Abstract
Purpose:
Meniscal injury and loss of meniscus tissue lead to osteoarthritis development. Therefore, novel biologic strategies are needed to enhance meniscus tissue repair. The purpose of this study was to identify a favorable culture medium for both bone marrow-derived mesenchymal stem cells (MSCs) and meniscal tissue, and to establish a novel meniscus tissue defect model that could be utilized for in vitro screening of biologics to promote meniscus repair.
Materials and Methods:
In parallel, we analyzed the biochemical properties of MSC-seeded meniscus-derived matrix (MDM) scaffolds and meniscus repair model explants cultured in different combinations of serum, dexamethasone (Dex), and TGF-β. Next, we combined meniscus tissue and MSC-seeded MDM scaffolds into a novel meniscus tissue defect model to evaluate effects of chondrogenic and meniscal media on the tissue biochemical properties and repair strength.
Results:
Serum-free medium containing TGF-β and Dex was the most promising formulation for experiments with MSC-seeded scaffolds, whereas serum-containing medium was the most effective for meniscus tissue composition and integrative repair. When meniscus tissue and MSC-seeded MDM scaffolds were combined into a defect model, the chondrogenic medium (serum-free with TGF-β and Dex) enhanced the production of proteoglycans and promoted integrative repair of meniscus tissue. As well, cross-linked scaffolds improved repair over the MDM slurry.
Conclusions:
The meniscal tissue defect model established in this paper can be used to perform in vitro screening to identify and optimize biological treatments to enhance meniscus tissue repair prior to conducting preclinical animal studies.
Keywords: fibrochondrogenic, tissue engineering, collagen, cartilage, growth factor, fibrochondrocyte, differentiation
Introduction
The menisci are critical knee structures that are essential for joint function and homeostasis by providing joint stability, congruity, load bearing capabilities, shock absorption, lubrication, and nutrition1–5. Meniscus injuries result in pain and disability, and also lead to the development of osteoarthritis (OA) in approximately two-thirds of meniscus-injured patients within 5–15 years6–13. Therefore, current treatment approaches focus on the preservation of meniscus tissue and repair of meniscus tears whenever possible, as well as replacing the lost or damaged tissue in an attempt to restore tissue function and biomechanics14. In vitro models show that meniscus repair can be successful in explanted tissue from either the avascular inner zone or the vascularized outer zone of the meniscus15. This suggests that tissue from both regions has the capacity to heal if provided with the appropriate environment. However, in patients, meniscus tissue healing is more favorable in the outer vascularized region and generally not recommended for tears in the inner avascular region, which are commonly treated with partial meniscectomy. Despite a focus on more conservative treatments, partial meniscectomy remains the most common orthopaedic procedure16. Thus, new biologic strategies are needed to promote improved meniscus tissue regeneration and repair.
Tissue engineering involves the use of cells, scaffolds, and biologic factors to repair or regenerate tissues. In particular for meniscus tissue engineering, primary meniscal cells17–24, adipose-derived stem cells25–27, synovial-derived stem cells28–30, meniscus-derived stem cells31, bone-marrow derived mesenchymal stem cells (MSCs)19, 22, 23, 26, 32–35, and MSCs co-cultured with meniscus cells36–39 have been investigated40–43. Stem cells are an attractive choice for meniscus tissue engineering because of their expansion capabilities, potential for fibrochondrogenic differentiation, ability to secrete repair-promoting growth factors, ability to migrate to sites of injury, and their role in immune system modulation31, 44–46. Furthermore, MSCs have been studied for meniscus tissue engineering applications and tissue repair19, 22, 23, 26, 32–35, 37, 40–42, 47. However, the optimal culture medium for MSCs co-cultured with meniscus tissue has not been established.
Biomaterial scaffolds are promising tools to augment meniscus repair procedures. In particular, scaffolds studied for meniscus tissue engineering have been composed of polymers19, 24, 28, 32, 34, 48–51, hydrogels25, 30, 43, extracellular matrix (ECM) components20, 26, 27, 52, tissue-derived materials18, 21, 29, 36, 43, 53, 54, and composites of the above materials22, 23, 32, 33, 35, 40, 41. Several preclinical studies have shown improved repair with MSC seeding of scaffolds prior to implantation into a meniscus defect22, 26, 34, 35, 41. Recently, we have shown that meniscus-derived matrix (MDM) scaffolds promote cellular infiltration of both endogenous meniscus cells and exogenous MSCs in an in vitro meniscus repair model54. In this model in which MDM scaffold cores are surrounded by meniscus tissue, the MDM scaffolds alone increased the integrative shear strength of repair when compared to control meniscus tissue. The seeding of exogenous MSCs onto the MDM scaffolds in this repair model system caused a trend towards higher shear strength of repair than control meniscus tissue54. The addition of MSCs to the MDM scaffolds presents an opportunity to deliver therapeutic agents to the meniscus defect but it may be necessary to use exogenous growth factors that are not present in the MDM scaffolds to further promote fibrochondrogenic differentiation of MSCs and enhance repair.
A variety of biologic factors have previously been used to promote and enhance meniscus tissue formation55. In particular for MSCs, the commonly used medium to promote fibrochondrogenic differentiation is composed of insulin/transferrin/selenium (ITS), dexamethasone (Dex), transforming growth factor (TGF)-β, proline, and ascorbic acid19, 22, 23, 31–35, 37–39, 47. On the other hand, meniscus growth medium is frequently composed of serum, proline, and ascorbic acid15, 54, 56–64. However, the optimal differentiation and growth conditions for co-culture of reparative MSCs and meniscus tissue have not been established and are needed to allow in vitro evaluation of biological and mechanical factors influencing integrative meniscus tissue repair. Therefore, the goals of the present study were 1) to identify a favorable culture medium for both MSC-seeded MDM scaffolds and meniscal tissue explants, and 2) to establish a novel meniscus tissue defect model for use in determining the effects of chondrogenic and meniscal media on the biochemical properties of the tissue and integrative repair. In parallel, we first analyzed the biochemical properties of MSC-seeded MDM scaffolds and the biochemical and mechanical properties of meniscus repair model explants cultured in different combinations of serum, Dex, and TGF-β. Subsequently, we combined the meniscal tissue and MSC-seeded MDM scaffolds into a novel meniscus tissue defect model to evaluate the effects of chondrogenic and meniscal media on the tissue biochemical properties and the shear strength of repair.
Materials and Methods
Experiment 1: Medium Optimization for MSCs Seeded in MDM Scaffolds and Meniscal Tissue
Media Preparation
The base medium for all samples was composed of high glucose Dulbecco’s Modified Eagle Medium (DMEM-HG; Sigma-Aldrich, St. Louis, MO), 1% antibiotic-antimycotic (PSF; Sigma-Aldrich), 1% non-essential amino acid solution (Sigma-Aldrich), 1% HEPES buffer solution (Sigma-Aldrich), 40 μg/mL proline (Sigma-Aldrich), and 50 μg/mL L-ascorbic acid 2-phosphate (Sigma-Aldrich). The following supplements were added to the base medium to generate four different media for testing in experiment 1: 1) 10% HyClone characterized fetal bovine serum (FBS; AVH79983, GE Healthcare Bio-Sciences, Pittsburgh, PA) (meniscal medium); 2) 100nM Dex (Sigma-Aldrich) and 1% ITS+ premix (Corning, Corning, NY); 3) 100nM Dex, 1% ITS+ premix, and 10ng/mL recombinant human TGF-β3 (R&D Systems, Minneapolis, MN) (chondrogenic medium); and 4) 10% FBS, 100nM Dex, and 10ng/mL rhTGF-β3.
