Abstract
Background
Memory and cognitive processes influence the amount of food consumed during a meal, yet the neurobiological mechanisms mediating these effects are poorly understood. The hippocampus (HPC) has recently emerged as a brain region that integrates feeding-relevant biological signals with learning and memory processes to regulate feeding. Here we investigate whether the gut-derived hormone, ghrelin, acts in the ventral HPC (vHPC) to increase meal size through interactions with gut-derived satiation signaling.
Methods
Interactions between vHPC ghrelin signaling, gut-derived satiation signaling, feeding, and interoceptive discrimination learning were assessed via rodent behavioral neuropharmacological approaches. Downstream neural pathways were identified using transsynaptic virus-based tracing strategies.
Results
Results reveal that vHPC ghrelin signaling counteracts the food intake-reducing effects produced by various peripheral biological satiation signals, including cholecystokinin, exendin-4 (a glucagon-like peptide-1 receptor agonist), amylin, and mechanical distension of the stomach. Results further show that vHPC ghrelin signaling may attenuate satiation processing by producing interoceptive cues that generalize to a perceived state of energy deficit. Neuroanatomical tracing results identify a multi-order connection from vHPC neurons to lateral hypothalamic area (LHA) orexin (aka, hypocretin)-producing neurons that project to the laterodorsal tegmental nucleus (LDTg) in the hindbrain. Lastly, results show that vHPC ghrelin signaling increases spontaneous meal size via downstream orexin receptor signaling in the LDTg.
Conclusions
We conclude that vHPC ghrelin signaling increases meal size by counteracting the efficacy of various gut-derived satiation signals. These effects occur via downstream orexin signaling to the hindbrain LDTg, thereby highlighting a novel hippocampus-hypothalamus-hindbrain pathway regulating meal size control.
Keywords: obesity, brainstem, lateral hypothalamus, food intake, reward, satiation
Introduction
The predominance of research on the neural control of energy balance has focused on the hypothalamus as a primary center regulating food intake and energy expenditure (1, 2). However, emerging findings highlight the importance of hindbrain and other extra-hypothalamic regions in food intake regulation, particularly in the control of cognitive aspects of feeding behavior related to reward and memory processes (3–7). The hippocampus (HPC), a brain region traditionally associated with declarative and visuospatial memory function (8, 9), has recently emerged as a critical brain region in the control of food intake and feeding-related cognitive processes [see (10–12) for review]. Moreover, evidence suggests that the HPC also contributes to satiation/meal size control, as HPC neurons are activated by within-meal gastrointestinal (GI) satiation signals, including cholecystokinin, gastric distension, intra-gastric nutrient infusion, and electrical stimulation of the gastric branch of the vagus nerve (13–16). The neurobiological mechanisms through which satiation signals communicate with and are influenced by HPC neural processing are unknown. The interaction between satiation signaling and the HPC may involve endocrine pathways, as leptin, GLP-1, and ghrelin influence feeding behavior, in part, via action in HPC neurons (17–19).
Ghrelin is a stomach-derived 28-amino acid “hunger hormone” that increases appetite and food intake via its seven transmembrane G-protein-coupled receptor, the type 1a growth hormone secretagogue receptor (GHSR1a) (20, 21). Ghrelin levels are elevated during energy restriction (22–24) and decrease following a meal (25–26). Previous work revealed that CNS ghrelin signaling promotes food intake via interactions with GI-derived vagally-mediated satiation signals. For example, peripheral ghrelin administration increases gastric emptying rate in both humans and rodent models (27, 28), and the hindbrain area postrema is one site mediating these effects (29). Further, the food restriction-induced elevation in circulating ghrelin levels is abolished by subdiaphragmatic vagotomy (30). Given that meal size is considered to be a primary determinant of overall caloric intake (31, 32), it is important to understand the neurobiological mechanisms through which ghrelin regulates satiation signaling.
GHSR1a is expressed in the dentate gyrus, CA1, CA2, and CA3 regions of the hippocampal formation, (19, 33–36) and GHSR1a activation in the ventral hippocampus subregion (vHPC) increases food intake (37, 38). However, the mechanisms mediating these outcomes are poorly understood. Based on research discussed above identifying a role for HPC ghrelin signaling in food intake control, combined with several recent reports that HPC neurons respond to vagally-mediated gastrointestinal signals (13, 14, 16, 39), we hypothesize that HPC ghrelin signaling promotes food intake, in part, by reducing the capacity of GI-derived satiation signals to terminate a meal. Moreover, in light of previous reports that CNS ghrelin signaling engages neurons in the lateral hypothalamic area (LHA) that produce the neuropeptide, orexin (aka, hypocretin), and that CNS ghrelin and orexin interact to increase feeding (19, 40, 41), we further hypothesize that HPC ghrelin counteracts satiation processing via downstream LHA orexin signaling.
Methods and Materials
Animals
Adult male Sprague-Dawley rats (Envigo; 320–450g on arrival) were individually housed with ad libitum access (except where noted) to water and chow (LabDiet 5001, LabDiet, St. Louis, MO) on 12 hr:12 hr light/dark cycle (lights on at 06:00h). All procedures involving animals were approved by the University of Southern California Institute of Animal Care and Use Committee.
Experiment 1: Interactions between vHPC ghrelin and gut-derived within-meal satiation signals
General Design
Following 24-hr food restriction, rats with bilaterally implanted vHPC cannulae received injections of either aCSF/vehicle or a subthreshold dose of ghrelin when injected alone (30 pmol/ 200 nl; dose selected from pilot work) 45 mins before intraperitoneal administration of a pharmacological treatments [vs. i.p. 0.9% sterile saline (1 ug/kg) control] or 1 hr before methylcellulose gavage (see Supplementary Methods for cannula implantation and drug preparation parameters). Food was returned immediately after i.p. injections/gavage. Treatments were separated by 2–3 intervening days using a counterbalanced within-subjects design (Experiments 1a, 1b) or using a counterbalanced mixed design with vHPC drug as a between-subjects variable and i.p. drug as a within-subjects variable (Experiments 1c, 1d). Chow intake and spillage were recorded at 30 mins, 1, 2, and 4 hours after injections.
Experiment 1a: CCK (n=8)
This food intake-reducing dose of i.p. CCK (3ug/kg, ip) was previously shown to depend on intact vagal afferent signaling (42).
Experiment 1b: methylcellulose gavage for mechanical stomach distension (43, 44)
2% methylcellulose (MC; viscosity 400 cP, Sigma-Aldrich) in 0.2% Tween-80 (Sigma-Aldrich) dissolved in warm DI water was stirred at room temperature. Animals (n=11) received an oral gavage administration of 15 mL of 2% MC or a sham gavage (no infusions).
Experiment 1c: Exendin-4 (n=20)
The Exendin-4 dose (3 ug/kg, ip) was selected because the food intake-reducing effects of i.p. Exendin-4 at this dose are dependent on vagal afferent signaling (45).
Experiment 1d: Amylin (n=23)
The amylin dose (50 ug/kg, ip) was selected because this dose reduces food intake via non-vagal mechanisms (46).
Experiment 2: Deprivation intensity discrimination (DID)
Experiment 2a: Deprivation intensity discrimination (DID) training
The DID task involves rats learning to use interoceptive energy status cues (0 vs. 24 h food restriction) as discriminative stimuli for a sucrose reinforcement. Training procedures follow previous publications (16, 47–49) (see Supplementary Figure 2a). Rats with HPC lesions are impaired in learning this discrimination problem (49, 50), indicating that the HPC is critical for this type of interoceptive discrimination memory process.
Experiment 2b: DID testing
At the end of training, rats from both groups were tested on 2 days under 0-h food restriction state, with one 24-h food restriction day without treatment intervening between the two test days. The first test day took place one day after the last 24-h food restricted training day. Testing was conducted during extinction, such that feeder operated, but no sucrose pellets were delivered to either group. On each of two test days, rats with cannulae targeting the vHPC received bilateral administration of aCSF or ghrelin (150 pmol/200 nl) approximately 60 mins prior to being placed in conditioning chambers, using a within-subjects design with order of treatments counterbalanced across Groups 0+ and 24+.
