Abstract
Background and Purpose:
Low frequency oscillations reflect brain injury but also contribute to normal behaviors. We examined hypotheses relating electroencephalography (EEG) measures, including low frequency oscillations, to injury and motor recovery post-stroke.
Methods:
Patients with stroke completed structural neuroimaging, a resting-state EEG recording, and clinical testing. A subset admitted to an inpatient rehabilitation facility (IRF) also underwent serial EEG recordings. The relationship that EEG measures (power and coherence with leads overlying ipsilesional primary motor cortex (iM1)) had with injury and motor status was assessed, focusing on delta (1–3 Hz) and high-beta (20–30 Hz) bands.
Results:
Across all patients (n=62), larger infarct volume was related to higher delta band power in bilateral hemispheres and to higher delta band coherence between iM1 and bilateral regions. In chronic stroke, higher delta power bilaterally correlated with better motor status. In subacute stroke, higher delta coherence between iM1 and bilateral areas correlated with poorer motor status. These coherence findings were confirmed in serial recordings from 18 patients in an IRF. Here, interhemispheric coherence between leads overlying iM1 and contralesional M1 (cM1) was elevated at IRF admission compared to healthy controls (n=22), declining to control levels over time. Decreases in interhemispheric coherence between iM1 and cM1 correlated with better motor recovery.
Conclusions:
Delta band coherence with iM1 related to greater injury and poorer motor status subacutely, while delta band power related to greater injury and better motor status chronically. Low frequency oscillations reflect both injury and recovery after stroke and may be useful biomarkers in stroke recovery and rehabilitation.
Keywords: EEG, rehabilitation, stroke, imaging, cerebrovascular disease/stroke
Introduction
The development of biomarkers1 to inform clinical decision-making and clinical trials is fast underway. Substantial evidence supports the utility of anatomical imaging measurements, e.g., infarct volume and extent of injury to the corticospinal tract (CST) injury2–5, as potential biomarkers based on the relationships these measures have with motor recovery. Biomarkers derived from functional imaging are also needed, as structural measurements do not fully capture the dynamic quality of the brain that arises from arrays of neural networks organized and operating across several distinct temporal and spatial scales6. Potential biomarkers developed from functional neuroimaging thus provide additional insights into stroke recovery beyond what can be learned using anatomical imaging7–9; however, such measures are generally at an earlier stage of development.
Electroencephalography (EEG) measures have advantages as potential biomarkers of brain function. EEG directly captures electrical potentials from underlying neural tissue10, has excellent temporal resolution, and is safe and accessible in complex medical environments. EEG spectral power and coherence are two common quantitative metrics that reflect synchronized oscillatory activity within and between brain regions, respectively, with the latter serving as an indicator of functional connectivity11.
Power and coherence measures in specific EEG frequency bandwidths characterize distinct brain states. Based on our prior work12, 13, current hypotheses focused on two frequency bands of interest: [1] Low frequency oscillations (LFOs) in the delta frequency band (1–3 Hz). Although LFOs are associated with brain injury13, 14, they also reflect other biological phenomena important to brain function in the healthy state15, e.g., movement parameters16, attentional processing17, and cognitive control18. Ramanathan et al recently found that task-related LFOs activity in motor cortex was a marker of skilled motor control in both rodents and humans, diminishing early after stroke and later reemerging in association with recovery of motor behaviors19. [2] High-beta frequency band (beta2, 20–30 Hz). Beta2 activity also reflects brain injury, decreasing after stroke13, 20, and is also related to brain activity in the healthy state, e.g., motor system function21, inhibitory signaling22, alertness23, and maintenance of a sensorimotor or cognitive state24. LFOs and beta2 EEG measures thus may serve as potential biomarkers of both injury and motor status.
The current study addressed three aims examining performance of EEG as a potential biomarker of injury and motor recovery post-stroke: [1] Functional EEG measures (power and coherence) were examined in relation to structural injury (infarct volume and percent corticospinal tract (CST) injury) and motor status. Past brain mapping work has shown that structural and functional plasticity both contribute to behavioral recovery after stroke25, 26. We hypothesized that measures of both structural injury and motor status would have significant associations with functional EEG measures of power and coherence, in both the delta and beta2 bands. [2] Stroke is not a singular entity, and measures of brain function vary across subpopulations, therefore relationships between EEG measures and injury and motor status were further examined as a function of (a) time-post stroke and (b) infarct topography, hypothesizing that biomarker performance, defined as the strength of associations that injury and motor status have with EEG-based measures and the capability of EEG to distinguish between different clinical phenotypes, would vary significantly in relation to both factors. [3] Because animal27 and human28 resting-state fMRI studies emphasize the importance of ipsilesional and contralesional primary motor cortex (iM1, cM1) connectivity in stroke recovery, we examined iM1-cM1 coherence over time in a subset of patients, hypothesizing that decreases in coherence in the delta frequency band and increases in coherence in the beta2 frequency band parallel better motor recovery.
Methods
The data that support findings of this study are available from the corresponding author upon reasonable request.
