Abstract
Neuroglia represent a diverse population of non-neuronal cells in the nervous systems, be that peripheral, central, enteric or autonomic nervous system. Arguably, these cells represent about half of the volume of the human brain. This volumetric ratio, and by extension glia to neurone ratio, not only widely differ depending on the size of the animal species brain and its positioning on the phylogenetic tree, but also vary between the regions of an individual brain. Neuroglia derived from a dual origin (ectoderm and mesodermal) and in an assorted morphology, yet their functional traits can be mainly classified into being keepers of homeostasis (water, ions, neurotransmitters, metabolites, fuels, etc.) and defenders (e.g., against microbial organisms, etc.) of the nervous system. As these capabilities go awry, neuroglia ultimately define their fundamental role in most, if not, all neuropathologies. This concept presented in this chapter serves as a general introduction into the world of neuroglia and subsequent topics covered by this book.
Keywords: Neuroglia, Origin, Morphology, Function, Homeostasis, Defence, Physiology, Pathophysiology
1.1. The Birth of the Concept of Homoeostatic Neuroglia
The complexity of the human brain is remarkable: a population of more than 200 billion (i.e. 2 × 1011) neural cells (neurones and neuroglia) is packed within a limited volume (average human brain occupies 1200–1400 cm3). These neural cells form complex networks, connected through 15–20 trillions of chemical and electrical synapses that provide for computing power of this organ. The logistical support underlying this highly complex organ is provided by a specific class of cells known as neuroglia.
The concept of connective tissue of the nervous system emerged in the nineteenth century [16, 47]; this concept was initially formalised by Rudolf Virchow who introduced the term neuroglia in the 1850s [100, 101]. According to Virchow the neuroglia was ‘…connective substance that forms in the brain, in the spinal cord and in the higher sensory nerves a type of putty (neuroglia), in which the nervous elements are embedded…’ [100]. Prominent neuroanatomists of the second half of the nineteenth century characterised the cellular nature of glia in great detail, and described many types of glial cells [16]. At the same time numerous theories have considered the functional role of neuroglia in the brain homeostasis, nutritional support, regulation of blood flow, sleep and conscience, as well as in neuropathology [6, 29, 30, 72, 79]. The first major type of glia, the astroglia, has been defined in 1895, when Michal von Lenhossék suggested to name a sub-population of parenchymal glia astrocytes, star-like cells (from Greek αστρoν κυτoς). At the same time the parenchymal glia was also sub-classified into protoplasmic (grey matter) and fibrous (white matter) cells [6]. The myelinating cells of the central nervous system (CNS) were first drawn by the Scottish pathologist William Ford Robertson [74, 75], and subsequently Pío del Río Hortega named them oligodendrocytes and recognised their myelinating function [24]. It was also Pío Del Río Hortega who identified and named microglia as the defensive cellular elements of the CNS, by demonstrating that these cells undergo remarkable metamorphosis in pathology and suggesting their role as ‘garbage collectors’ [21–23]. Finally, in the 1980s the fourth type of neuroglia, the NG2 glia (also known as oligodendrocyte progenitor cells or polydendrocytes), was discovered by William Stallcup and colleagues, after they developed an antibody to a chondroitin sulphate proteoglycan, dubbed NG2 [88]. Based on their developmental origin (neuroepithelial or mesodermal), neuroglia of the CNS have been classified as macroglia (astrocytes, oligodendrocytes, NG2) and microglia, respectively.
1.2. The Definition of Neuroglia
The definition of neuroglia is based on the unifying fundamental function of these cells, which, regardless of their origin, is homeostasis of the nervous system. This function is fundamental for both physiological context, when glial cells perform their routine housekeeping duties, as well as for pathological context, when glial cells can undergo reactive remodelling in order to preserve, repair and restore brain homeostasis. Failure in this function results in the development of the neurological disease and damage to the nervous tissue. Therefore, neuroglia can be defined as homeostatic and defensive cells of the nervous system, represented by highly heterogeneous cellular populations of different origin, structure and function [94].
In this sense neuroglia are the ultimate supportive cells of the nervous system, keeping it in a functional state. This reflects upon evolution of the nervous system, which resulted in the division of labour: the information processing and electrical excitability became confined to the neuronal networks, whereas homeostatic support and defence became the sole prerogative of the neuroglia [95]. This homeostatic support occurs at all levels of organisation of the nervous system: at molecular level (control over homoeostasis of ions, neurotransmitters, protons, reactive oxygen species, metabolites, etc.), at cellular level (astrocytes involvement in neurogenesis), at network level (both astroglia and microglia regulate synaptogenesis, synaptic maturation and extinction), connectome level (which is maintained by myelinating oligodendroglia and Schwann cells), organ level (astrocytes control blood-brain barrier and glymphatic flow and regulate functional hyperaemia) and systemic level (glial cells emerge as central chemoceptors and contribute to systemic control over ventilation, ion homeostasis and energy metabolism); for comprehensive coverage of neuroglial homeostatic capabilities see [1, 2, 7, 19, 31, 34, 37, 41, 45, 46, 49, 50, 59, 68, 70, 93, 96, 97, 103, 105].
