction on post-scan positive and negative affect, (FDR correction, p=.04)36. Using regularized regression, to model the large number of predictor variables (p=24)38 (Supplement), we then identified predictors of post-scan affect, including the above demographic, clinical, and neural measures, accounting for pre-scan affect. We report non-zero coefficients identified in this model, and parameters from standard regression analyses showing the association strengths.
Additional analyses
T-tests examined relationships between medication, symptom severity, and neural measures with each tDCS montage in participants with Bipolar Disorder(Supplement). To test the specificity of tDCS effects on neural measures to RE, similar analyses were performed using neural measures to reward-related prediction error and ANOVAs (FDR-corrected threshold, p=0.01; Supplement). For t-tests and reward related prediction error repeated measures ANOVA we report degrees of freedom, test statistics, and p-values.
Results
Effect of tDCS on primary neural measures
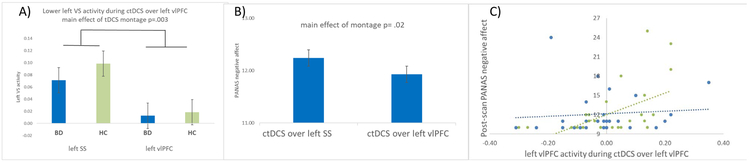
There was a main effect of tDCS montage on reward expectancy-related left VS activity (F(1,51)=9.61,p=.003), with lower activity during cathodal tDCS over the left vlPFC than left somatosensory cortex in all participants (Table2, Figure3A). There was no effect of montage on any other primary neural measures; and no effects of group, montage×group interaction, age, gender, or counterbalance order on primary neural measures (Table2).
Figure 3.
A) Bar graphs with standard error of left ventral striatal activity (F(1,51)=9.61, p=.003) to reward expectancy during cathodal tDCS over the left vlPFC versus the left SS in Bipolar Disorder and healthy participants. B) Bar graph with standard error of pre-scan negative affect and post-scan negative affect (PANAS scores). Main effect of montage on post scan negative affect controlling for pre scan negative affect, following cathodal tDCS over the left vlPFC versus the left SS in all participants (F(1,49)=5.57, p=.02). C) The relationship between left vlPFC activity during cathodal tDCS over the left vlPFC and post-scan negative affect following cathodal tDCS over the left vlPFC. Bipolar Disorder participants in blue and healthy participants in green. Left vlPFC activity explained 8.2% of the variance in post-scan negative affect. Abbreviation: ctDCS= cathodal transcranial direct current stimulation, VS= ventral striatum, vlPFC = ventrolateral prefrontal cortex, SS = somatosensory cortex, Panas= Positive and negative affect schedule.
Effect of tDCS on secondary neural measures
There was a main effect of tDCS montage on reward expectancy-related activity in left BA 24 (F(1,51)=7.15,p=.01); right BA24 (F(1,51)=8.24,p=.006); and left BA32 (F(1,51)=8.58,p=.005): activity in these regions was lower with cathodal tDCS over the left vlPFC than over the left somatosensory cortex; and a group by tDCS montage interaction (F(1,51)=8.86,p=.004) on reward expectancy-related right amygdala activity, with greater activity with cathodal tDCS over the left vlPFC than over the left somatosensory cortex in Bipolar Disorder, and lower activity with cathodal tDCS over the left vlPFC than over the left somatosensory cortex in healthy, participants (Table2, Supplement Figure 9). These findings were primarily driven by montage effects in healthy participants (Supplement Figure 9). There were no other significant effects of montage, group, or montage×group interaction.
Effect of tDCS on post-scan affect
Pre-scan negative affect did not differ significantly across groups for left vlPFC (t(56)=−.87, p=.390) and left somatosensory cortex tDCS (t(56)=−.80,p=.428). Negative affect was lower post versus pre-scan in all participants for each montage. For post-scan negative affect, controlling for pre-scan negative affect, age, gender, IQ, and counterbalance order, there was a main effect of montage (F(1,49)=5.57,p=.02), with lower negative affect after cathodal tDCS over the left vlPFC than over the left somatosensory cortex (Figure3B). PANAS negative affect descriptions that were strongly correlated with total negative affect scores and impacted by cathodal tDCS over the left vlPFC were: irritable, distressed, upset, scared, nervous, jittery, and afraid (all rs>.696). There was no effect of group (F(1,49)=.31,p=.579) or tDCS montageXgroup interaction (F(1,49)=1.04,p=.313) on post-scan negative affect. There was no effect of montage (F(1,49)=2.44,p=.124), group (F(1,49)=2.87,p=.097), or tDCS montageXgroup interaction (F(1,49=.83,p=.367) on post-scan positive affect, controlling for pre-scan positive affect, age, gender, IQ, and counterbalance order.
