Abstract
Attention Deficit Hyperactivity Disorder (ADHD) is associated with pervasive impairments in attention and cognitive control. Although brain circuits underlying these impairments have been extensively investigated with resting-state fMRI, little is known about task-evoked functional brain circuits and their relation to cognitive control deficits and inattention symptoms in children with ADHD. Children with ADHD and age, gender and head motion matched typically-developing (TD) children completed a Go/NoGo fMRI task. We used multivariate and dimensional analyses to investigate impairments in two core cognitive control systems: (i) cingulo-opercular “salience” network (SN) anchored in the right anterior insula, dorsal anterior cingulate cortex (rdACC), and ventrolateral prefrontal cortex (rVLPFC) and (ii) dorsal fronto-parietal “central executive” (FPN) network anchored in right dorsolateral prefrontal cortex (rDLPFC) and posterior parietal cortex (rPPC). We found that multivariate patterns of task-evoked effective connectivity between brain regions in SN and FPN distinguished the ADHD and control groups, with rDLPFC–rPPC connectivity emerging as the most distinguishing link. Task-evoked rdACC–rVLPFC connectivity was positively correlated with NoGo accuracy, and negatively correlated with severity of inattention symptoms. Brain-behavior relationships were robust against potential age, gender and head motion confounds. Our findings highlight aberrancies in task-evoked modulation of SN and FPN connectivity in children with ADHD. Crucially, cingulo-frontal connectivity was a common locus of deficits in cognitive control and clinical measures of inattention symptoms. Our study provides insights into a parsimonious systems neuroscience model of cognitive control deficits in ADHD, and suggests specific circuit biomarkers for predicting treatment outcomes in childhood ADHD.
Keywords: human, fMRI, attention, response inhibition, gPPI, anterior insula
Attention-deficit-hyperactivity-disorder (ADHD) is one of the most common neurodevelopmental disorders 1, and is characterized by deficits in attention and cognitive control 2–6. Although decades of brain imaging research has focused on anomalies in discrete brain regions 7, 8, ADHD has been increasingly viewed as a disorder stemming from disturbance in functional connectivity of brain networks 9. Cognitive function relies on dynamic interactions between brain regions 10 and cognitive deficits reflect dysfunction of large-scale neuronal systems characterized by altered regional interactions in specific brain networks 11. Previous studies have highlighted abnormal intrinsic functional connectivity as a key neurobiological feature of childhood ADHD 12–15. However, the specific focus on resting-state fMRI has precluded knowledge of impairments in dynamic engagement of functional circuits and their relation to impaired information processing. Here we address this gap by investigating task-evoked modulation of the cingulo-opercular “salience” network and dorsal fronto-parietal “central executive” (SN, FPN), two intrinsically coupled brain networks that play a critical role in cognitive control and their relation to cognitive control abilities and inattention symptoms in children with ADHD.
More than two decades of functional neuroimaging research have revealed a similar cortical activation pattern during a variety of cognitive demanding tasks, including the Go/No-Go task and Stop-signal tasks that require cognitive control and flexibly switching from response to inhibition 16, 17. This common activation pattern underlying cognitive control is anchored in a core set of nodes in frontal, cingulate and parietal cortices, with consistent evidence for involvement of anterior insula (AI), ventrolateral prefrontal cortex (VLPFC), dorsolateral prefrontal cortex (DLPFC), dorsal anterior cingulate cortex (dACC)/pre-supplementary motor cortex (preSMA), and posterior parietal cortex (PPC) 17–19. This core cognitive control system also shows convergent overlap with connectivity patterns observed in resting-state fMRI 20–23. This convergence has led to the view of control systems anchored in two large-scale brain networks: (i) SN, anchored in the AI, dACC, and VLPFC, and (ii) FPN, anchored in the DLPFC and PPC 21, 23–25. The right AI and dACC are important for monitoring behaviorally salient events in the environment and modulating other brain networks to facilitate cognitive control processes 18, 26–30 while the right VLPFC has been implicated more directly in inhibition of prepotent responses 18, 31–33. In contrast, the DLPFC and PPC are more important for active maintenance and manipulation of task-relevant information during demanding cognitive tasks 34–38.
Deficits in cognitive control are a core feature of ADHD 39, 40, and are accompanied by abnormal neural responses in multiple frontal and parietal cortical regions 41–44. Meta-analyses of fMRI studies of cognitive control have found consistent under-activation in individuals with ADHD, relative to controls, in the right and left VLPFC and AI, dACC and SMA 43. Poor performance on tasks requiring cognitive control has also been linked to decreased gray matter volume in the VLPFC, AI, and dACC in individuals with ADHD 45, 46. Taken together, these findings point to deficits in multiple frontal control regions; however, a principled systems neuroscience approach to modeling task-related functional circuits associated with core cognitive control systems has been lacking in studies of childhood ADHD.