This ultimate homeostatic capability of neuroglia underlies their fundamental role in neuropathology, the latter being broadly defined as a homeostatic failure of the nervous system. Environmental stress and/or pathological insults trigger glial homeostatic response (when glial cells attempt to keep homeostatic equilibrium or steady state) and glial reactivity, which represents an evolutionary conserved programme of glial cells remodelling aimed at mounting defence of the nervous tissue. Neuroglial reactivity is manifested in reactive astrogliosis, microglial activation and Wallerian degeneration (for oligodendrocytes). At the same time glial asthenia or atrophy, which is observed in numerous neurological conditions, facilitates evolution of the disease because of compromised homeostatic and defensive capabilities. Although the fundamental role of neuroglia in neuropathology has been predicted by prominent neurologists of the nineteenth and the beginning of the twentieth centuries (such as Rudolf Virchow, Carl Frommann, Alois Alzheimer, Nicolas Achucarro and Franz Nissl), the pathophysiological role of glia begun to be universally recognised only in the recent decade; for references and concepts see [11, 12, 53, 66, 67, 76, 81, 84–86, 99]. The concept of astrotauopathology, recently introduced by Kovacz [51], supports the notion that the neuroglia emerges in the limelight when considering the evolution of neurological diseases.
1.3. Glial Numbers
The numerical preponderance of glial cells in the brains and spinal cords of different species and glial to neurone ratio (GNR) have been a matter of the most common fallacy over recent decades. The notion of glial cells outnumbering neurones in the human brain by a factor of 10 or even 50 [10, 18, 44] represented an undisputed general knowledge that has been repeatedly proclaimed in glial literature (for critical analysis see for example [39, 98, 102]). The story of exceedingly high number of glial cells in the human brain goes back to Franz Nissl [58]; this idea became rather popular and reached the climax in writings of Robert Galambos who considered that neuroglia represent the primary seat of intelligence, consciousness, emotions and are overall responsible for our ‘humanity’. ‘Glia is … conceived as genetically charged to organize and program neuron activity so that the best interests of the organism will be served; the essential product of glia action is visualized to be what we call innate and acquired behavioural responses. In this scheme, neurons in large part merely execute the instructions glia give them’ [28]. The notion was further promoted by the finding that the Einstein’s brain had a rather higher glia to neurone ratio in his associated cortex than that found in the control human population [25], leading to speculations that this could be the reason for his remarkable intellectual abilities (https://www.theguardian.com/science/2007/feb/21/neuroscience.highereducation) (https://www.npr.org/templates/story/story.php?storyId=126229305). The public myth of glia has extended into that of an untapped part of the brain that we may not use, perhaps gloriously captured in Starbuck’s The Way I see it? (http://www.stevekmccoy.com/blog/2005/08/starbucks_the_w) #236 quote ‘Scientists tell us we only use 5% of our brains. But if they only used 5% of their brains to reach that conclusion, then why should we believe them?’ Of course, based on any functional imaging, this myth has been debunked and the authors would like to assure the readers that we had used the vast majority if not all of our brains to write this chapter.
None of these concepts had experimental confirmation. Exceptionally high glia to neurone ratio of the human brain was not related to actual cell counts; to the contrary most of stereological investigations produced the GNR values in the neocortex somewhere around 1.5 (see Table 1 in [42, 102]), with the number of neuronal counts in the range of 20–30 billion and glial cells in the range of 27–38 billion. In the cerebellum, which contains the largest number of brain neurones (around 70 billion) the number of glial cells is much smaller, with GNR not exceeding 0.1 [3, 4]. These stereological data obviously made the total GNR estimate of 10:1 unrealistic. Further advances in defining the glial numbers are associated with the application of isotopic fractionation technique, which counts nuclei of neurones and non-neuronal cells in the homogenates of the nervous tissue [8, 40, 54]. This technique demonstrated that the total numbers of neuronal and non-neuronal cells in the human brain are more or less on par, both being in the range of ~80–100 billion. After subtracting the population of endothelial cells which may account for about 20% of all non-neuronal cells, the true number of glial can be estimated at ~60 billion and total GNR for the whole brain is therefore less than 1:1. The density of glia is quite different in various brain areas. For example, the GNR varies between 1.2 in the grey matter of the occipital cortex and 3.6 in the grey matter of frontal cortical regions [73, 82], it is technically an infinity in the white matter that does not contain neuronal cell bodies, and hence inclusion of white matter counts increases the total GNR in the cortex to ~3. As already alluded above the GNR in cerebellum is very low probably not exceeding 0.1. Much higher GNR values were reported for the striatum (3.7:1), for the superior colliculus (10:1), for the ventral pallidum (12.2:1), for the lateral vestibular nucleus (30–50:1), while for the globus pallidus a very high GNR of 160:1 has been calculated from stereological counts [5, 8, 64, 71, 80, 91, 102]. Similarly, the GNR for the spinal cord was determined at 5:1 in cynomolgus monkey and almost 7:1 in humans [9].
