Abstract
Given the prevalence and the rising incidence of hepatocellular carcinoma (HCC) in older adults worldwide, there is an urgent need to improve our understanding of the implications of treatment modalities in this population. The care of older patients with HCC is challenging due to the lack of evidence-based recommendations in this population. The current treatment approach for older patients relies on extrapolation of data from clinical trials conducted mostly in younger patients or fit older adults. Further, in the last few years, the arsenal of systemic treatments has increased with currently seven FDA-approved therapies available for patients with advanced HCC. Therefore, understanding how to apply current data to this unique and diverse patient population is necessary. This review will aim to shed light on the approach to older adults with HCC through an assessment of available data in the literature.
Keywords: hepatocellular carcinoma, older adult, multimodality treatment, geriatric assessment
1. Epidemiology
Hepatocellular carcinoma (HCC) is rapidly becoming one of the most prevalent cancers worldwide and the third leading cause of cancer-related deaths.1–3 As of 2012, there were over 14 million cases of HCC worldwide, and the number is expected to rise to 22 million over the next two decades.4 Currently, the median age of patients with HCC is 63 years (median age of diagnosis ranges between 56–74).5 Incidence rates have also increased by 8% in persons 65–69 years old and by 3% in persons 70 years or older.6–8 HCC is increasingly relevant to older adults, especially in Europe and US, where later onset HCC may be related to risk factors acquired with aging, such as acquired hepatitis, fatty liver disease, and comorbidities.5
HCC accounts for over 90% of primary liver malignancies and predominantly occurs in patients with hepatitis and/or cirrhosis.2 In the United States, Hepatitis C virus (HCV) infection is the most common cause of HCC, whereas in developing countries and Asia, Hepatitis B virus (HBV) infection is most common.2,9,10 Despite effective treatments for HBV and HCV, the HCC risk is not completely eliminated, and HCC continues to prevail due to underlying cirrhosis and other risk factors, some of which are not clear.7,11,12 These include rise in non-alcoholic fatty liver disease (NAFLD), resulting from the increasing incidence of obesity, insulin resistance, hyperlipidemia, and metabolic syndrome.13–18
Given the prevalence and rising incidence of HCC in older adults, we need to understand the unique issues that arise when treating this cancer in older adults and the implications of treatment modalities in this population. Many prior clinical trials have excluded older or unfit patients, but several retrospective reviews and meta-analyses suggest that selected older patients derive similar benefit to their younger counterparts from active treatment of HCC.19–24 Standard management for older patients with HCC is yet to be defined, especially in vulnerable and frail older adults.
2.0. Geriatric assessment in older adults with HCC
Physiological and functional status are poorly correlated to chronological age alone in older patients with cancer.25,26,27 Therefore, a systematic evaluation of the functional age of older adults with cancer is necessary prior to making treatment decision. Performance status scores (Eastern Cooperative Oncology Group (ECOG) and Karnofsky) are inadequate tools to evaluate the functional status in patients who are ≥65 years.26,28,29 The comprehensive geriatric assessment (CGA) of older patients should include evaluation of several domains: physical function, cognition, comorbidities, polypharmacy, nutrition, psychological status, social support, and geriatric syndromes.25–27 This complex evaluation identifies deficits and abnormalities not detected by routine medical interview and physical examination. It may also aid in estimating survival, assist decision-making, and predict treatment related complications and toxicities.26,28–38
Several efforts have been made in order to find simple and feasible tools that fit the daily practice in oncology, resulting in evidence-based recommendations.39–41 Based on the result of CGA, patients are categorized as fit, vulnerable or frail. Appropriate patient stratification, CGA-based interventions, and adapted treatments to functional status avoids under- or overtreatment, preserves quality of life and contributes to physical and mental well-being in older patients with cancer.33–38 Thus, future studies in HCC should focus on older adults and incorporate CGA to determine the factors that affect treatment in this population.
3.0. Overview of HCC management.
The Barcelona Clinic Liver Cancer (BCLC) staging and treatment algorithm guides management of HCC.42,43 Given the multi-modality treatment options for HCC, a multidisciplinary team-approach is vital in therapeutic decision-making.2,42 In early stage HCC (Stage 0 or A), patients may be offered liver resection, liver transplant or local ablative therapies.42,43 Those with intermediate stage HCC (Stage B) may be offered trans-arterial chemoembolization (TACE), and those with more advanced stage HCC (Stage C) may be offered systemic therapy.42,43 For patients with end stage liver disease (Stage D), best supportive care is recommended.2,42,43 Along with the CGA, we should account for older patients with varying liver dysfunction and disease burden. In this regard, scores, such as Cancer of the Liver Italian Program (CLIP),44 allow us to understand prognosis and help us weigh the risks and benefits from cancer-directed treatments in older adults. The CLIP score is based on the Child-Pugh stage, tumor burden, α-fetoprotein level (AFP), and portal vein thrombosis, resulting in a score from 0–6 that correlates with a median survival from 1 month to 42.5 months (Table 1).44 Although this helps with prognostication of survival based on tumor characteristics and liver function, neither functional age nor other comorbidities are built into any treatment algorithm for HCC. These guidelines lack guidance on how to treat patients who are vulnerable and/or have comorbidities.
Table 1.
Cancer of the Liver Italian Program (CLIP) scoring system for HCC.44
| Score | 0 | 1 | 2 |
|---|---|---|---|
| Child-Pugh Score | A | B | C |
| Tumor morphology | Unilobular and extension ≤50% | Multinodular and extension ≤50% | Massive or extension >50% |
| AFP (ng/ml) | <400 | ≥400 | ---- |
| Portal vein thrombosis | No | Yes | ---- |
|
Based on total score, median survival: 0 = 42.5 months 1 = 32.0 months 2 = 16.5 months 3 = 4.5 months 4 = 2.5 months 5–6 = 1 month | |||
AFP = alfa-fetoprotein; ng/ml = nanogram/milliliter
Localized HCC
4.1. Liver Resection
The only curative treatments for HCC are surgical resection and liver transplant. Liver resection may achieve a 5-year survival of 60–80% and is the best option for those with early stage disease and preserved liver function.42 The Italian Liver Cancer group performed a 20-year, multicenter, retrospective cohort and nested case-control study, which included 614 older patients (70+ years) and 1104 younger patients (<70 years), found that older patients were less often treated with liver resection or TACE and more often treated with ablative therapies when compared to younger patients.19 However, when older patients were treated with liver resection, median OS was 52 months in older adults versus 47 months in younger adults (p=0.070).19 Two meta-analyses have shown similar findings in patients who underwent liver resections for HCC. Mizuguchi et al included five studies with 470 older patients and 1094 younger patients who underwent liver resection.20 There was no difference in post-surgical morbidity and 3-year and 5-year survival when comparing older and younger adults (p=0.471, test for heterogeneity p=0.007) or mortality (p=0.888, test for heterogeneity p=0.219).20 Hung et al assessed 6341 surgically resected patients in a meta-analysis.45 The older group (70+ years) had higher 1-year survival but similar 3-year and 5-year survival after liver resection compared with the younger group; post-operative complication rates were similar.45
Another retrospective study of 262 patients with HCC compared surgical resection in older (70+) versus younger adults.46 Older patients had a higher frailty score (modified Frailty Index, mean, 1.14 vs 0.51, p<0.001), more comorbidities (comorbidities ≥4: 28% vs 14%, p=0.005), and had more non-viral-induced HCC (65% vs 19%; p<0.001).46 The overall complication rate and duration of stay were similar (p > 0.05).46 Higher mortality rates were observed in the older adults (8% vs 2%, p=0.011).46 Multiple logistic regression revealed that MELD ≥11 (Odds ratio [OR] 2.415; p=0.480) and positive surgical margin (Odds ratio 2.549; p=0.024) were independent predictors for major complications. The 5-year OS rate was 62% in older versus 68.5% younger adults (p=0.712), and the 5-year DFS rate 30.4% vs 38.8%, respectively (p=0.323).46 Multiple Cox regression found that albumin < 4 gram/dL (Hazard ratio (HR) 2.533; p=0.002) and the presence of vascular invasion (HR 2.417; p=0.004) were independent predictors of poor survival.46
A Japanese study prospectively investigated whether a CGA may predict post-operative complications in older patients with HCC.47 Seventy-one patients with a median age of 77 years (range: 70–89) underwent R0 hepatic resection.47 CGA was done pre-operatively and then postoperatively at one, three, and six months.47 Postoperative complications developed in 25% of patients, with median length of hospitalization due to complications of thirteen days (range: 6–189).47 Patients with complications had significantly lower median G8 and mini-nutritional assessments (MNA) scores, higher incidence of cirrhosis, longer median duration of operation and more blood loss during surgery.47 In multivariate logistic regression analysis, only the G8 score <14 was an independent predictor for complications (OR 24.4, 95% CI 1.66–157.08; p=0.0198).47
Overall, this data suggests the process of selecting older adults who benefit from surgical resection is complex. Fit older adults with low volume disease may benefit with long-term improvements in survival. However, older patients who are vulnerable or frail may be at higher risk for worse short-term outcomes. Therefore, geriatric assessments would help us better select patients for liver resection. Simple geriatric screening tools, such as G8, completed by the patient or caregivers within 5–10 minutes, can predict adverse events in older patients with HCC. Further risk factors, such as serum albumin, lower G8 and lower MNA scores, may help us identify patients who are frail or vulnerable who may benefit from pre-rehabilitation or supportive care.