Subjects
Patients with a radiologically confirmed stroke (ischemic or intracerebral hemorrhage) aged ≥18 years participated. Exclusion criteria were contraindication to MRI, substantial communication deficits, and history of cranial surgery that would introduce an EEG breach rhythm. Motor impairment was measured using the Upper Extremity Fugl-Meyer scale (UEFM). The subset of patients recruited from an inpatient rehabilitation facility (IRF) also underwent serial EEG recording and clinical assessments over time: around IRF admission, IRF discharge, and 90-days post-stroke. An additional clinical measure, the Functional Independence Measurement Motor subscale (FIM-motor), was scored at IRF admission and discharge. This subset is included in both cross-sectional and serial EEG studies reported. Healthy right-hand dominant age-matched controls were also studied. This study protocol received approval from the University of California, Irvine Institutional Review Board. All subjects provided written informed consent.
Magnetic Resonance Imaging
Neuroimaging was acquired on a 3-Tesla Philips Achieva scanner. Anatomical imaging included a high-resolution T1-weighted scan using a three-dimensional magnetization-prepared rapid gradient echo sequence (repetition time (TR)=8.1–8.5ms, echo time (TE)=3.7–3.9ms, 150 slices, voxel size=1×1×1mm3) and a T2-weighted fluid-attenuated inversion recovery scan (TR=9,000–11,000ms, TE=120–125ms, 31–33 slices, voxel size=0.58×0.58×5mm3).
Structural injury due to stroke was primarily assessed using a global injury measurement (infarct volume) and secondarily using a motor-specific measurement (percent CST injury). Lesion volume29 and percent CST injury5, 12 were measured using methods previously validated in stroke.
EEG Acquisition & Processing
Individuals completed at least one 3-minute awake, resting-state EEG recording using a dense-array 256 lead Hydrocel net (Electrical Geodesics, Eugene, OR) as previously described12. EEG data were collected at a sampling rate of 1,000 Hz using a high input impedance Net Amp 300 amplifier and Net Station 4.5.3 software (Electrical Geodesics Inc.). Consistent with past work12, raw and unfiltered EEG data were exported to MATLAB 8.5.0 (Mathworks, Natick, MA) for offline processing. Data were re-referenced to the mean signal across all leads. Of the 256 leads, 64 overlying cheek and neck regions were removed, leaving 192 leads for further analysis. A 50 Hz low-pass filter was applied with the remaining data segmented into 1-second non-overlapping epochs and mean detrended. Epochs consistent with muscle artifact were removed during visual inspection. Data underwent an Infomax independent components analysis using EEGLAB to remove ocular and cardiac artifacts30 and then transformed to electrode space for a second round of visual inspection.
EEG Coherence & Power
Coherence values are reported as the analogue of the squared correlation coefficient. Values range from 0 (indicative of random amplitude ratios and phase differences between signals across time) to 1 (indicating consistent amplitude ratios and phase differences ratios across time). A spectral analysis was performed by submitting the EEG time series to a discrete Fast Fourier transform. Power at all leads and coherence values between pairs of leads were computed across a 1–30 Hz range. Relative power was calculated by dividing power in a given frequency band for each electrode by the total power summed over the 1–30 Hz range. Data for patients with infarct in the right hemisphere were flipped across the midline so that the left hemisphere corresponded to the ipsilesional hemisphere for all patients. Left (ipsilesional) M1 served as the primary seed region for coherence measurements and included electrode C3 and the surrounding six leads. In the IRF cohort, power and coherence measurements were focused exclusively on leads overlying iM1 and cM1.
To determine whether measures of delta power reflect bursts of frontal intermittent rhythmic delta activity, data from the 46 leads overlying the frontal lobe were interrogated to measure the number of delta bursts, deemed present within a 1-second epoch when delta power exceeded a threshold defined as one standard deviation above the mean value of delta power across all epochs in all 62 patients with stroke.
Statistical Analysis
Spearman correlation coefficients were computed to assess associations between EEG data, structural injury, and motor impairment using Matlab and JMP 8.0.2 (SAS Institute, Cary, NC). Calculations involving dense-array EEG, which contains many leads for power and electrode pairs for coherence, introduce a large number of statistical tests. We addressed type 1 error using two approaches: [1] an omnibus approach across all analyses combined, which involved dividing the number of significant leads across all analyses by the total number of leads tested using a 5% criterion, i.e. ≥5% of leads tested across all analyses must show significance to argue against type 1 error, and [2] a strategy for each separate analysis adapted from Murias et al31. We defined an individual EEG map as significant if at least 10% of leads or electrode pairs in the map demonstrated a significant association (uncorrected p≤0.05). Since 192 leads were analyzed in each EEG map, a threshold of 19 significant (p≤ 0.05) leads for computations involving EEG power and 18 significant (p≤ 0.05) lead pairs for computations involving EEG coherence was necessary to declare an EEG map as showing a significant relationship with injury or motor status. For EEG maps demonstrating a significant relationship with injury and/or motor status, data from significant leads were compared between stroke patients and controls using two-tailed t-tests. Two-sample t-tests or Wilcoxon rank sums tests were done to examine differences in injury and clinical measures between stroke subgroups: subacute (3–30 days post-stroke) vs. chronic (>90 days post-stroke), and subcortical (infarct limited to subcortical white matter) vs. cortical (infarct involving both the cerebral cortex and subcortical white matter). Spearman rank partial correlation coefficients between CST and EEG measures were computed to control for lesion volume.