Evolution of the nervous system paralleled with an increase in GNR, which however was not entirely linear and was not directly related to the intelligence. The nervous system of invertebrates has, as a rule, relatively smaller numbers of glial cells, with a GNR between 0.01:1 and 0.2:1 (50 glial cells derived from neuronal/epithelial progenitors and six glial cells that are mesodermally derived per 302 neurones in Caenorhabditis elegans [63, 89]; 10 glial cells per 400–700 neurones in every ganglion of the leech [20]; ~9000 glial cells per 90,000 neurones in the CNS of Drosophila [26, 52]). At the same time, the buccal ganglion of the great ramshorn snail Planorbis corneus contains 298 for example, in the cortex, the GNR is about 0.3–0.4 in rodents, ~1.1 in cat, ~1.2 in horse, 0.5–1.0 in rhesus monkey, 2.2 in Göttingen minipig, ~1.5 in humans and as high as 4–8 in elephants and the fin whale [15, 27, 38, 43, 55, 65, 92]. The largest absolute number of glial cells has been counted in the neocortex of whales [27, 56]; stereological cell counts in the neocortex of the long-finned pilot whale (Globicephala melas) brain determined there are approximately 37.2 billion neurones and 127 billion glial cells and this gives a GNR of 3.4 [56]. The largest GNR was found in the neocortex of the common Minke whale (Balaenoptera acutorostrata), which contained ~12.8 billion neurones and 98 billion of glia giving therefore a GNR of ~7.6 [27].
1.4. Classification and Main Functions
Neuroglia (Fig. 1.1, see also [94]) are classified into glia of the peripheral nervous system (PNS) and of the CNS. The glial cells of the PNS originate (similarly to peripheral neurones) from the neural crest and are classified into:
Schwann cells [48] associated with sensory, motor, sympathetic and parasympathetic axons; Schwann cells are further subdivided into (i) myelinating Schwann cells that myelinate peripheral axons; (ii) non-myelinating Schwann cells that surround multiple non-myelinating axons and (iii) perisynaptic Schwann cells, which enwrap peripheral synapses and maintain homoeostasis in the perisynaptic milieu.
Satellite glial cells [35, 36], which are surrounding neurones in sensory, sympathetic and parasympathetic ganglia. These satellite glial cells control local homeostasis and are capable of reactive remodelling in pathology.
Olfactory ensheathing cells [77], which are a part of the olfactory system. These cells extend very fine processes that enclose large numbers of unmyelinated olfactory axons
Enteric glia [32, 33], represented by homeostatic glial cells of the enteric nervous system.
Fig. 1.1.
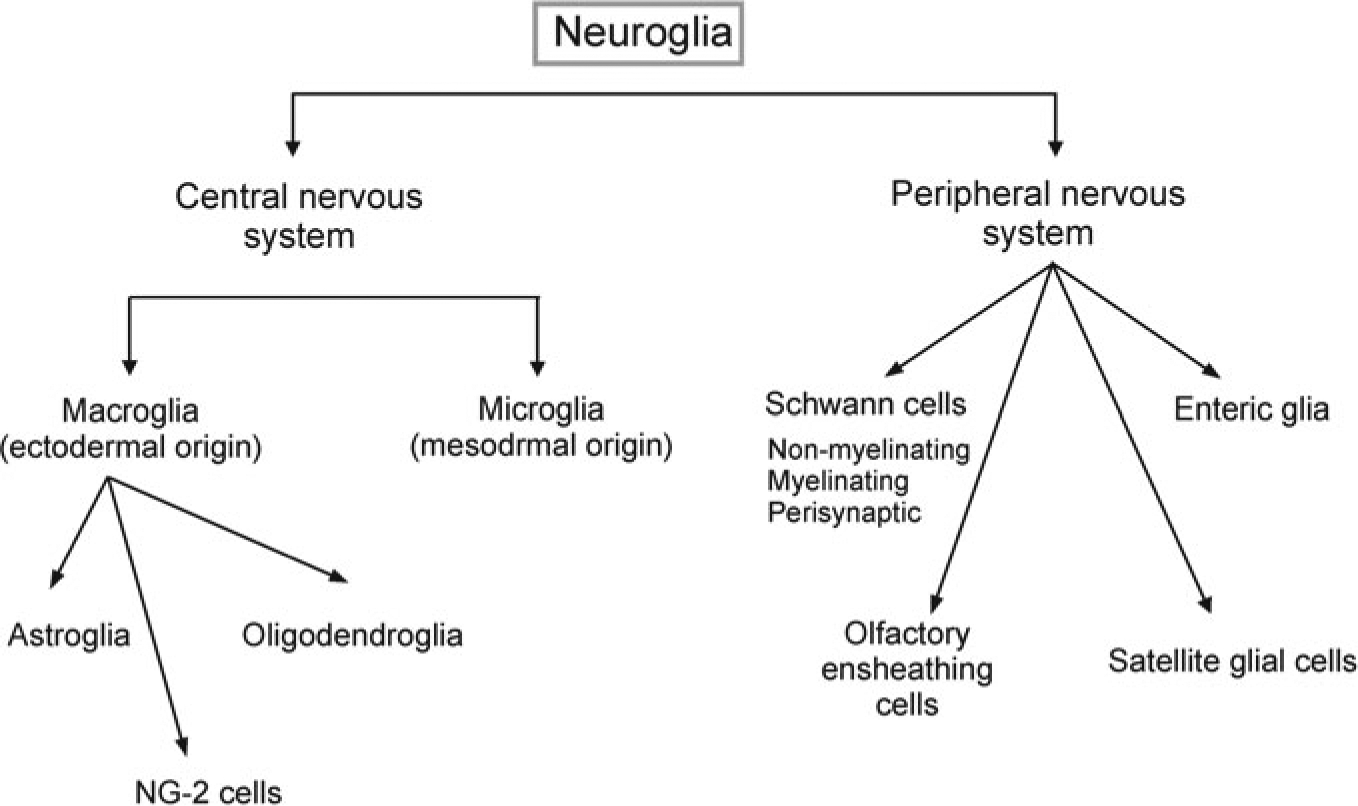
Classification of neuroglia
Neuroglia of the CNS are subdivided into macroglia (cells of ectodermal, neuroepithelial origin) and microglia (cells of mesodermal, myeloid origin). The macroglia is further classified into:
-
1Astroglia or astrocytes. Astrocytes are heterogeneous population of primary homeostatic glia residing throughout the brain and the spinal cord, in both grey and white matter. Astroglia include [94, 96]:
- protoplasmic astrocytes of grey matter;
- fibrous astrocytes of white matter
- surface-associated astrocytes associated with the cortical surface in the posterior prefrontal and amygdaloid cortex;
- Velate astrocytes, which are localised in the parts of the brain that are densely packed with small neurones, for example in the olfactory bulb or in the granular layer of the cerebellar cortex;