4.2. Liver Transplant
Liver transplant is recommended for patients with HCC that meet the Milan criteria (one lesion <5cm or up to three lesions each <3cm) or the University of California San Francisco criteria (1 tumor ≤6.5cm or maximum of 3 lesions with the largest ≤4.5cm and total tumor diameter ≤8cm).43,45,48,49 Transplant is an ideal treatment for HCC as it offers potential to cure both the underlying liver disease and the HCC, with the 5-year survival being 65–80% in a highly selected patient population.42
Data for survival benefit of transplant in older versus younger adults are mixed. Some early registry data from the United Network for Organ Sharing (UNOS) during 1990 found lower survival in adults >65 (60% vs 72% 1-year survival), and other single institutional series during the 1990s suggested inferior long-term survival rates after transplant in older adults.50–54 However, subsets of older adults with preserved synthetic function or lower pre-transplant bilirubin had similar survival to younger patients, suggesting that carefully selected older adults may benefit from transplant.53
Other analyses have demonstrated similar five-year mortality and graft loss in selected older adults ≥70 years compared with younger adults.23,24,55 A large meta-analysis of patients receiving liver transplant for all causes from 2000–2018 found that 23,660 older (65+) patients and 218,827 younger (<65) patients had similar long-term survival and graft loss rates.22 Internationally, transplantation guidelines do not exclude patients from liver transplant based on chronological age, but assessment should be based on functional age,48 comorbidities and performance status,42 with an expected survivorship of >50% at 5 years after a transplant.49 Despite these recommendations, only a small proportion of older patients are offered a liver transplant (3.1% of transplants in 2014 in the United States and 1.3% of transplants in 2015 in Europe were assigned to patients 70+ years).22
Studies of frailty in patients awaiting liver transplant show older patients have higher rates of frailty than younger patients; and frailty is associated with about 2-fold increased risk of waitlist mortality.56,57 The benefit of transplant should be weighed at the individual level regardless of chronological age for fit older adults with HCC with minimal comorbidities, excellent functional status, and an increased predicted life expectancy. Measures of frailty may guide selection of patients as well as interventions in patients awaiting liver transplantation.
4.3. Local Ablative Therapies
Several local ablation techniques are used in the treatment of early stage HCC (Table 2). Radiofrequency ablation (RFA) uses thermal energy to induce necrosis and has the most supporting evidence of efficacy.42 In single tumors <3 cm, RFA may achieve 3-year survival rates of 76%58 with a low procedure-related mortality rate of 0.15%.59 A Cochrane meta-analysis compared surgery (average age 51–56 years) with RFA (average age 49–72 years) in early stage HCC and found that there was no difference in all-cause mortality between the two treatment approaches.60 The cancer-related mortality was lower in the surgery group (17.4% versus 37.4%); however, serious adverse events were higher (23.3% versus 1.7%) with resection (odds ratio 17.96, 95% CI 2.28–141.60).60 Microwave ablation (MWA), an alternative treatment that has larger ablation volumes and less affected by vessel proximity, has similar efficacy to RFA.61
Table 2.
Local ablative therapies in patients with localized HCC.
| Study | Survival | Survival in Older adults |
|
|---|---|---|---|
| Radiofrequency ablation (RFA) | Cucchetti et al58 | 3-year OS: 76% | n/a |
| Mirici-Cappa et al19 | mOS: 42 mo | n/a | |
| SURF trial62 | 3-year RFS: surgery 49.8% vs RFA 47.7% (p=0.793) | n/a | |
| Transarterial chemoembolization (TACE) | Llovet et al63 | 2-year survival: 63% vs 27% control (p=0.009) | n/a |
| Lo et al65 | 3-year survival: 26% vs 3% control (p=0.002) | n/a | |
| Zhou et al97 | n/a | mOS: 21 mo (70.4 +/− 4.6 years) |
|
| Mirici-Cappa et al19 | n/a | mOS: 26 mo (74.6 +/− 3.9 years) |
|
| Selective internal radiation therapy (SIRT) | SARAH study67 | mOS: 8.0 mo vs 9.9 mo sorafenib (p=0.18) | n/a |
| Golfieri et al68 | n/a | mOS: 14.5 mo older vs 12.8 mo younger (p=0.942) (74.3 years ((70–87)) |
|
| Stereotactic body radiotherapy (SBRT) | Rajyaguru et al98 | 5-year OS: 29.8% RFA vs 19.3% SBRT (p<0.001) | n/a |
| Teraoka et al69 | n/a | mOS: 52 mo vs NR younger (p=0.27) (79 years (75–93)) |
|
OS = overall survival, mOS = median overall survival, mo = months, vs = versus, NR = not reached, n/a = not available
The Italian Liver Cancer group retrospective series as well as a meta-analysis by Hung, et al, showed similar survival among older patients who underwent RFA when compared to younger adults.19,21 There was no difference in the complication rates between the two groups.21 Most recently, the SURF trial randomized patients with HCC (<3 tumors, <3cm) to surgery versus RFA, which showed 3-year RFS was 49.8% with surgery versus 47.7% with RFA (HR 0.96; 95% CI: 0.72–1.28; p=0.793).62 In this study, all patient were < 80 years old with median age of 68 and 69, respectively.62 Length of hospital stay with surgery was higher than RFA (p<0.01), and no mortality due to the procedures was observed.62 When considering these treatments for older adults with BCLC Stage A HCC, local ablations should be considered due to their safety and efficacy compared to more aggressive surgical approaches.