Predictors of post-scan affect after tDCS over left vlPFC and left somatosensory cortex
As there was no effect of tDCS montage on post-scan positive affect (above), analyses focused on identifying predictors of post-scan negative affect. Across all participants, lower negative affect after cathodal tDCS over the left vlPFC, controlling for pre-scan negative affect, was predicted by lower left vlPFC activity during cathodal tDCS over the left vlPFC (exp coeff=2.248), lower pre-scan negative affect (exp coeff=.0705), and having cathodal tDCS over the left vlPFC on the second (exp coeff=−.686). Standard regression analysis showed that following cathodal tDCS over the left vlPFC, these three variables explained 21.8% of the variance in post-scan negative affect (F(3,54)=45.12,p=.004), with left vlPFC activity t=2.38,p=.021 alone explaining 8.2% (more than 1/3 of the variance; Figure3C). Following cathodal tDCS over the left somatosensory cortex, only pre-scan negative affect (exp coeff=.446) and age (exp coeff= −.0427) were non-zero predictors of post-scan negative affect (F(1,54)=21.34,p<.001).
Relationships with medication
There were no significant relationships between psychotropic medication use (taking/not taking) and reward expectancy-related neural measures during cathodal tDCS over either the left vlPFC or the left somatosensory cortex (all ps > .059).
Discussion
We aimed to determine whether acute cathodal tDCS over the left vlPFC impacted reward expectancy-related activity and functional connectivity in reward circuitry, and affect, in Bipolar Disorder and healthy adults. We show for the first time that acute cathodal tDCS over the left vlPFC relative to over the left somatosensory cortex significantly reduced reward expectancy-related bilateral VS activity. Furthermore, negative affect was significantly lower after cathodal tDCS over the left vlPFC than over the left somatosensory cortex, after controlling for pre-scan negative affect; and lower post-scan negative affect was associated with lower reward expectancy-related left vlPFC activity during cathodal tDCS over the left vlPFC, but not left somatosensory cortex.
There is uncertainty about the efficacy of tDCS because some electrode montages disperse current throughout the cortex rather than targeting neural circuitry of interest20. Our findings indicate, however, that cathodal tDCS significantly impacted primary and secondary neural measures when targeted over the left vlPFC versus the left somatosensory cortex. Our findings thus add to the increasing literature indicating focal effects of cathodal tDCS on reward circuitry26. Interestingly, there were no effects of cathodal tDCS over the left vlPFC on reward expectancy-related left vlPFC activity as measured by BOLD fMRI; instead, effects were on activity in connected regions, including left VS, and bilateral rACC, dACC and amygdala. These findings are consistent with other studies showing effects of tDCS on regions downstream from stimulated cortical areas39.
All effects of cathodal tDCS over left vlPFC versus left somatosensory cortex were on primary and secondary neural measures to reward expectancy, with no significant findings to reward-related prediction error (Supplement and Supplement Table 1). One explanation for these reward expectancy-specific findings relates to the activity-selectivity hypothesis, where tDCS is thought to preferentially modulate neural networks with heightened activity, e.g., during task performance, rather than at rest20. Indeed, whole-brain analyses (Supplement Tables2-4) revealed that, while both Bipolar Disorder and healthy participants showed significantly greater activity to reward related prediction error than reward expectancy in right VS during the control tDCS condition, as predicted by previous studies showing VS activity to reward related prediction error 10, both groups showed significantly greater reward expectancy- than reward related prediction error-related left vlPFC functional connectivity across the brain during control tDCS. Thus, the greater impact on primary and secondary neural measures of cathodal tDCS over left vlPFC during reward expectancy than during reward related prediction error might have resulted from participants showing significantly greater left vlPFC-reward circuitry functional connectivity to reward expectancy than reward related prediction error, as is evident for patterns of functional connectivity during the control tDCS condition.