In contrast to task-based fMRI studies, several studies have used resting-state fMRI connectivity analyses to examine cognitive control networks and found altered SN and FPN connectivity in children with ADHD 12, 13, 15, 47. A recent resting-state fMRI study has shown that aberrant interactions between large-scale cognitive control networks, including SN and FPN, are related to clinical symptoms in children with ADHD, such as inattention, suggesting that functional interactions in SN and FPN are a robust and clinically relevant neurobiological feature of childhood ADHD 12. However, it is not known whether the SN and FPN also show aberrant task-evoked modulation in response to attentionally demanding cognitive control tasks. Previous studies have reported both hypo- and hyper-connectivity between frontal, motor, parietal and striatal regions in children and adolescents with ADHD performing cognitive control tasks 48–51, but no consensus has emerged about deficits in fronto-opercular-parietal circuits and their relation to behavioral deficits that characterize ADHD. Here we investigate a specific cognitive control circuit model and test the hypothesis that the SN and FPN are functionally impaired in childhood ADHD and that the degree of impairments in task-evoked functional brain networks predicts cognitive control ability and clinical inattention symptoms more generally.
We investigated differences in brain activation and connectivity between children with ADHD and typically developing (TD) children in a Go/NoGo fMRI task. We first examined group differences in brain responses associated with NoGo trials. Next, we analyzed task-evoked connectivity between key nodes of the SN and FPN associated with NoGo correct 52, 53. Crucially, to facilitate correspondence with previous intrinsic connectivity analysis of ADHD, the five core cortical nodes of the SN and FPN – rAI, rdACC, rVLPFC, rDLPFC, and rPPC – were determined using independent resting-state fMRI studies 30. This choice of ROIs is based on right hemispheric dominance in cognitive control 18, 54 and right hemispheric functional abnormality related to cognitive control deficit in ADHD 7, 41, 42. Previous studies in neurotypical adults have demonstrated that task-evoked regional interactions in these key nodes of the SN and FPN are modulated by cognitive demands and correlated with cognitive control abilities across different task paradigms and datasets 26, 28, 30, 55. Our approach and selection of ROIs therefore provides a principled approach to the investigation of cognitive control systems in childhood ADHD.
There are four key components to our study. First, we used machine learning algorithms to determine whether task-evoked effective connectivity between SN and FPN nodes could distinguish children with ADHD and TD children, and then identified the most significant distinguishing links after correction for multiple comparisons. Second, we trained a multivariate non-linear regression model to investigate whether SN-FPN links could predict cognitive control ability as assessed by performance on the NoGo task, and then identified the most significant predictive links after correction for multiple comparisons. Third, we examined whether the strength of task-evoked connectivity also predicts severity of inattention symptoms in children with ADHD. We hypothesized that task-evoked effective connectivity between SN and FPN nodes would distinguish between children with ADHD and TD children. We further hypothesized that task-evoked connectivity between SN and FPN would predict cognitive control abilities and clinical symptoms in children with ADHD. Finally, we replicated the relationship between task-evoked connectivity of rdACC and rVLPFC, and cognitive performance as well as inattention symptom using ROIs obtained from previous meta-analyses of cognitive control 18, and demonstrated the robustness of our findings.
Methods & Materials
Datasets
fMRI data was acquired from 46 children and adolescents with ADHD and 51 TD children and adolescents (8–17 years old) who took part in the International Study to Predict Optimized Treatment in ADHD (iSPOT-A) trial, which is approved by local institutional review board. All participants and/or their guardians consented to participate the study. A detailed ADHD diagnosis procedure can be found in a previous study 56. In brief, ADHD diagnosis was confirmed using the Mini International Neuropsychiatric Interview 57 and Attention Deficit/Hyperactivity Disorder Rating Scale (ADHD-RS) 58. Symptom severity was rated using the ADHD-RS. While subtypes of ADHD were determined for individuals with ADHD, the small sample size in each subtype group prevented further analysis of the specificity of each subtype. All participants were free of medication during testing and at least 5 half-live washout period was applied.
After screening for the completeness and quality of behavioral, neuroimaging and clinical data, the final sample include 27 children with ADHD and 30 TD children with matched age, gender and head movement (Supplemental Table S1). The criterion can be found in the Supplementary Methods.
Cognitive control task
Participants completed one run of the Go/NoGo task during scan in which they respond to the word “press” when it is presented in GREEN, and inhibit response when presented in RED. Each stimulus was presented for 500 ms with an inter-stimulus interval of 750 ms. There were 180 Go and 60 NoGo stimuli. Stimuli were presented in pseudorandom order and NoGo was not repeated more than 3 times in a row. Details of task can be found in previous studies 59, 60.
MRI acquisition
fMRI data was acquired using an 8-channel head coil in a 3T GE Signa HDx scanner and echo planar imaging sequence (TR=2.5s). Details of protocol can be found in Supplemental Methods.
fMRI Preprocessing
A standard preprocessing procedure was implemented using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8), including realignment, co-registration to structural MRI, slice-timing correction, normalization to the Montreal Neurological Institute space and smoothing using a 8-mm full-width half-maximum Gaussian kernel.
General linear model (GLM)
The model includes NoGo correct and NoGo error. Go trials were treated as a baseline.
Regions of Interest (ROIs)
Five ROIs of SN and FPN were made as spheres with 6mm radius with their centers located at rdACC (x=7, y=18, z=33), rAI (x=37, y=16, z=−2), rDLPFC (x=50, y=18, z=44), rVLPFC (x=42, y=26, z=14), and rPPC (x=48, y=−52, z=50), determined from a previous study using independent component analysis on a separate resting-state fMRI data 30. Critically, ROIs were selected independently of the task-fMRI dataset we examined, thus facilitating data analysis in an unbiased and principled manner 26, 55.