4.4. Trans-arterial Chemoembolization
Trans-arterial chemoembolization (TACE) is a method of delivering chemotherapy directly to the tumor followed by embolization of the arterial supply to the tumor in order to induce necrosis.63 Trans-arterial embolization (TAE) or “bland embolization” is done without chemotherapy and may be better tolerated in older adults with borderline liver function.64 TACE demonstrated an improved survival over bland embolization63 and over best supportive care65 in two randomized controlled trials (RCTs). Therefore, it is recommended in the BCLC algorithm for intermediate stage (B) HCC with preserved liver function and patent portal vein.42 The median OS in older studies was about 20 months, but with careful selection and optimal delivery, survival is currently up to 30–40 months.2
A retrospective multicenter analysis of 548 patients (27% >75 years) revealed that older patients undergoing TACE did not have increased risk of complications and had similar survival to younger patients.66 A meta-analysis found that the cohort of older patients had an increased 1-year and 3-year survival after TACE compared with the younger cohort, with a similar 5-year survival and complication rate.21 The Italian liver group study also noted similar survival after TACE in older and younger patient groups with a median OS of 26 months in the older group and 5-year survival of 6.4%.19 Based on these data, TACE offers a relatively effective and safe treatment option for older adults with intermediate stage (B) HCC and may be considered in older adults with adequate renal and liver function.
4.5. Selective Internal Radiation Therapy
Selective internal radiation therapy (SIRT) is the injection of radiolabelled Yttrium-90 microspheres into the hepatic artery to induce tumor necrosis.67 Cohort studies have suggested varying tumor response rates from 35 – 90% with SIRT.2,42 The SARAH study, a RCT of intermediate to advanced HCC, compared SIRT with sorafenib, which is a tyrosine kinase inhibitor (TKI) and the first systemic treatment approved for HCC.67 Although survival benefit of SIRT (8.0 months) over sorafenib (9.9 months) was not demonstrated, quality of life was significantly better in the SIRT group.67 A retrospective analysis conducted across eight European centers included 128 older (mean age 74 years) and 197 younger patients who received SIRT between 2003 and 2009.68 SIRT induced similar toxicities in both groups, without any differences detected in survival between the two groups.68 SIRT may be an option for older adults who would otherwise require several TACE procedures because of the size of their lesions or who are ineligible for TACE due to portal vein thrombosis. SIRT decreases the burden of travel on patients and caregivers, does not require a hospital stay, and thus avoids hospital-related complications, such as hospital delirium.
4.6. Stereotactic Body Radiotherapy
Single center studies have suggested that stereotactic body radiotherapy (SBRT) can achieve tumor control rates of >90% in tumors < 5 cm after 12 months.43 In older adults, SBRT appears to have similar outcomes when compared to younger adults based on a single center study analyzing 54 patients ≥75 years and 63 patients <75 years.69 This study found no difference in median OS or adverse events, and the 3-year disease control rate in both groups was 98%, although progression-free survival (PFS) was shorter in the older group.69 SBRT may still be a suitable option when RFA is not possible, such as larger tumors or a challenging location of the tumors.43 Furthermore, given SBRT is non-invasive and does not require anesthesia, SBRT is a feasible option for older adults with comorbidities and decreased functional status.
5.0. Systemic Therapy
The last two years have produced an unprecedented growth in systemic therapy options for HCC (Table 3). In published clinical trials leading to the approval of six first- and second-line targeted agents and immunotherapies, the median age ranged from 62 to 68 years.70–74 Although many of these studies included older adults, few prospective randomized clinical trials have reported subgroup analyses in older adults or investigated therapeutic options for HCC in this specific population.75
Table 3.
Clinical trials of FDA-approved treatments in patients with advanced HCC.
| Median Age (y) (range) |
Overall Survival |
Time to Progression |
Objective Response Rate |
Median Duration of Treatment |
Survival in Older adults |
|
|---|---|---|---|---|---|---|
| First-line | ||||||
| Sorafenib vs Placebo76 | 64.9 (n/a) vs 66.3 (n/a) | 10.7 mo vs 7.9 mo (HR 0.69; 95% CI: 0.55–0.87; p<0.001) | 5.5 mo vs 2.8 mo (p<0.001) | n/a | 5.3 mo | n/a |
| Lenvatinib vs Sorafenib70 (non-inferiority) | 63 (20–88) vs 62 (22–88) [65y- <75y: 30%; 75+: 13%] | 13.6 mo vs 12.3 mo (HR 0.92; 95% CI: 0.79–1.06; p n/a) | 8.9 mo vs 3.7 mo (HR 0.63; 95% CI: 0.53 –0.73; p<0.0001) | 24.1 % vs 9.2% (p<0.0001) | 5.7 mo | mOS: 14.6 mo vs 13.4 mo (HR 0.84; 95% CI: 0.661.07) |
| Second-line | ||||||
| Regorafenib vs placebo71 (tolerated prior sorafenib > 20 days at > 400 mg/day) | 64 (54–71) vs 62 (55–68) | 10.6 mo vs 7.8 mo (HR 0.63; 95% CI: 0.500.79; p<0.0001) | 3.1 mo vs 1.5 mo (HR 0.46; 95% CI: 0.37–0.56; p<0.0001 | 11% vs 4% (p=0.0047) | 3.6 mo | mOS: HR was 0.74 (95% CI: 0.490.87) |
| Cabozantinib vs placebo72 (2nd and 3d line) | 64 (22–86) vs 64 (24–86) | 10.2 vs 8 mo (HR 0.76; 95% CI: 0.63–0.92; p=0.005) | 5.2 mo vs 1.9 mo (HR 0.44; 95% CI: 0.36–0.52; p<0.001) | 4% vs <1% (p=0.009) | 3.8 mo | mOS: HR 0.46 (95% CI: 0.350.59) |
| Ramucirumab vs placebo94 (AFP >400 ng/mL) | 64 (58–73) vs 64 (56–71) | 8.5 mo vs 7.3 mo (HR 0.710; 95% CI: 0.531–0.949; p=0.0199) | 2.8 mo vs 1.6 mo (HR 0.452; 95% CI: 0.339–0.603; p<.0001) | 5% vs 1% (p=0.1697) | 12 weeks | mOS: HR 0.641 (0.4290.957) |
| Nivolumab73 | Expansion phase: 64 (56–70) [65+: 47%] | Not reached; 6-mo OS rate was 83% | 4.1 mo (95% CI: 3.75.5) | 20% | n/a | n/a |
| Pembrolizumab vs placebo88 | (18–91) vs (23–89) | 13.9 mo vs 10.6 mo (HR 0.781; CI 95%: 0.6110.998; p=0.0238) | 3.0 mo vs 2.8 mo (HR 0.718; CI 95%: 0.5700.904; p=0.0022) | 18.3% vs 4.4% (p=0.00007) | n/a | n/a |
Mo = months, y = years, HR = hazard ratio, CI = Confidence interval, vs = versus, n/a = not available.
5.1. TKIs
5.1.1. First-line TKIs
Sorafenib.
Sorafenib is a multi-kinase inhibitor with activity across vascular endothelial growth factor-2 (VEGF-2) and BRAF.43 The SHARP trial established sorafenib as the standard of care in advanced HCC in a multicenter, phase 3, double-blind, placebo-controlled trial.76 A total of 602 patients with advanced HCC and Child-Pugh A cirrhosis were randomized to sorafenib 400 mg twice daily versus placebo.76 The median age in the sorafenib arm was 64.9 years (+/−11.1) and 66.3 years (+/− 10.1) in the placebo arm.76 The median OS was 10.7 months in the sorafenib group and 7.9 months in the placebo group (HR 0.69; 95% CI: 0.55–0.87; p<0.001).76 Higher toxicity, such as diarrhea, weight loss, hand-foot skin reaction, anorexia, and voice changes, was observed with sorafenib compared to placebo (p<0.001).76
The SHARP study did not publish a subgroup analysis on patients 65 years or older; however, several retrospective studies have been reported (Table 4).77–82 These retrospective studies demonstrated that the efficacy of sorafenib is preserved in older adults with HCC with OS, TTP, and PFS either not statistically different from younger adults or with historical controls.77,78,80,82
Table 4.