Experiment 3: Identification of downstream targets of vCA1 to LHA projections
Experiment 3a: Neural tract tracing and immunohistochemistry
To drive expression of Cre recombinase in 1st order targets of vCA1 neurons, rats (n=5) received a unilateral 200nl pressure co-injection of AAV1-hSyn-Cre-WPRE-hGH and CTB-488 (CTB for postmortem injection site confirmation) in the vCA1 of the HPC. Next, animals received a unilateral 200nl pressure injection of a Cre-dependent anterograde tracer, AAV1-CAG-Flex-tdTomato-WPRE-rBG, in the lateral hypothalamic area (LHA) (see Supplemental Methods for coordinates). This Cre-mediated transsynaptic anterograde tracing approach allows us to identify downstream targets of vCA1 HPC to LHA second-order projections by mapping axons of LHA neurons that receive synaptic input from vCA1 neurons (16, 51). Following a 3-week survival period, animals housed in 6:00am lights off were both food restricted and received i.p. injections of saline (n=2) or CCK (n=3) 90-minutes before perfusion and tissue was harvest and processed as described in Supplementary Methods. Representative images for orexin protein expression in the LDTg obtained from these animals were confined to Swanson Atlas level 48–50 (52).
Experiment 3b: Fluorescence in situ hybridization (FISH) and immunohistochemistry
In control rats (n=3), tissue was harvested following transcardial perfusion for mRNA analyses (see Supplemental Methods for details). Representative images for GHSR1a and VGLUT1 mRNA expression in the vCA1 subregion of HPC and for ORX1-R and GLP1-R mRNA expression in the LDTg obtained from these animals were confined to Swanson Atlas levels 36–38 and 48–50 (52), respectively.
Experiment 4: Interactions between vHPC GHSR1a and LDTg ORX-1R signaling on spontaneous meal pattern parameters
Meal pattern analyses (measuring cumulative food intake, meal frequency, and meal size) were conducted in chambers equipped with automated food intake monitors (Med Associates Inc.; see Supplementary Methods for details). Immediately prior to the dark cycle, animals received LDTg bilateral administration of either 50% DMSO/aCSF or a dose of an ORX-1R antagonist, SB-334867, subthreshold for food intake effects alone (1 ug/200 nl; dose based on (40) and our pilot work), immediately followed by vHPC bilateral administration of either aCSF or ghrelin (150 pmol/200 nl total, 75 pmol/100 nl per side; dose selected to have hyperphagic effects when administered alone (37)). Animals were then placed in the chamber for 15-hrs and food intake was continuously monitored. Treatments were counterbalanced using a within-subjects design and were separated by 2–3 days where animals were returned to their home cage between treatments.
Statistical Analyses
Statistical analyses used multifactorial repeated measures analysis of variance (rANOVA), except for Experiment 3 (one-way ANOVA) and Experiment 2a (unpaired Student t-tests). Fisher LSD (Experiment 2b) and Newman-Keuls (Experiments 1 and 4) post hoc comparisons were used when significant main effects and/or interactions were obtained. Results are presented as mean ± SE. Statistical significance was set at p<0.05. Statistical analyses were conducted with computer software (Statistica V7; Statsoft).
Results
Experiment 1: vHPC ghrelin signaling counteracts the food intake-reducing effects of meal-derived gastrointestinal satiation signals
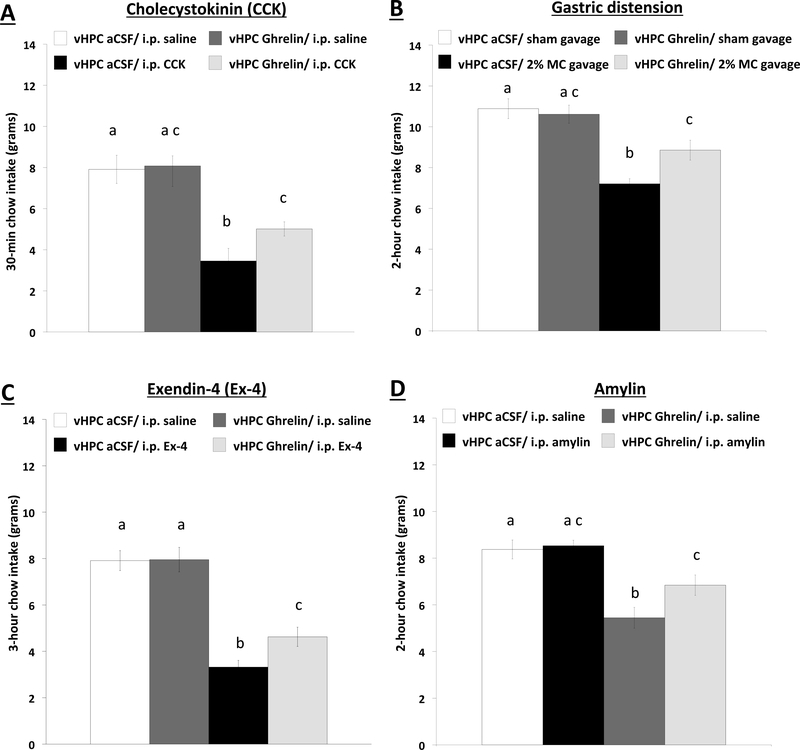
In 24-hour food restricted animals, a subthreshold dose of ghrelin for feeding effects alone administered to the vHPC attenuates the food intake reduction during refeeding produced from the satiation signals CCK (i.p., 3ug/kg, Experiment 1a), gastric distension (Experiment 1b), Exendin-4 (3ug/kg, Experiment 1c), and amylin (50ug/kg. Experiment 1d) (Figure 1) (statistical results in Supplemental Results). Given that three of these peripheral meal-derived satiation signal treatments were designed based on vagus nerve requirement for food intake reduction (CCK, distension, Exendin-4) whereas one treatment’s effects do not require the vagus (amylin), results indicate that the enhancement of meal size associated with vHPC ghrelin signaling involves interactions with both paracrine (vagal) and non-vagal endocrine pathways. Collective results from Experiment 1 demonstrate that vHPC ghrelin signaling, at a dose that has no effects on feeding alone, attenuates the anorexigenic effects of an array of vagally- and non-vagally-mediated within-meal satiation signals.
Figure 1.
Cumulative home cage chow intake in 24-hour food restricted rats at (a) 30-min following administration of a subthreshold dose of vHPC ghrelin (30pmol/200nl) and i.p. CCK (3 ug/kg) injections (n=9); at (b) 2-hour following vHPC ghrelin administration and gastric distension via 15 mL 2% MC oral gavage (n=13); at (c) 3-hour following vHPC ghrelin administration and i.p. Ex-4 (3 ug/kg) injections (n=20); and at (d) 2-hour following vHPC ghrelin administration and i.p. amylin (50 ug/kg) injections (n=23). Data are mean ± SEM; Means with different letters are statistically significant at p<0.05. vHPC: ventral hippocampus; aCSF: artificial cerebral spinal fluid; i.p.: intraperitoneal; CCK: cholecystokinin; MC: methylcellulose; Ex-4: Exendin-4.
Experiment 2: vHPC ghrelin signaling produces interoceptive cues that generalize to 24-hr food restriction
Based on findings that an intact hippocampus is required for discriminating between low and high levels of food restriction in rats (49, 50), and that both peripheral (i.p.) and central administration of ghrelin produce an energy state that generalizes to 24-hr food restriction (48), we hypothesized that vHPC ghrelin signaling counteracts satiation signaling by producing interoceptive cues that generalize to a state of energy restriction. Indeed, results revealed that vHPC ghrelin administration in nonrestricted rats produced an interoceptive state similar to 24hr food restriction, as evident from increased anticipatory appetitive responding in Group 24+ following ghrelin compared to aCSF, and vice versa for rats in Group 0+ (consistent with the performance for each group on 24-hr food-restricted training days at the end of the training phase) (statistical results in Supplemental Results). Overall, results show that vHPC ghrelin signaling counteracts satiation processing, in part, by producing an internal perception of energy deficit similar to that experienced following 24-hrs without food.