MSC Seeding of MDM Scaffolds
MDM scaffolds were generated from skeletally mature female porcine medial menisci that were minced, frozen overnight at −80°C, and then lyophilized (FreeZone 2.5 L, Labconco, Kansas City, MO) for 24 hours, as previously described54. The meniscus tissue was then pulverized (5 min pre-cool, 5 cycles of 1 min at 5 Hz and 2 min cool) in a 6770-freezer mill (SPEX SamplePrep, Metuchen, NJ) and sieved to remove particles larger than 500 μm. The MDM powder was suspended in ultra-pure distilled water to an 8% by weight solution. The solution was then homogenized (PRO260, PRO Scientific Inc., Oxford, CT) at 30,000 rpm for 2 cycles of 2 min run, 2 min cool on ice54, 65 and transferred to a 5mL syringe. The homogenized solution was then injected into a delrin mold to generate scaffolds that were 4 mm in diameter and 2 mm thick. The scaffolds were frozen overnight at −80°C, lyophilized for 24 hours, and sterilized with ethylene oxide.
Human bone marrow-derived mesenchymal stem cells (MSCs) were isolated from excess bone marrow aspirate from de-identified adult bone marrow donors in a Duke University IRB-exempt waste tissue protocol as previously described54, 65. Cells were pooled into a superlot (n=3 donors) and cultured in MSC expansion medium containing low glucose Dulbecco’s Modified Eagle Medium (DMEM-LG; Sigma-Aldrich), 10% FBS, 1% PSF, and 2 ng/mL recombinant human fibroblast growth factor (FGF; R&D Systems) at 37°C and 5% CO2.
Passage 4 MSCs were resuspended in expansion medium to achieve 7.8 × 106 cells/mL. MDM scaffolds were placed into individual wells of a 24-well polystyrene ultra-low attachment plate (Corning) and 16 μL of cell suspension was pipetted onto the top of each scaffold. The cell suspension was allowed to wick into the scaffold for 10 minutes, resulting in 1.25×105 cells seeded per scaffold (Figure 1A). MSC expansion medium (1 mL per well) was carefully added to avoid disturbing scaffolds. Seeded scaffolds were pre-cultured for 7 days at 37°C and 5% CO2 prior to being allocated into one of the four media formulations described above (n=6/group). Scaffolds were then cultured at 37°C and 5% CO2 in the different media formulations for 28 days.
Figure 1.
Schematic of cell seeded scaffolds and in vitro meniscus repair model explants utilized in experiment 1. (A) Meniscus-derived matrix (MDM) scaffolds were seeded with bone marrow-derived mesenchymal stem cells (MSCs). (B) Meniscus tissue repair model explants were generated by harvesting 8 mm diameter meniscus explants from porcine mensci. Then a 3 mm diameter inner core was removed and re-inserted. The MSC-seeded MDM scaffolds and meniscus tissue repair model explants were cultured in four different experimental media: 1) serum (meniscal), 2) dexamethasone (Dex) + ITS, 3) Dex + TGF-β3 + ITS (chondrogenic), and 4) serum + Dex + TGF-β3.
Meniscus Tissue Repair Model Explants
Medial meniscus tissue was harvested from female porcine knee joints obtained from a local abattoir. Explants were harvested from the centerline of medial menisci to generate 8 mm diameter punches that were cut to 2 mm thickness using a cutting block. An inner 3 mm diameter core was removed from the explant and immediately returned to the defect to generate meniscus repair model explants containing a meniscus tissue core surrounded by a meniscus tissue ring (Figure 1B), as described previously15, 56–62. The meniscus repair model explants (n=6/group) were incubated at 37°C and 5% CO2 for 28 days in the four different media described above.
Outcome Measures
Cell Viability.
Mitochondrial dehydrogenase activity, which correlates to the number of metabolically active cells, was measured using the BioVision Quick Cell Proliferation Assay Kit. MSC-seeded scaffolds and meniscus repair model explants were incubated in fresh media containing 1% WST-1 reagent at the end of culture. Absorbance was measured at 450 nm with a reference wavelength of 620 nm66.
Histology.
Histological analyses were performed to assess the structure and composition of the MSC-seeded MDM scaffolds and meniscus tissue repair model explants that were cultured in different media formulations. Samples were fixed in 10% neutral-buffered formalin at 4°C overnight, dehydrated, cleared using xylene and embedded in paraffin overnight at 60°C. Samples were then sectioned and stained with Harris hematoxylin with glacial acetic acid (Poly Scientific, Bay Shore, NY), 0.02% aqueous fast green solution (Electron Microscopy Sciences), and 0.1% Safranin O solution (Sigma-Aldrich).
Integrative Shear Strength of Repair.
A push–out test was performed on the meniscus tissue repair model explants15, 56, 57, 59–62 to determine the shear strength of repair at the interface between the inner core and outer ring of meniscus tissue. Images of the samples were captured using a Genie camera (Teledyne Dalsa, Waterloo, ON, Canada) with a 50 mm lens (Tamron, Cologne, Germany), and Image J (NIH) was used to determine average sample thickness. Each sample was placed in the center of a custom made fixture with a 4 mm diameter hole in the center and placed in a mechanical testing frame (840L TestResources, Shakopee, MN). A 2 mm diameter piston was attached to a load cell and centered directly over the tissue inner core. A 0.25 g tare load was applied until tissue equilibration and then the inner core was pushed-out at a rate of 0.0833 mm/sec. Force-displacement curves were generated and the shear strength of repair was calculated as the peak force divided by the surface area of the interface.
Biochemical Analyses.
MSC-seeded scaffolds and meniscus repair model inner cores were digested in 0.5 mL or 1.5 mL, respectively, of 125 μg/mL papain overnight at 65°C54, 67, 68. DNA content was measured using the picogreen assay (Invitrogen, Carlsbad, CA). Sulfated glycosaminoglycan (sGAG) content was measured using the dimethylmethylene blue assay69 and bovine chondroitin sulfate as a standard (Sigma-Aldrich). Collagen content was assessed with the hydroxyproline (OHP) assay70, using trans-4-hydroxy-L-proline (Sigma-Aldrich) as a standard, as previously described54, 67, 68.
Experiment 2: Development of Meniscus Tissue Defect Model and Medium Optimization for Co-culture of MSCs and Meniscus Tissue
Media Preparation
Based on the results from experiment 1, we tested the serum-containing medium (meniscal) and serum-free medium supplemented with ITS, Dex, and TGF-β (chondrogenic) in a novel meniscus tissue defect model that combines both MSC-seeded MDM scaffolds and meniscus tissue. These media were chosen for subsequent analyses given that the serum-containing medium appeared to be the most favorable for the meniscus tissue explants, and the serum-free medium supplemented with ITS, Dex, and TGF-β was most favorable for the MSC-seeded MDM scaffolds.
Scaffold Preparation and MSC Seeding
As shown in Figure 2A, three different types of MDM scaffolds were used for these experiments: a slurry, prefabricated non cross-linked (non X-linked), and prefabricated cross-linked (X-linked) scaffold rings. In order to make the slurry, MDM powder was sterilized with ethylene oxide, and was suspended under sterile conditions in ultra-pure distilled water and homogenized to create a 16% by weight MDM slurry. In parallel, MDM powder was reconstituted to a 16% by weight solution and injected into a 3 mm wide gel casting apparatus (BioRad, Hercules, CA) using a 5 mL syringe. The scaffold in the gel casting apparatus was frozen overnight at −80°C and lyophilized for 24 hours to generate an MDM sheet. This sheet was divided, and half was left untreated and half was dehydrothermally cross-linked by heating at 120°C for 24 hours in a dry oven54, 71. Using a biopsy punch, 4 mm diameter scaffolds were removed from both non cross-linked and cross-linked MDM sheets. A 3 mm inner core was removed from each of these 4 mm scaffolds using a biopsy punch to obtain a prefabricated scaffold ring with an inner diameter of 3 mm and an outer diameter of 4 mm. All prefabricated scaffolds were gas-sterilized using ethylene oxide.
Figure 2.