Retrospective studies of sorafenib in older adults with advanced HCC.
| Author (n=older adults) |
Median Age (years) (range) |
Baseline Characteristics | Overall Survival |
Time to Progression |
Toxicity / Dosing | |
|---|---|---|---|---|---|---|
| Patient | Disease | |||||
| Wong 201182 (n=35) | 73 (70–85) | ECOG 0: 25.7% ECOG 1: 60% DM: 31.4% CVD: 45.7% |
CPA 62.9% CPB: 37.1% BCLC C: 91.4% Cirrhosisetiology: HCV 11.4% HBV 65.7% |
5.32 mo | 2.99 mo | Grade 3–4: 68.6% Dose interruption: 42.9% |
| Di Costanzo 201380 (n=60) | 73 (70–85) | ECOG 0: 81.7% ECOG 1: 18.3% DM: 31.7% CVD: 46.7% Average Albumin: 3.7 |
CPA 90% CPB: 10% BCLC C: 76.7% CLIP <3: 60% Cirrhosisetiology: HCV 78.3% HBV 8.3% Non-viral 8.3% |
16 mo | 12 mo | Grade 3–4: 15.7% |
| Montella 201377 (n=60) | 76 (70–90) | ECOG: n/a DM: 28% CVD: 45% |
CPA 73% CPB: 22% BCLC C: 22% Cirrhosisetiology: HCV 68% HBV 5% EtOH 1% |
10 mo | 7 mo | No Grade 2–4 registered Reduced dose: 81.7% At 2 months: lADLs: 15% improved 15% reduced; ADLs: 9% improved 4% reduced |
| Edeline 201578 (n=51 ) | Median age (range)–n/a 70–74: 53% 75–79: 35% 80+: 12% |
ECOG 1: 49% HTN: 54.9% CCI: 51%, 29.4%, 19.6% |
CPB: 7.7% Cirrhosisetiology: HCV 5.9% HBV 5.9% EtOH 54.9% NASH 35.3% |
12.6 mo | 5.6 mo | Grade 3+: 51% Reduced dose: 58.8% Hospitalization: 13.7% |
| Willet 201781 (n=51 ) | 78 (75–92) | ECOG 0: 49% ECOG 1: 49% HTN: 68.6% Average Albumin: 3.6 Median CCI: 14 |
CPA 84.3% CPB: 3.9% BCLC C: 74.5% Cirrhosisetiology: HCV 5.9% HBV 5.9% EtOH 54.9% NASH 35.3% |
15 mo | n/a | Grade 3–4: 66.7% Reduced dose: 41.2% Discontinuation due to toxicity: 60.8% |
| Arora 201879 (n=31 ) | 70 (65–93) | ECOG 0: 38.7% ECOG 1: 51.6% Hispanic: 74% |
CPA 71% CPB: 29% BCLC C: 86.1% Cirrhosisetiology: HCV 61% EtOH 52% |
13.5 mo | 6.2 mo | Grade 3: Fatigue (9.7%) HFS (16.1%) Reduced dose: 70% |
n = number, ECOG = Eastern Cooperative Oncology Group, DM = Diabetes mellitus, CVD = cardiovascular disease, CPA = Child-Pugh A cirrhosis, CPB = Child-Pugh B cirrhosis, BCLC = Barcelona Clinic Liver Cancer, CLIP = Cancer of the Liver Italian Program, HCV = hepatitis C virus, HBC = hepatitis B virus, mo = months, n/a = not available, EtOH = alcoholic, IADLs = instrumental activities of daily living, ADLs = activities of daily living, HTN = hypertension, CCI = Charlson Comorbidity Index, NASH = non-alcoholic steatohepatitis, HFS = hand-foot syndrome.
Lenvatinib.
Ten years after the approval of sorafenib, lenvatinib, an oral multi-kinase inhibitor that has additional targets including VEGF1–3, fibroblast growth factor (FGFR) 1–4, platelet-derived growth factor (PDGF) receptor alpha, RET and KIT, was approved for first-line treatment of HCC.70 The REFLECT trial, an open-label, phase 3, multicenter, non-inferiority trial recruited patients with unresectable HCC, with a median age of 62 years (20–88), with 30% of patients 65 to < 75 and 13% were 75+ years of age.70 Patients received oral lenvatinib (12 mg/day for bodyweight ≥60 kg or 8 mg/day for bodyweight <60 kg) or sorafenib 400 mg twice daily.70 The primary endpoint of this study was OS, which showed non-inferiority of lenvatinib when compared to sorafenib.70 The subgroup analysis showed similar benefit from lenvatinib in patients <65 years versus 65+ years.70 Although not powered for response rate, lenvatinib (24.1%) showed a higher response rate than sorafenib (9.2%) that was statistically significant (p<0.0001).70
The REFLECT study reported a higher rate of hand-foot syndrome among patients treated with sorafenib, while higher rates of hypertension was observed with lenvatinib.76 Although subgroup analyses in regards to age and adverse events are not reported in HCC, studies of lenvatinib in older adults with thyroid cancer showed that younger lenvatinib-treated patients experienced significantly longer time to first dose reduction (3.7 versus 1.5 months) and lower proportion of grade ≥ 3 treatment-related adverse events (67% v 89%; p<0.001) compared with older patients.83
In summary, at this time, there are no predictive biomarkers to enable a more personalized choice between the two drugs. Clinicians must consider toxicity profiles when selecting appropriate treatment for older adults with HCC, along with careful evaluation of a patient’s comorbidities (i.e., uncontrolled HTN, coronary artery disease, cerebrovascular disease, etc.) that may increase risk of toxicities. Therefore, in older adults with HCC, consider starting at a reduced dose of sorafenib (400 mg daily) or lenvatinib (8 mg daily) with up-titration to full dose as tolerated and close monitoring (i.e., every 1–2 weeks) due to increased toxicity, especially in vulnerable or frail older adults.
5.1.2. Second-line TKIs
Regorafenib.
Regorafenib is an oral pan-kinase inhibitor that blocks both angiogenic (VEGF-1, VEGF-2) and oncogenic (KIT, RET and BRAF) kinases.84 Regorafenib is now a therapeutic option in HCC patients with Child-Pugh A cirrhosis that have progressed on first-line therapy with sorafenib.43,71 Efficacy of regorafenib was demonstrated in the phase III RESORCE trial in which patients with advanced HCC and with Child-Pugh A cirrhosis were randomized to regorafenib or placebo as second-line treatment.71 Regorafenib had a statistically significant median OS of 10.6 months versus 7.8 months with placebo (HR 0.63; 95% CI: 0.50–0.79; p<0.0001).71 The median age was 64 years (54–71) in the regorafenib arm versus 62 years (55–68) in the placebo arm.71 For patients 65+ years, the HR for median OS was 0.74 (95% CI: 0.49–0.87), suggesting that older fit patients derived benefit from regorafenib.71
All patients on the study had tolerated sorafenib prior to enrolling on this study.71 Sixty-eight percent of regorafenib-treated patients and 54% of placebo treated patients required dose reductions due to drug toxicity and discontinuation of the treatment was seen in 10% and 4% respectively.71 The most common grade 3/4 treatment-related adverse events were hypertension (15%), palmar-plantar erythema (13%), fatigue (9%) and diarrhea (3%).71 Toxicity profile in older adults with HCC has not been published. The ReDOS study demonstrated improved tolerability with a dose-escalation strategy in metastatic colorectal cancer,85 which should be further investigated in HCC. Until more data is available, regorafenib may be offered to older patients who tolerated first-line sorafenib, with a consideration to start regorafenib at 80 mg and titrating upward as tolerated.
Cabozantinib.