Experiment 3: Orexin neurons in the LHA are a relay connecting the vHPC (field CA1) to the LDTg
Based on our previous work showing that the orexigenic effects of unilateral vHPC ghrelin administration are abolished following contralateral lesion of the LHA (19), we hypothesized that the LHA is a monosynaptic downstream mediator of vHPC ghrelin effects on meal size control. We utilized a transsynaptic anterograde tracing approach combined with immunohistochemistry and in situ hybridization to confirm that vCA1 pyramidal neurons synaptically target LHA orexin neurons, and that the LDTg is a likely 2nd-order targets of vHPC GHSR1a -> LHA orexin signaling.
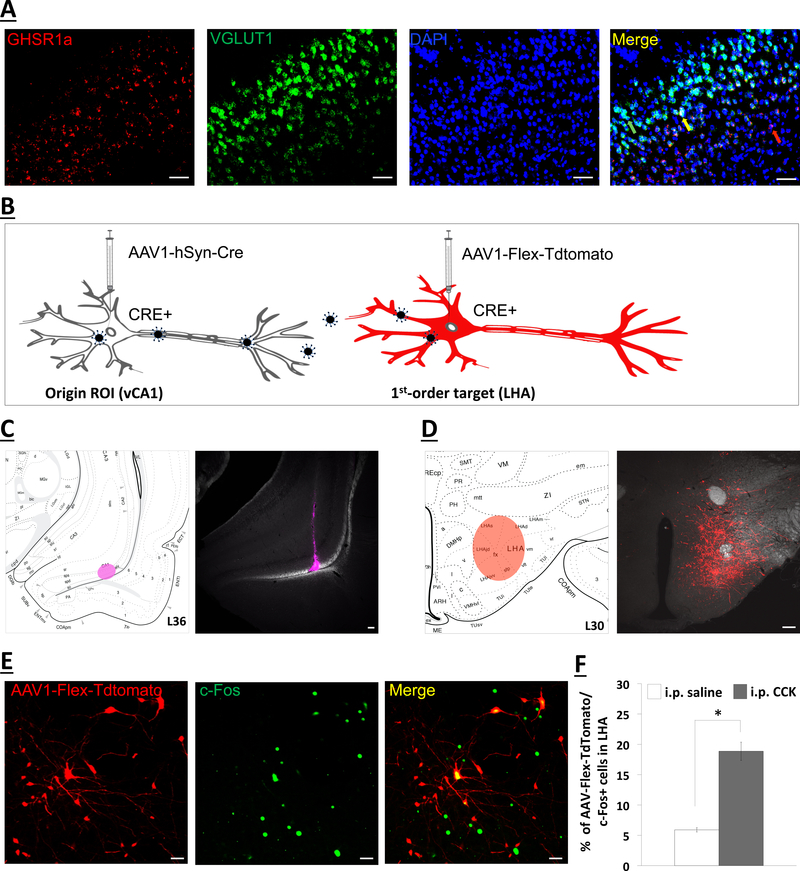
Fluorescence in situ hybridization analyses revealed GHSR1a mRNA expression in the ventral CA1 field (vCA1), and further, that the vCA1 GHSR1a expression is predominantly isolated within the glutamatergic (VGLUT1-expressing) projection neurons of the pyramidal layer (Figure 3a). These pyramidal vCA1 glutamatergic neurons have previously been shown to densely innervate the LHA (19, 53), as well as the medial prefrontal cortex (18) and lateral septum (54, 55). To identify second-order targets of the vCA1 neurons that monosynaptically communicate to LHA, we utilized a dual-synaptic virus-based pathway tracing approach (Figure 3b) (16, 51). A unilateral iontophoretic co-injection of AAV2/1-hSyn-Cre and CTB-488 (imaged in AF647 channel to confirm injection site) targeted to the vCA1 induces Cre recombinase expression in neurons at the injection site (vCA1 HPC) and in first-order (but not second-order) neurons (Figure 3c, left) (16, 51). This was followed by a unilateral and ipsilateral pressure injection of AAV1-CAG-FLEX-TdTomato (a Cre-dependent anterograde tracer) targeted to the LHA (Figure 3d, right). Five animals were confirmed as double hits in both vCA1 HPC (Figure 3c) and LHA (Figure 3d) injection sites. In these rats, i.p. CCK-8 (3ug/kg) administration 90-min before tissue harvest induced c-Fos protein immunoreactivy in dorsal perifornical LHA (dpLHA) neurons that colocalize with Cre-expressing neurons (AAV-Flex-Tdtomato) receiving direct synaptic input from the vCA1 (Figure 3e). Quantitative analyses indicate i.p. CCK-8 significantly increased the percentage of AAV-Flex-Tdtomato cells that co-express c-Fos protein in the dpLHA relative to i.p. saline treatment (Figure 3f; F[1,3]=4.6, p=0.007). Collectively, these findings indicate that CCK-8, a vagally-mediated satiation signal, engages dpLHA neurons that receive direct synaptic input from vCA1 glutamatergic neurons.
Figure 3.
(a) Representative images show GHSR1a mRNA (red) and VGLUT1 mRNA (green) expression in vCA1 cell bodies (DAPI nuclear stain; blue) (n=3). A representative red arrow depicts GHSR1a mRNA that is not vGLUT1; green arrow for vGLUT1 mRNA that is not GHSR1a; yellow arrow for vGLUT1/GHSR1a+ mRNA. Scale bar: 50μm. (b) Schematic illustration of Cre-mediated anterograde tracing method (51). The AAV2/1-hSyn-Cre drives Cre expression in origin region of interest (ROI) infected at the injection site (left), as well as in first-order (but not second-order) neurons based on virion release from first-order axon terminals. The AAV-Flex-Tdtomato, a Cre-dependent anterograde tracer, will express red fluorescence in Cre+ neurons, including first-order neurons (right). (c) Right panel shows an ipsilateral and unilateral 200nl pressure vCA1 co-injection site of AAV-hSyn-Cre and CTB (far red) (n=5). Left panel shows schematic representative injection site in vCA1 HPC. (d) Right panel shows an ipsilateral and unilateral 200nl pressure injection site of a Cre-dependent anterograde tracer (red; AAV1-Flex-Tdtomato) in the lateral hypothalamic area (LHA). Left panel shows schematic representative injection site in LHA. Scale bar: 100μm. (e) Intraperitoneal injections of CCK (n=3) increases the percentage of AAV-Flex-TdTomato cells that colocalize with c-Fos positive immunoreactive cells in the LHA vs. saline (n=2). (f) Representative images of immunohistochemical staining of peripheral-CCK induced c-Fos protein (green) colocalize with neurons in the LHA that receive direct input from the vCA1 (red; AAV1-Flex-Tdtomato). Scale bar: 25μm. Data are mean 507 ± SEM; *p<0.05 vs saline treatment. i.p.: intraperitoneal; CCK: cholecystokinin; GHSR1a: type 1a growth hormone secretagogue receptor.