- Radial glia, which are the pluripotent neural cells precursors that generally disappear at birth in mammals
- Radial astrocytes, which include Bergmann glia in the cerebellum, Müller glia of the retina, radial glia-like neural stem cells of the neurogenic niches and tanycytes of the hypothalamus, hypophysis and the raphe part of the spinal cord;
- Pituicytes, which are the glial cells of the neurohypophysis;
- Gomori astrocytes rich in iron and positive for Gomori’s chrome alum hematoxylin staining identified in the hypothalamus and in the hippocampus;
- Perivascular and marginal astrocytes, which are placed near the pia mater, where they form endfeet with blood vessels. These astrocytes do not establish contacts with neurones and their main function is in establishing the pial and perivascular glia limitans barriers.
-
Ependymocytes, choroid plexus cells and retinal pigment epithelial cells. These cells line up the ventricles and the subretinal space; the choroid plexus cells produce the cerebrospinal fluid. Ependymocytes possess small movable processes (microvilli and kinocilia), which by rhythmic movements produce a stream of cerebrospinal fluid.
- Interlaminar astrocytes;
- Polarised astrocytes;
-
Varicose projection astrocytes.Function of these hominoid astroglia remain unknown.
Parenchymal astrocytes of the human brain are substantially larger and more complex compared with astroglial cells of rodents, and have distinct gene expression pattern [60–62, 87, 104]. Human protoplasmic astrocytes have about 10 times more primary processes and a more complex secondary process arborisation, with an average volume about 16.5 times larger than that of the corresponding astrocytes in a rat brain [61]. The larger human protoplasmic astrocytes also have extended outreach onto neuronal structures, on average contacting and encompassing up to 2 million synapses residing within astrocytic territorial domains, significantly more than the integrating capacity of rodent protoplasmic astrocytes, which covers ~20,000–120,000 synaptic contacts [13, 61]. Similarly, human fibrous astrocytes have a 2.14-fold larger domain compared to that in rodents [61].
-
2Oligodendroglia or oligodendrocytes, the myelinating cells of the CNS are subdivided into 4 classes [94]:
- Type I oligodendrocytes are most numerous in the cortex and grey matter; they have small rounded somata and fine branching processes that myelinate 30 or more small diameter axons;
- Type II oligodendrocytes are similar to type I, but have parallel arrays of intermediate length internodes (100–250 μm), and are most common in white matter, such as the corpus callosum, optic nerve, cerebellum and spinal cord;
- Type III oligodendrocytes have larger (than type I and II) irregular cell bodies, with one or more thick primary processes that myelinate a small number of large diameter axons with long internodes (250–500 μm). These cells are localised in the cerebral and cerebellar peduncles, the medulla oblongata, and the spinal cord funiculi;
- Type IV oligodendrocytes, are somewhat similar to Schwann cells, being directly associated with a large diameter axon to form a single long internodal myelin sheath (as long as 1000 μm), and are restricted to tracts containing the largest diameter axons near the entrance/exit of nerve roots into the CNS.
-
3
NG-2 glia also known as oligodendrocyte progenitor cells or OPCs, or synantocytes, or polydendrocytes [14, 57]. The NG2 glia can have homeostatic role and contribute to adulthood myelination, albeit their functions are yet to be better characterised.