An additional ROI set of rdACC (x=4, y=28, z=36) and rVLPFC (x=50, y=16, z=18), determined from a meta-analysis study of cognitive control 18, was used for replication.
Task-based connectivity analysis
The general psychophysiological interaction (gPPI) 52, 53 was used to analyze interactions between ROIs on NoGo correct. Method details can be found in Supplemental Methods.
Task-based connectivity differentiates TD and ADHD children
To examine whether task-based connectivity in the SN and FPN could successfully differentiate TD children and children with ADHD, we conducted multivariate classification analysis using linear support vector machine (SVM). The PPI weights on all pairs of seed-target ROIs were used as feature to predict group identity of each child (TD or ADHD). The model was evaluated using the leave-one-out cross validation (LOOCV). Each time, one data point was selected as a test set and the rest of data were used as a training set. The training set was then used to train a SVM model, which was then applied to the test set for classification. This procedure was repeated N times with each data point used exactly once as a test set. The significance of classification accuracy was evaluated using permutation (500 times).
Next, we conducted univariate analysis (two sample t-test) to examine specific links that are different between the two group and corrected using False Discovery Rate (FDR).
Task-based connectivity predicts cognitive control ability
To examine whether task-based connectivity in the SN and FPN could account for individual differences in cognitive control ability, we conducted multivariate regression analysis using nonlinear support vector regression (SVR). The PPI weights on all pairs of seed-target ROIs were used as features to predict NoGo accuracy. The model was evaluated using the aforementioned LOOCV. Pearson’s correlations were used to evaluate prediction performance.
Next, to examine which specific link accounts for individual variability in cognitive control, we examined correlation between PPI weights of each link on NoGo correct and NoGo accuracy and corrected using FDR. In addition, we examined whether the relationship is stable across two groups and in each group separately. Multiple linear regression was conducted to control for confounding effects of age, gender and head motion.
Task-based connectivity in relation to inattention symptom
For the links whose PPI weights are correlated to individuals’ cognitive control ability, we further examined whether the PPI weights of the same links are correlated to the severity of inattention score from the ADHD-RS. Multiple linear regression was conducted to control for the effect confounds, including age, gender and head motion.
Results
Go/NoGo task performance
Both children with ADHD and TD children performed the task with high levels of accuracy (Supplemental Table S2). There were no significant differences in Go Accuracy, NoGo Accuracy, Go RT and NoGo Error RT (all ps>0.05, t-test and permutation test). However, additional analyses with a larger group, in which we did not excluded participants with large head motion, revealed that children with ADHD display marginally significant deficits in NoGo accuracy (p=0.05, effect size=0.42) (Supplemental Results). This effect size is similar to the weighted mean effect size of 0.51 reported in a previous meta-analysis of behavioral studies comparing ADHD and control groups 40. These results indicate that children with ADHD show modest deficits on the Go/NoGo task; however, participants who met movement criteria for inclusion in the fMRI analyses showed similar performance on the Go/NoGo task as their TD peers.
We then examined post-error slowing as it is a critical aspect of cognitive control 61, 62. Specifically, we compared RT on Go trials after error NoGo trials versus RT on Go trials after correct NoGo trials. In TD controls, RT on Go trials after correct NoGo trials (390±74ms) was not significantly different from RT on Go trials after error NoGo trials (370±72ms) (p=0.63). In the ADHD group, RT on Go trials after correct NoGo trials (425±114ms) was not significantly different from RT on Go trials after error NoGo trials (528±337ms) (p=0.22). The lack of post-error adjustment is due to good overall performance levels in the GNG task. High NoGo accuracy leads to small number of error NoGo trials and even fewer Go trials after error NoGo trials, which also explains large standard deviation for RT on Go trials after error NoGo trials. These results suggest that children with ADHD do not differ from TD controls in post-error slowing on the Go/NoGo task, likely due to the lack of sufficient trials to probe post-error adjustments.
Whole brain activation on correct and error NoGo trials
On correct NoGo trials, there was significantly greater activation in DLPFC, VLPFC, frontal pole, AI, preSMA/dACC, striatum and PPC in both children with ADHD and TD children (p<0.01, FDR corrected, Supplemental Figure S1). On NoGo error trials, there were significantly greater activation in AI, preSMA/dACC, VLPFC and PPC in TD children (p<0.01, FDR corrected) but no significant activation in children with ADHD (Supplemental Figure S2).
Differences in whole brain activation between ADHD and TD groups
There was greater activation on NoGo error trials in AI and dACC/preSMA in TD children than children with ADHD (activation height p<0.01 and cluster p<0.05, Supplemental Figure S3). There was no significant difference in NoGo correct trials between the two groups. Notably, there was no significant group difference in default mode network (DMN).
Multivariate task-evoked effective connectivity between SN and FPN distinguish children with ADHD from TD children
To determine whether task-evoked connectivity between the SN and FPN contains useful signal to differentiate children with ADHD and TD children, we trained a linear SVM model and found that multivariate task-evoked effective connectivity between SN and FPN could distinguish children with ADHD from TD with an LOOCV testing accuracy of 64% (p<0.05).
We then determined which specific links between SN and FPN nodes differ between the two groups and found that task-evoked effective connectivity between the rDLPFC (seed) and rPPC (target) was significantly greater in TD than ADHD children (p<0.05, FDR corrected).