All participants demonstrated significantly lower post-scan negative affect after cathodal tDCS over the left vlPFC versus the left somatosensory cortex, controlling for pre-scan negative affect. Furthermore, there was a significant positive association between post-scan negative affect after, and reward expectancy-related left vlPFC activity during, cathodal tDCS over the left vlPFC. While reward expectancy-related left vlPFC activity was not significantly different during cathodal tDCS over the left vlPFC versus left somatosensory cortex, the specificity of the relationship between lower post-scan negative affect after, and reward expectancy-related left vlPFC activity during, cathodal tDCS over the left vlPFC suggests that there may have been a more subtle impact of cathodal tDCS over left vlPFC on reward expectancy-related left vlPFC activity, where lower left vlPFC activity resulted in lower negative affect post tDCS. The reduction in negative affect included descriptive components associated with hypo/mania and depression, e.g., irritable, distressed, upset, rather than descriptions associated primarily with depression, e.g., guilt and shame. These indicate an impact of cathodal tDCS over the left vlPFC predominantly on affective components relating to arousal and irritability, characterizing hypo/mania rather than depression. Post-scan positive affect was not differentially affected by the two montages, suggesting that cathodal tDCS over the left vlPFC impacted negative, but not positive, hypo/mania-related affect. This finding may reflect the role of the left vlPFC in impulsive decision-making, associated impatience, and negative affect, when unable to delay gratification4.
The overall findings regarding the impact of cathodal tDCS over the left vlPFC on neural measures and affect were similar in Bipolar Disorder and healthy participants, possibly reflecting the remission status of Bipolar Disorder participants, and suggesting a perturbation of the physiological relationships between neural and affective measures in all participants by cathodal tDCS over this region. The impact of tDCS over the left vlPFC may be greater in participants with Bipolar Disorder in hypo/manic or mixed mood episode, given higher levels of arousal and irritability, and likely associated reward circuity reward expectancy-related activity and functional connectivity in the latter. The effect of montage was more pronounced on primary than secondary neural measures in participants with Bipolar Disorder, however, and one secondary measure, reward expectancy-related right amygdala activity, was not lower during cathodal tDCS over left vlPFC versus left somatosensory cortex in these participants. The relative absence of effects on secondary neural measures in participants with Bipolar Disorder may result from aberrant connectivity between left vlPFC and secondary neural regions (rACC, dACC, amygdala) implicated in reward and emotional regulation in these participants14. Together with the effects of cathodal tDCS over left vlPFC on negative affect, these findings highlight a need for future clinical trials of cathodal tDCS over left vlPFC in Bipolar Disorder, informed by a mechanistic understanding of neural circuity-affect relationships in Bipolar Disorder in the present proof of concept study. Having cathodal tDCS over left vlPFC on the second scan was a predictor of lower negative affect post cathodal tDCS over left vlPFC. Although difficult to explain, this finding may suggest that effects on negative affect were more apparent after repeated tDCS and may call for the use of multi-session tDCS in future clinical trials.
The absence of a sham tDCS condition could be seen as a limitation here; however, previous studies indicate that participants can often distinguish between actual and sham tDCS40. Furthermore, given our specific hypothesis regarding the left vlPFC, it was important to include a tDCS condition that controlled for the general impact of cathodal tDCS over the left hemisphere. The placement of the left vlPFC electrode was determined using a tight-fitting cap with a 5.8cm electrode. While there is a possibility that the electrode shifted during scanning, this was unlikely due to the rigid cap and chin strap employed, which ensured that the cap and electrode remained fixed in their original positions. Although we did not include a non-tDCS baseline scan because of participant burden, this can be included in future studies. We employed conventional rather than high definition (HD) montages to target the left vlPFC and left somatosensory cortex. While the latter are thought to have more focal effects on neural circuity of interest 20, neurotargeting showed a more focal and higher magnitude electric field at the left vlPFC by cathodal tDCS over the left vlPFC than over the left somatosensory cortex. These findings thus suggest that the montages we employed produced the hypothesized effects on neural circuitry of interest. While studies employed higher currents, we chose −1mA, as there are paradoxical excitatory effects of higher-dose cathodal tDCS29. Additionally, using techniques such as EEG can provide more fine-grained examination of the impact of targeted cathodal tDCS on connectivity measures such as coherence among regions of interest in reward circuitry. A more nuanced affect measure may add variance and facilitate detection of between-group differences regarding the impact of tDCS on affect in Bipolar Disorder and healthy participants. Many Bipolar Disorder participants were taking psychotropic medications, but these did not impact neural measures (Supplement).