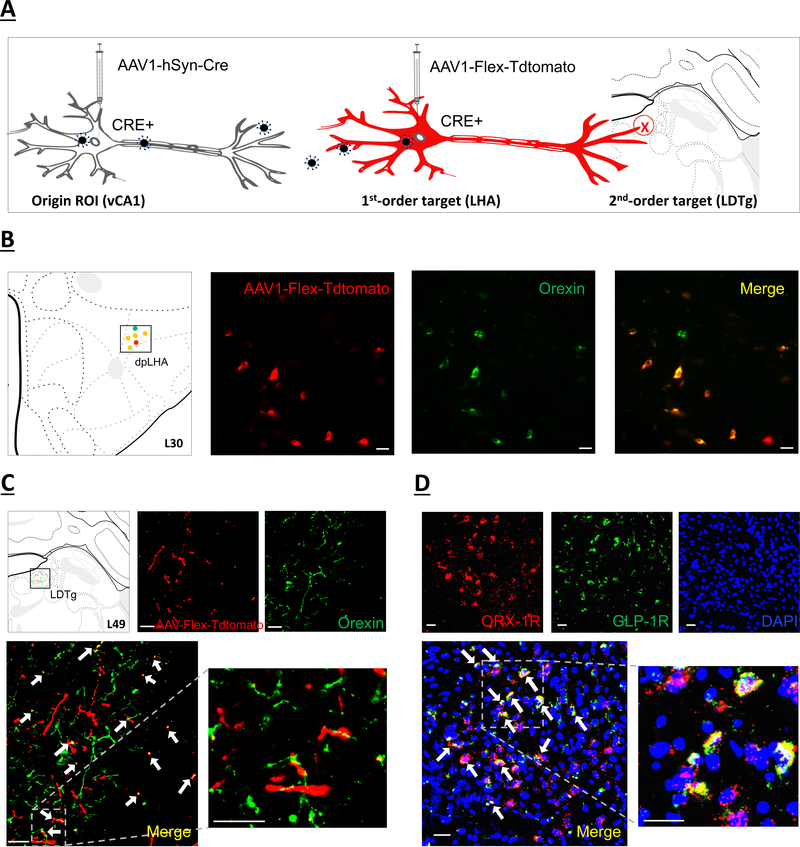
Given previous work identifying interactions between ghrelin and orexin signaling in promoting appetite (19, 56), we hypothesized that LHA orexin neurons receive input from the GHSR1a-expressing vCA1 glutamatergic neurons. Results from the five double hit animals reveal a population of LHA orexin immunoreactive neurons that are colocalized with Tdtomato (i.e., Cre-expressing neurons that receive vCA1 synaptic input) (Figure 4b), and further, we identified Cre-expressing (Tdtomato+) putative axon terminal fields in the LDTg that overlap with orexin immunoreactive terminal fields (Figure 4c). Based on previous results demonstrating that GLP-1-producing neurons in the nucleus tractus solitarius (NTS) project to the LDTg to reduce food intake via a selective reduction in meal size (57), we next examined whether ORX-1R and GLP-1 receptor (GLP-1R) colocalize in LDTg neurons. FISH analyses identify substantial colocalization of ORX-1R and GLP-1R mRNA expression in the LDTg, with 59.6% of ORX-1R mRNA-positive cells containing GLP-1R mRNA (representative image; Figure 3e). Schematics of a representative double hit animal IHC and FISH analyses are displayed in Figures 3 and 4. Overall, these results indicate that GHSR1a is expressed in vCA1 glutamatergic projections neurons in the pyramidal layer, and that these neurons communicate monosynaptically to LHA orexin neurons that project to the LDTg.
Figure 4.
(a) Schematic illustration of identifying second-order targets using a Cre-mediated multisynaptic anterograde tracing method (51). The AAV2/1-hSyn-Cre drives Cre expression in origin region of interest (ROI) infected at the injection site (left), as well as in first-order (but not second-order) neurons based on virion release from origin ROI axon terminals. The AAV-Flex-Tdtomato, a Cre-dependent anterograde tracer, will express red fluorescence in Cre+ neurons (center) and label axon terminals in second-order brain regions (right). (b) orexin immunofluorescent neurons (green) colocalize with neurons in the dpLHA that receive direct synaptic input from the vCA1 (red; AAV1-Flex-Tdtomato). Scale bar: 25μm. Left panel shows schematic representation in Swanson Atlas level 30. (c) Overlap of second-order RFP-immunoreactive axon terminal fields in the LDTg (red) of LHA neurons that receive direct input from the vCA1 and orexin axon terminal fields (green). Arrows, co-expression of RFP and orexin terminal fields. Scale bar: 25μm. Left panel shows schematic representation of terminal field overlap in Swanson Atlas level 49. (d) Representative images show ORX-1R (red) GLP-1R (green) mRNA expression in LDTg cell bodies (DAPI nuclear stain; blue) (n=3). Arrows, co-expression of GLP-1R and ORX-1R mRNA cells. Scale bar: Scale bar: 25μm. dpLHA: dorsal perifornical region of the lateral hypothalamic area; LDTg: laterodorsal tegmental nucleus; ORX-1R: orexin-1 receptor; GLP-1R: glucagon-like peptide-1 receptor.
Experiment 4: vHP ghrelin signaling increases meal size via downstream orexin signaling in the LDTg
Having identified LDTg ORX-1R signaling as a putative 2nd-order target of vHPC GHSR1a signaling, we next examined whether this pathway is relevant for vHPC ghrelin effects on meal size control. This was examined using a within-subjects neuropharmacological approach that utilized automated food intake monitors for analyses of spontaneous meal patterning in free-feeding rats. Results reveal that vHPC ghrelin administration just prior to the onset of the nocturnal feeding cycle increased 2hr cumulative food intake via a selective increase in meal size, and that this effect was blocked by LDTg ORX-1R antagonist administration (statistical results in Supplementary Results). Overall, these results demonstrate that vHPC ghrelin signaling promotes food intake by increasing spontaneous meal size, and that these effects require downstream ORX-1R signaling in the LDTg.
Discussion
Meal size control involves bidirectional communication between gastrointestinal-derived biological signals and the brain. Research on the how the brain regulates satiation processing has predominantly focused on the hypothalamus and the hindbrain (4), with data from decerebrate rats highlighting the sufficiency of hindbrain processing for critical aspects of meal size control (4, 58–60). However, meal size control is potently influenced by memory for recent eating occasions (61–64) and social factors (65) and therefore must undoubtedly involve processing in brain substrates that regulate higher-order cognitive functions. The ventral hippocampus (vHPC) has recently been linked with mnemonic control of feeding behavior (10, 66, 67). Here we expand this previous work and reveal that the stomach-derived endocrine signal ghrelin acts in the vHPC to counteract the intake-reducing effects of various gut-derived satiation signals, including CCK, Exendin-4, amylin, and mechanical distension of the stomach. Considering that amylin, unlike CCK, Exendin-4, and distension, acts to reduce food intake via non-vagal mechanisms (46), our results demonstrate that vHPC ghrelin signaling attenuates the efficacy of meal-derived satiation signals that communicate to the brain via multiple signaling pathways.
One mechanism through which vHPC ghrelin signaling can override satiation signaling is by producing a perceived internal state of energy depletion. For example, damage to the hippocampus is associated with interoceptive agnosia in human amnesic patients who will consume multiple consecutive meals without altering self-reported levels of hunger and fullness (68, 69). Similarly in rats, both selective HPC lesions (49) and consumption of a Western diet (70) impair the ability of interoceptive energy status cues to exert discriminative responding over conditioned appetitive behavior in the deprivation intensity discrimination task (DID). Ghrelin is a candidate signal for communicating an interoceptive state of energy restriction to hippocampal neurons, as both peripheral and intracerebroventricular ghrelin administration generalizes to a state of 24hr food restriction in the DID task (48). Indeed, results from the present study identify the vHPC as a site mediating these effects. Following vHPC ghrelin treatment, nonrestricted rats tested in the DID task demonstrated anticipatory appetitive responding consistent with their performance on 24hr-restricted training days, indicating that the treatment produced a perceived state of 24hr energy restriction. These results cannot simply be explained by vHPC increasing appetitive behavior generally speaking, as rats in Group 0+ reduced appetitive responding following vHPC ghrelin relative to vehicle treatment, an outcome mirroring their performance during training where internal cues arising from 24hrs food restriction were trained as a nonreinforced condition for this group.
It is also possible that ghrelin promotes an interoceptive perception of energy deficit through GHSR1a expressed on vagal afferent terminals, as capsaicin-induced elimination of vagal afferent signaling attenuates peripheral pharmacological ghrelin-induced hyperphagia (71). However, another study demonstrated that intraperitoneal administration of ghrelin stimulated eating in rats with subdiaphragmatic vagal deafferentation (72), suggesting that vagal signaling may not be required for the present results. Indeed, we recently demonstrated that the learned ability to use interoceptive hunger and satiety cues as discriminative stimuli for food reinforcement in the DID task is sustained following total subdiaphragmatic vagotomy (16). Therefore, present data revealing the ability of vHPC ghrelin signaling to provide an interoceptive “hunger-like” cue likely involves non-vagal mechanisms (e.g., BBB ghrelin transport from vasculature to vHPC), although this hypothesis requires further examination.