Multivariate patterns of task-evoked effective connectivity predict NoGo task accuracy
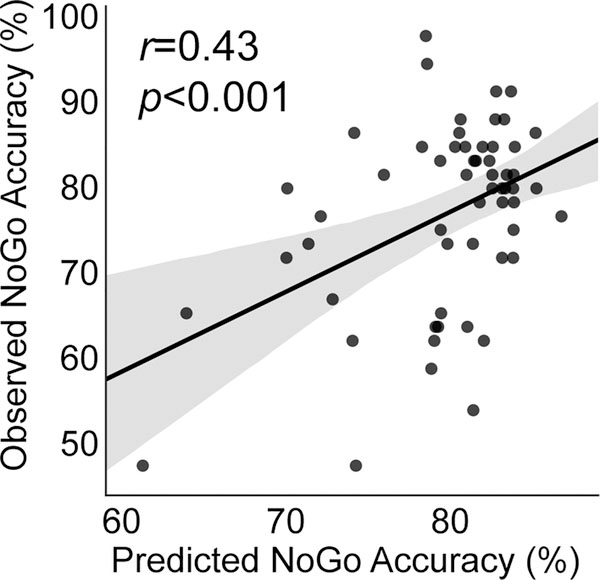
Next, we examined whether multivariate connectivity patterns could predict individual NoGo task accuracy. We used a dimensional approach in which both TD children and children with ADHD were included. We trained a nonlinear SVR model and found that predicted NoGo accuracy was significantly correlated with observed NoGo accuracy (r=0.43, p<0.001, Figure 1).
Figure 1.
Support vector regression analysis with cross-validation revealed that multivariate patterns of task-evoked effective connectivity between SN and FPN nodes accurately predict NoGo task accuracy in combined ADHD and TD groups (r=0.43, p<0.001).
Specific task-evoked effective connectivity links between SN and FPN nodes that predict NoGo task accuracy in children with ADHD and in TD children
We further examined whether specific links were related to individual cognitive control abilities and found that effective connectivity between rdACC (seed) and rVLPFC (target) was significantly and positively correlated with NoGo accuracy in the combined group (r=0.47, p<0.05, FDR corrected) (Figure 2A, 2B). Additional analysis using age, gender and head motion as confounds confirmed that rdACC-rVLPFC connectivity was the only significant predictor in the combined group (p=0.0004, Table 1). Further analysis revealed the same significant correlation in the ADHD (r=0.55, p=0.003, Figure 2C) and TD group (r=0.42, p=0.02, Figure 2D). These results held when age, gender and head motion were included as confounds (ADHD group: p=0.0004; TD group: p=0.07; Table 1).
Figure 2.
(A) Predictive power of task-evoked effective connectivity between rdACC and rVLPFC nodes in the SN and FPN nodes. rdACC-rVLPFC connectivity was significantly correlated with NoGo accuracy (p<0.05, FDR corrected). (B, C, D) rdACC-rVLPFC connectivity was significantly correlated with NoGo accuracy in pooled data across the two groups (r=0.47, p<0.005), in the TD group (r=0.42, p<0.05), and in the ADHD group (r=0.55, p<0.005).
Table 1.
Multiple linear regression analysis showed that psychophysiological interaction (PPI) between rdACC and rVLPFC on NoGo is the most robust predictor for NoGo Accuracy.
| beta | t value | p value | |
|---|---|---|---|
| Control + ADHD | |||
| rdACC-rVLPFC PPI on NoGo | 0.024 | 3.8 | 0.0004*** |
| Gender | −0.04 | −1.2 | 0.23 |
| Age | 0.01 | 1.56 | 0.13 |
| Framewise displacement | −0.47 | −1.24 | 0.22 |
| Control | |||
| rdACC-rVLPFC PPI on NoGo | 0.02 | 1.9 | 0.07 |
| Gender | 0.05 | 1.21 | 0.24 |
| Age | −0.01 | −1.01 | 0.33 |
| Framewise displacement | −0.87 | 1.88 | 0.07 |
| ADHD | |||
| rdACC-rVLPFC PPI on NoGo | 0.03 | 4.15 | 0.0004*** |
| Gender | −0.07 | −1.69 | 0.11 |
| Age | 0.02 | 2.74 | 0.01 |
| Framewise displacement | −0.33 | −0.63 | 0.54 |
p < 0.001
To examine whether the relation between effective connectivity of rdACC-rVLPFC and NoGo accuracy is different between ADHD and controls, we performed a non-parametric permutation test. Specifically, in each permutation, we randomly shuffled ADHD and control labels across the two groups, computed correlation coefficients in the permutated groups separately, and calculated the difference in correlation coefficients between the two permutated groups. We repeated permutation for 500 times to generate a null distribution of correlation coefficient differences from the sample and determined the p value of the correlation coefficient differences from the original data. We found that the correlation coefficient difference between ADHD and TD groups was not significantly different (p=0.29).