Schematic of scaffold preparation and meniscus tissue defect model utilized in experiment 2. (A) MDM scaffolds were prepared by reconstituting MDM powder to 16% to generate a slurry that was either seeded with MSCs to generate a cell-seeded slurry or was injected into a cassette, frozen, and lyophilized to form a MDM sheet. The MDM sheet was divided and left non-cross-linked (Non X-link) or was dehydrothermally cross-linked (X-link). Scaffolds were obtained using a 4 mm diameter biopsy punch. A 3 mm diameter core was removed from each of these prefabricated scaffolds. (B) The meniscus tissue defect model was generated using 8 mm diameter meniscus explants from which a 4 mm diameter core was removed. Next, a 3 mm diameter inner core tissue was cut from this tissue and the resulting tissue ring (4 mm outer diameter and 3 mm inner diameter) was discarded to generate the tissue defect. The defect was filled with non X-link or X-link scaffolds or slurry and the inner 3 mm diameter tissue core was replaced. Meniscus tissue defect model explants were cultured in either meniscal or chondrogenic medium.
Passage 4 MSCs were suspended in expansion medium to achieve a cell suspension of 1 × 107 cells/mL. The non X-linked and X-linked MDM scaffold rings were seeded with 1.6 × 105 cells per scaffold by placing 16μl of cell suspension in the center of the MDM ring and allowing the cell suspension to wick through the MDM scaffolds. The MSC-seeded scaffolds (n = 6/group) were pre-cultured in 1 mL of expansion medium at 37°C and 5% CO2 for 7 days prior to implantation into the meniscus tissue defect model.
Meniscus Tissue Defect Model
As shown in Figure 2B, 8 mm diameter explants were harvested from the centerline of porcine medial menisci. A 4 mm diameter core was removed from these 8 mm explants. Then, we explanted a 3 mm inner core from this 4 mm tissue. The resulting 4 mm outer diameter and 3 mm inner diameter tissue ring was discarded, leaving a 0.5 mm meniscus tissue defect that was filled with either the prefabricated non X-linked or X-linked MSC-seeded scaffolds, or MDM slurry containing 1.6 × 105 MSCs (n = 6/group). Next, the 3 mm inner core meniscus tissues were replaced in all samples, taking care that the prefabricated scaffold ring surrounded the inner core and scaffold material filled the gap between the inner core tissue and outer ring meniscus tissue. Meniscus tissue defect model samples were cultured in meniscal medium (serum) or chondrogenic medium (serum-free + Dex + TGF-β) for 14 days (n = 3/group) at 37°C and 5% CO2.
Outcome Measures
Integrative Shear Strength of Repair.
A push-out test was performed to assess the integrative shear strength of repair15, 54, 56, 57, 59–62 between the inner core meniscus tissue and MDM scaffolds or slurry using an ElectroForce 3220 Series III mechanical test instrument (TA Instruments, New Castle, DE), as described above.
Biochemical Analyses.
After mechanical testing, the inner core tissue of all meniscus tissue defect model samples was digested in 1 mL of papain54, 67, 68 after which DNA, sGAG69, and OHP content54, 67, 68, 70 were measured as detailed above.
Statistical Analyses
Statistical analyses were performed using Statistica 7.0 (Tibco). For experiment 1, MSC-seeded scaffolds and tissue repair model explants were analyzed by one-way ANOVA and Fisher LSD post hoc tests to determine significant effects of the different media formulations on all outcome measures (α=0.05). For experiment 2, factorial ANOVA and Fisher LSD post hoc tests were performed to determine significant group differences, as well as the interactive effects of medium formulation and scaffold type in the meniscus tissue defect model.
Results
Experiment 1: Medium Optimization for MSCs Seeded in MDM Scaffolds and Meniscal Tissue
Biochemical Analyses
MSC-seeded scaffolds had the highest DNA content when cultured in serum-free medium containing Dex and TGF-β (Figure 3A), and the DNA content was significantly reduced in scaffolds cultured without TGF-β (p < 0.00001). The addition of serum to the medium (serum, Dex, and TGF-β) decreased the DNA content as compared to Dex and TGF-β (p < 0.00001). However, DNA content was significantly higher in the scaffolds cultured in serum, Dex, and TGF-β as compared to both groups cultured in the absence of TGF-β (p < 0.00001). In addition, cell metabolic activity was highest in the MSC-seeded scaffolds cultured in serum-free medium with Dex and TGF-β (Figure 3B), and there was a significant decrease in metabolic activity in MSC-seeded scaffolds cultured with Dex alone (p < 0.00001). Furthermore, cell metabolic activity was the lowest in the serum-containing medium and upon supplementation with Dex and TGF-β there was a significant increase in metabolic activity (p < 0.01). The sGAG content of the MSC-seeded scaffolds was significantly elevated in scaffolds cultured in Dex and TGF-β, as compared to scaffolds cultured in all other media (Figure 3C, p< 0.00001). In addition, collagen content was highest in MSC-seeded scaffolds cultured in Dex and TGF-β (Figure 3D) and the addition of serum caused a significant decrease in the collagen content (p < 0.00005). There was a significant decrease in collagen content in scaffolds cultured in Dex alone, as compared to Dex and TGF-β (p < 0.0005). Collagen content was lowest in the scaffolds treated with serum alone; however, there was a trend towards an increase in collagen content with the addition of Dex and TGF-β (p = 0.06).
Figure 3.
The effects of different combinations of serum, dexamethasone (Dex), and TGF-β3 on the (A) DNA content, (B) cell metabolic activity [based on the absorbance (abs) of WST-1], (C) sulfated glycosaminoglycan (sGAG) content, and (D) collagen (OHP) content of the MSC-seeded MDM scaffolds. The serum-free media contain ITS. Data are expressed as the mean + SEM. Groups not sharing the same letters have p-values < 0.05.
Meniscus tissue DNA content was highest in samples cultured in serum or in serum + Dex + TGF-β (Figure 4A). DNA content was significantly lower in samples cultured without serum (p< 0.01), and these effects were independent of the presence of TGF-β. Furthermore, cell metabolic activity was highest in the meniscus repair model explants cultured with serum, or with serum + Dex + TGF-β (Figure 4B). Cell metabolic activity was significantly lower in samples cultured without serum (p < 0.00001). Meniscus tissue cultured in Dex alone had the lowest metabolic activity, but metabolic activity was significantly increased with TGF-β supplementation (p < 0.005). On the other hand, sGAG content was significantly elevated in meniscus tissues cultured in Dex and TGF-β, as compared to all other media (Figure 4C, p <0.001). No significant differences were detected in the collagen content of the meniscus tissue repair model explants (Figure 4D).
Figure 4.
The effects of different combinations of serum, dexamethasone (Dex), and TGF-β3 on the (A) DNA content, (B) cell metabolic activity [based on the absorbance (abs) of WST-1], (C) sulfated glycosaminoglycan (sGAG) content, and (D) collagen (OHP) content of the meniscus repair model explants. The serum-free media contain ITS. Data are expressed as the mean + SEM. Groups not sharing the same letters have p-values < 0.05.
Histological Analyses
Histological analyses revealed that the scaffolds and meniscus tissue were composed of a predominantly collagen extracellular matrix (Figure 5). In the MSC-seeded scaffolds, TGF-β + Dex and serum + Dex + TGF-β resulted in a denser ECM, as compared to the scaffolds cultured with serum or Dex alone. Strong proteoglycan staining was detectable in both the scaffolds and meniscus tissue cultured in Dex and TGF-β. However, the proteoglycan staining was diminished in scaffolds and tissues cultured in the presence of serum, Dex, and TGF-β.
Figure 5.
Histological staining of the MSC-seeded MDM scaffolds and meniscus tissue in different combinations of serum, dexamethasone (Dex), and TGF-β3. The serum-free media contain ITS. Sections were stained with Safranin-O (red: proteoglycans), fast green (blue: collagen), and hematoxylin (black: nuclei). Scale bar is 100 μm.