In the present study, vHPC ghrelin administration increased 2hr cumulative intake through a specific effect on meal size without influencing meal frequency. These findings are consistent with present data revealing that vHPC GHSR1a activation attenuates the efficacy of various satiation signals and produces an interceptive state similar to 24hr food restriction, as hyperphagia following periods of acute food restriction in rats is based predominantly on increased meal size and not frequency (73, 74). However, our data differ from previous work showing that activation of GHSR in the vHPC increases both meal size and meal frequency in rats (37), although we note that this discrepancy could be due to differences between testing animals during light (37) versus dark/nocturnal (present study) cycle. Taken together, previous work and present findings suggest that vHPC ghrelin signaling enhances meal size independent of light/dark cycle, whereas upregulating this system may only increase meal frequency at times in which animals are not normally feeding (light cycle).
To identify downstream neural pathways through which vHPC ghrelin signaling enhances meal size, we utilized a dual-synaptic virus-based pathway tracing strategy (51). The LHA was selected as a first-order target for Cre-dependent anterograde tracer administration as previous work established the dpLHA as a direct synaptic target from pyramidal projection neurons in vCA1 HPC (53, 75), and here we confirm that these pyramidal neurons express GHSR1a. Results revealed that dpLHA neurons that receive synaptic input from the vCA1 HPC are activated by peripheral CCK, and are predominantly orexin-producing neurons. While these data suggest that orexin neurons responsive to CCK also receive synaptic input from vCA1 neurons, this was not confirmed in the present study due to methodological limitations for co-labeling of orexin, TdTomato, and c-Fos signals. Previous literature has shown that in addition to feeding, orexin neurons in the dpLHA are involved in arousal and stress-related functions (76–80). Thus, it is possible that the capacity of vHPC ghrelin signaling to override gut-derived satiation signals and increase meal size is mediated, in part, by enhanced dpLHA orexin-mediated arousal or stress. However, this remains to be directly tested.
We further reveal that LHA orexin neurons that receive vCA1 input communicate downstream to the hindbrain LDTg, and that LDTg orexin signaling is required for vHPC ghrelin-induced elevations in spontaneous meal size. These findings complement other recent results identifying the LDTg as an emerging region of importance in food intake control. For example, the LDTg has many reciprocal projections with feeding-relevant forebrain and midbrain regions, including the LHA and the ventral tegmental nucleus (VTA) (81, 82). Further, LDTg neurons and glial cells express receptors for peptides that have established effects on food intake (such as amylin, orexin, ghrelin, GLP-1) (34, 57, 83, 84), and this brain region has been shown to modulate both homeostatic (energy deficit-based) and reward-based feeding behaviors (57, 81–83, 85–90). The LDTg also has afferent and efferent connections with local hindbrain nuclei involved in satiation processing and oral motor control, including the NTS, parabrachial nucleus, paramedian reticular formation (82, 90). The mechanisms through which LDTg neurons interact with these local hindbrain substrates to regulate meal size control remains poorly understood.
While anatomical studies have previously identified orexin neuron projections in the LDTg (91–93), to our knowledge the functional role of LDTg ORX-1R signaling to feeding behavior has not been previously explored. Present data show that orexin input to the LDTg plays a role in food intake and satiation signaling, as ORX-1R blockade in the LTDg eliminated the effects of vHPC ghrelin to enhance cumulative food intake via an increase in meal size. These results are consistent with recent work showing that CNS-ghrelin mediated hyperphagia involves downstream orexin signaling (40), an effect that is counteracted by activation of leptin receptor-expressing neurotensin neurons (41). Our fluorescence in situ hybridization analyses further identified substantial ORX-1R and GLP-1R mRNA colocalization in the LDTg. Given that gastric distension activates GLP-1-producing neurons in the NTS (94), and that LDTg GLP-1R activation reduces meal size in free-feeding rats (57), future work could explore whether LDTg ORX-1R signaling functionally interacts with GLP-1R signaling in the LDTg to regulate meal size.
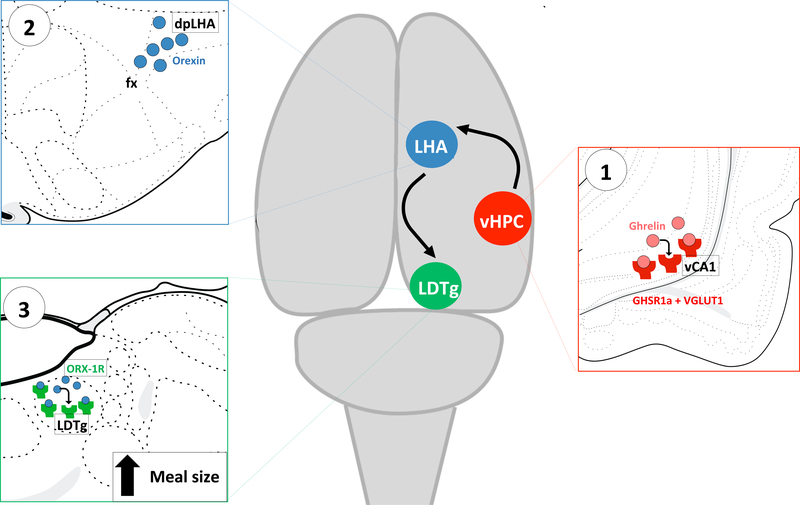
Meal size control is potently influenced by the integration of various complex cognitive and mnemonic variables, including the external context/environment, interoceptive energy status cues, social factors, and previous learned experiences. The present results illuminate a novel descending forebrain-hindbrain circuitry through which such integration putatively occurs. Specifically, results provide evidence that vHPC ghrelin signaling increases meal size by counteracting the efficacy of various gut-derived satiation signals. Given that ghrelin levels are elevated not only during energy restriction but also in anticipation of eating (23, 95), we hypothesize that vHPC ghrelin signaling represents a key component of a cognitive override system that allows for the consumption of larger meals at times when eating is convenient and/or an extended period without eating is anticipated to follow the meal (e.g., habitual meals, meal entrainment models). Our additional findings further identify the downstream neural pathways mediating these effects, through which vCA1 GHSR1a-expressing neurons communicate to LHA orexin neurons that project to the LDTg (Figure 6). Collective results add to an emerging body of research identifying brain systems traditionally involved with memory and cognition in the control of feeding behavior
Figure 6.
Schematic illustration of the novel multi-order hippocampus-hypothalamic-hindbrain circuit. (1) GHSR1a is expressed in vCA1 glutamatergic projection neurons (red) in the pyramidal layer, which communicate monosynaptically to (2) dpLHA orexin neurons (blue) that project to (3) LDTg ORX-1R in the hindbrain (green). This pathway is critical for promoting meal size through interactions between vCA1 HPC ghrelin and downstream LHA orexin signaling to the LDTg. GHSR1a: type 1a growth hormone secretagogue receptor; dpLHA: dorsal perifornical region of the lateral hypothalamic area; LDTg: laterodorsal tegmental nucleus; ORX-1R: orexin-1 receptor.
Supplementary Material
Figure 2.
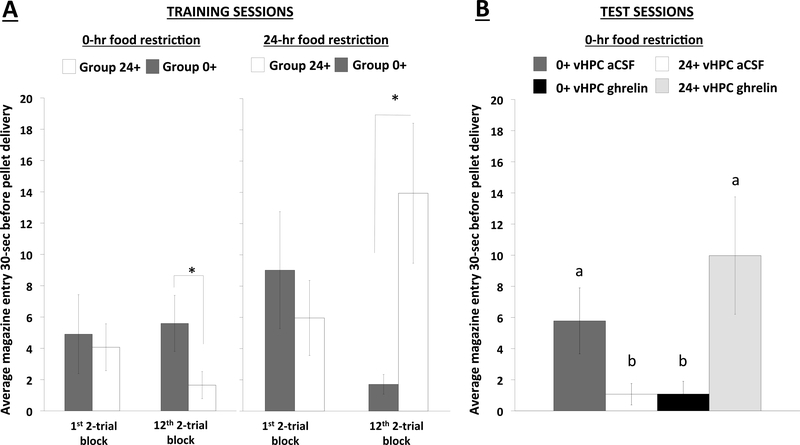
(a) Mean magazine entries during the last 30-seconds before pellet delivery for the first and twelfth (last session before testing) two-trial blocks of deprivation intensity discrimination training sessions for Group 0+ (n=7) and 24+ (n=10) under alternating 0-hr (left panel) and 24-hr (right panel) food restriction. (b) Mean photobeam interruptions following vHPC ghrelin (150pmol/200nl) testing under 0-hr food restriction. Data are mean ± SEM; (a) *p<0.05 vs non-reinforced group. (b) Means with different letters are statistically significant at p<0.05. vHPC: ventral hippocampus; aCSF: artificial cerebral spinal fluid.