Task-evoked effective connectivity between rdACC and rVLPFC predicts inattention symptoms in children with ADHD
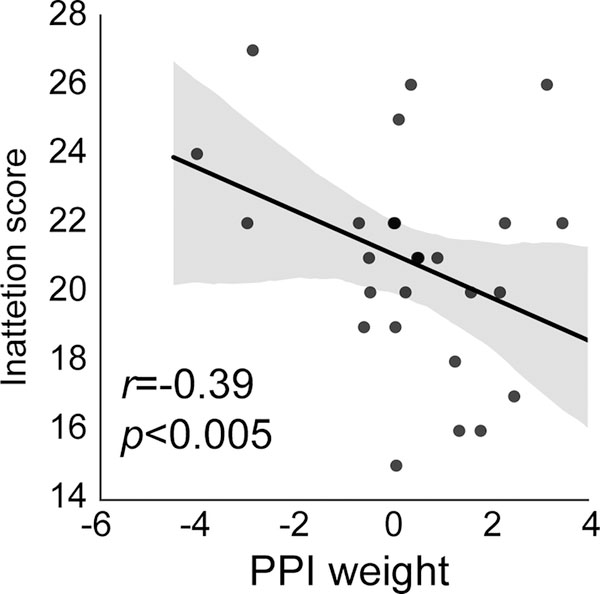
Then we examined whether task-evoked effective connectivity between rdACC and rVLPFC could also predict severity of inattention symptoms in children with ADHD. We found a significant correlation between rdACC-rVLPFC effective connectivity and inattention scores (r=−0.39, p=0.04, Figure 3). This result held when age, gender and head motion were included as potential confounds (p=0.04, Table 2).
Figure 3.
Task-evoked effective connectivity between rdACC and rVLPFC was significantly and negatively correlated with inattention symptoms in children with ADHD (r=−0.39, p<0.005).
Table 2.
Multiple linear regression analysis showed that effective connectivity between rdACC and rVLPFC was the most robust predictor of inattention symptoms in children with ADHD.
| beta | t value | p value | |
|---|---|---|---|
| ADHD | |||
| rdACC-rVLPFC PPI on NoGo | −0.65 | −2.13 | 0.04* |
| Gender | −0.3 | −0.21 | 0.84 |
| Age | −0.36 | −1.27 | 0.22 |
| Framewise displacement | −4.63 | −0.24 | 0.81 |
p < 0.05
Replication of the relationship between task-evoked effective connectivity between rdACC and rVLPFC and NoGo task accuracy as well as inattention symptoms
We conducted the same analysis using a different set of rdACC and rVLPFC ROIs determined based on a previous meta-analysis study 18 and replicated the brain-behavior and brain-symptom correlation findings (Supplemental Figure S4, S5, Table S3, S4). See Supplemental Results for details.
Discussion
ADHD is associated with prominent deficits in attention and cognitive control. Here we found that multivariate patterns of task-evoked connectivity accurately distinguished the ADHD and TD groups, and accurately predicted individual task performance. Effective connectivity between the rdACC and rVLPFC was correlated with NoGo accuracy and inattention symptoms in children with ADHD. Our findings demonstrate that task-evoked connectivity associated with SN and FPN provide informative neurobiological signatures for distinguishing ADHD from controls, and prediction of clinical symptoms in affected individuals.
Task-evoked effective connectivity between SN and FPN distinguish ADHD and TD children
We focused on five key cortical nodes of the SN and FPN comprised of the rAI, rdACC, rVLPFC, rDLPFC and rPPC. These brain regions are commonly activated in a wide range of cognitive control tasks 17, 18. While previous studies have investigated interactions between these key nodes in SN and FPN during cognitive control in neurotypical adults 26, 28, it is not known whether task-evoked interactions between these two networks carry useful neurobiological signatures of childhood ADHD. We found that the strength of task-evoked effective connectivity between rDLPFC and rPPC on correct NoGo trials was significantly weaker in children with ADHD and TD children. The rDLPFC and rPPC are the two core nodes of the FPN, which is tightly associated with working memory as well as planning and controlling goal-directed behavior 38, 63. Our finding demonstrates that fronto-parietal communication during cognitive control is particularly weak in children with ADHD, in comparison to their TD peers. Importantly, our findings suggest that a parsimonious systems neuroscience framework involving just two core cognitive control networks contains a constrained theoretically-meaningful feature space to distinguish children with ADHD from controls.
Task-evoked effective connectivity in SN and FPN predicts cognitive control abilities in children with ADHD and in TD children
Our findings showed that multivariate pattern of effective connectivity between nodes in the SN and FPN could accurately predict NoGo task accuracy on unseen data. This result provides evidence that children’s performance during cognitive control task relies on modulation of functional circuits linking key SN and FPN nodes. We also found that effective connectivity between rdACC and rVLPFC during correct NoGo trials was significantly and positively correlated with NoGo accuracy. This suggests that the greater the interaction between the rdACC and rVLPFC during successful cancellation of action, the better the child’s cognitive control ability.
The rdACC is implicated in a diverse set of cognitive functions, including response selection and action control 64, error detection 65, 66 and conflict and performance monitoring 67–69. The rVLPFC has an essential role in stimulus triggered response inhibition 18, 27, 70, though its unique contribution to inhibitory control process per se is still debated 71, 72. One possibility here is that interactions between the rdACC and rVLPFC may facilitate context-dependent modulation of response inhibition processes initiated by the rVLPFC 18, 27. Crucially, our findings demonstrate that interactions between nodes in the SN and FPN play an important role in children’s ability to implement cognitive control.