Mechanical Analyses
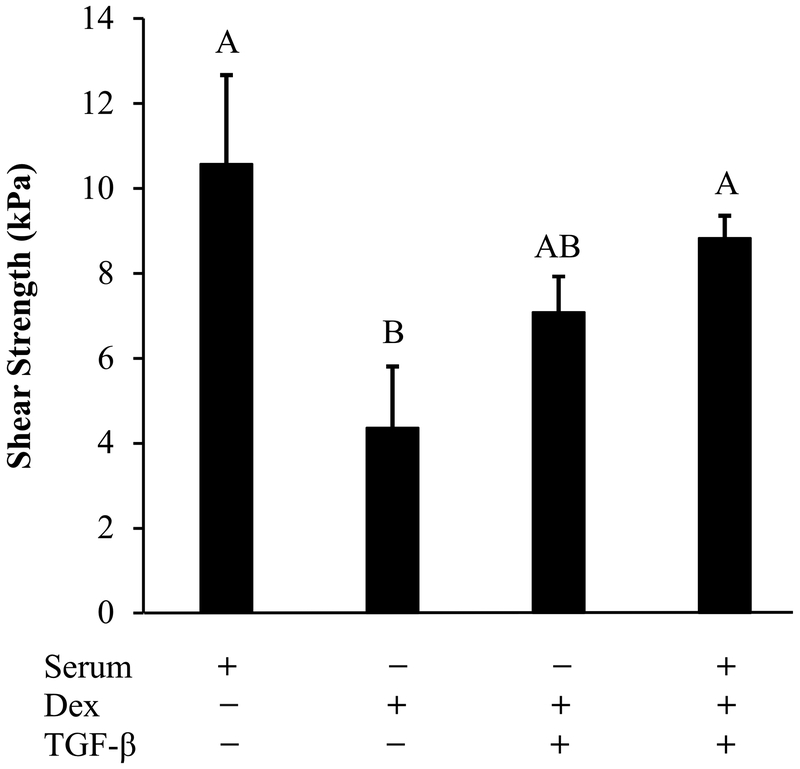
The integrative shear strength of repair was highest in the meniscus repair model explants cultured with serum (Figure 6). The tissues cultured with Dex alone had the lowest shear strength of repair and had significantly reduced strength of repair when compared to explants cultured in either serum or serum + Dex + TGF-β (p < 0.05).
Figure 6.
The effects of different combinations of serum, dexamethasone (Dex), and TGF-β3 on the integrative shear strength of repair of the meniscus tissue repair model explants. The serum-free media contain ITS. Data are expressed as the mean + SEM. Groups not sharing the same letters have p-values < 0.05.
Experiment 2: Development of Meniscus Tissue Defect Model and Medium Optimization for Co-culture of MSCs and Meniscus Tissue
Biochemical Analyses
In the meniscus tissue defect model, no significant differences were observed in the DNA content of the inner core tissue due to either culture medium or scaffold type (Figure 7A). On the other hand, the chondrogenic medium caused a significant increase in the sGAG content of the tissue, as compared to the meniscal medium (Figure 7B, p = 0.05). However, there was no effect of scaffold type on the sGAG content of the inner core meniscus tissue. In addition, no significant differences were noted in the collagen content across culture media or scaffold types (Figure 7C). There were no interactive effects of medium formulation and scaffold type on the biochemical properties.
Figure 7.

The effects of meniscal (serum) or chondrogenic (Dex + TGF-β3 + ITS) medium and different types of MSC-seeded scaffolds [slurry or prefabricated non cross-linked (Non X-link) or prefabricated cross-linked (X-link) scaffolds] on (A) DNA content, (B) sulfated glycosaminoglycan (sGAG) content, and (C) collagen (OHP) content of meniscus tissue defect inner cores. Data are expressed as mean ± SEM. sGAG content is higher in the chondrogenic medium than the meniscal medium (p = 0.05). No significant differences are detected by scaffold type.
Mechanical Analyses
The integrative shear strength of repair was significantly increased in meniscus tissue defect model explants that were cultured in the chondrogenic medium, as compared to explants cultured in the meniscal medium (Figure 8, p < 0.001). In addition, there was a significant effect of scaffold type, revealing that defects treated with the prefabricated X-linked scaffolds had a higher shear strength of repair than meniscus defects treated with the slurry (p < 0.05). There were no interactive effects of medium formulation and scaffold type on the shear strength of repair.
Figure 8.
The effects of meniscal (serum) or chondrogenic (Dex + TGF-β3 + ITS) medium and different types of MSC-seeded scaffolds [slurry or prefabricated non-cross-linked (Non X-link) or prefabricated cross-linked (X-link) scaffolds] on the integrative shear strength of repair of meniscus tissue defect model explants. Data are expressed as mean ± SEM. The chondrogenic medium improves shear strength of repair over the meniscal medium (p < 0.001). Meniscal defects treated with the X-link prefabricated scaffolds have a higher shear strength of repair than defects treated with the slurry (p < 0.05).
Discussion
In the present study, we established favorable culture conditions for in vitro meniscus tissue engineering utilizing bone marrow-derived MSCs, which will allow efficient and rapid screening of factors that may modulate meniscus tissue repair. The serum-free medium containing both TGF-β and Dex, which is commonly utilized for MSC chondrogenesis19, 22, 23, 31–35, 37–39, 47, was the most promising formulation for future experiments with MSC-seeded MDM scaffolds. In contrast, the serum-containing medium, which is commonly used for meniscus tissue culture15, 54, 56–64, was the most effective medium for meniscus tissue culture and integrative repair of native meniscus tissue. When native meniscus tissue and MSC-seeded MDM scaffolds were combined in a co-culture meniscus tissue defect model, the chondrogenic medium (serum-free medium containing both TGF-β and Dex) enhanced proteoglycan production and promoted the integrative repair of meniscus tissue.
Serum-free medium supplemented with both TGF-β and Dex yielded the most favorable biochemical properties for the MSC-seeded scaffolds from experiment 1. Our results suggest that TGF-β and Dex promoted MSC proliferation and increased production of the major meniscus ECM components, collagens and proteoglycans. In particular, the elevated DNA and sGAG content in the Dex and TGF-β treated samples appears to be driven predominantly by TGF-β, since MSC-seeded scaffolds cultured with Dex alone did not show these increases. However, samples treated with TGF-β alone would be required to determine if there is a synergistic effect between Dex and TGF-β. Prior work screening 32 different media formulations for MSC chondrogenesis revealed that differentiation medium composed of TGF-β and Dex promoted the most favorable chondrogenic gene expression72, which is consistent with our findings. In other prior studies, the addition of TGF-β to chondrogenic medium containing Dex upregulated type II collagen and aggrecan gene expression and type I collagen and GAG production in MSC cell pellets37. Likewise, transduction of MSCs with cDNA encoding TGF-β increased expression of type I and II collagen and sGAG production23. In this study, we tested the effects of Dex alone as it has previously been shown to increase sGAG and collagen production by MSCs73. Furthermore, Dex treatment of MSCs has been shown to suppress catabolism, including aggrecanase activity74 and matrix metalloproteinase (MMP)-13 expression, and the pro-inflammatory mediator prostaglandin E275, factors which are key mediators of joint tissue degradation and OA progression.
Serum-supplemented medium resulted in the lowest DNA content, metabolic activity and proteoglycan and collagen content of the MSC-seeded MDM scaffolds. Furthermore, the addition of serum to the Dex and TGF-β containing medium attenuated the beneficial effects of TGF-β on collagen and proteoglycan content and decreased the DNA content. Prior work demonstrated that serum prevented MSC aggregate formation76. More recent studies have shown that serum-free MSC chondrogenic medium increased type II collagen and sGAG production, but the addition of serum caused increased type I collagen expression and staining77, suggesting a fibrochondrogenic phenotype. However, in this study, the general suppression of metabolic activity and ECM production in the serum-containing medium suggests the presence of inhibitors of TGF-β and/or Dex signaling in the serum that prevent MSC proliferation and differentiation.