Figure 5.
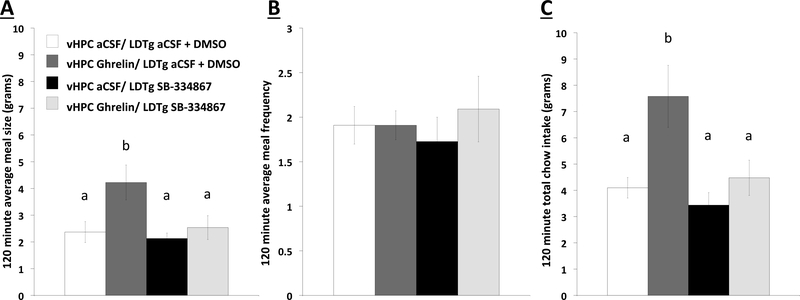
Meal pattern analysis (n=11) following vHPC ghrelin (150pmol/200nl) and LDTg subthreshold dose of orexin 1-receptor antagonist (SB-334867; 1ug/200nl) administration: (a) 2 hr average meal size, (b) 2 hr average meal frequency, and (c) 2 hr total chow intake. Data are mean ± SEM; Means with different letters are statistically significant at p<0.05. LDTg: laterodorsal tegmental nucleus; DMSO: dimethyl sulfoxide.
KEY RESOURCES TABLE.
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Antibody | Rabbit anti-orexin | Phoenix Pharmaceuticals | RRIF: AB_10013632 | |
| Antibody | Mouse anti-CTB | Abcam | RRID: AB 300499 | |
| Antibody | Rabbit anti-RFP | Rockland | RRID: AB 2209751 | |
| Antibody | Rabbit anti-cFos | Cell Signaling | RRID: AB_2106617 | |
| Antibody | Donkey anti-mouse IgG-AlexaFluor 488 | Jackson Immunoresearch | RRID: AB_2341099 | |
| Antibody | Donkey anti-rabbit IgG-Cy3 | Jackson Immunoresearch | RRID: AB_2307443 | |
| Antibody | Donkey anti-goat IgG-AlexaFluor 750 | Abcam | Cat #: ab175744 | |
| Bacterial or Viral Strain | AAV1-hSyn-Cre-WPRE-hGH | Addgene | RRID: Addgene_105553 | |
| Bacterial or Viral Strain | AAV1 -CAG-Flex-T dT omato-WPR E-rBG | Addgene | RRID: Addgene_51503 | |
| Chemical Compound or Drug | Protease inhibitor cocktail | Sigma | Cat #: P8340–5ML | |
| Chemical Compound or Drug | Donkey serum | Jackson Immunoresearch | Cat #: 017–000-121 | |
| Chemical Compound or Drug | Triton-X 100 | Sigma | Cat #: X100–500ML | |
| Chemical Compound or Drug | Glycerol | EMD Millipore | Cat #: GX0185–5 | |
| Chemical Compound or Drug | Ketaset (Ketamine), 100mg/mL | Henry Schein Animal Health | Cat #: 010177 | |
| Chemical Compound or Drug | Anased (Xylazine) Injection, 100mg/mL | Henry Schein Animal Health | Cat #: 033198 | |
| Chemical Compound or Drug | Aceproject (Acepromazine) Injection, 10mg/mL | Henry Schein Animal Health | Cat #: 003845 | |
| Chemical Compound or Drug | Paraformaldehyde | Alfa Aesar | Cat # A11313 | |
| Chemical Compound or Drug | Sodium hydroxide | EMD Millipore | Cat #: SX0590–1 | |
| Chemical Compound or Drug | Sodium tetraborate | Alfa Aesar | Cat #: 40114 | |
| Chemical Compound or Drug | Methylcellulose, 400 cP | Sigma-Aldrich | CAS #: 9004–67-5 | |
| Chemical Compound or Drug | Tween-80 | Sigma-Aldrich | CAS #: 9005–65-6 | |
| Chemical Compound or Drug | EDTA Tetrasodium | EMD Millipore | Cat #: EX0550–5 | |
| Chemical Compound or Drug | Trizma hydrochloride | Sigma Aldrich | Cat #: T3252–250G | |
| Chemical Compound or Drug | Proteinase K | Sigma Aldrich | Cat #: P2308–100MG | |
| Chemical Compound or Drug | Triethanolamine | Sigma Aldrich | Cat #: T58300–1KG | |
| Chemical Compound or Drug | Acetic anhydride | EMD Millipore | Cat #: AX0080–6 | |
| Chemical Compound or Drug | Citric acid trisodium | VWR | Cat #: 0101–500G | |
| Chemical Compound or Drug | ProLong Gold Antifade mounting medium | Cell Signaling | Cat #: 9071S | |
| Chemical Compound or Drug | aCSF | Harvard Apparatus | Cat #: 59–7316 | |
| Chemical Compound or Drug | DMSO | MP Biomedicals, LLC | Cat #: 196055 | |
| Commercial Assay Or Kit | GHSR1a probe | Advanced Cell Diagnostics | Cat #: 431991-C2 | |
| Commercial Assay Or Kit | Glucagon-like peptide-1 receptor probe | Advanced Cell Diagnostics | Cat #: 315221-C2 | |
| Commercial Assay Or Kit | hyocretin (orexin) receptor-1 probe | Advanced Cell Diagnostics | Cat #: 444761 | |
| Commercial Assay Or Kit | vGLUT1 probe | Advanced Cell Diagnostics | Cat #: 317001-C2 | |
| Commercial Assay Or Kit | RNAscope Fluorescent Multiplex Reagent Kit | Advanced Cell Diagnostics | Cat #: 320851 | |
| Commercial Assay Or Kit | RNAscope Wash Buffer | Advanced Cell Diagnostics | Cat #: 320058 | |
| Peptide, Recombinant Protein | Ghrelin | Bachem | Cat #: 4033076 | |
| Peptide, Recombinant Protein | Cholecystokinin | Bachem | Cat #: 4003323 | |
| Peptide, Recombinant Protein | Exendin-4 | Bachem | Cat #: 4027457 | |
| Peptide, Recombinant Protein | Amylin | Bachem | Cat #: 4030201 | |
| Peptide, Recombinant Protein | SB-334867 | Tocris Bioscience | Cat #: 1960 |
Acknowledgments
Funding and Disclosure
This study was supported by the National Institute of Health grants: DK104897 (SK), DK118402 (SK), DK116558 (AS), DK118000 (EN), and DK118944 (CL). The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods SC, Seeley RJ (2000): Adiposity signals and the control of energy homeostasis. Nutrition. 16:894–902. [DOI] [PubMed] [Google Scholar]
- 2.Leibowitz SF, Wortley KE (2004): Hypothalamic control of energy balance: different peptides, different functions. Peptides. 25:473–504. [DOI] [PubMed] [Google Scholar]
- 3.Narayanan NS, Guarnieri DJ, DiLeone RJ (2010): Metabolic hormones, dopamine circuits, and feeding. Front Neuroendocrinol. 31:104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grill HJ, Hayes MR (2012): Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 16:296–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu CM, Kanoski SE (2018): Homeostatic and non-homeostatic controls of feeding behavior: Distinct vs. common neural systems. Physiol Behav. 193:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu TM, McCutcheon JE, Roitman MF (2018): Parallels and Overlap: The Integration of Homeostatic Signals by Mesolimbic Dopamine Neurons. Front Psychiatry. 9:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azevedo EP, Pomeranz L, Cheng J, Schneeberger M, Vaughan R, Stern SA, et al. (2019): A Role of Drd2 Hippocampal Neurons in Context-Dependent Food Intake. Neuron. 102:873–886 e875. [DOI] [PubMed] [Google Scholar]
- 8.Eichenbaum H (2001): The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav Brain Res. 127:199–207. [DOI] [PubMed] [Google Scholar]
- 9.Moser EI, Moser MB, McNaughton BL (2017): Spatial representation in the hippocampal formation: a history. Nat Neurosci. 20:1448–1464. [DOI] [PubMed] [Google Scholar]
- 10.Kanoski SE, Grill HJ (2017): Hippocampus Contributions to Food Intake Control: Mnemonic, Neuroanatomical, and Endocrine Mechanisms. Biol Psychiatry. 81:748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parent MB, Darling JN, Henderson YO (2014): Remembering to Eat: Hippocampal Regulation of Meal Onset. Am J Physiol Regul Integr Comp Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson TL, Jones S, Roy M, Stevenson RJ (2019): The Cognitive Control of Eating and Body Weight: It’s More Than What You “Think”. Front Psychol. 10:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min DK, Tuor UI, Koopmans HS, Chelikani PK (2011): Changes in differential functional magnetic resonance signals in the rodent brain elicited by mixed-nutrient or protein-enriched meals. Gastroenterology. 141:1832–1841. [DOI] [PubMed] [Google Scholar]
- 14.Min DK, Tuor UI, Chelikani PK (2011): Gastric distention induced functional magnetic resonance signal changes in the rodent brain. Neuroscience. 179:151–158. [DOI] [PubMed] [Google Scholar]