Task-evoked effective connectivity in SN and FPN predicts clinical inattention symptoms in children with ADHD
Task-evoked effective connectivity between the rdACC and rVLPFC not only predicted children’s cognitive control abilities, but also inattention symptoms as assessed by the ADHD-RS in children with ADHD. The ADHD-RS is widely used in assessing severity of inattention symptoms in childhood ADHD and provides a clinically useful tool to assess individual levels of impairment 73. Importantly, this relationship was not influenced by head motion during scanning. Crucially, we demonstrated the robustness of this finding by a replication using different ROIs determined by a previous meta-analysis study 18. The current finding is an advance over intrinsic connectivity studies 12, 13, 74, 75 as it more directly links aberrant brain network interactions during task-evoked cognitive control to a core clinical symptom of childhood ADHD.
Two neurocognitive mechanisms underlie the observed brain-symptom relationship. One is related to reactive control. Inattention can lead to failure or sluggishness in detection of NoGo stimuli or initiation of response inhibition. The second relates to proactive control. Attention deficits can jeopardize preparatory process for response inhibition. The rdACC and rVLPFC are differentially implicated in reactive and proactive control with the rdACC playing a greater role in regulating behavioral adaption and persistence 76, 77 and the rVLPFC more involved in inhibitory control 18, 27. We suggest that impairments in both reactive and proactive control arising from aberrant rdACC-rVLPFC circuits might contribute to inattention. While rDLPFC-rPPC connectivity weight was significantly different between ADHD and TD controls, it was rdACC-rVLPFC connectivity weights that were significantly correlated with NoGo performance and clinical symptoms. This dissociation speaks to the importance of both categorical and dimensional analyses in uncovering distinct neurobiological features underlying heterogeneity in ADHD.
Conclusion
We demonstrated that aberrant task-evoked effective connectivity between the SN and FPN is a distinguishing neurobiological signature of childhood ADHD, and can predict cognitive control ability and inattention symptoms in children with ADHD with a replication using a different set of ROIs. Our findings highlight aberrant interactions between key regions in the SN and FPN as an important neurobiological feature of childhood ADHD that contribute to both impaired experimentally derived measures of cognitive control and clinical symptoms of inattention.
Supplementary Material
Acknowledgements
The International Study to Predict Optimized Treatment in Attention-deficit hyperactivity disorder (iSPOT-A) was sponsored by Brain Resource Company Operations Pty Ltd LIMCA data were funded by the National Health and Medical Research Council funded project grant (APP1008080) awarded to MSK and LMW. LMW was the academic Principal Investigator for iSPOT-A from 2009 to 2013. Tracey Tsang was the iSPOT-A trial coordinator. Sheryl Foster was the senior MRI Research Coordinator at Westmead Hospital and specialist radiographer who oversaw the scanning of subjects for iSPOT-A and LIMCA. This research was supported by National Institutes of Health grants EB022907 (VM), NS086085 (VM), MH121069 (VM, WC), and MH105625 (WC), Stanford Departmental Innovator grant (WC) and Stanford Children Health Research Institute grant (WC).
Footnotes
Financial Disclosures
There are no biomedical financial interests or potential conflicts of interest.
Reference
- 1.Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. The American journal of psychiatry 2007; 164(6): 942–948. [DOI] [PubMed] [Google Scholar]
- 2.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychological bulletin 1997; 121(1): 65–94. [DOI] [PubMed] [Google Scholar]
- 3.Castellanos FX, Tannock R. Neuroscience of attention-deficit/hyperactivity disorder: the search for endophenotypes. Nature reviews Neuroscience 2002; 3(8): 617–628. [DOI] [PubMed] [Google Scholar]
- 4.Nigg JT, Stavro G, Ettenhofer M, Hambrick DZ, Miller T, Henderson JM. Executive functions and ADHD in adults: evidence for selective effects on ADHD symptom domains. Journal of abnormal psychology 2005; 114(4): 706–717. [DOI] [PubMed] [Google Scholar]
- 5.Pennington BF, Ozonoff S. Executive functions and developmental psychopathology. Journal of child psychology and psychiatry, and allied disciplines 1996; 37(1): 51–87. [DOI] [PubMed] [Google Scholar]
- 6.Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and biobehavioral reviews 2007; 31(7): 977–986. [DOI] [PubMed] [Google Scholar]
- 7.Cortese S, Kelly C, Chabernaud C, Proal E, Di Martino A, Milham MP et al. Toward systems neuroscience of ADHD: a meta-analysis of 55 fMRI studies. The American journal of psychiatry 2012; 169(10): 1038–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of child psychology and psychiatry, and allied disciplines 2006; 47(10): 1051–1062. [DOI] [PubMed] [Google Scholar]
- 9.Castellanos FX, Proal E. Large-scale brain systems in ADHD: beyond the prefrontal-striatal model. Trends in cognitive sciences 2012; 16(1): 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences 2010; 14(6): 277–290. [DOI] [PubMed] [Google Scholar]
- 11.Menon V Large-scale brain networks and psychopathology: a unifying triple network model. Trends in cognitive sciences 2011; 15(10): 483–506. [DOI] [PubMed] [Google Scholar]