For serial EEG analyses, associations between EEG and motor recovery were evaluated using Pearson correlation coefficients. Student and paired t-tests were performed to ascertain group (patients vs. controls) differences in EEG metrics and within-subject EEG and behavioral change over time, respectively. To address multiple comparisons, alpha was adjusted using Bonferroni correction. The EEG metric of interest was iM1-cM1 coherence, and an alpha value of 0.025 was used at each time point to correct for analysis across two frequency bands (delta and beta2).
Results
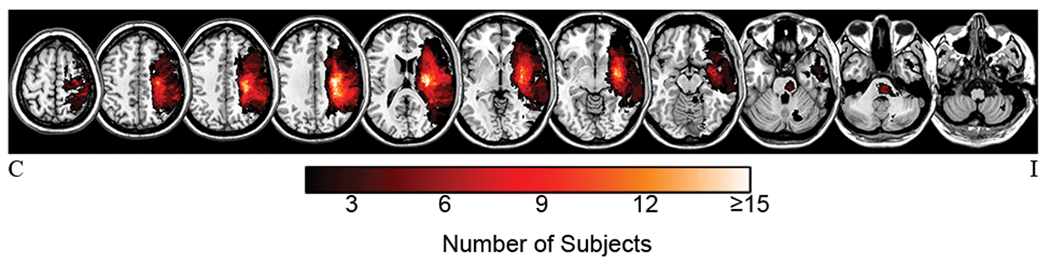
By design, patients showed considerable heterogeneity with respect to infarct location (Figure 1), motor impairment, and time post-stroke (Table 1). Of all EEG epochs inspected for artifact, 96.5 ± 4.9% were retained for analysis. Cross-sectional and serial data involved a total of 62 and 18 patients, respectively.
Figure 1.
Infarct masks from the 62 patients overlaid on T1-weighted images. Brighter colors signify increasing voxel damage frequency. Bilateral injury (not shown) occurred in two patients.
Table 1.
Patient Characteristics (n=62)
| Measure | Value | Range |
|---|---|---|
| Side of stroke (R/L) | 35/27 | |
| Dominant hemisphere injured (Y/N) | 28/34 | |
| Stroke type (ischemic/hemorrhagic) | 46/16 | |
| Sex (male/female) | 42/20 | |
| Time post-stroke (months) | 11.6±20.9 | 0.1–114.5 |
| Age (years) | 56.6±14.5 | 23–86 |
| Upper Extremity Fugl-Meyer | 42.9±16.2 | 8–66 |
| NIH Stroke Scale | 3 [2–5] | 0–12 |
| Infarct volume (cc) | 19.7±24.8 | 0.029–100.6 |
| Percent injury to corticospinal tract | 44.3±35.3 | 0–100 |
Values expressed as mean±SD or median [IQR].
Cross-sectional EEG Studies
EEG & INJURY.
Total infarct volume was examined in relation to delta and beta2 band EEG measures across all 62 patients (Table 2). In the delta band, infarct volume positively related to EEG power in leads overlying a large bilateral fronto-parietal area, and to coherence with iM1 in bilateral leads especially over contralesional fronto-parietal areas (Figure 2A). In the beta2 band, the direction of the relationship with power was reversed, being negatively correlated with infarct volume throughout leads overlying bilateral frontal regions.
Table 2.
EEG Associations with Injury & Behavior
| Delta band (1–3 Hz) | Beta2 band (20–30 Hz) | |||
|---|---|---|---|---|
| Power | Coherence with iM1 | Power | Coherence with iM1 | |
| Infarct Volume | ||||
| All patients | 94 (94+ / 0-) | 46 (46+ / 0-) | 36 (0+ / 36-) | |
| Subacute | 21 (21+ / 0-) | 65 (65+ / 0-) | 22 (0+ / 22-) | 18 (18+ / 0-) |
| Chronic | 78 (78+ / 0-) | 18 (16+ / 2-) | ||
| Subcortical | 74 (74+ / 0-) | 77 (0+ / 77-) | ||
| Cortical | ||||
| CST Injury | ||||
| All patients | 56 (53+ / 3+)* | 21 (9+ / 13-)* | ||
| Subacute | 52 (52+ / 0-) | |||
| Chronic | 23 (19+ / 4-)* | |||
| Subcortical | 54 (53+ / 1-) | |||
| Cortical | 20 (18+ / 2-)* | |||
| UEFM | ||||
| All patients | 45 (45+ / 0-) | 43 (0+ / 43-) | ||
| Subacute | 30 (0+ / 30-) | |||
| Chronic | 65 (65+ / 0-) | |||
| Subcortical | 67 (67+ / 0-) | 55 (1+ / 54-) | ||
| Cortical | ||||
Significant relationships (post multiple comparison correction) between EEG and injury/behavior. Numbers indicate the quantity of leads depicting a significant relationship (Spearman rho) between EEG and injury/behavioral measures; + and - symbols signify the direction of the correlation;
denotes significant associations between EEG and CST injury after correcting for lesion volume.