Cabozantinib is an inhibitor of the MET, AXL and VEGF receptors 1, 2 and 3.86 It is a second- or third-line systemic therapeutic option for patients with Child-Pugh A cirrhosis with advanced HCC.72 The double-blind, placebo-controlled phase III CELESTIAL trial led to its FDA approval in January 2019.72 Median OS was 10.2 versus 8 months (HR 0.76; 95% CI: 0.63–0.92; p=0.005) and median PFS 5.2 months versus 1.9 months (HR 0.44; 95% CI: 0.36–0.52; p<0.001), favoring cabozantinib.72 Median age in the cabozantinib arm was 64 years (22–86) versus 64 years (24–86) in the placebo arm.72
An exploratory sub-group analysis showed that in patients 65+ years, there was benefit in terms of PFS (HR 0.74; 95% CI: 0.56–0.97) and OS (HR 0.46; 95% CI: 0.35–0.59), both favoring cabozantinib over placebo.72 While 62% of patients required a dose reduction, while 16% discontinued cabozantinib due to toxicity.72 Grade 3/4 toxicity occurred in 68% with the main adverse events being palmar-plantar erythema (17%), hypertension (16%) and diarrhea (10%).72 Toxicity data specifically in older adults has not yet been reported. However, it is reasonable to start at 40 mg daily and then titrate up to full dose of 60 mg as tolerated, with close monitoring for toxicity.
5.2. Immunotherapy
Nivolumab.
Nivolumab is a fully human IgG4 monoclonal antibody directed against programmed cell death protein 1 (PD-1), blocking the PD-1 interaction with PD-ligand 1 (PD-L1) and PD-ligand (PD-L2), which results in immune activation.87 Safety and efficacy of nivolumab as a monotherapy in advanced HCC was investigated in the CheckMate 040, a phase I/II non-randomized trial in patients who were sorafenib naïve, sorafenib refractory or intolerant.73 Globally, 262 patients were enrolled, with a median age of 64 years in the dose-expansion phase (the primary endpoint was ORR).73 Forty-seven percent of patients in the expansion phases were older than 65 years.73 However, there were no age-specific data on toxicity or efficacy published for this trial.
Treatment with nivolumab resulted in ORR of 20% in dose expansion phase (nivolumab at 3 mg/kg every 2 weeks), irrespective of the line of therapy and etiology.73 The 6-month OS rate was 83% in the dose-expansion phase.73 Additional analyses showed a median duration of response of 17 months in a sorafenib-naïve group (n=80) and 19 months in patients previously treated with sorafenib (n=182).73 Nivolumab showed a manageable safety profile, similar to prior reports in other diseases.73 This led to the FDA granting accelerated approval for the use of nivolumab in patients with advanced HCC previously treated with sorafenib. Building on the favorable results of CheckMate 040, a randomized phase III trial (CheckMate 459) is underway to investigate nivolumab versus sorafenib as first-line treatment in patients with advanced HCC (NCT02576509).
Pembrolizumab.
More recently, pembrolizumab, another fully human monoclonal IgG4 antibody targeting PD-1 has been investigated in HCC. The single-arm, multi-center phase II trial KEYNOTE-224 assessed safety and efficacy of pembrolizumab in 104 patients with advanced HCC, who received first-line sorafenib.74 Based on KEYNOTE-224, the FDA granted accelerated approval to pembrolizumab for patients with advanced HCC who have been previously treated with sorafenib. To confirm these findings, a large randomized phase 3 trial (KEYNOTE-240) aimed to assess pembrolizumab versus placebo as a second-line therapy in advanced HCC, with a median age of 67 year (18–91) and 68 years (23–89), respectively.88 The primary endpoints were median OS and median PFS (with a pre-specified statistical significance met if p=0.0174 and 0.0020, respectively).88 Median OS was 13.9 months with pembrolizumab versus 10.6 months with placebo (HR 0.781; CI 95%: 0.611–0.998; p=0.0238); and median PFS was 3.0 versus 2.8 months, respectively (HR 0.718; CI 95%: 0.570–0.904; p=0.0022).88 ORR was 18.3 % with pembrolizumab versus 4.4 % with placebo (p=0.00007).88 Toxicities were similar to most immune checkpoint inhibitors: elevated liver enzymes, fatigue, nausea, diarrhea and decreased appetite.88 Although the study did not meet the pre-specified statistical plan for significance, the magnitude of the hazard ratio for survival demonstrates benefit.88 Therefore, as studies with pembrolizumab continue to mature, this continues to be another option for patients with HCC.
As we await final results of larger studies, efficacy results of immune checkpoint inhibitors are encouraging. Biomarkers, such as PDL-1, are currently not utilized to determine which patients may benefit from immune checkpoint inhibitors.89 Until this is further validated, immune checkpoint inhibitors may be a good option for older patients intolerant to TKIs or for older adults in whom additional oral medications may be challenging (polypharmacy, noncompliance, cognitive deficits, etc.). Current data from pivotal studies across different tumor types do not show a significant difference in efficacy or toxicity rates in older patients when compared to younger adults, but toxicity management may need to be more tailored in older adults.90“93 Future studies with immune checkpoint inhibitors should incorporate subgroup analyses of older adults in regards to efficacy, safety, and quality of life.
5.3. Ramucirumab
Ramucirumab, a human IgG1 monoclonal antibody that inhibits ligand activation of VEGFR2, is approved for second-line treatment in HCC. The REACH-2 is the first positive phase III trial done in a biomarker-selected patient population (AFP of ≥400 ng/mL) previously received sorafenib.94 Median age in this study was 63 years (55–71).94 Median OS was better with ramucirumab versus placebo (8.5 months versus 7.3 months (HR 0.710; 95% CI: 0.531–0.949; p=0.0199).94 Ramucirumab was well-tolerated, as most common adverse events were fatigue (27%), peripheral edema (25%), hypertension (25%), and decreased appetite (23%).94 Although age-specific survival and toxicity are not available at this time, this may be a good option for older adults with HCC who develop or are at risk for toxicities from TKIs, have difficulty with oral home medication regimens or have a contraindication to immunotherapy.
5.4. Clinical trials in HCC
The efficacy of PD-1/PD-L1 inhibitors may be enhanced if used in combination with other systemic therapies. At present, several clinical trials are ongoing with various combinations of PD-1 inhibitors and other immune checkpoint inhibitors, molecular targeted agents and local therapies. Given the increase in the rates of older patients with HCC, and the unique challenges their treatment poses, it is essential for studies to incorporate CGA and focus on subgroup analyses of older adults in regards to efficacy, adverse events, and patient reported outcomes that are specific to the older patient population.
6.0. Incorporation of CGA into the care of older adults with HCC
To the best of our knowledge, there are limited prospective trials investigating systemic therapies for HCC specifically in the older adult population. The development of effective therapeutic strategies in older adults with HCC represents an unmet need, particularly given the rising incidence of HCC in those aged 75 to 85.95 As outlined in the large clinical trials evaluating systemic therapies, patient stratification was exclusively based on chronological age, without including any form of CGA. Thus, we need to advocate for older adults to be enrolled onto clinical trials, as well as work with pharmaceutical industries to design interventional studies that incorporate CGA.
We do see a trend over the last ten years that subgroup analyses deliberately assess efficacy in older adults; however, we cannot stop there. Clinical trials should also report and publish safety data and patient reported outcomes in older adults, so we can further understand implications of these treatments in this population. Furthermore, since larger studies mostly include very fit older adults without significant comorbidities and organ dysfunction, we need to design studies with CGA dedicated to all older patients, including vulnerable/frail. For example, the GO2 phase III trial was designed to find the optimal dosing of oxaliplatin/capecitabine in frail and older adults with advanced gastroesophageal cancer.96 In addition to demonstrating similar survival with lower doses, this study evaluated toxicity and quality of life, showing that lower doses correlated with less toxicity and improved quality of life.96 This study is one example of the feasibility and relevance of novel endpoints, such as toxicity and quality of life, in studies of vulnerable/frail older adults, which may be more meaningful in older adults with gastrointestinal cancers.