Microglia originate from the foetal macrophages that migrate into the neural tube very early in the embryonic development; arguably, microglia represent the first parenchymal glia to populate neural tissue in development. Microglial cells carry numerous physiological functions, including shaping neuronal synaptic connectivity, removing of redundant or apoptotic neurones in the development and regulating synaptic transmission [45, 46, 90]. Microglia form the main defence system of the CNS through evolutionary conserved programme of activation (microgliosis) which can produce numerous neuroprotective and neurotoxic phenotypes [78, 83].
In terms of numbers, the most numerous glia are oligodendrocytes and NG2 cells combined (40–60%), with astrocytes accounting for 20–40% and microglia for ~10% of neuroglia population, although there is, of course, a considerable variability between the brain regions, developmental stage and species.
1.5. Envoi and Outlook
One of the two goals of this chapter is to serve as a general introduction into the world of Neuroglia. The other goal is to pique an interest of the reader into subsequent chapters in this book. As we tersely reviewed Neuroglia we establish the origin of these cells, their classification and their general functions in homeostasis and defence of the brain. In the following chapters, we explore the role of these cells in the progression of neuropathologies, especially neurodegenerative disorders. For a long time, the neurone-centric view dominated neuropathological thinking, and only recently the role of glia has been reassessed and the perception is mounting of specific importance of neuroglia that to a very large extent defines the progression and outcome of most (if not all) neurological diseases.
Acknowledgments
VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PT, UK; Faculty of Health and Medical Sciences, Center for Basic and Translational Neuroscience, University of Copenhagen, 2200 Copenhagen, Denmark; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain.
Margaret S. Ho, School of Life Science and Technology, ShanghaiTech University, Shanghai 201210, China
Robert Zorec, Laboratory of Neuroendocrinology-Molecular Cell Physiology, Faculty of Medicine, Institute of Pathophysiology, University of Ljubljana, Ljubljana, Slovenia; Celica BIOMEDICAL, Ljubljana, Slovenia.
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA.
References
- 1.Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25 [DOI] [PubMed] [Google Scholar]
- 2.Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53 [DOI] [PubMed] [Google Scholar]
- 3.Andersen BB, Korbo L, Pakkenberg B (1992) A quantitative study of the human cerebellum with unbiased stereological techniques. J Comp Neurol 326:549–560 [DOI] [PubMed] [Google Scholar]
- 4.Andersen K, Andersen BB, Pakkenberg B (2012) Stereological quantification of the cerebellum in patients with Alzheimer’s disease. Neurobiol Aging 33(197):e111–e120 [DOI] [PubMed] [Google Scholar]
- 5.Andrade-Moraes CH, Oliveira-Pinto AV, Castro-Fonseca E, da Silva CG, Guimaraes DM, Szczupak D, Parente-Bruno DR, Carvalho LR, Polichiso L, Gomes BV, Oliveira LM, Rodriguez RD, Leite RE, Ferretti-Rebustini RE, Jacob-Filho W, Pasqualucci CA, Grinberg LT, Lent R (2013) Cell number changes in Alzheimer’s disease relate to dementia, not to plaques and tangles. Brain 136:3738–3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andriezen WL (1893) The neuroglia elements of the brain. Br Med J 2:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468:232–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Jacob Filho W, Lent R, Herculano-Houzel S (2009) Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol 513:532–541 [DOI] [PubMed] [Google Scholar]
- 9.Bahney J, von Bartheld CS (2018) The cellular composition and Glia-neuron ratio in the spinal cord of a human and a nonhuman primate: comparison with other species and brain regions. Anat Rec (Hoboken) 301:697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bear MF, Connors BW, Paradiso MA (2007) Exploring the brain. Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 11.Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butt AM, Kiff J, Hubbard P, Berry M (2002) Synantocytes: new functions for novel NG2 expressing glia. J Neurocytol 31:551–565 [DOI] [PubMed] [Google Scholar]
- 15.Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B (2007) Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 290:330–340 [DOI] [PubMed] [Google Scholar]
- 16.Chvatal A, Verkhratsky A (2018) Early history of neuroglial research: personalities. Neuroglia 1:245–257 [Google Scholar]
- 17.Colombo JA (2018) Interlaminar glia and other glial themes revisited: pending answers following three decades of glial research. Neuroglia 1:7–20 [Google Scholar]
- 18.Darlington CL (2009) The female brain. CRC Press, Boca Raton [Google Scholar]