Conclusion
We show for the first time that acute cathodal tDCS over the left vlPFC, relative to acute cathodal tDCS over a control region, significantly reduces reward expectancy-related reward circuitry activity and is associated with lower post tDCS negative affect in remitted Bipolar Disorder and healthy participants. We show proof of concept for the potential use of cathodal tDCS over the left vlPFC as an intervention for Bipolar Disorder. Building on this concept, future studies can determine the extent to which chronic administration of cathodal tDCS over the left vlPFC has sustained effects on mood in hypo/manic individuals with Bipolar Disorder, to prepare for randomized clinical trials examining the efficacy of this intervention in the disorder.
Supplementary Material
Acknowledgements
We would like to acknowledge the participants for their contributions to this study.
Financial Support
This work was supported by the National Institute of Mental Health (M.L.P. and H.W.C., grant number R21 MH108421) and the Pittsburgh Foundation (M.L.P.)
Footnotes
Conflict of Interest
M.A.B., H.W.C., S.G., R.S., E.K.E., B.A.C., B.D.G., and M.L.P. have no financial interests or potential conflicts of interest. The study sponsors had no role in study design, data collection, analysis, interpretation of data, trial design, patient recruitment, or any aspect pertinent to the study; sponsors were also not involved in the writing of the report or in the decision to submit the paper for publication. The corresponding author had full access to all data in the study and the final responsibility for the decision to submit for publication.
Data Sharing
Individual data along with a data dictionary defining each field are available at the NIMH data archive repository, updates made every six months to study end, https://data-archive.nimh.nih.gov/. Following data use certification scientists can access these data.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
- 1.Collins PY, Patel V, Joestl SS, March D, Insel TR, Daar AS et al. Grand challenges in global mental health. Nature 2011; 475(7354): 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meyer B, Johnson SL, Carver CS. Exploring behavioral activation and inhibition sensitivities among college students at risk for bipolar spectrum symptomatology. Journal of Psychopathology and Behavioral Assessment 1999; 21(4): 275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alloy LB, Abramson LY, Walshaw PD, Cogswell A, Grandin LD, Hughes ME et al. Behavioral Approach System and Behavioral Inhibition System sensitivities and bipolar spectrum disorders: prospective prediction of bipolar mood episodes. Bipolar Disord 2008; 10(2): 310–322. [DOI] [PubMed] [Google Scholar]
- 4.Giovanelli A, Hoerger M, Johnson SL, Gruber J. Impulsive responses to positive mood and reward are related to mania risk. Cognition & emotion 2013; 27(6): 1091–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry 2013; 170(5): 533–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jimura K, Chushak MS, Braver TS. Impulsivity and self-control during intertemporal decision making linked to the neural dynamics of reward value representation. Journal of Neuroscience 2013; 33(1): 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim SH, Yoon H, Kim H, Hamann S. Individual differences in sensitivity to reward and punishment and neural activity during reward and avoidance learning. Social cognitive and affective neuroscience 2015; 10(9): 1219–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. Reward processing in healthy offspring of parents with bipolar disorder. JAMA psychiatry 2014; 71(10): 1148–1156. [DOI] [PubMed] [Google Scholar]
- 9.Bermpohl F, Kahnt T, Dalanay U, Hägele C, Sajonz B, Wegner T et al. Altered representation of expected value in the orbitofrontal cortex in mania. Human brain mapping 2010; 31(7): 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chase HW, Fournier JC, Bertocci MA, Greenberg T, Aslam H, Stiffler R et al. A pathway linking reward circuitry, impulsive sensation-seeking and risky decision-making in young adults: identifying neural markers for new interventions. Transl Psychiatry 2017; 7(4): e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SW, O’Doherty JP, Shimojo S. Neural computations mediating one-shot learning in the human brain. PLoS Biol 2015; 13(4): e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BJ, Monterosso JR, Wakslak CJ, Bechara A, Read SJ. A meta-analytical review of brain activity associated with intertemporal decisions: Evidence for an anterior-posterior tangibility axis. Neuroscience & Biobehavioral Reviews 2018; 86: 85–98. [DOI] [PubMed] [Google Scholar]
- 13.Davidson RJ, Shackman AJ, Maxwell JS. Asymmetries in face and brain related to emotion. Trends Cogn Sci 2004; 8(9): 389–391. [DOI] [PubMed] [Google Scholar]