- 15.Wang G-J, Yang J, Volkow ND, Telang F, Ma Y, Zhu W, et al. (2006): Gastric stimulation in obese subjects activates the hippocampus and other regions involved in brain reward circuitry. PNAS Proceedings of the National Academy of Sciences of the United States of America. 103:15641–15645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suarez AN, Hsu TM, Liu CM, Noble EE, Cortella AM, Nakamoto EM, et al. (2018): Gut vagal sensory signaling regulates hippocampus function through multi-order pathways. Nat Commun. 9:2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanoski SE, Hayes MR, Greenwald HS, Fortin SM, Gianessi CA, Gilbert JR, et al. (2011): Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology. 36:1859–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu TM, Noble EE, Liu CM, Cortella AM, Konanur VR, Suarez AN, et al. (2018): A hippocampus to prefrontal cortex neural pathway inhibits food motivation through glucagon-like peptide-1 signaling. Molecular psychiatry. 23:1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, et al. (2015): Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. Elife. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Wang P, Zheng H, Smith RG (2004): Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proc Natl Acad Sci U S A. 101:4679–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Howard AD, Feighner SD, Cully DF, Arena JP, Liberator PA, Rosenblum CI, et al. (1996): A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 273:974–977. [DOI] [PubMed] [Google Scholar]
- 22.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS (2001): A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 50:1714–1719. [DOI] [PubMed] [Google Scholar]
- 23.Drazen DL, Vahl TP, D’Alessio DA, Seeley RJ, Woods SC (2006): Effects of a fixed meal pattern on ghrelin secretion: evidence for a learned response independent of nutrient status. Endocrinology. 147:23–30. [DOI] [PubMed] [Google Scholar]
- 24.Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, et al. (2009): Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 164:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Callahan HS, Cummings DE, Pepe MS, Breen PA, Matthys CC, Weigle DS (2004): Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 89:1319–1324. [DOI] [PubMed] [Google Scholar]
- 26.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. (2001): Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 86:4753–4758. [DOI] [PubMed] [Google Scholar]
- 27.Cao SG, Wu H, Cai ZZ (2016): Dose-dependent effect of ghrelin on gastric emptying in rats and the related mechanism of action. Kaohsiung J Med Sci. 32:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levin F, Edholm T, Schmidt PT, Gryback P, Jacobsson H, Degerblad M, et al. (2006): Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 91:3296–3302. [DOI] [PubMed] [Google Scholar]
- 29.Cabral A, Cornejo MP, Fernandez G, De Francesco PN, Garcia-Romero G, Uriarte M, et al. (2017): Circulating Ghrelin Acts on GABA Neurons of the Area Postrema and Mediates Gastric Emptying in Male Mice. Endocrinology. 158:1436–1449. [DOI] [PubMed] [Google Scholar]
- 30.Williams DL, Grill HJ, Cummings DE, Kaplan JM (2003): Vagotomy dissociates short- and long-term controls of circulating ghrelin. Endocrinology. 144:5184–5187. [DOI] [PubMed] [Google Scholar]
- 31.Woods SC (2004): Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. American Journal of Physiology - Gastrointestinal & Liver Physiology. 286:G7–13. [DOI] [PubMed] [Google Scholar]
- 32.Moran TH (2004): Gut peptides in the control of food intake: 30 years of ideas. Physiol Behav. 82:175–180. [DOI] [PubMed] [Google Scholar]
- 33.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, et al. (2006): Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 9:381–388. [DOI] [PubMed] [Google Scholar]
- 34.Guan XM, Yu H, Palyha OC, McKee KK, Feighner SD, Sirinathsinghji DJ, et al. (1997): Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res Mol Brain Res. 48:23–29. [DOI] [PubMed] [Google Scholar]
- 35.Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006): Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 494:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perello M, et al. (2014): Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J Comp Neurol. 522:3644–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanoski SE, Fortin SM, Ricks KM, Grill HJ (2013): Ghrelin Signaling in the Ventral Hippocampus Stimulates Learned and Motivational Aspects of Feeding via PI3K-Akt Signaling. Biol Psychiatry. 73:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hsu TM, Noble EE, Reiner DJ, Liu CM, Suarez AN, Konanur VR, et al. (2018): Hippocampus ghrelin receptor signaling promotes socially-mediated learned food preference. Neuropharmacology. 131:487–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Gong Y, Wang H, Sun X, Guo F, Gao S, et al. (2014): The stimulating effect of ghrelin on gastric motility and firing activity of gastric-distension-sensitive hippocampal neurons and its underlying regulation by the hypothalamus. Exp Physiol. 99:123–135. [DOI] [PubMed] [Google Scholar]
- 40.Cone JJ, McCutcheon JE, Roitman MF (2014): Ghrelin acts as an interface between physiological state and phasic dopamine signaling. J Neurosci. 34:4905–4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brown JA, Bugescu R, Mayer TA, Gata-Garcia A, Kurt G, Woodworth HL, et al. (2017): Loss of Action via Neurotensin-Leptin Receptor Neurons Disrupts Leptin and Ghrelin-Mediated Control of Energy Balance. Endocrinology. 158:1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moran TH, Baldessarini AR, Salorio CF, Lowery T, Schwartz GJ (1997): Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol. 272:R1245–1251. [DOI] [PubMed] [Google Scholar]
- 43.Scarpignato C, Capovilla T, Bertaccini G (1980): Action of caerulein on gastric emptying of the conscious rat. Arch Int Pharmacodyn Ther. 246:286–294. [PubMed] [Google Scholar]
- 44.Bozkurt A, Oktar BK, Kurtel H, Alican I, Coskun T, Yegen BC (1999): Capsaicin-sensitive vagal fibres and 5-HT3-, gastrin releasing peptide- and cholecystokinin A-receptors are involved in distension-induced inhibition of gastric emptying in the rat. Regul Pept. 83:81–86. [DOI] [PubMed] [Google Scholar]
- 45.Kanoski SE, Fortin SM, Arnold M, Grill HJ, Hayes MR (2011): Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology. 152:3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz TA, Mollet A, Rushing PA, Riediger T, Scharrer E (2001): The anorectic effect of a chronic peripheral infusion of amylin is abolished in area postrema/nucleus of the solitary tract (AP/NTS) lesioned rats. Int J Obes Relat Metab Disord. 25:1005–1011. [DOI] [PubMed] [Google Scholar]
- 47.Kanoski SE, Walls EK, Davidson TL (2007): Interoceptive “satiety” signals produced by leptin and CCK. Peptides. 28:988–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davidson TL, Kanoski SE, Tracy AL, Walls EK, Clegg D, Benoit SC (2005): The interoceptive cue properties of ghrelin generalize to cues produced by food deprivation. Peptides. 26:1602–1610. [DOI] [PubMed] [Google Scholar]
- 49.Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE (2010): Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 124:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy PJ, Shapiro ML (2004): Retrieving memories via internal context requires the hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 24:6979–6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zingg B, Chou XL, Zhang ZG, Mesik L, Liang F, Tao HW, et al. (2017): AAV-Mediated Anterograde Transsynaptic Tagging: Mapping Corticocollicular Input-Defined Neural Pathways for Defense Behaviors. Neuron. 93:33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Swanson LW (2003): Brain Maps: Structure of the Rat Brain (Vol 3). 3rd ed: Academic Press. [Google Scholar]
- 53.Cenquizca LA, Swanson LW (2006): Analysis of direct hippocampal cortical field CA1 axonal projections to diencephalon in the rat. Journal of Comparative Neurology. 497:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Swanson LW, Cowan WM (1979): The connections of the septal region in the rat. J Comp Neurol. 186:621–655. [DOI] [PubMed] [Google Scholar]