This study focused specifically on bone marrow-derived MSCs. Due to inherent differences in stem cells isolated from different sites, further studies with stem cells from other sources will be needed to identify favorable medium formulations for in vitro co-culture with meniscus tissue. Additionally, in this study, MSCs were cultured on a MDM scaffold but our findings are consistent with other studies of MSCs in alginate, agarose, and type I collagen-GAG matrices23, 37, 72–76. This suggests that the chondrogenic medium is able to drive MSC differentiation across a variety of different scaffolds.
In contrast to the MSC-seeded MDM scaffolds, the meniscus repair model explants had the highest DNA content, metabolic activity, and integrative shear strength of repair when cultured in serum supplemented medium. However, we did not detect a significant difference in the shear strength of repair between media containing serum and medium containing Dex + TGF-β. Previously, we have shown that serum promotes meniscus cell proliferation and accumulation in a micro-wound repair model61. In general, meniscus tissue culture experiments are performed with 10% serum, and the effects of TGF-β supplementation on tissue repair were rather minimal in most prior experiments. In bovine meniscal repair model explants, 10 ng/mL TGF-β3 had no effect on strength of repair until 8 weeks of treatment63, suggesting that longer culture times may be required to see beneficial effects of TGF-β3 on tissue repair. On the other hand, 50 ng/mL TGF-β1 treatment of in vitro meniscal defects for 8 weeks did not affect cell density or proliferation64. In addition, we have previously shown that 1 ng/mL TGF-β1 promotes cellular accumulation at the repair interface and integrative meniscus repair but 10 ng/mL TGF-β1 did not58, 61, suggesting a dose response to TGF-β1 treatment. Since both TGF-β1 and TGF-β3 isoforms promote fibrochondrogenic differentiation of MSCs19, 31, 78, 79, in this study we chose to test the effect of 10 ng/mL TGF-β3 as this is the most commonly used concentration of TGF-β3 in chondrogenic medium19, 31.
In the meniscus tissue repair model explants, TGF-β and Dex increased sGAG content of the tissue. This effect is likely driven by TGF-β since Dex treatment alone did not enhance sGAG tissue content. The suppressed proteoglycan content upon the addition of serum to the TGF-β and Dex medium further suggests that there may be inhibitors of TGF-β signaling in the serum. Our findings regarding TGF-β-mediated upregulation of sGAG production are consistent with prior studies, which showed that meniscal cells in monolayer, cells in alginate beads and poly-glycolic acid (PGA) scaffolds, and meniscal explants increase proteoglycan production in response to TGF-β treatment24, 80–82. Transduction of meniscus cells with TGF-β or recombinant TGF-β treatment also increased collagen production as measured by radiolabeling24, 81, 83. However, we did not detect differences in collagen content in our meniscus repair model explants with the different media treatments. This may be due to subtle changes in total tissue collagen content, as compared to measuring new collagen synthesis by radiolabel incorporation.
While glucocorticoid treatment is frequently used as a conservative treatment for OA pain, few studies have directly examined the effects of the synthetic glucocorticoid steroid Dex on meniscus tissue. In our study, Dex treatment showed the lowest DNA content, cell metabolic activity, sGAG content, and shear strength of repair in the meniscus repair model explants. Prior work has shown that Dex treatment of isolated meniscus cells induced autophagy and apoptosis and also upregulated the expression of aggrecanases and matrix metalloproteinases84. Dex has also been shown to cause a significant decrease in medial meniscus cell viability in the presence of blood85. However, in an ex vivo impact injury of porcine knee joints, Dex treatment suppressed meniscus IL-1β and MMP-3 expression and upregulated miR140, which is a negative regulator of aggrecanase activity86. Most recently, in a rabbit surgical drill injury model, Dex treatment caused a downregulation in the meniscal expression of inflammatory and degradative markers85. Given these conflicting findings, further studies are necessary to understand the full spectrum of effects of Dex treatment on meniscus tissue.
Based on the beneficial effects of the serum-free medium containing TGF-β and Dex (chondrogenic medium) on the MSC-seeded scaffolds and the proteoglycan content of the meniscus tissue, and the positive effects of the serum-containing medium (meniscal medium) on the meniscus repair model explants, we evaluated the effects of both of these media on our meniscus tissue defect model. In this experiment, we did not evaluate the effects of serum, Dex, and TGF-β containing medium, which showed intermediate results in experiment 1, and thus may be a good medium formulation to consider for future experiments. Nonetheless, in this experiment when the MSC-seeded MDM scaffolds were placed into the meniscus tissue defect and co-cultured, the chondrogenic medium increased sGAG content and integrative shear strength of repair. The elevated proteoglycan content in this defect model is consistent with our findings in experiment 1. The increase in the shear strength with the chondrogenic medium is in contrast with the tissue repair results from experiment 1. While this difference could be due to the mixed species co-culture of human MSCs with porcine meniscus tissue, it more likely suggests an important contribution of the MSCs to meniscus repair. Previously, we have shown that MSC-seeded MDM scaffolds trended towards improved integrative repair when cultured as an inner core surrounded by a meniscus tissue ring in meniscal medium, but this repair was not significantly improved over a native tissue core54. In the current study, the chondrogenic medium containing TGF-β and Dex improved integrative meniscus repair, suggesting that exogenous growth factors are necessary to enhance MSC-mediated repair using the MDM scaffold. These findings are consistent with prior work using MSC-seeded scaffolds composed of tissue-derived matrix, which required chondrogenic medium containing TGF-β to promote matrix gene expression and ECM production33, 47, 87, 88.
Several studies have been performed evaluating co-cultures of MSCs and meniscus cell pellets36–39, 89. These studies revealed that there is a synergy between the meniscus cells and MSCs that promotes type I collagen and GAG production and suppresses expression of hypertrophic genes during culture with chondrogenic medium37–39, 89. Furthermore, co-cultures were partially protected against the pro-inflammatory cytokine IL-1, allowing retention of a fibrochondrogenic phenotype39. On the other hand, 50:50 co-cultures of MSCs and meniscus cells seeded on a 3D collagen scaffold and cultured in meniscal medium had the highest sGAG retention and mechanical properties and diminished hypertrophy, as compared to all other co-culture ratios36. While these studies used either the meniscal or chondrogenic medium, our experiments directly compared the effects of these media formulations on cell proliferation, ECM production, and strength of repair. In addition to utilizing MSCs to directly promote cell-mediated repair, MSCs can also be utilized to deliver growth factors or other therapeutics through viral transduction23, 83, 90. Therefore, future work could explore the use of genetically modified MSCs to deliver therapeutics to further improve meniscus healing.
Prior in vitro meniscus repair models58 created either a biopsy punch defect that was filled with a reparative scaffold54, 63, 91 or a meniscal tear that was reintegrated and evaluated for repair56–63, similar to our meniscal repair model system in experiment 1. In our current study, we established a novel meniscus tissue defect model that created a 0.5 mm defect that can be filled with a reparative scaffold and evaluated for integration between two meniscus tissue explants. Although circular defects are not natural defects that occur during meniscal injuries, this model allows quantification of tissue healing (through push-out testing) between two pieces of tissue that are bridged by a tissue engineered scaffold, which is not feasible with a naturally occurring defect. As compared to a surgically created tear, our meniscal tissue defect model more closely mimics clinical meniscal defects that are generally left after a meniscus tear debridement that could benefit from tissue-engineered repair strategies to augment tissue healing. In addition to adding MSCs to biomaterial scaffolds, it is possible to modulate the properties of scaffolds through various material processing and cross-linking strategies. In our meniscus tissue defect model, the prefabricated X-linked scaffolds had improved integrative shear strength of repair, as compared to the defects filled with MDM slurry. Overall, the prefabricated scaffolds trended towards a higher shear strength of repair, which may be due to the enhanced mechanical integrity of the prefabricated scaffolds and/or improved retention of the scaffolds. Previous studies have shown that cross-linking of cartilage-derived scaffolds may be necessary to increase the mechanical properties of the scaffolds and prevent cell-mediated retraction away from the tissue interface71. However, in the current study we were unable to see a significant difference in shear strength between the non X-linked and X-linked scaffolds, but this may be due to the small sample size used in these experiments. However, in our prior work comparing scaffolds of varying MDM concentrations and cross-linking in our meniscus tissue repair model with meniscal medium, we also did not detect a difference in shear strength with dehydrothermal cross-linking54. Nevertheless, there was improved integrative shear strength of repair in the higher percentages of MDM54, laying the ground work for testing 16% MDM in our meniscus tissue defect model.