Figure 2.

Low frequency oscillations relate to stroke injury. Delta band power and coherence showed significant associations with infarct volume (A). Positive correlations between delta band power and injury occurred in both subacute and chronic stroke groups (B) while delta coherence with ipsilesional M1 positively correlated with injury in the subacute stroke group only (C). Colors reflect magnitude and direction of association at each EEG lead; color bar, Spearman’s rho. Black dots (C) indicate ipsilesional M1.
Extent of CST injury was significantly related to coherence with iM1. In the delta band, the relationship was positive, bilaterally, with higher delta coherence related to greater CST injury. In contrast, beta2 coherence showed scattered regions of both positive and negative associations. A significant positive association (ρ=0.37, p=0.005) was present between lesion volume and CST injury; however, the above CST-EEG association remained significant after accounting for infarct volume. Extent of CST injury was not related to EEG power, in either frequency band, even in leads overlying iM1.
EFFECT OF TIME AFTER STROKE.
To examine injury findings with respect to time post-stroke, patients were divided into two groups: subacute (n=24), who were 12.6±6.4 days post-stroke, and chronic (n=36), who were 19.5±24.6 months post-stroke. Two outliers (71 and 76 days post-stroke) were excluded from this analysis. Groups did not differ by infarct volume (Z=−0.84, p=0.40), extent of CST injury (Z=1.02, p=0.31), or UEFM score (Z=1.00, p=0.32).
Total infarct volume positively correlated with delta power in the subacute group within a narrow strip of leads overlying bilateral peri-Rolandic areas, and in the chronic group across a larger area that extended across bilateral fronto-parietal areas (Figure 2B). For beta2 power, the direction of the relationship with power was again reversed, with larger infarct volumes correlating with lower beta2 power in leads overlying contralesional fronto-parietal areas in the subacute group; this relationship was absent in the chronic group.
Total infarct volume positively correlated with delta coherence with iM1 in broad bilateral areas in the subacute group, and in a smaller contralesional area in the chronic group (Figure 2C). Beta2 coherence with iM1 showed a positive correlation with infarct volume in scattered regions in the subacute group that was absent in the chronic group.
Extent of CST injury was positively related to delta coherence with iM1. Significant leads were distributed across broad bilateral regions in the subacute group and a smaller ipsilesional region in the chronic group. Lesion volume and CST injury positively correlated in the subacute (ρ=0.52, p=0.03) chronic (ρ=0.34, p=0.05) groups. In the chronic group, the above CST-EEG association remained significant after correcting for infarct volume.
EFFECT OF INFARCT TOPOGRAPHY.
Infarct volume differed between subcortical (n=35, 4.7±7.2 cc) and cortical (n=27, 39.1±26.0 cc) groups (Z=5.94, p<0.0001), but CST injury (Z=1.31, p=0.19) and UEFM score (Z=−0.75, p=0.46) did not.
In the subcortical group, total infarct volume negatively correlated with beta2 power in broadly distributed leads bilaterally and positively correlated with delta coherence with iM1 in leads overlying bilateral fronto-parietal regions. These associations were not observed in the cortical group.
In both groups, CST injury positively correlated with delta coherence with iM1. In the subcortical group, this was present in contralesional>ipsilesional leads; in the cortical group, in leads overlying ipsilesional fronto-parietal regions. Lesion volume and CST injury demonstrated a significant positive correlation in the subcortical group (ρ=0.58, p=0.001) but not in the cortical group (ρ=0.24, p=0.22). After accounting for infarct volume, the CST-EEG association observed in the subcortical group was not significant.
EEG & MOTOR STATUS.
Results were also examined in relation to UEFM score. Associations between motor impairment and EEG measurements occurred exclusively in the delta frequency band. Higher UEFM scores (less motor impairment) positively correlated with delta power in leads overlying ipsilesional>contralesional areas, a pattern seen in the entire cohort and in the chronic (Figure 3A) and subcortical subgroups.
Figure 3.

Low frequency oscillations relate to post-stroke motor status. Greater delta band power related to higher UEFM score (less impairment) in the chronic but not subacute phase (A), while greater delta band coherence with ipsilesional M1 related to lower UEFM score (more impairment) in the subacute but not chronic phase (B). Colors reflect magnitude and direction of association at each EEG lead; color bar, Spearman’s rho. Black dots (B) indicate ipsilesional M1.
Higher UEFM scores negatively correlated with delta coherence with iM1 in contralesional>ipsilesional areas, a pattern seen in the entire cohort, and the subacute (Figure 3B) and subcortical subgroups.
EEG COMPARISONS WITH CONTROLS.