- 12.Cai W, Chen T, Szegletes L, Supekar K, Menon V. Aberrant Time-Varying Cross-Network Interactions in Children With Attention-Deficit/Hyperactivity Disorder and the Relation to Attention Deficits. Biological psychiatry Cognitive neuroscience and neuroimaging 2018; 3(3): 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2008; 63(3): 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fair DA, Posner J, Nagel BJ, Bathula D, Dias TG, Mills KL et al. Atypical default network connectivity in youth with attention-deficit/hyperactivity disorder. Biol Psychiatry 2010; 68(12): 1084–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sripada C, Kessler D, Fang Y, Welsh RC, Prem Kumar K, Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Human brain mapping 2014; 35(9): 4693–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Human brain mapping 2005; 25(1): 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swick D, Ashley V, Turken U. Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. NeuroImage 2011; 56(3): 1655–1665. [DOI] [PubMed] [Google Scholar]
- 18.Cai W, Ryali S, Chen T, Li CS, Menon V. Dissociable roles of right inferior frontal cortex and anterior insula in inhibitory control: evidence from intrinsic and task-related functional parcellation, connectivity, and response profile analyses across multiple datasets. The Journal of neuroscience : the official journal of the Society for Neuroscience 2014; 34(44): 14652–14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy BJ, Wagner AD. Cognitive control and right ventrolateral prefrontal cortex: reflexive reorienting, motor inhibition, and action updating. Ann N Y Acad Sci 2011; 1224: 40–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE. Intrinsic and task-evoked network architectures of the human brain. Neuron 2014; 83(1): 238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC et al. A core system for the implementation of task sets. Neuron 2006; 50(5): 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America 2005; 102(27): 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience : the official journal of the Society for Neuroscience 2007; 27(9): 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. NeuroImage 2007; 37(1): 343–360. [DOI] [PubMed] [Google Scholar]
- 25.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America 2007; 104(26): 11073–11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cai W, Chen T, Ryali S, Kochalka J, Li CS, Menon V. Causal Interactions Within a Frontal-Cingulate-Parietal Network During Cognitive Control: Convergent Evidence from a Multisite-Multitask Investigation. Cerebral cortex 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai W, Chen T, Ide JS, Li CR, Menon V. Dissociable Fronto-Operculum-Insula Control Signals for Anticipation and Detection of Inhibitory Sensory Cue. Cerebral cortex 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Michels L, Supekar K, Kochalka J, Ryali S, Menon V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory-visual attention. The European journal of neuroscience 2015; 41(2): 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain structure & function 2010; 214(5–6): 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America 2008; 105(34): 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aron AR, Poldrack RA. Cortical and subcortical contributions to Stop signal response inhibition: role of the subthalamic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience 2006; 26(9): 2424–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai W, Leung HC. Rule-guided executive control of response inhibition: functional topography of the inferior frontal cortex. PloS one 2011; 6(6): e20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH et al. Executive “brake failure” following deactivation of human frontal lobe. Journal of cognitive neuroscience 2006; 18(3): 444–455. [DOI] [PubMed] [Google Scholar]
- 34.D’Esposito M, Postle BR. The cognitive neuroscience of working memory. Annual review of psychology 2015; 66: 115–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman-Rakic PS. Cellular basis of working memory. Neuron 1995; 14(3): 477–485. [DOI] [PubMed] [Google Scholar]
- 36.Myers NE, Stokes MG, Nobre AC. Prioritizing Information during Working Memory: Beyond Sustained Internal Attention. Trends in cognitive sciences 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human brain mapping 2005; 25(1): 46–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postle BR. Working memory as an emergent property of the mind and brain. Neuroscience 2006; 139(1): 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pievsky MA, McGrath RE. The Neurocognitive Profile of Attention-Deficit/Hyperactivity Disorder: A Review of Meta-Analyses. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists 2018; 33(2): 143–157. [DOI] [PubMed] [Google Scholar]
- 40.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol Psychiatry 2005; 57(11): 1336–1346. [DOI] [PubMed] [Google Scholar]
- 41.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA psychiatry 2013; 70(2): 185–198. [DOI] [PubMed] [Google Scholar]
- 42.McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychological medicine 2014; 44(4): 869–880. [DOI] [PubMed] [Google Scholar]
- 43.Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J et al. Structural and Functional Brain Abnormalities in Attention-Deficit/Hyperactivity Disorder and Obsessive-Compulsive Disorder: A Comparative Meta-analysis. JAMA psychiatry 2016; 73(8): 815–825. [DOI] [PubMed] [Google Scholar]
- 44.Schulz KP, Li X, Clerkin SM, Fan J, Berwid OG, Newcorn JH et al. Prefrontal and parietal correlates of cognitive control related to the adult outcome of attention-deficit/hyperactivity disorder diagnosed in childhood. Cortex; a journal devoted to the study of the nervous system and behavior 2017; 90: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Depue BE, Burgess GC, Bidwell LC, Willcutt EG, Banich MT. Behavioral performance predicts grey matter reductions in the right inferior frontal gyrus in young adults with combined type ADHD. Psychiatry research 2010; 182(3): 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAlonan GM, Cheung V, Chua SE, Oosterlaan J, Hung SF, Tang CP et al. Age-related grey matter volume correlates of response inhibition and shifting in attention-deficit hyperactivity disorder. Br J Psychiatry 2009; 194(2): 123–129. [DOI] [PubMed] [Google Scholar]