In conclusion, the chondrogenic serum-free medium containing both TGF-β and Dex promoted integrative meniscus repair in meniscus tissue defects repaired with bone marrow-derived MSC-seeded MDM scaffolds. With the ability to modulate the differentiation of MSCs to provide an abundant source of fibrochondrogenic cells, and an MDM scaffold that supports the growth and differentiation of MSCs that can integrate with meniscal defects to promote repair, there is great promise for successful meniscus tissue engineering and improved tissue repair. In addition to cell-based repair, MSCs can be tailored to deliver therapeutic agents to the meniscus defect to promote cellular differentiation, tissue repair, and/or suppress the pro-inflammatory microenvironment following joint injury23, 83, 90, 92, 93. Finally, the meniscal tissue defect model that was established in this paper can be used to perform in vitro screening to identify and optimize biological and mechanical treatments that may be utilized to enhance meniscus tissue repair prior to moving into preclinical animal studies.
Acknowledgements
We thank Dr. Jocelyn Wittstein for clinical advice and expertise.
Funding
This work was supported in part by NIH grants AG028716 and AR073221, a VA Rehabilitation Research Service Merit Review Award, and an Orthopaedic Research and Education Foundation grant with funding provided by the Musculoskeletal Transplant Foundation.
Footnotes
Declaration of Interests
The authors of this manuscript have no conflicts of interests related to the content of this study.
References
- 1.Blake MH, Johnson DL. Knee meniscus injuries: Common problems and solutions. Clin Sports Med 2018:37(2): 293–306. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--part i: Tibial surface of the knee. J Biomech Eng 1983:105(3): 216–225. [DOI] [PubMed] [Google Scholar]
- 3.Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee stability. J Bone Joint Surg Am 1981:63(4): 570–585. [PubMed] [Google Scholar]
- 4.Wojtys EM, Chan DB. Meniscus structure and function. AAOS Instructional Course Lectures 2005:54:323–330. [PubMed] [Google Scholar]
- 5.Carter TE, Taylor KA, Spritzer CE, Utturkar GM, Taylor DC, Moorman CT 3rd, Garrett WE, Guilak F, McNulty AL, DeFrate LE. In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech 2015:48(8): 1461–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badlani JT, Borrero C, Golla S, Harner CD, Irrgang JJ. The effects of meniscus injury on the development of knee osteoarthritis: Data from the osteoarthritis initiative. Am J Sports Med 2013:41(6): 1238–1244. [DOI] [PubMed] [Google Scholar]
- 7.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy-the Journal of Arthroscopic and Related Surgery 2005:21(11): 1366–1369. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DJ, Zhang YQ, Niu JB, Tu X, Amin S, Clancy M, Guermazi A, Grigorian M, Gale D, Felson DT. The association of meniscal pathologic changes with cartilage loss in symptomatic knee osteoarthritis. Arthritis Rheum 2006:54(3): 795–801. [DOI] [PubMed] [Google Scholar]
- 9.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: Osteoarthritis. Am J Sports Med 2007:35(10): 1756–1769. [DOI] [PubMed] [Google Scholar]
- 10.Lohmander LS, Roos H, Dahlberg L, Hoerrner LA, Lark MW. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res 1994:12(1): 21–28. [DOI] [PubMed] [Google Scholar]
- 11.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: Prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum 1998:41(4): 687–693. [DOI] [PubMed] [Google Scholar]
- 12.Sharma L, Eckstein F, Song J, Guermazi A, Prasad P, Kapoor D, Cahue S, Marshall M, Hudelmaier M, Dunlop D. Relationship of meniscal damage, meniscal extrusion, malalignment, and joint laxity to subsequent cartilage loss in osteoarthritic knees. Arthritis Rheum 2008:58(6): 1716–1726. [DOI] [PubMed] [Google Scholar]
- 13.Wyland DJ, Guilak F, Elliott DM, Setton LA, Vail TP. Chondropathy after meniscal tear or partial meniscectomy in a canine model. J Orthop Res 2002:20(5): 996–1002. [DOI] [PubMed] [Google Scholar]
- 14.Doral MN, Bilge O, Huri G, Turhan E, Verdonk R. Modern treatment of meniscal tears. EFORT Open Rev 2018:3(5): 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. Am J Sports Med 2007:35(5): 754–762. [DOI] [PubMed] [Google Scholar]
- 16.Garrett WE Jr., Swiontkowski MF, Weinstein JN, Callaghan J, Rosier RN, Berry DJ, Harrast J, Derosa GP. American board of orthopaedic surgery practice of the orthopaedic surgeon: Part-ii, certification examination case mix. J Bone Joint Surg Am 2006:88(3): 660–667. [DOI] [PubMed] [Google Scholar]
- 17.Adesida AB, Mulet-Sierra A, Laouar L, Jomha NM. Oxygen tension is a determinant of the matrix-forming phenotype of cultured human meniscal fibrochondrocytes. PLoS One 2012:7(6): e39339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monibi FA, Bozynski CC, Kuroki K, Stoker AM, Pfeiffer FM, Sherman SL, Cook JL. Development of a micronized meniscus extracellular matrix scaffold for potential augmentation of meniscal repair and regeneration. Tissue Eng Part C Methods 2016:22(12): 1059–1070. [DOI] [PubMed] [Google Scholar]
- 19.Baker BM, Mauck RL. The effect of nanofiber alignment on the maturation of engineered meniscus constructs. Biomaterials 2007:28(11): 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinek V, Ueblacker P, Braun K, Nitschke S, Mannhardt R, Specht K, Gansbacher B, Imhoff AB. Second generation of meniscus transplantation: In-vivo study with tissue engineered meniscus replacement. Arch Orthop Trauma Surg 2006:126(4): 228–234. [DOI] [PubMed] [Google Scholar]
- 21.Wu J, Ding Q, Dutta A, Wang Y, Huang YH, Weng H, Tang L, Hong Y. An injectable extracellular matrix derived hydrogel for meniscus repair and regeneration. Acta Biomater 2015:16:49–59. [DOI] [PubMed] [Google Scholar]
- 22.Zellner J, Pattappa G, Koch M, Lang S, Weber J, Pfeifer CG, Mueller MB, Kujat R, Nerlich M, Angele P. Autologous mesenchymal stem cells or meniscal cells: What is the best cell source for regenerative meniscus treatment in an early osteoarthritis situation? Stem Cell Res Ther 2017:8(1): 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, Spector M, Evans CH. Genetically enhanced engineering of meniscus tissue using ex vivo delivery of transforming growth factor-beta 1 complementary deoxyribonucleic acid. Tissue Engineering 2007:13(9): 2227–2237. [DOI] [PubMed] [Google Scholar]
- 24.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res 2005:23(5): 1184–1190. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki H, Rothrauff BB, Alexander PG, Lin H, Gottardi R, Fu FH, Tuan RS. In vitro repair of meniscal radial tear with hydrogels seeded with adipose stem cells and tgf-beta3. Am J Sports Med 2018:46(10): 2402–2413. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Fernandez ML, Perez-Castrillo S, Sanchez-Lazaro JA, Prieto-Fernandez JG, Lopez-Gonzalez ME, Lobato-Perez S, Colaco BJ, Olivera ER, Villar-Suarez V. Assessment of regeneration in meniscal lesions by use of mesenchymal stem cells derived from equine bone marrow and adipose tissue. Am J Vet Res 2016:77(7): 779–788. [DOI] [PubMed] [Google Scholar]
- 27.Oda S, Otsuki S, Kurokawa Y, Hoshiyama Y, Nakajima M, Neo M. A new method for meniscus repair using type i collagen scaffold and infrapatellar fat pad. J Biomater Appl 2015:29(10): 1439–1448. [DOI] [PubMed] [Google Scholar]
- 28.Fox DB, Warnock JJ, Stoker AM, Luther JK, Cockrell M. Effects of growth factors on equine synovial fibroblasts seeded on synthetic scaffolds for avascular meniscal tissue engineering. Res Vet Sci 2010:88(2): 326–332. [DOI] [PubMed] [Google Scholar]