The mean values for EEG power and coherence did not differ between patients and controls (n=22, 16 females, aged 57.3±15.5 years), with a single exception: healthy controls demonstrated greater beta2 band coherence with M1 compared to the subacute stroke group (n=24, t=−3.06, p=0.004) in leads previously identified as significant in an association between lesion volume and beta2 coherence.
INTERMITTENCY OF DELTA POWER.
Delta power in frontal leads was examined and found to be generally not intermittent as demonstrated by evaluation of delta burst frequency. Across patients, 10.4±3.4% (range: 2.7–17.2%) of epochs contained a delta burst. Delta burst percentages did not vary between subacute (9.9±3.9%) and chronic (10.7±3.0%) groups (t=−0.82, p=0.41) or between subcortical (10.6±3.5%) and cortical (10.3±3.3%) groups (t=0.35, p=0.73). Across the healthy controls, 11.0±2.6% (range: 6.9–16.6%) of epochs contained a delta burst in frontal leads. The percentage of delta bursts did not significantly differ between patients and controls (t=−0.72, p=0.47).
EFFECTS OF MULTIPLE COMPARISONS.
Although a conservative threshold was used to define the number of EEG leads needed to declare that an EEG map showed a significant relationship with injury or motor status in the above analyses, concern for type I error was also addressed using an omnibus approach across all analyses performed. A total of 11,430 statistical tests were done on electrodes across all analyses combined, including across coherence, power, frequency bands, and subgroups. A total of 1,080 electrodes, or 9.45%, were found to have a significant relationship, arguing against a role for type I error to explain current findings.
Serial EEG Studies
A total of 18 patients completed multiple EEG recordings during their IRF stay. Median time post-stroke at enrollment was 10 days (range: 5–71); IRF length of stay, 16 days (range: 10–116). Patients made substantial FIM-motor gains (FIM-motor at IRF admit: 38.8±13.4 points, FIM-motor at IRF discharge: 73.4±11.1 points, FIM-motor change 34.6±14.2 points (range: 4–58)) and UEFM gains (UEFM at IRF admit: 39.4± 20.8 points, UEFM at IRF discharge: 46.7±17.1 points, UEFM change: 7.7±7.7 points (range: 0–28)) during their IRF stay. Patients completing UEFM testing at 90-days post-stroke also demonstrated clinically meaningful change from the time of IRF admission (n=17, UEFM at IRF admission: 38.5±20.5 points, UEFM at 90-days post-stroke: 53.6±15.7 points, UEFM change:15.1±13.9 points (range: −1–48)).
At the time of IRF admission, patients demonstrated significantly elevated delta iM1-cM1 coherence (0.22±0.10) compared to controls (0.12±0.11, t=3.64, p=0.0008). In those patients that completed a 90-day follow-up (n=17), delta iM1-cM1 coherence was comparable to controls (0.19±0.12, t=1.87, p=0.07). A decrease in delta iM1-cM1 coherence occurring during the IRF stay correlated with FIM-motor score improvement during the same time period (r=−0.70, p=0.001; Figure 4A), but not with UEFM score improvement. A decrease in delta iM1-cM1 coherence over 90-days post-stroke correlated with UEFM gains during this time (r=−0.57, p=0.02, Figure 4B). Because changes in power might modulate coherence, post-hoc analyses examined potential associations between change in iM1-cM1 coherence and change in bilateral delta power across the two time periods. A significant association was found between change in delta iM1-cM1 coherence and delta band cM1 power during IRF stay (r=0.68, p=0.002), although change in delta band cM1 power during this period was not related to motor recovery.
Figure 4.

(A) Greater gains in FIM-motor score from IRF admission to discharge correlated with larger decreases in delta band iM1-cM1 coherence (n=18). (B) Greater gains in UEFM scores from IRF admission to 90-days post-stroke correlated with decreased delta iM1-cM1 coherence (n=17).
Discussion
Structural and functional plasticity contribute to behavioral recovery following stroke. The current study examined candidate EEG measures as potential biomarkers of stroke injury and motor recovery using cross-sectional and serial data. Delta iM1 coherence related to greater injury and poorer behavioral status in the subacute period, while delta power related to greater injury and better behavioral status in the chronic period. Reductions in delta coherence between iM1 and cM1 over time correlated with motor recovery. These findings highlight LFOs as a potential biomarker of stroke recovery.
Delta Band Power
Across all patients, larger infarct volume correlated with higher delta power across bilateral hemispheres (Figure 2A). At least two parallel biological processes were apparent, both apparent late after stroke: (1) a more anterior, bilateral, injury-related process where higher delta power was associated with larger injury but was unrelated to motor status; and (2) a more posterior, ipsilesional, behavior-related process where higher delta power was related to better motor status (Figures 2, 3).
In the subacute phase, LFOs reflected stroke injury, consistent with the classic association between brain injury and delta waves. Larger subacute injury was associated with higher delta power in leads overlying bilateral motor cortices, a location concordant with the motor status of the population studied. Only 10.4 % of epochs contained a delta burst– these LFOs are not simply frontal intermittent rhythmic delta activity.