- 19.Deitmer JW, Rose CR (1996) pH regulation and proton signalling by glial cells. Prog Neurobiol 48:73–103 [DOI] [PubMed] [Google Scholar]
- 20.Deitmer JW, Rose CR, Munsch T, Schmidt J, Nett W, Schneider HP, Lohr C (1999) Leech giant glial cell: functional role in a simple nervous system. Glia 28:175–182 [PubMed] [Google Scholar]
- 21.Del Rio-Hortega P (1919) El tercer elemento de los centros nerviosos. I. La microglia en estado normal. II. Intervencíon de la microglia en los procesos patológicos. III. Naturaleza probable de la microglia. Bol de la Soc esp de biol 9:69–120 [Google Scholar]
- 22.Del Rio-Hortega P (1920) La microglia y su transformacíon en células en bastoncito y cuerpos gránulo-adiposos. Trab del Lab de invest biol 18:37 [Google Scholar]
- 23.Del Rio-Hortega P (1932) Microglia In: Penfield W (ed) Cytology and cellular pathology of the nervous system, vol 2 Hoeber, New York, pp 482–534 [Google Scholar]
- 24.Del Río-Hortega P (1921) Estudios sobre la neuroglia. La glia de escasas radiaciones oligodendroglia. Biol Soc Esp Biol 21:64–92 [Google Scholar]
- 25.Diamond MC, Scheibel AB, Murphy GM Jr, Harvey T (1985) On the brain of a scientist: Albert Einstein. Exp Neurol 88:198–204 [DOI] [PubMed] [Google Scholar]
- 26.Edwards TN, Meinertzhagen IA (2010) The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol 90:471–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eriksen N, Pakkenberg B (2007) Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 290:83–95 [DOI] [PubMed] [Google Scholar]
- 28.Galambos R (1961) A glia-neural theory of brain function. Proc Natl Acad Sci USA 47:129–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golgi C (1870) Sulla sostanza connettiva del cervello (nevroglia). Rendiconti del R. Instituto Lombardo di Scienze e Lettere. serie 2, 3:275–277 [Google Scholar]
- 30.Golgi C (1903) Opera Omnia. Hoepli, Milano [Google Scholar]
- 31.Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329:571–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grubisic V, Gulbransen BD (2017) Enteric glia: the most alimentary of all glia. J Physiol 595:557–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grubisic V, Verkhratsky A, Zorec R, Parpura V (2018) Enteric glia regulate gut motility in health and disease. Brain Res Bull 136:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Halassa MM, Florian C, Fellin T, Munoz JR, Lee SY, Abel T, Haydon PG, Frank MG (2009) Astrocytic modulation of sleep homeostasis and cognitive consequences of sleep loss. Neuron 61:213–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanani M (2005) Satellite glial cells in sensory ganglia: from form to function. Brain Res Rev 48:457–476 [DOI] [PubMed] [Google Scholar]
- 36.Hanani M (2010) Satellite glial cells in sympathetic and parasympathetic ganglia: in search of function. Brain Res Rev 64:304–327 [DOI] [PubMed] [Google Scholar]
- 37.Hansen DB, Garrido-Comas N, Salter M, Fern R (2015) HCO3–independent pH regulation in astrocytes in situ is dominated by V-ATPase. J Biol Chem 290:8039–8047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hawkins A, Olszewski J (1957) Glia/nerve cell index for cortex of the whale. Science 126:76–77 [DOI] [PubMed] [Google Scholar]
- 39.Herculano-Houzel S, Dos Santos SE (2018) You do not mess with the Glia. Neuroglia 1:193–219 [Google Scholar]
- 40.Herculano-Houzel S, Lent R (2005) Isotropic fractionator: a simple, rapid method for the quantification of total cell and neuron numbers in the brain. J Neurosci 25:2518–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hertz L, Dringen R, Schousboe A, Robinson SR (1999) Astrocytes: glutamate producers for neurons. J Neurosci Res 57:417–428 [PubMed] [Google Scholar]
- 42.Hilgetag CC, Barbas H (2009) Are there ten times more glia than neurons in the brain? Brain Struct Funct 213:365–366 [DOI] [PubMed] [Google Scholar]
- 43.Jelsing J, Nielsen R, Olsen AK, Grand N, Hemmingsen R, Pakkenberg B (2006) The postnatal development of neocortical neurons and glial cells in the Gottingen minipig and the domestic pig brain. J Exp Biol 209:1454–1462 [DOI] [PubMed] [Google Scholar]
- 44.Kandel ER, Schwartz JH, Jessell TM (2000) Principles of neural science. McGrawhill, New York [Google Scholar]
- 45.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553 [DOI] [PubMed] [Google Scholar]
- 46.Kettenmann H, Kirchhoff F, Verkhratsky A (2013) Microglia: new roles for the synaptic stripper. Neuron 77:10–18 [DOI] [PubMed] [Google Scholar]
- 47.Kettenmann H, Verkhratsky A (2008) Neuroglia: the 150 years after. Trends Neurosci 31:653–659 [DOI] [PubMed] [Google Scholar]
- 48.Kidd GJ, Ohno N, Trapp BD (2013) Biology of Schwann cells. Handb Clin Neurol 115:55–79 [DOI] [PubMed] [Google Scholar]
- 49.Kirischuk S, Kettenmann H, Verkhratsky A (2007) Membrane currents and cytoplasmic sodium transients generated by glutamate transport in Bergmann glial cells. Pflugers Arch 454:245–252 [DOI] [PubMed] [Google Scholar]