- 55.van Groen T, Wyss JM (1990): Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol. 302:515–528. [DOI] [PubMed] [Google Scholar]
- 56.Cone JJ, Roitman JD, Roitman MF (2015): Ghrelin regulates phasic dopamine and nucleus accumbens signaling evoked by food-predictive stimuli. J Neurochem. 133:844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reiner DJ, Leon RM, McGrath LE, Koch-Laskowski K, Hahn JD, Kanoski SE, et al. (2018): Glucagon-Like Peptide-1 Receptor Signaling in the Lateral Dorsal Tegmental Nucleus Regulates Energy Balance. Neuropsychopharmacology. 43:627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seeley RJ, Grill HJ, Kaplan JM (1994): Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci. 108:347–352. [PubMed] [Google Scholar]
- 59.Grill HJ, Norgren R (1978): Chronically decerebrate rats demonstrate satiation but not bait shyness. Science. 201:267–269. [DOI] [PubMed] [Google Scholar]
- 60.Grill HJ, Kaplan JM (1992): Sham feeding in intact and chronic decerebrate rats. Am J Physiol. 262:R1070–1074. [DOI] [PubMed] [Google Scholar]
- 61.Ferriday D, Brunstrom JM (2008): How does food-cue exposure lead to larger meal sizes? Br J Nutr. 100:1325–1332. [DOI] [PubMed] [Google Scholar]
- 62.Higgs S (2002): Memory for recent eating and its influence on subsequent food intake. Appetite 39:159–166 [DOI] [PubMed] [Google Scholar]
- 63.Brunstrom JM, Burn JF, Sell NR, Collingwood JM, Rogers PJ, Wilkinson LL, et al. , (2012): Episodic memory and appetite regulation in humans. PLoS One 7:e50707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whitelock V, Robinson E (2018): Remembered Meal Satisfaction, Satiety, and Later Snack Food Intake: A Laboratory Study. Nutrients. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Higgs and Thomas (2016): Social influences on eating, Current Opinion in Behavioral Sciences 9, 1–6. [Google Scholar]
- 66.Hannapel R, Ramesh J, Ross A, LaLumiere RT, Roseberry AG, Parent MB (2019): Postmeal Optogenetic Inhibition of Dorsal or Ventral Hippocampal Pyramidal Neurons Increases Future Intake. eNeuro. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sweeney P, Yang Y (2015): An excitatory ventral hippocampus to lateral septum circuit that suppresses feeding. Nature communications. 6:10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rozin P, Dow S, Moscovitch M, Rajaram S (1998): What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychol Sci. 9:392–396. [Google Scholar]
- 69.Higgs S, Williamson AC, Rotshtein P, Humphreys GW (2008): Sensory-specific satiety is intact in amnesics who eat multiple meals. Psychol Sci. 19:623–628. [DOI] [PubMed] [Google Scholar]
- 70.Sample CH, Martin AA, Jones S, Hargrave SL, Davidson TL (2015): Western-style diet impairs stimulus control by food deprivation state cues: Implications for obesogenic environments. Appetite. 93:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H, et al. (2002): The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 123:1120–1128. [DOI] [PubMed] [Google Scholar]
- 72.Arnold M, Mura A, Langhans W, Geary N (2006): Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 26:11052–11060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Del Prete E, Balkowski G, Scharrer E (1994): Meal pattern of rats during hyperphagia induced by longterm food restriction is affected by diet composition. Appetite. 23:79–86. [DOI] [PubMed] [Google Scholar]
- 74.Dess NK, Schreiber KR, Winter GM, Chapman CD (2018): Taste as a marker for behavioral energy regulation:Replication and extension of meal pattern evidence from selectively bred rats. Behav Processes. 153:9–15. [DOI] [PubMed] [Google Scholar]
- 75.Hahn JD, Swanson LW (2012): Connections of the lateral hypothalamic area juxtadorsomedial region in the male rat. J Comp Neurol. 520:1831–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Estabrooke IV, McCarthy MT, Ko E, Chou TC, Chemelli RM, Yanagisawa M, et al. (2001): Fos expression in orexin neurons varies with behavioral state. J Neurosci. 21:1656–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harris GC, Aston-Jones G (2006): Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29:571–577. [DOI] [PubMed] [Google Scholar]
- 78.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. (2010): Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 1314:74–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aston-Jones G, Smith RJ, Moorman DE, Richardson KA (2009): Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology. 56 Suppl 1:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, et al. (2005): Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci U S A. 102:19168–19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dickson SL, Egecioglu E, Landgren S, Skibicka KP, Engel JA, Jerlhag E (2011): The role of the central ghrelin system in reward from food and chemical drugs. Mol Cell Endocrinol. 340:80–87. [DOI] [PubMed] [Google Scholar]
- 82.Cornwall J, Cooper JD, Phillipson OT (1990): Afferent and efferent connections of the laterodorsal tegmental nucleus in the rat. Brain Res Bull. 25:271–284. [DOI] [PubMed] [Google Scholar]
- 83.Reiner DJ, Mietlicki-Baase EG, Olivos DR, McGrath LE, Zimmer DJ, Koch-Laskowski K, et al. (2017): Amylin Acts in the Lateral Dorsal Tegmental Nucleus to Regulate Energy Balance Through Gamma-Aminobutyric Acid Signaling. Biol Psychiatry. 82:828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cabral A, Fernandez G, Perello M (2013): Analysis of brain nuclei accessible to ghrelin present in the cerebrospinal fluid. Neuroscience. 253:406–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, et al. (2012): Input-specific ontrol of reward and aversion in the ventral tegmental area. Nature. 491:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schmidt HD, Famous KR, Pierce RC (2009): The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 30:1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA (2007): Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 12:6–16. [DOI] [PubMed] [Google Scholar]
- 88.Jerlhag E, Janson AC, Waters S, Engel JA (2012): Concomitant release of ventral tegmental acetylcholine and accumbal dopamine by ghrelin in rats. PLoS One. 7:e49557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, et al. (2009): Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A. 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Semba K, Fibiger HC (1992): Afferent connections of the laterodorsal and the pedunculopontine tegmental nuclei in the rat: a retro- and antero-grade transport and immunohistochemical study. J Comp Neurol. 323:387–410. [DOI] [PubMed] [Google Scholar]
- 91.Hong EY, Yoon YS, Lee HS (2011): Differential distribution of melanin-concentrating hormone (MCH)- and hypocretin (Hcrt)-immunoreactive neurons projecting to the mesopontine cholinergic complex in the rat. Brain Res. 1424:20–31. [DOI] [PubMed] [Google Scholar]
- 92.Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. (1998): Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 18:9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, et al. (2001): Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 435:6–25. [DOI] [PubMed] [Google Scholar]
- 94.Hayes MR, Bradley L, Grill HJ (2009): Endogenous hindbrain glucagon-like peptide-1 receptor activation contributes to the control of food intake by mediating gastric satiation signaling. Endocrinology. 150:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hsu TM, Suarez AN, Kanoski SE (2016): Ghrelin: A link between memory and ingestive behavior. Physiol Behav. 162:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.