In the chronic phase, positive associations between injury and delta power were observed in leads overlying M1 and involved widespread bilateral areas (Figure 2B), particularly a large posterior ipsilesional area where higher delta power also correlated with better motor status (Figure 3A). In chronic stroke, posterior ipsilesional LFOs was not simply a marker of neural injury as higher delta power corresponded to better motor status. Chronic phase LFOs might reflect adaptive plasticity related to attentional processing, task complexity, or cognitive control, each of which has also been linked with LFOs17, 18. The positive association between delta power and motor status in the chronic stroke group represents a behaviorally favorable association and might therefore reflect an effective compensatory mechanism.
Delta Band Coherence
Across all patients, larger infarct volume also correlated with higher delta coherence with iM1 (Figure 2A). This association was observed in subacute stroke group but not in the chronic group (Figure 2C). As with delta power, two parallel processes were apparent: (1) an anterior, bilateral, injury-related process where higher coherence early after stroke was associated with larger injury but unrelated to motor status and (2) a more posterior, contralesional, behavior-related process where higher coherence was related to poorer motor behavior (Figures 2, 3). These findings align with prior EEG work in traumatic brain injury32 that found higher delta coherence across long distances with greater injury extent.
The positive associations that delta coherence has with injury and motor status might reflect an ineffective compensatory response. Normally, delta coherence plays an important role when large-scale, distant cortical networks coordinate their neural activity33, particularly in the context of modulating attention34 or motivation35. One interpretation of the coherence findings is that with greater injury and larger deficits subacutely post-stroke, iM1 increases inter-regional delta band communication, perhaps in relation to distressed attention and motivation, but this response is insufficient to improve motor status.
In the IRF cohort, decreases in delta band iM1-cM1 coherence over time parallel better motor recovery. Our findings complement previous work that reported disruptions in normal resting-state low-frequency brain rhythms early after stroke that later resolved in conjunction with behavioral recovery36, 37. Although we cannot determine whether these reductions in delta band iM1-cM1 coherence over time reflect spontaneous versus treatment-induced recovery, these findings nevertheless represent behaviorally relevant neuroplasticity in a motor-specific circuit similar to processes previously described in fMRI and PET studies25, 26, and so may be a potential biomarker of stroke recovery measurable at the bedside.
Infarct Topography
Results differed sharply according to infarct topography. Stronger delta band associations in the subcortical group is consistent with prior studies, which found that subcortical injury produces delta activity while isolated cortical injury does not38, and that delta waves arising from white matter lesions are localized to the area of ipsilesional cortex overlying the lesion39. These findings underscore the potential of EEG biomarkers to capture upstream cortical plasticity after subcortical injury.
Healthy Control Comparisons
Cross-sectional data (n=62) did not reveal EEG power and coherence differences between patients and controls, apart from one exception. Our interpretation of this finding is that in this cohort, primarily composed of patients with mild to moderate motor impairment, neurophysiological events occurring after stroke as captured by EEG take place within the frame of normal events and within the normal range defined by the control group. EEG measures from the stroke cohort therefore represent a combination of individual up- and down-regulation within this normal range. Delta bursts in frontal leads were equally common when comparing patients with healthy controls, also supporting this reasoning.
Beta2 Band Findings
Although prior studies from our group in patients with acute stroke emphasized the relevance of both delta and beta2 bands for understanding stroke-related injury and behavior12, 13, current findings were sparse in the beta2 band. Consistent with the well-established relationship between brain injury and attenuation of beta waves13, 20, we observed lower beta2 power with greater infarct volume. Beta activity in the 20–30 Hz band relates to cortical output from layer V pyramidal neurons40, suggesting that beta band EEG measures might serve as a potential biomarker of injury to CST which also originates from layer V. Here, beta2 band coherence with iM1 significantly related to the extent of CST injury.
Motor status (cross-sectional data) and motor recovery (serial data) did not correlate with either beta2 band power or coherence. These findings match work by Rossiter et al41 but not Thibaut et al42. Discrepancies in these findings may reflect the populations studied or the method used to assess the motor system and might be addressed by directly matching clinical measures across future studies of stroke recovery.
Strengths and Weaknesses
This study had several strengths, including serial EEG measurements in patients with subacute stroke admitted to an IRF. The confirmation of cross-sectional findings with the serial data supports this as a proof of principle study; however, the modest sample size (n=18) in the serial data set is consistent with a pilot study; therefore, we await additional investigation to confirm those findings. The use of dense array EEG provides good spatial resolution, which facilitated identification of spatially distinct EEG-related processes. In comparison to most other EEG studies in stroke, the large sample size, consisting of all individuals completing a three-minute resting-state EEG recording including consecutive consenting patients admitted to an IRF over a three-year recruitment period, provided the study with a diverse population, enabling evaluation of EEG measures as potential biomarkers with respect to time post-stroke, motor status, and injury topography. The study also had several important weaknesses. Source localization, head modeling, and surface Laplacian transformations were not applied, which while potentially informative also introduce their own limitations and distortions10. Issues of volume conduction and/or extended slow-wave activity from average referencing may exist, generating concern of EEG power driving coherence. Here, power and coherence associations were largely absent with (with three exceptions). Finally, recordings were obtained at rest. Recent work by Bönstrup et al. reported diminished LFOs in motor cortices during movement preparation of a grip task in patients with acute stroke in comparison to control subjects43. Increases in LFOs over time related to motor function (grip strength) improvement and motor recovery (UEFM and Nine Hole Peg scores). Movement-related LFOs acquired during event-related recordings therefore provide additional insights to what is learned using resting-state EEG16, 19, 40, 41 and should be pursued in future studies.