- 29.Liang Y, Idrees E, Szojka ARA, Andrews SHJ, Kunze M, Mulet-Sierra A, Jomha NM, Adesida AB. Chondrogenic differentiation of synovial fluid mesenchymal stem cells on human meniscus-derived decellularized matrix requires exogenous growth factors. Acta Biomater 2018:80(131–143. [DOI] [PubMed] [Google Scholar]
- 30.Shimomura K, Rothrauff BB, Hart DA, Hamamoto S, Kobayashi M, Yoshikawa H, Tuan RS, Nakamura N. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials 2018:192:346–354. [DOI] [PubMed] [Google Scholar]
- 31.Mauck RL, Martinez-Diaz GJ, Yuan X, Tuan RS. Regional multilineage differentiation potential of meniscal fibrochondrocytes: Implications for meniscus repair. Anat Rec (Hoboken) 2007:290(1): 48–58. [DOI] [PubMed] [Google Scholar]
- 32.Achatz FP, Kujat R, Pfeifer CG, Koch M, Nerlich M, Angele P, Zellner J. In vitro testing of scaffolds for mesenchymal stem cell-based meniscus tissue engineering-introducing a new biocompatibility scoring system. Materials (Basel) 2016:9(4):276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shimomura K, Rothrauff BB, Tuan RS. Region-specific effect of the decellularized meniscus extracellular matrix on mesenchymal stem cell-based meniscus tissue engineering. Am J Sports Med 2017:45(3): 604–611. [DOI] [PubMed] [Google Scholar]
- 34.Koch M, Achatz FP, Lang S, Pfeifer CG, Pattappa G, Kujat R, Nerlich M, Angele P, Zellner J. Tissue engineering of large full-size meniscus defects by a polyurethane scaffold: Accelerated regeneration by mesenchymal stromal cells. Stem Cells Int 2018:8207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zellner J, Hierl K, Mueller M, Pfeifer C, Berner A, Dienstknecht T, Krutsch W, Geis S, Gehmert S, Kujat R et al. Stem cell-based tissue-engineering for treatment of meniscal tears in the avascular zone. J Biomed Mater Res B Appl Biomater 2013:101(7): 1133–1142. [DOI] [PubMed] [Google Scholar]
- 36.McCorry MC, Puetzer JL, Bonassar LJ. Characterization of mesenchymal stem cells and fibrochondrocytes in three-dimensional co-culture: Analysis of cell shape, matrix production, and mechanical performance. Stem Cell Res Ther 2016:7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui XF, Hasegawa A, Lotz M, D’Lima D. Structured three-dimensional co-culture of mesenchymal stem cells with meniscus cells promotes meniscal phenotype without hypertrophy. Biotechnology and Bioengineering 2012:109(9): 2369–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matthies NF, Mulet-Sierra A, Jomha NM, Adesida AB. Matrix formation is enhanced in co-cultures of human meniscus cells with bone marrow stromal cells. J Tissue Eng Regen Med 2013:7(12): 965–973. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury A, Bezuidenhout LW, Mulet-Sierra A, Jomha NM, Adesida AB. Effect of interleukin-1beta treatment on co-cultures of human meniscus cells and bone marrow mesenchymal stromal cells. BMC Musculoskelet Disord 2013:14:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu H, Adesida AB, Jomha NM. Meniscus repair using mesenchymal stem cells - a comprehensive review. Stem Cell Res Ther 2015:6:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korpershoek JV, de Windt TS, Hagmeijer MH, Vonk LA, Saris DB. Cell-based meniscus repair and regeneration: At the brink of clinical translation?: A systematic review of preclinical studies. Orthop J Sports Med 2017:5(2): 2325967117690131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niu W, Guo W, Han S, Zhu Y, Liu S, Guo Q. Cell-based strategies for meniscus tissue engineering. Stem Cells Int 2016:4717184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz JA, Wang W, Goldstein T, Grande DA. Tissue engineered meniscus repair: Influence of cell passage number, tissue origin, and biomaterial carrier. Cartilage 2014:5(3): 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barry FP, Murphy JM. Mesenchymal stem cells: Clinical applications and biological characterization. Int J Biochem Cell Biol 2004:36(4): 568–584. [DOI] [PubMed] [Google Scholar]
- 45.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006:98(5): 1076–1084. [DOI] [PubMed] [Google Scholar]
- 46.Glenn JD, Whartenby KA. Mesenchymal stem cells: Emerging mechanisms of immunomodulation and therapy. World J Stem Cells 2014:6(5): 526–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YC, Chen RN, Jhan HJ, Liu DZ, Ho HO, Mao Y, Kohn J, Sheu MT. Development and characterization of acellular extracellular matrix scaffolds from porcine menisci for use in cartilage tissue engineering. Tissue Eng Part C Methods 2015:21(9): 971–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Patel JM, Merriam AR, Culp BM, Gatt CJ Jr., Dunn MG. One-year outcomes of total meniscus reconstruction using a novel fiber-reinforced scaffold in an ovine model. Am J Sports Med 2016:44(4): 898–907. [DOI] [PubMed] [Google Scholar]
- 49.Verdonk P, Beaufils P, Bellemans J, Djian P, Heinrichs EL, Huysse W, Laprell H, Siebold R, Verdonk R, Actifit Study G. Successful treatment of painful irreparable partial meniscal defects with a polyurethane scaffold: Two-year safety and clinical outcomes. Am J Sports Med 2012:40(4): 844–853. [DOI] [PubMed] [Google Scholar]
- 50.Rothrauff BB, Numpaisal PO, Lauro BB, Alexander PG, Debski RE, Musahl V, Tuan RS. Augmented repair of radial meniscus tear with biomimetic electrospun scaffold: An in vitro mechanical analysis. J Exp Orthop 2016:3(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Warnecke D, Stein S, Haffner-Luntzer M, de Roy L, Skaer N, Walker R, Kessler O, Ignatius A, Durselen L. Biomechanical, structural and biological characterisation of a new silk fibroin scaffold for meniscal repair. J Mech Behav Biomed Mater 2018:86:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pan Z, Wu Y, Zhang X, Fu Q, Li J, Yang Y, Yu D, Xu Y, Lu X, Sun H et al. Delivery of epidermal growth factor receptor inhibitor via a customized collagen scaffold promotes meniscal defect regeneration in a rabbit model. Acta Biomater 2017:62:210–221. [DOI] [PubMed] [Google Scholar]
- 53.Monibi FA, Cook JL. Tissue-derived extracellular matrix bioscaffolds: Emerging applications in cartilage and meniscus repair. Tissue Eng Part B Rev 2017:23(4): 386–398. [DOI] [PubMed] [Google Scholar]
- 54.Ruprecht JC, Waanders TD, Rowland CR, Nishimuta JF, Glass KA, Stencel J, DeFrate LE, Guilak F, Weinberg JB, McNulty AL. Meniscus-derived matrix scaffolds promote the integrative repair of meniscal defects. Sci Rep 2019:9(1): 8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makris EA, Hadidi P, Athanasiou KA. The knee meniscus: Structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 2011:32(30): 7411–7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthritis Cartilage 2007:15(9): 1053–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McNulty AL, Estes BT, Wilusz RE, Weinberg JB, Guilak F. Dynamic loading enhances integrative meniscal repair in the presence of interleukin-1. Osteoarthritis Cartilage 2010:18(6): 830–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNulty AL, Guilak F. Integrative repair of the meniscus: Lessons from in vitro studies. Biorheology 2008:45(3–4): 487–500. [PMC free article] [PubMed] [Google Scholar]
- 59.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor alpha. Arthritis Rheum 2007:56(9): 3033–3042. [DOI] [PubMed] [Google Scholar]