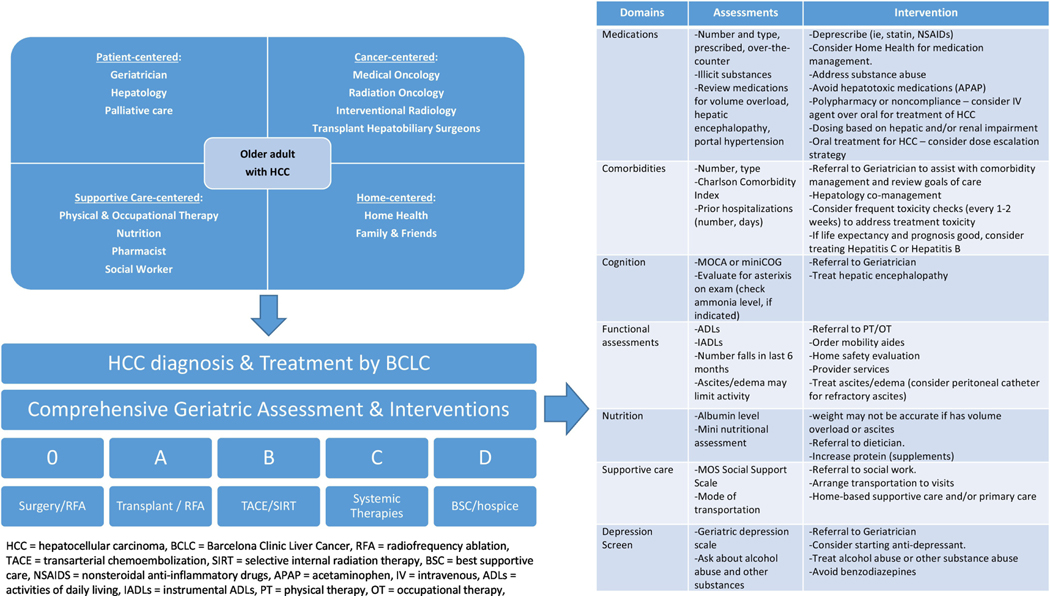
In older adults, the competing comorbid condition of cirrhosis makes HCC a challenging disease. Therefore, along with the geriatric assessment and patient preferences, we must also consider disease-related prognostic scores, such CLIP,44 which will help us determine if the patient would benefit from cancer-directed treatments. Therefore, the multi-disciplinary team-based approach needs to be expanded beyond the oncologic clinicians we typically included in traditional multi-disciplinary tumor boards (Figure 1). Further, as cancer specialists may have time constraints or lack of specialized training in geriatrics, screening tools such as the G8, can help us identify patients who may be vulnerable or frail, and would benefit from a comprehensive assessment by a geriatrician or geriatric oncologist. These screening tools also help to identify domains that need direct interventions in older adults with HCC. For example, cirrhosis is a complex competing comorbidity, often with sequelae of sarcopenia, hypoalbuminemia, ascites, gastrointestinal bleeding, and encephalopathy.57 In addition to cancer treatments, these need to be co-managed by the entire treatment team as well. Therefore, incorporation of such assessments into the treatment algorithm for HCC is necessary for directed interventions (Figure 1).
Figure 1.
Revised treatment paradigm for older adults with HCC
7.0. Conclusions
Generally, older adults with HCC and good performance status seem to tolerate and benefit from standard treatments, including surgery, transplant, local ablative therapies, and systemic treatment, often with multi-modality treatment plans. Geriatric screening tools and CGA need to be prospectively studied as well as incorporated into clinical trials, so we can have a better understanding of how to predict outcomes and safety in older patients whether they be fit, vulnerable or frail. Such clinical interventions and research endeavors are essential to optimize and personalize care for our growing population of older adults with HCC.
Acknowledgements:
SPA: NIH CA054174, NIA AG044271
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest and Disclosures:
Conflicts of interest: all authors: none
Disclosures:
SPA: Advisory Board for Exelixis, Speakers Bureau for Bayer and Exelixis; Research: Beigene, Caris, Halozyme, Lexicon, Lilly.
GL: No disclosures
SC: Research: Roche
RFD: Advisory Board and consulting for Exelixis
DO: No disclosures
MGR: No disclosures
GTM: No disclosures
DD: No disclosures
ED: Advisory Board: Boston Medical; Research funding: Pfizer, Lilly
References:
- 1.Waller LP, Deshpande V, Pyrsopoulos N: Hepatocellular carcinoma: A comprehensive review. World journal of hepatology 7:2648–2663, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Reig M, Bruix J: Hepatocellular carcinoma. Lancet 391:1301–1314, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Center MM, Jemal A: International trends in liver cancer incidence rates. Cancer Epidemiol Biomarkers Prev 20:2362–8, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Ghouri YA, Mian I, Rowe JH: Review of hepatocellular carcinoma: Epidemiology, etiology, and carcinogenesis. J Carcinog 16:1, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang JD, Altekruse SF, Nguyen MH, et al. : Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer 123:81–89, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asahina Y, Tsuchiya K, Tamaki N, et al. : Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology 52:518–27, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Ryerson AB, Eheman CR, Altekruse SF, et al. : Annual Report to the Nation on the Status of Cancer, 1975–2012, featuring the increasing incidence of liver cancer. Cancer 122:1312–37, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SEER: Surveillance, Epidemiology, and End Results Program. Incidence-SEER 9 Regs Research Data with Delay-Adjustment, Malignant Only, Nov. 2016 Sub (1975–2014) <Katrina/Rita Population Adjustments Bethesda, MD, National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch, 2017 [Google Scholar]
- 9.Lok AS, McMahon BJ: Chronic hepatitis B. Hepatology 45:507–39, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Lavanchy D: Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11:97–107, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2018. CA Cancer J Clin 68:7–30, 2018 [DOI] [PubMed] [Google Scholar]
- 12.Su TH, Tseng TC, Kao JH: HCC risk in patients with HBV-related cirrhosis receiving nucleos(t)ide analogues therapy: Is HCC prevented or delayed? Hepatology 67:1634–1635, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Baffy G, Brunt EM, Caldwell SH: Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol 56:1384–91, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Zoller H, Tilg H: Nonalcoholic fatty liver disease and hepatocellular carcinoma. Metabolism 65:1151–60, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Dongiovanni P, Romeo S, Valenti L: Hepatocellular carcinoma in nonalcoholic fatty liver: role of environmental and genetic factors. World journal of gastroenterology 20:12945–12955, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Serag HB: Hepatocellular carcinoma. N Engl J Med 365:1118–27, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Schlesinger S, Aleksandrova K, Pischon T, et al. : Diabetes mellitus, insulin treatment, diabetes duration, and risk of biliary tract cancer and hepatocellular carcinoma in a European cohort. Ann Oncol 24:2449–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welzel TM, Graubard BI, Quraishi S, et al. : Population-attributable fractions of risk factors for hepatocellular carcinoma in the United States. Am J Gastroenterol 108:1314–21, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mirici-Cappa F, Gramenzi A, Santi V, et al. : Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut 59:387–396, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Mizuguchi T, Kawamoto M, Meguro M, et al. : Impact of aging on morbidity and mortality after liver resection: a systematic review and meta-analysis. Surg Today 45:259–70, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Hung AK, Guy J: Hepatocellular carcinoma in the elderly: Meta-analysis and systematic literature review. World J Gastroenterol 21:12197–210, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez Gavara C, Esposito F, Gurusamy K, et al. : Liver transplantation in elderly patients: a systematic review and first meta-analysis. HPB (Oxford) 21:14–25, 2019 [DOI] [PubMed] [Google Scholar]
- 23.Aduen JF, Sujay B, Dickson RC, et al. : Outcomes after liver transplant in patients aged 70 years or older compared with those younger than 60 years. Mayo Clin Proc 84:973–8, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson GC, Quillin RC 3rd, Wima K, et al. : Is liver transplantation safe and effective in elderly (>70 years) recipients? A case-controlled analysis. HPB (Oxford) 16:1088–94, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soto-Perez-de-Celis E, Li D, Yuan Y, et al. : Functional versus chronological age: geriatric assessments to guide decision making in older patients with cancer. Lancet Oncol 19:e305–e316, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Loh KP, Soto-Perez-de-Celis E, Hsu T, et al. : What Every Oncologist Should Know About Geriatric Assessment for Older Patients With Cancer: Young International Society of Geriatric Oncology Position Paper. J Oncol Pract 14:85–94, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamaker ME, Wildes TM, Rostoft S: Time to Stop Saying Geriatric Assessment Is Too Time Consuming. J Clin Oncol 35:2871–2874, 2017 [DOI] [PubMed] [Google Scholar]