- 50.Kofuji P, Newman EA (2004) Potassium buffering in the central nervous system. Neuroscience 129:1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovacs GG, Ferrer I, Grinberg LT, Alafuzoff I, Attems J, Budka H, Cairns NJ, Crary JF, Duyckaerts C, Ghetti B, Halliday GM, Ironside JW, Love S, Mackenzie IR, Munoz DG, Murray ME, Nelson PT, Takahashi H, Trojanowski JQ, Ansorge O, Arzberger T, Baborie A, Beach TG, Bieniek KF, Bigio EH, Bodi I, Dugger BN, Feany M, Gelpi E, Gentleman SM, Giaccone G, Hatanpaa KJ, Heale R, Hof PR, Hofer M, Hortobagyi T, Jellinger K, Jicha GA, Ince P, Kofler J, Kovari E, Kril JJ, Mann DM, Matej R, McKee AC, McLean C, Milenkovic I, Montine TJ, Murayama S, Lee EB, Rahimi J, Rodriguez RD, Rozemuller A, Schneider JA, Schultz C, Seeley W, Seilhean D, Smith C, Tagliavini F, Takao M, Thal DR, Toledo JB, Tolnay M, Troncoso JC, Vinters HV, Weis S, Wharton SB, White CL 3rd, Wisniewski T, Woulfe JM, Yamada M, Dickson DW (2016) Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131:87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U (2017) The glia of the adult Drosophila nervous system. Glia 65:606–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E (2013) Astrocytes: emerging stars in leukodystrophy pathogenesis. Transl Neurosci 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lent R, Azevedo FA, Andrade-Moraes CH, Pinto AV (2012) How many neurons do you have? Some dogmas of quantitative neuroscience under revision. Eur J Neurosci 35:1–9 [DOI] [PubMed] [Google Scholar]
- 55.Lidow MS, Song ZM (2001) Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol 435:263–275 [DOI] [PubMed] [Google Scholar]
- 56.Mortensen HS, Pakkenberg B, Dam M, Dietz R, Sonne C, Mikkelsen B, Eriksen N (2014) Quantitative relationships in delphinid neocortex. Front Neuroanat 8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishiyama A, Komitova M, Suzuki R, Zhu X (2009) Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity. Nat Rev Neurosci 10:9–22 [DOI] [PubMed] [Google Scholar]
- 58.Nissl F (1898) Nervenzellen und graue Substanz. Munch Med Wochenschr 45:988–992; 1023,–1029; 1060–1062 [Google Scholar]
- 59.Noda M, Hiyama TY (2015) The Nax channel: what it is and what it does. Neuroscientist 21:399–412 [DOI] [PubMed] [Google Scholar]
- 60.Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553 [DOI] [PubMed] [Google Scholar]
- 63.Oikonomou G, Shaham S (2011) The glia of Caenorhabditis elegans. Glia. 59:1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pakkenberg B, Gundersen HJ (1988) Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc 150:1–20 [DOI] [PubMed] [Google Scholar]
- 65.Pakkenberg B, Gundersen HJ (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320 [PubMed] [Google Scholar]
- 66.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345 [DOI] [PubMed] [Google Scholar]
- 68.Pellerin L, Magistretti PJ (2012) Sweet sixteen for ANLS. J Cereb Blood Flow Metab 32:1152–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pentreath VW, Radojcic T, Seal LH, Winstanley EK (1985) The glial cells and glia-neuron relations in the buccal ganglia of Planorbis corneus (L.): cytological, qualitative and quantitative changes during growth and ageing. Philos Trans R Soc Lond B Biol Sci 307:399–455 [DOI] [PubMed] [Google Scholar]
- 70.Pfrieger FW (2010) Role of glial cells in the formation and maintenance of synapses. Brain Res Rev 63:39–46 [DOI] [PubMed] [Google Scholar]
- 71.Ponomarev VS (1966) Glial index in vestibular nuclei of humans, macacos, and dogs. Arkh Anat Gistol Embriol 51:100–104 [PubMed] [Google Scholar]
- 72.Ramón y Cajal S (1895) Algunas conjeturas sobre el mechanismoanatomico de la ideacion, asociacion y atencion. Imprenta y Libreria de Nicolas Moya [Google Scholar]
- 73.Ribeiro PF, Ventura-Antunes L, Gabi M, Mota B, Grinberg LT, Farfel JM, Ferretti-Rebustini RE, Leite RE, Filho WJ, Herculano-Houzel S (2013) The human cerebral cortex is neither one nor many: neuronal distribution reveals two quantitatively different zones in the gray matter, three in the white matter, and explains local variations in cortical folding. Front Neuroanat 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robertson W (1899) On a new method of obtaining a black reaction in certain tissue-elements of the central nervous system (platinum method). Scott Med Surg J 4:23 [Google Scholar]
- 75.Robertson W (1900) A microscopic demonstration of the normal and pathological histology of mesoglia cells. J Ment Sci 46:733–752 [Google Scholar]
- 76.Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2016) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience 323:170–182 [DOI] [PubMed] [Google Scholar]
- 77.Ruitenberg MJ, Vukovic J, Sarich J, Busfield SJ, Plant GW (2006) Olfactory ensheathing cells: characteristics, genetic engineering, and therapeutic potential. J Neurotrauma 23:468–478 [DOI] [PubMed] [Google Scholar]