- 60.McNulty AL, Weinberg JB, Guilak F. Inhibition of matrix metalloproteinases enhances in vitro repair of the meniscus. Clin Orthop Relat Res 2009:467(6): 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Riera KM, Rothfusz NE, Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Interleukin-1, tumor necrosis factor-alpha, and transforming growth factor-beta 1 and integrative meniscal repair: Influences on meniscal cell proliferation and migration. Arthritis Res Ther 2011:13(6): R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. J Orthop Res 2008:26(4): 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ionescu LC, Lee GC, Garcia GH, Zachry TL, Shah RP, Sennett BJ, Mauck RL. Maturation state-dependent alterations in meniscus integration: Implications for scaffold design and tissue engineering. Tissue Eng Part A 2011:17(1–2): 193–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Izal I, Ripalda P, Acosta CA, Forriol F. In vitro healing of avascular meniscal injuries with fresh and frozen plugs treated with tgf-beta1 and igf-1 in sheep. Int J Clin Exp Pathol 2008:1(5): 426–434. [PMC free article] [PubMed] [Google Scholar]
- 65.Rowland CR, Colucci LA, Guilak F. Fabrication of anatomically-shaped cartilage constructs using decellularized cartilage-derived matrix scaffolds. Biomaterials 2016:91:57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishimuta JF, Levenston ME. Response of cartilage and meniscus tissue explants to in vitro compressive overload. Osteoarthritis Cartilage 2012:20(5): 422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Collins AT, Hatcher CC, Kim SY, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL. Selective enzymatic digestion of proteoglycans and collagens alters cartilage t1rho and t2 relaxation times. Ann Biomed 2019:47(1):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC 3rd, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, McNulty AL. Relationship between t1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech 2017:55:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta 1986:883(2): 173–177. [DOI] [PubMed] [Google Scholar]
- 70.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem 1996:29(3): 225–229. [DOI] [PubMed] [Google Scholar]
- 71.Rowland CR, Lennon DP, Caplan AI, Guilak F. The effects of crosslinking of scaffolds engineered from cartilage ecm on the chondrogenic differentiation of mscs. Biomaterials 2013:34(23): 5802–5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jakobsen RB, Ostrup E, Zhang X, Mikkelsen TS, Brinchmann JE. Analysis of the effects of five factors relevant to in vitro chondrogenesis of human mesenchymal stem cells using factorial design and high throughput mrna-profiling. PLoS One 2014:9(5): e96615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Derfoul A, Perkins GL, Hall DJ, Tuan RS. Glucocorticoids promote chondrogenic differentiation of adult human mesenchymal stem cells by enhancing expression of cartilage extracellular matrix genes. Stem Cells 2006:24(6): 1487–1495. [DOI] [PubMed] [Google Scholar]
- 74.Florine EM, Miller RE, Porter RM, Evans CH, Kurz B, Grodzinsky AJ. Effects of dexamethasone on mesenchymal stromal cell chondrogenesis and aggrecanase activity: Comparison of agarose and self-assembling peptide scaffolds. Cartilage 2013:4(1): 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tangtrongsup S, Kisiday JD. Effects of dexamethasone concentration and timing of exposure on chondrogenesis of equine bone marrow-derived mesenchymal stem cells. Cartilage 2016:7(1): 92–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnstone B, Hering TM, Caplan AI, Goldberg VM, Yoo JU. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp Cell Res 1998:238(1): 265–272. [DOI] [PubMed] [Google Scholar]
- 77.Ho ST, Tanavde VM, Hui JH, Lee EH. Upregulation of adipogenesis and chondrogenesis in msc serum-free culture. Cell Med 2011:2(1): 27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mueller MB, Fischer M, Zellner J, Berner A, Dienstknecht T, Prantl L, Kujat R, Nerlich M, Tuan RS, Angele P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of tgf-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010:192(3): 158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barry F, Boynton RE, Liu B, Murphy JM. Chondrogenic differentiation of mesenchymal stem cells from bone marrow: Differentiation-dependent gene expression of matrix components. Exp Cell Res 2001:268(2): 189–200. [DOI] [PubMed] [Google Scholar]
- 80.Collier S, Ghosh P. Effects of transforming growth-factor-beta on proteoglycan synthesis by cell and explant cultures derived from the knee-joint meniscus. Osteoarthritis and Cartilage 1995:3(2): 127–138. [DOI] [PubMed] [Google Scholar]
- 81.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng 2005:11(7–8): 1141–1148. [DOI] [PubMed] [Google Scholar]
- 82.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage 2004:12(9): 736–744. [DOI] [PubMed] [Google Scholar]
- 83.Goto H, Shuler FD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: Enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a tgfbeta(1)gene. Osteoarthritis Cartilage 2000:8(4): 266–271. [DOI] [PubMed] [Google Scholar]
- 84.Shen C, Gu W, Cai GQ, Peng JP, Chen XD. Autophagy protects meniscal cells from glucocorticoids-induced apoptosis via inositol trisphosphate receptor signaling. Apoptosis 2015:20(9): 1176–1186. [DOI] [PubMed] [Google Scholar]
- 85.Heard BJ, Barton KI, Agbojo OM, Chung M, Sevick JL, Bader TJ, Martin CR, Shrive NG, Hart DA. Molecular response of rabbit menisci to surgically induced hemarthrosis and a single intra-articular dexamethasone treatment. J Orthop Res 2019. 37(9):2043–2052. [DOI] [PubMed] [Google Scholar]
- 86.Genemaras AA, Ennis H, Bradshaw B, Kaplan L, Huang CC. Effects of anti-inflammatory agents on expression of early responsive inflammatory and catabolic genes in ex vivo porcine model of acute knee cartilage injury. Cartilage 2018:9(3): 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yuan X, Wei Y, Villasante A, Ng JJD, Arkonac DE, Chao PG, Vunjak-Novakovic G. Stem cell delivery in tissue-specific hydrogel enabled meniscal repair in an orthotopic rat model. Biomaterials 2017:132(59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rothrauff BB, Shimomura K, Gottardi R, Alexander PG, Tuan RS. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta Biomater 2017:49:140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Saliken DJ, Mulet-Sierra A, Jomha NM, Adesida AB. Decreased hypertrophic differentiation accompanies enhanced matrix formation in co-cultures of outer meniscus cells with bone marrow mesenchymal stromal cells. Arthritis Res Ther 2012:14(3): R153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cucchiarini M, McNulty AL, Mauck RL, Setton LA, Guilak F, Madry H. Advances in combining gene therapy with cell and tissue engineering-based approaches to enhance healing of the meniscus. Osteoarthritis Cartilage 2016:24(8): 1330–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M. Regional differences in the healing potential of the meniscus-an organ culture model to eliminate the influence of microvasculature and the synovium. Knee 2004:11(4): 271–278. [DOI] [PubMed] [Google Scholar]
- 92.Rowland CR, Glass KA, Ettyreddy AR, Gloss CC, Matthews JRL, Huynh NPT, Guilak F. Regulation of decellularized tissue remodeling via scaffold-mediated lentiviral delivery in anatomically-shaped osteochondral constructs. Biomaterials 2018:177:161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glass KA, Link JM, Brunger JM, Moutos FT, Gersbach CA, Guilak F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials 2014:35(22): 5921–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]