- 28.Repetto L, Fratino L, Audisio RA, et al. : Comprehensive geriatric assessment adds information to Eastern Cooperative Oncology Group performance status in elderly cancer patients: an Italian Group for Geriatric Oncology Study. J Clin Oncol 20:494–502, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Jolly TA, Deal AM, Nyrop KA, et al. : Geriatric assessment-identified deficits in older cancer patients with normal performance status. Oncologist 20:379–85, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klepin HD, Geiger AM, Tooze JA, et al. : Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood 121:4287–94, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamaker ME, Vos AG, Smorenburg CH, et al. : The value of geriatric assessments in predicting treatment tolerance and all-cause mortality in older patients with cancer. Oncologist 17:1439–49, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giantin V, Valentini E, Iasevoli M, et al. : Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. J Geriatr Oncol 4:208–17, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Hamaker ME, Schiphorst AH, ten Bokkel Huinink D, et al. : The effect of a geriatric evaluation on treatment decisions for older cancer patients--a systematic review. Acta Oncol 53:289–96, 2014 [DOI] [PubMed] [Google Scholar]
- 34.Decoster L, Kenis C, Van Puyvelde K, et al. : The influence of clinical assessment (including age) and geriatric assessment on treatment decisions in older patients with cancer. J Geriatr Oncol 4:235–41, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Mohile SG, Velarde C, Hurria A, et al. : Geriatric Assessment-Guided Care Processes for Older Adults: A Delphi Consensus of Geriatric Oncology Experts. J Natl Compr Canc Netw 13:1120–30, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Extermann M, Boler I, Reich RR, et al. : Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118:3377–86, 2012 [DOI] [PubMed] [Google Scholar]
- 37.Hurria A, Mohile S, Gajra A, et al. : Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults With Cancer. J Clin Oncol 34:2366–71, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurria A, Togawa K, Mohile SG, et al. : Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29:3457–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology Summary. J Oncol Pract 14:442–446, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohile SG, Dale W, Somerfield MR, et al. : Practical Assessment and Management of Vulnerabilities in Older Patients Receiving Chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol 36:2326–2347, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wildiers H, Heeren P, Puts M, et al. : International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol 32:2595–603, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol 69:182–236, 2018 [DOI] [PubMed] [Google Scholar]
- 43.Vogel A, Cervantes A, Chau I, et al. : Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv238–iv255, 2018 [DOI] [PubMed] [Google Scholar]
- 44.Kudo M, Chung H, Osaki Y: Prognostic staging system for hepatocellular carcinoma (CLIP score): its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J Gastroenterol 38:207–15, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ni JY, Liu SS, Xu LF, et al. : Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: A meta-analysis. Journal of Cancer Research and Clinical Oncology 139:653–659, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tan E, Sandroussi C: Liver resection in elderly patients over the age of 70 with primary liver cancer: A retrospective analysis. Journal of Clinical Oncology 37:e15647-e15647, 2019 [Google Scholar]
- 47.Kaibori M, Ishizaki M, Matsui K, et al. : Geriatric assessment as a predictor of postoperative complications in elderly patients with hepatocellular carcinoma. Langenbecks Arch Surg 401:205–14, 2016 [DOI] [PubMed] [Google Scholar]
- 48.Martin P, DiMartini A, Feng S, et al. : Evaluation for Liver Transplantation in Adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation, 2014, pp 1144–1165 [DOI] [PubMed] [Google Scholar]
- 49.McCaughan GW, Munn SR: Liver transplantation in Australia and New Zealand. Liver Transpl 22:830–8, 2016 [DOI] [PubMed] [Google Scholar]
- 50.Keswani RN, Ahmed A, Keeffe EB: Older age and liver transplantation: a review. Liver Transpl 10:957–67, 2004 [DOI] [PubMed] [Google Scholar]
- 51.Collins BH, Pirsch JD, Becker YT, et al. : Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation 70:780–3, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Bilbao I, Dopazo C, Lazaro JL, et al. : Our experience in liver transplantation in patients over 65 yr of age. Clin Transplant 22:82–8, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Levy MF, Somasundar PS, Jennings LW, et al. : The elderly liver transplant recipient: a call for caution. Ann Surg 233:107–13, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Egeli T, Unek T, Agalar C, et al. : Survival Outcomes After Liver Transplantation in Elderly Patients: A Single-Center Retrospective Analysis. Transplant Proc 51:1143–1146, 2019 [DOI] [PubMed] [Google Scholar]
- 55.Sahai T, Cholankeril G, Cholankeril R, et al. : Overall survival of liver transplantation for hepatocellular carcinoma in the elderly. Journal of Clinical Oncology 34:e15639-e15639, 2016 [Google Scholar]
- 56.Haugen CE, McAdams-DeMarco M, Holscher CM, et al. : Multicenter Study of Age, Frailty, and Waitlist Mortality Among Liver Transplant Candidates. Ann Surg, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai JC, Feng S, Terrault NA, et al. : Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 14:1870–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cucchetti A, Piscaglia F, Cescon M, et al. : Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol 59:300–7, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Bertot LC, Sato M, Tateishi R, et al. : Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. European Radiology 21:2584–96, 2011 [DOI] [PubMed] [Google Scholar]
- 60.Majumdar A, Roccarina D, Thorburn D, et al. : Management of people with early-or very early-stage hepatocellular carcinoma: an attempted network meta-analysis. Cochrane Database Syst Rev 3:CD011650, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Facciorusso A, Di Maso M, Muscatiello N: Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: A systematic review and meta-analysis. Int J Hyperthermia 32:339–44, 2016 [DOI] [PubMed] [Google Scholar]
- 62.Izumi N, Hasegawa K, Nishioka Y, et al. : A multicenter randomized controlled trial to evaluate the efficacy of surgery vs. radiofrequency ablation for small hepatocellular carcinoma (SURF trial). Journal of Clinical Oncology 37:4002–4002, 2019 [Google Scholar]
- 63.Llovet JM, Real MI, Montana X, et al. : Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–9, 2002 [DOI] [PubMed] [Google Scholar]