Conclusions
Biomarkers have the potential to provide valuable information about patients that may assist researchers and clinicians in identifying specific biological subgroups and/or those that may benefit from one treatment approach versus another. Given the heterogeneity of stroke, biomarkers have the potential to significantly impact the field of stroke rehabilitation. This study aimed to identify EEG biomarkers of brain function after stroke with respect to injury, motor status, time post-stroke, and recovery. The strongest findings emerged with respect to LFOs. Biomarker findings were distinct between extent of injury and impairment, underscoring that biomarkers after stroke are not one-size-fits all1. Delta waves reflect more than one biological phenomenon15 and so might serve as a link between injury and plasticity. LFO data acquired at the bedside using EEG may be useful biomarkers in the study of stroke recovery.
The informative potential of EEG, combined with its portability and accessibility, may offer clinicians an additional tool to incorporate in their practice to enhance patient prognostication, treatment allocation, and assessment of therapeutic response. However, implementation of EEG biomarkers in a clinical setting depends on answering a crucial next set of questions starting with external validation of current results. The current study adds to the increasing body of data describing the clinical potential of EEG-based biomarkers in stroke rehabilitation and recovery.
Acknowledgments
JC and SC were responsible for the study conception and design, manuscript preparation, and figures. JC, JW, KK, AM, AW, RS, SC were responsible for the data acquisition/analysis.
Sources of Funding
This work received funding from the NIH: K24HD074722 (SC), T32AR047752 (JC), K99HD091375 (JC), and the Institute for Clinical and Translational Science at the University of California, Irvine (5M011RR-00827–29).
Footnotes
Disclosures
Dr. Cramer has been a consultant for Abbvie, Constant Therapeutics, MicroTransponder, Neurolutions, Regenera, SanBio, Stemedica, Fujifilm Toyama Chemical Co., Biogen, and TRCare.
References
- 1.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehab Neural Re. 2017;31:864–876 [DOI] [PubMed] [Google Scholar]
- 2.Puig J, Pedraza S, Blasco G, Daunis IEJ, Prados F, Remollo S, et al. Acute damage to the posterior limb of the internal capsule on diffusion tensor tractography as an early imaging predictor of motor outcome after stroke. AJNR Am J Neuroradiol. 2011;32:857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riley JD, Le V, Der-Yeghiaian L, See J, Newton JM, Ward NS, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng W, Wang J, Chhatbar PY, Doughty C, Landsittel D, Lioutas VA, et al. Corticospinal tract lesion load: An imaging biomarker for stroke motor outcomes. Ann Neurol. 2015;78:860–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy JM, Tran G, Quinlan EB, Cramer SC. Neuroimaging identifies patients most likely to respond to a restorative stroke therapy. Stroke. 2018;49:433–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett DS, Bullmore E. Small-world brain networks. Neuroscientist. 2006;12:512–523 [DOI] [PubMed] [Google Scholar]
- 7.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The prep algorithm predicts potential for upper limb recovery after stroke. Brain : a journal of neurology. 2012;135:2527–2535 [DOI] [PubMed] [Google Scholar]
- 8.Nouri S, Cramer SC. Anatomy and physiology predict response to motor cortex stimulation after stroke. Neurology. 2011;77:1076–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke Quinlan E, Dodakian L, See J, McKenzie A, Le V, Wojnowicz M, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Annals of Neurology. 2015;77:132–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nunez PL, Nunez MD, Srinivasan R. Multi-scale neural sources of eeg: Genuine, equivalent, and representative. A tutorial review. Brain Topogr. 2019;32:193–214 [DOI] [PubMed] [Google Scholar]
- 11.Srinivasan R, Winter WR, Ding J, Nunez PL. Eeg and meg coherence: Measures of functional connectivity at distinct spatial scales of neocortical dynamics. J Neurosci Methods. 2007;166:41–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu J, Quinlan EB, Dodakian L, McKenzie A, Kathuria N, Zhou RJ, et al. Connectivity measures are robust biomarkers of cortical function and plasticity after stroke. Brain. 2015:awv156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu J, Srinivasan R, Burke Quinlan E, Solodkin A, Small SL, Cramer SC. Utility of eeg measures of brain function in patients with acute stroke. J Neurophysiol. 2016;115:2399–2405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanciullacci C, Bertolucci F, Lamola G, Panarese A, Artoni F, Micera S, et al. Delta power is higher and more symmetrical in ischemic stroke patients with cortical involvement. Front Hum Neurosci. 2017;11:385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assenza G, Di Lazzaro V. A useful electroencephalography (eeg) marker of brain plasticity: Delta waves. Neural Regen Res. 2015;10:1216–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall TM, de Carvalho F, Jackson A. A common structure underlies low-frequency cortical dynamics in movement, sleep, and sedation. Neuron. 2014;83:1185–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez ME, Pusil S, Pereda E, Maestu F, Barcelo F. Dynamic low frequency eeg phase synchronization patterns during proactive control of task switching. NeuroImage. 2019;186:70–82 [DOI] [PubMed] [Google Scholar]