- 14.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry 2014; 171(8): 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in cognitive sciences 2011; 15(2): 56–67. [DOI] [PubMed] [Google Scholar]
- 16.Paulus MP, Frank LR. Anterior cingulate activity modulates nonlinear decision weight function of uncertain prospects. NeuroImage 2006; 30(2): 668–677. [DOI] [PubMed] [Google Scholar]
- 17.Baxter MG, Murray EA. The amygdala and reward. Nature reviews neuroscience 2002; 3(7): 563. [DOI] [PubMed] [Google Scholar]
- 18.Weiss SA, Bikson M. Open questions on the mechanisms of neuromodulation with applied and endogenous electric fields. Frontiers in human neuroscience 2014; 8: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindman LJ, Lippold OC, Redfearn JW. The Action of Brief Polarizing Currents on the Cerebral Cortex of the Rat (1) during Current Flow and (2) in the Production of Long-Lasting after-Effects. The Journal of physiology 1964; 172: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bikson M, Name A, Rahman A. Origins of specificity during tDCS: anatomical, activity-selective, and input-bias mechanisms. Front Hum Neurosci 2013; 7: 688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dondé C, Neufeld NH, Geoffroy PA. The Impact of Transcranial Direct Current Stimulation (tDCS) on Bipolar Depression, Mania, and Euthymia: a Systematic Review of Preliminary Data. Psychiatric Quarterly 2018: 1–13. [DOI] [PubMed] [Google Scholar]
- 22.Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. The British journal of psychiatry : the journal of mental science 2012; 200(1): 52–59. [DOI] [PubMed] [Google Scholar]
- 23.Galvez V, Alonzo A, Martin D, Mitchell PB, Sachdev P, Loo CK. Hypomania induction in a patient with bipolar II disorder by transcranial direct current stimulation (tDCS). The journal of ECT 2011; 27(3): 256–258. [DOI] [PubMed] [Google Scholar]
- 24.Bai S, Dokos S, Ho KA, Loo C. A computational modelling study of transcranial direct current stimulation montages used in depression. NeuroImage 2014; 87: 332–344. [DOI] [PubMed] [Google Scholar]
- 25.Arul-Anandam AP, Loo C, Mitchell P. Induction of hypomanic episode with transcranial direct current stimulation. The journal of ECT 2010; 26(1): 68–69. [DOI] [PubMed] [Google Scholar]
- 26.Weber MJ, Messing SB, Rao H, Detre JA, Thompson-Schill SL. Prefrontal transcranial direct current stimulation alters activation and connectivity in cortical and subcortical reward systems: a tDCS-fMRI study. Hum Brain Mapp 2014; 35(8): 3673–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vergallito A, Riva P, Pisoni A, Lauro LJR. Modulation of negative emotions through anodal tDCS over the right ventrolateral prefrontal cortex. Neuropsychologia 2018. [DOI] [PubMed] [Google Scholar]
- 28.Lang N, Nitsche MA, Paulus W, Rothwell JC, Lemon RN. Effects of transcranial direct current stimulation over the human motor cortex on corticospinal and transcallosal excitability. Experimental brain research 2004; 156(4): 439–443. [DOI] [PubMed] [Google Scholar]
- 29.Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. The Journal of physiology 2013; 591(Pt 7): 1987–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Hao Y, Zhou J, Fried PJ, Wang X, Zhang J et al. Direct current stimulation over the human sensorimotor cortex modulates the brain's hemodynamic response to tactile stimulation. The European journal of neuroscience 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dmochowski JP, Datta A, Bikson M, Su Y, Parra LC. Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng 2011; 8(4): 046011. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of personality and social psychology 1988; 54(6): 1063. [DOI] [PubMed] [Google Scholar]
- 33.Bertocci M, Bebko G, Mullin B, Langenecker S, Ladouceur C, Almeida J et al. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychological medicine 2012; 42(7): 1417–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage 2003; 19(3): 1233–1239. [DOI] [PubMed] [Google Scholar]
- 35.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 2012; 61(4): 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narum SR. Beyond Bonferroni: Less conservative analyses for conservation genetics. Conservation Genetics 2006; 7(5): 783–787. [Google Scholar]
- 37.IBM. SPSS Statistics 24. 2016. [Google Scholar]
- 38.Tibshirani R Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological) 1996; 58(1): 267–288. [Google Scholar]
- 39.Fiori V, Kunz L, Kuhnke P, Marangolo P, Hartwigsen G. Transcranial direct current stimulation (tDCS) facilitates verb learning by altering effective connectivity in the healthy brain. NeuroImage 2018; 181: 550–559. [DOI] [PubMed] [Google Scholar]
- 40.Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimul 2012; 5(2): 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.