- 64.Tsochatzis EA, Fatourou E, O’Beirne J, et al. : Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol 20:3069–77, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lo CM, Ngan H, Tso WK, et al. : Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology 35:1164–71, 2002 [DOI] [PubMed] [Google Scholar]
- 66. Cohen MJ, Levy I, Barak O, et al. : 1030 TRANS-ARTERIAL CHEMOEMBOLIZATION (TACE) IS SAFE AND EFFICIENT FOR ELDERLY PATIENTS WITH ADVANCED HEPATOCELLULAR CARCINOMA (HCC): RESULTS FROM AN INTERNATIONAL META-DATABASE. Journal of Hepatology 56:3403, 2012 [Google Scholar]
- 67.Vilgrain V, Pereira H, Assenat E, et al. : Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 18:1624–1636, 2017 [DOI] [PubMed] [Google Scholar]
- 68.Golfieri R, Bilbao JI, Carpanese L, et al. : Comparison of the survival and tolerability of radioembolization in elderly vs. younger patients with unresectable hepatocellular carcinoma. J Hepatol 59:753–61, 2013 [DOI] [PubMed] [Google Scholar]
- 69.Teraoka Y, Kimura T, Aikata H, et al. : Clinical outcomes of stereotactic body radiotherapy for elderly patients with hepatocellular carcinoma. Hepatol Res 48:193–204, 2018 [DOI] [PubMed] [Google Scholar]
- 70.Kudo M, Finn RS, Qin S, et al. : Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase S non-inferiority trial. Lancet S91:1163–1173, 2018 [DOI] [PubMed] [Google Scholar]
- 71.Bruix J, Qin S, Merle P, et al. : Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase S trial. Lancet 389:56–66, 2017 [DOI] [PubMed] [Google Scholar]
- 72.Abou-Alfa GK, Meyer T, Cheng AL, et al. : Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med 379:54–63, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Khoueiry AB, Sangro B, Yau T, et al. : Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389:2492–2502, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu AX, Finn RS, Edeline J, et al. : Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 19:940–952, 2018 [DOI] [PubMed] [Google Scholar]
- 75.Arellano LM, Arora SP: Systemic Treatment of Advanced Hepatocellular Carcinoma in Older Adults. J Nat Sci 4, 2018 [PMC free article] [PubMed] [Google Scholar]
- 76.Llovet JM, Ricci S, Mazzaferro V, et al. : Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–90, 2008 [DOI] [PubMed] [Google Scholar]
- 77.Montella L, Addeo R, Cennamo G, et al. : Sorafenib in elderly patients with advanced hepatocellular carcinoma: a case series. Oncology 84:265–72, 2013 [DOI] [PubMed] [Google Scholar]
- 78.Edeline J, Crouzet L, Le Sourd S, et al. : Sorafenib use in elderly patients with hepatocellular carcinoma: caution about use of platelet aggregation inhibitors. Cancer Chemother Pharmacol 75:215–9, 2015 [DOI] [PubMed] [Google Scholar]
- 79.Arora SP, Ketchum NS, Gelfond J, et al. : Comparative efficacy and safety of sorafenib in elderly versus non-elderly patients with advanced hepatocellular carcinoma (HCC) with varying liver dysfunction. Journal of Clinical Oncology 36:430–430, 2018. 29227726 [Google Scholar]
- 80.Di Costanzo GG, Tortora R, De Luca M, et al. : Impact of age on toxicity and efficacy of sorafenib-targeted therapy in cirrhotic patients with hepatocellular carcinoma. Med Oncol 30:446, 2013 [DOI] [PubMed] [Google Scholar]
- 81.Williet N, Clavel L, Bourmaud A, et al. : Tolerance and outcomes of sorafenib in elderly patients treated for advanced hepatocellular carcinoma. Dig Liver Dis 49:1043–1049, 2017 [DOI] [PubMed] [Google Scholar]
- 82.Wong H, Tang YF, Yao TJ, et al. : The outcomes and safety of single-agent sorafenib in the treatment of elderly patients with advanced hepatocellular carcinoma (HCC). Oncologist 16:1721–8, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brose MS, Worden FP, Newbold KL, et al. : Effect of Age on the Efficacy and Safety of Lenvatinib in Radioiodine-Refractory Differentiated Thyroid Cancer in the Phase III SELECT Trial. J Clin Oncol 35:2692–2699, 2017 [DOI] [PubMed] [Google Scholar]
- 84.Wilhelm SM, Dumas J, Adnane L, et al. : Regorafenib (BAY 73–4506): a new oral multikinase inhibitor of angiogenic, stromal and oncogenic receptor tyrosine kinases with potent preclinical antitumor activity. Int J Cancer 129:245–55, 2011 [DOI] [PubMed] [Google Scholar]
- 85.Bekaii-Saab TS, Ou FS, Ahn DH, et al. : Regorafenib dose-optimisation in patients with refractory metastatic colorectal cancer (ReDOS): a randomised, multicentre, open-label, phase 2 study. Lancet Oncol, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yakes FM, Chen J, Tan J, et al. : Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 10:2298–308, 2011 [DOI] [PubMed] [Google Scholar]
- 87.Guo L, Zhang H, Chen B: Nivolumab as Programmed Death-1 (PD-1) Inhibitor for Targeted Immunotherapy in Tumor. J Cancer 8:410–416, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finn RS, Ryoo B-Y, Merle P, et al. : Results of KEYNOTE-240: phase 3 study of pembrolizumab (Pembro) vs best supportive care (BSC) for second line therapy in advanced hepatocellular carcinoma (HCC). Journal of Clinical Oncology 37:4004–4004, 2019 [Google Scholar]
- 89.Chen CL, Pan QZ, Zhao JJ, et al. : PD-L1 expression as a predictive biomarker for cytokine-induced killer cell immunotherapy in patients with hepatocellular carcinoma. Oncoimmunology 5:e1176653, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaultier De Saint Basile H, Poisson C, Arrondeau J, et al. : [Efficacy and toxicity of immune checkpoint inhibitors in elderly patients - 5th edition of the congress of pharmacology of anticancer drugs]. Bull Cancer 105:1202–1208, 2018 [DOI] [PubMed] [Google Scholar]
- 91.Kanesvaran R, Cordoba R, Maggiore R: Immunotherapy in Older Adults With Advanced Cancers: Implications for Clinical Decision-Making and Future Research. American Society of Clinical Oncology Educational Book:400–414, 2018 [DOI] [PubMed] [Google Scholar]
- 92.Bhandari S, Gill AS, Perez CA, et al. : Management of immunotherapy toxicities in older adults. Semin Oncol 45:226–231, 2018 [DOI] [PubMed] [Google Scholar]
- 93.Muchnik E, Loh KP, Strawderman M, et al. : Immune Checkpoint Inhibitors in Real-World Treatment of Older Adults with Non-Small Cell Lung Cancer. J Am Geriatr Soc 67:905–912, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu AX, Kang YK, Yen CJ, et al. : Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 20:282–296, 2019 [DOI] [PubMed] [Google Scholar]
- 95.Altekruse SF, McGlynn KA, Reichman ME: Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol 27:1485–91, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hall PS, Swinson D, Waters JS, et al. : Optimizing chemotherapy for frail and elderly patients (pts) with advanced gastroesophageal cancer (aGOAC): The GO2 phase III trial. Journal of Clinical Oncology 37:4006–4006, 2019 [Google Scholar]
- 97.Zhou Y: Drug-eluting bead transarterial chemoembolization is efficient and well-tolerated in treating elderly Chines hepatocellular carcinoma patients. Int J Clin Exp Pathol 11:4867–4878, 2018 [PMC free article] [PubMed] [Google Scholar]
- 98.Rajyaguru DJ, Borgert AJ, Smith AL, et al. : Radiofrequency Ablation Versus Stereotactic Body Radiotherapy for Localized Hepatocellular Carcinoma in Nonsurgically Managed Patients: Analysis of the National Cancer Database. J Clin Oncol 36:600–608, 2018 [DOI] [PubMed] [Google Scholar]