- 47.Barber AD, Jacobson LA, Wexler JL, Nebel MB, Caffo BS, Pekar JJ et al. Connectivity supporting attention in children with attention deficit hyperactivity disorder. NeuroImage Clinical 2015; 7: 68–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hwang S, White SF, Nolan ZT, Craig Williams W, Sinclair S, Blair RJ. Executive attention control and emotional responding in attention-deficit/hyperactivity disorder--A functional MRI study. NeuroImage Clinical 2015; 9: 545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ma I, van Holstein M, Mies GW, Mennes M, Buitelaar J, Cools R et al. Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex; a journal devoted to the study of the nervous system and behavior 2016; 82: 225–236. [DOI] [PubMed] [Google Scholar]
- 50.van Rooij D, Hartman CA, Mennes M, Oosterlaan J, Franke B, Rommelse N et al. Altered neural connectivity during response inhibition in adolescents with attention-deficit/hyperactivity disorder and their unaffected siblings. NeuroImage Clinical 2015; 7: 325–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vloet TD, Gilsbach S, Neufang S, Fink GR, Herpertz-Dahlmann B, Konrad K. Neural mechanisms of interference control and time discrimination in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry 2010; 49(4): 356–367. [PubMed] [Google Scholar]
- 52.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage 1997; 6(3): 218–229. [DOI] [PubMed] [Google Scholar]
- 53.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage 2012; 61(4): 1277–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proceedings of the National Academy of Sciences of the United States of America 1999; 96(14): 8301–8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Supekar K, Menon V. Developmental maturation of dynamic causal control signals in higher-order cognition: a neurocognitive network model. PLoS computational biology 2012; 8(2): e1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elliott GR, Blasey C, Rekshan W, Rush AJ, Palmer DM, Clarke S et al. Cognitive Testing to Identify Children With ADHD Who Do and Do Not Respond to Methylphenidate. Journal of attention disorders 2017; 21(14): 1151–1160. [DOI] [PubMed] [Google Scholar]
- 57.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry 1998; 59 Suppl 20: 22–33;quiz 34–57. [PubMed] [Google Scholar]
- 58.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. The ADHD rating scale IV:Checklists, norms, and clinical interpretation. Guilford Press: New York, 1998. [Google Scholar]
- 59.Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using Standardized fMRI Protocols to Identify Patterns of Prefrontal Circuit Dysregulation that are Common and Specific to Cognitive and Emotional Tasks in Major Depressive Disorder: First Wave Results from the iSPOT-D Study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2013; 38(5): 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams LM, Rush AJ, Koslow SH, Wisniewski SR, Cooper NJ, Nemeroff CB et al. International Study to Predict Optimized Treatment for Depression (iSPOT-D), a randomized clinical trial: rationale and protocol. Trials 2011; 12: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu S, Ide JS, Zhang S, Li CS. Anticipating conflict: Neural correlates of a Bayesian belief and its motor consequence. NeuroImage 2015; 119: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li CS, Yan P, Chao HH, Sinha R, Paliwal P, Constable RT et al. Error-specific medial cortical and subcortical activity during the stop signal task: a functional magnetic resonance imaging study. Neuroscience 2008; 155(4): 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in cognitive sciences 2003; 7(9): 415–423. [DOI] [PubMed] [Google Scholar]
- 64.Rushworth MF, Walton ME, Kennerley SW, Bannerman DM. Action sets and decisions in the medial frontal cortex. Trends in cognitive sciences 2004; 8(9): 410–417. [DOI] [PubMed] [Google Scholar]
- 65.Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science 1998; 280(5364): 747–749. [DOI] [PubMed] [Google Scholar]
- 66.Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science 2005; 307(5712): 1118–1121. [DOI] [PubMed] [Google Scholar]
- 67.Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in cognitive sciences 2004; 8(12): 539–546. [DOI] [PubMed] [Google Scholar]
- 68.Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, affective & behavioral neuroscience 2007; 7(4): 367–379. [DOI] [PubMed] [Google Scholar]
- 69.MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288(5472): 1835–1838. [DOI] [PubMed] [Google Scholar]
- 70.Rae CL, Hughes LE, Anderson MC, Rowe JB. The prefrontal cortex achieves inhibitory control by facilitating subcortical motor pathway connectivity. The Journal of neuroscience : the official journal of the Society for Neuroscience 2015; 35(2): 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aron AR, Cai W, Badre D, Robbins TW. Evidence Supports Specific Braking Function for Inferior PFC. Trends in cognitive sciences 2015; 19(12): 711–712. [DOI] [PubMed] [Google Scholar]
- 72.Hampshire A, Sharp D. Inferior PFC Subregions Have Broad Cognitive Roles. Trends in cognitive sciences 2015; 19(12): 712–713. [DOI] [PubMed] [Google Scholar]
- 73.Chan E, Fogler JM, Hammerness PG. Treatment of Attention-Deficit/Hyperactivity Disorder in Adolescents: A Systematic Review. JAMA 2016; 315(18): 1997–2008. [DOI] [PubMed] [Google Scholar]
- 74.Elton A, Alcauter S, Gao W. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Human brain mapping 2014; 35(9): 4531–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McCarthy H, Skokauskas N, Mulligan A, Donohoe G, Mullins D, Kelly J et al. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA psychiatry 2013; 70(12): 1329–1337. [DOI] [PubMed] [Google Scholar]
- 76.Kolling N, Wittmann MK, Behrens TE, Boorman ED, Mars RB, Rushworth MF. Value, search, persistence and model updating in anterior cingulate cortex. Nature neuroscience 2016; 19(10): 1280–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature 2012; 488(7410): 218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.