- 78.Savage JC, Picard K, Gonzalez-Ibanez F, Tremblay ME (2018) A brief history of microglial ultrastructure: distinctive features, phenotypes, and functions discovered over the past 60 years by electron microscopy. Front Immunol 9:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schleich CL (1894) Schmerzlose Operationen: Örtliche Betäubung mit indiffrenten Flüssigkeiten Psychophysik des natürlichen und künstlichen Schlafes, Julius Springer, Berlin, p 256 [Google Scholar]
- 80.Schroder KF, Hopf A, Lange H, Thorner G (1975) Morphometrical-statistical structure analysis of human striatum, pallidum and subthalamic nucleus. J Hirnforsch 16:333–350 [PubMed] [Google Scholar]
- 81.Seifert G, Steinhauser C (2013) Neuron-astrocyte signaling and epilepsy. Exp Neurol 244:4–10 [DOI] [PubMed] [Google Scholar]
- 82.Sherwood CC, Stimpson CD, Raghanti MA, Wildman DE, Uddin M, Grossman LI, Goodman M, Redmond JC, Bonar CJ, Erwin JM, Hof PR (2006) Evolution of increased glia-neuron ratios in the human frontal cortex. Proc Natl Acad Sci USA 103:13606–13611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sierra A, Beccari S, Diaz-Aparicio I, Encinas JM, Comeau S, Tremblay ME (2014) Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast 2014:610343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20:160–172 [DOI] [PubMed] [Google Scholar]
- 86.Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sosunov AA, Wu X, Tsankova NM, Guilfoyle E, McKhann GM 2nd, Goldman JE (2014) Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. J Neurosci 34:2285–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stallcup WB (1981) The NG2 antigen, a putative lineage marker: immunofluorescent localization in primary cultures of rat brain. Dev Biol 83:154–165 [DOI] [PubMed] [Google Scholar]
- 89.Stout RF Jr, Verkhratsky A, Parpura V (2014) Caenorhabditis elegans glia modulate neuronal activity and behavior. Front Cell Neurosci 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME (2017) Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol 595:1929–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thorner G, Lange H, Hopf A (1975) Morphometrical-statistical structure analysis of human striatum, pallidus and subthalamic nucleus. II. Globus pallidus. J Hirnforsch 16:401–413 [PubMed] [Google Scholar]
- 92.Tower DB (1954) Structural and functional organization of mammalian cerebral cortex; the correlation of neurone density with brain size; cortical neurone density in the fin whale (Balaenoptera physalus L.) with a note on the cortical neurone density in the Indian elephant. J Comp Neurol 101:19–51 [DOI] [PubMed] [Google Scholar]
- 93.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A (2011) The role of microglia in the healthy brain. J Neurosci 31:16064–16069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwel, Chichester, p 560 [Google Scholar]
- 95.Verkhratsky A, Nedergaard M (2016) The homeostatic astroglia emerges from evolutionary specialization of neural cells. Philos Trans R Soc Lond B Biol Sci 371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Verkhratsky A, Nedergaard M, Hertz L (2015) Why are astrocytes important? Neurochem Res 40:389–401 [DOI] [PubMed] [Google Scholar]
- 98.Verkhratsky A, Oberheim Bush NA, Nedergaard M, Butt AM (2018) The special case of human astrocytes. Neuroglia 1:21–29 [Google Scholar]
- 99.Verkhratsky A, Parpura V, Pekna M, Pekny M, Sofroniew M (2014) Glia in the pathogenesis of neurodegenerative diseases. Biochem Soc Trans 42:1291–1301 [DOI] [PubMed] [Google Scholar]
- 100.Virchow R (1856) Ueber das granulirte Ansehen der Wandungen der Gehirnventrikel In: Virchow R (ed) Gesammelte Abhandlungen zur wissenschaftlichen Medicin. Meidinger Sohn & Comp., Frankfurt A.M., pp 885–891 [Google Scholar]
- 101.Virchow R (1858) Die Cellularpathologie in ihrer Begründung auf physiologische und pathologische Gewebelehre 20 Vorlesungen, gehalten während d. Monate Febr., März u. April 1858 im Patholog. Inst. zu Berlin. August Hirschwald, Berlin, 440 pp [Google Scholar]
- 102.von Bartheld CS, Bahney J, Herculano-Houzel S (2016) The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J Comp Neurol 524:3865–3895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK (2010) Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104:3042–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Y, Sloan SA, Clarke LE, Caneda C, Plaza CA, Blumenthal PD, Vogel H, Steinberg GK, Edwards MS, Li G, Duncan JA 3rd, Cheshier SH, Shuer LM, Chang EF, Grant GA, Gephart MG, Barres BA (2016) Purification and characterization of progenitor and mature human astrocytes reveals transcriptional and functional differences with mouse. Neuron 89:37–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zorec R, Horvat A, Vardjan N, Verkhratsky A (2015) Memory formation shaped by astroglia. Front Integr Neurosci 9:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