- 19.Ramanathan DS, Guo L, Gulati T, Davidson G, Hishinuma AK, Won SJ, et al. Low-frequency cortical activity is a neuromodulatory target that tracks recovery after stroke. Nat Med. 2018;24:1257–1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman B, Claassen J. Quantitative eeg for the detection of brain ischemia. Critical care. 2012;16:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roopun AK, Middleton SJ, Cunningham MO, LeBeau FE, Bibbig A, Whittington MA, et al. A beta2-frequency (20–30 hz) oscillation in nonsynaptic networks of somatosensory cortex. Proc Natl Acad Sci U S A. 2006;103:15646–15650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picazio S, Veniero D, Ponzo V, Caltagirone C, Gross J, Thut G, et al. Prefrontal control over motor cortex cycles at beta frequency during movement inhibition. Curr Biol. 2014;24:2940–2945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spironelli C, Manfredi M, Angrilli A. Beta eeg band: A measure of functional brain damage and language reorganization in aphasic patients after recovery. Cortex. 2013;49:2650–2660 [DOI] [PubMed] [Google Scholar]
- 24.Engel AK, Fries P. Beta-band oscillations--signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165 [DOI] [PubMed] [Google Scholar]
- 25.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–287 [DOI] [PubMed] [Google Scholar]
- 26.Ward NS. Restoring brain function after stroke - bridging the gap between animals and humans. Nat Rev Neurol. 2017;13:244–255 [DOI] [PubMed] [Google Scholar]
- 27.van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burke E, Dodakian L, See J, McKenzie A, Riley JD, Le V, et al. A multimodal approach to understanding motor impairment and disability after stroke. J Neurol. 2014;261:1178–1186 [DOI] [PubMed] [Google Scholar]
- 30.Delorme A, Sejnowski T, Makeig S. Enhanced detection of artifacts in eeg data using higher-order statistics and independent component analysis. Neuroimage. 2007;34:1443–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murias M, Swanson JM, Srinivasan R. Functional connectivity of frontal cortex in healthy and adhd children reflected in eeg coherence. Cerebral cortex (New York, N.Y. : 1991). 2007;17:1788–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thatcher RW, Biver C, McAlaster R, Salazar A. Biophysical linkage between mri and eeg coherence in closed head injury. NeuroImage. 1998;8:307–326 [DOI] [PubMed] [Google Scholar]
- 33.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480 [DOI] [PubMed] [Google Scholar]
- 34.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320:110–113 [DOI] [PubMed] [Google Scholar]
- 35.Knyazev GG. Eeg delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36:677–695 [DOI] [PubMed] [Google Scholar]
- 36.Caliandro P, Vecchio F, Miraglia F, Reale G, Della Marca G, La Torre G, et al. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabil Neural Repair. 2017;31:81–94 [DOI] [PubMed] [Google Scholar]
- 37.Dubovik S, Pignat JM, Ptak R, Aboulafia T, Allet L, Gillabert N, et al. The behavioral significance of coherent resting-state oscillations after stroke. Neuroimage. 2012;61:249–257 [DOI] [PubMed] [Google Scholar]
- 38.Steriade M, Gloor P, Llinas RR, Lopes de Silva FH, Mesulam MM. Report of ifcn committee on basic mechanisms. Basic mechanisms of cerebral rhythmic activities. Electroencephalography and clinical neurophysiology. 1990;76:481–508 [DOI] [PubMed] [Google Scholar]
- 39.Ball GJ, Gloor P, Schaul N. The cortical electromicrophysiology of pathological delta waves in the electroencephalogram of cats. Electroencephalography and clinical neurophysiology. 1977;43:346–361 [DOI] [PubMed] [Google Scholar]
- 40.Baker SN, Olivier E, Lemon RN. Coherent oscillations in monkey motor cortex and hand muscle emg show task-dependent modulation. J Physiol. 1997;501 ( Pt 1):225–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rossiter HE, Boudrias MH, Ward NS. Do movement-related beta oscillations change after stroke? J Neurophysiol. 2014;112:2053–2058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thibaut A, Simis M, Battistella LR, Fanciullacci C, Bertolucci F, Huerta-Gutierrez R, et al. Using brain oscillations and corticospinal excitability to understand and predict post-stroke motor function. Front Neurol. 2017;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonstrup M, Krawinkel L, Schulz R, Cheng B, Feldheim J, Thomalla G, et al. Low-frequency brain oscillations track motor recovery in human stroke. Ann Neurol. 2019;86:853–865 [DOI] [PubMed] [Google Scholar]