Abstract
Astroglial cells are involved in most if not in all pathologies of the brain. These cells can change the morpho-functional properties in response to pathology or innate changes of these cells can lead to pathologies. Overall pathological changes in astroglia are complex and diverse and often vary with different disease stages. We classify astrogliopathologies into reactive astrogliosis, astrodegeneration with astroglial atrophy and loss of function, and pathological remodelling of astrocytes. Such changes can occur in neurological, neurodevelopmental, metabolic and psychiatric disorders as well as in infection and toxic insults. Mutation in astrocyte-specific genes leads to specific pathologies, such as Alexander disease, which is a leukodys-trophy. We discuss changes in astroglia in the pathological context and identify some molecular entities underlying pathology. These entities within astroglia may repent targets for novel therapeutic intervention in the management of brain pathologies.
Keywords: Astrocyte, Neuropathology, Alexander disease, Stroke, Psychiatric diseases, Metabolic diseases, Neurotrauma, Infectious diseases, Systemic Inflammation, Sepsis-associated encephalopathy, Toxic encephalopathy, Hepatic encephalopathy, Autistic spectrum disorders, Epilepsy
7.1. Prologue: Neuroglia in Neurological Diseases
The role of neuroglia in neurological disorders have been widely accepted by leading neuroanatomists and neurologists of the nineteenth century, from Rudolf Virchow (who indicated that the ‘interstitial tissue of the brain and spinal marrow are one of the most frequent seats of morbid change’ [222]) to Carl Fromann, Alois Alzheimer and Nicolas Achucarro [1, 6, 8, 77], to name but a few. The neuropathological philosophy of the twentieth century was dominated by neurono-centric views, while the last decade witnessed the resurgence of neurogliopathology. Recently, the pathological potential of neuroglia in general, and astrocytes in particular, has been extensively studied and the fundamental principles of astrogliopathology have been defined [33, 74, 79, 152, 156, 173, 191, 192, 216, 219, 220, 234].
Neuroglial cells are primary homeostatic and defensive cells of the nervous system; and naturally, all types of glia are contributing to neuropathological developments. Astrocytes are a part of neural networks; they interact with neurones, with other glia and with blood vessels, thus, maintaining the structural and functional integrity of the neural tissue. Astrocytes are indispensable for maintaining neuronal functional and neuronal survival both in physiology and in pathology [214]. Therefore, astroglial failure creates a disease-permissive landscape and underlies neuronal malfunction, neuronal death and neurological deficits.
7.2. Principles of Astrogliopathology
Pathological changes in astroglia in neurological diseases are complex and diverse (Fig. 7.1). These changes can be generic or disease-specific. They often vary at different disease stages. In the context of human pathology, changes are affected by age and comorbidity. Astrogliopathology is classified into (i) reactive astrogliosis; (ii) astrodegeneration with astroglial atrophy and loss of function; and (iii) pathological remodelling of astrocytes (Fig. 7.1, [74, 156, 220]); all these pathological reactions occur together or in isolation.
Fig. 7.1.
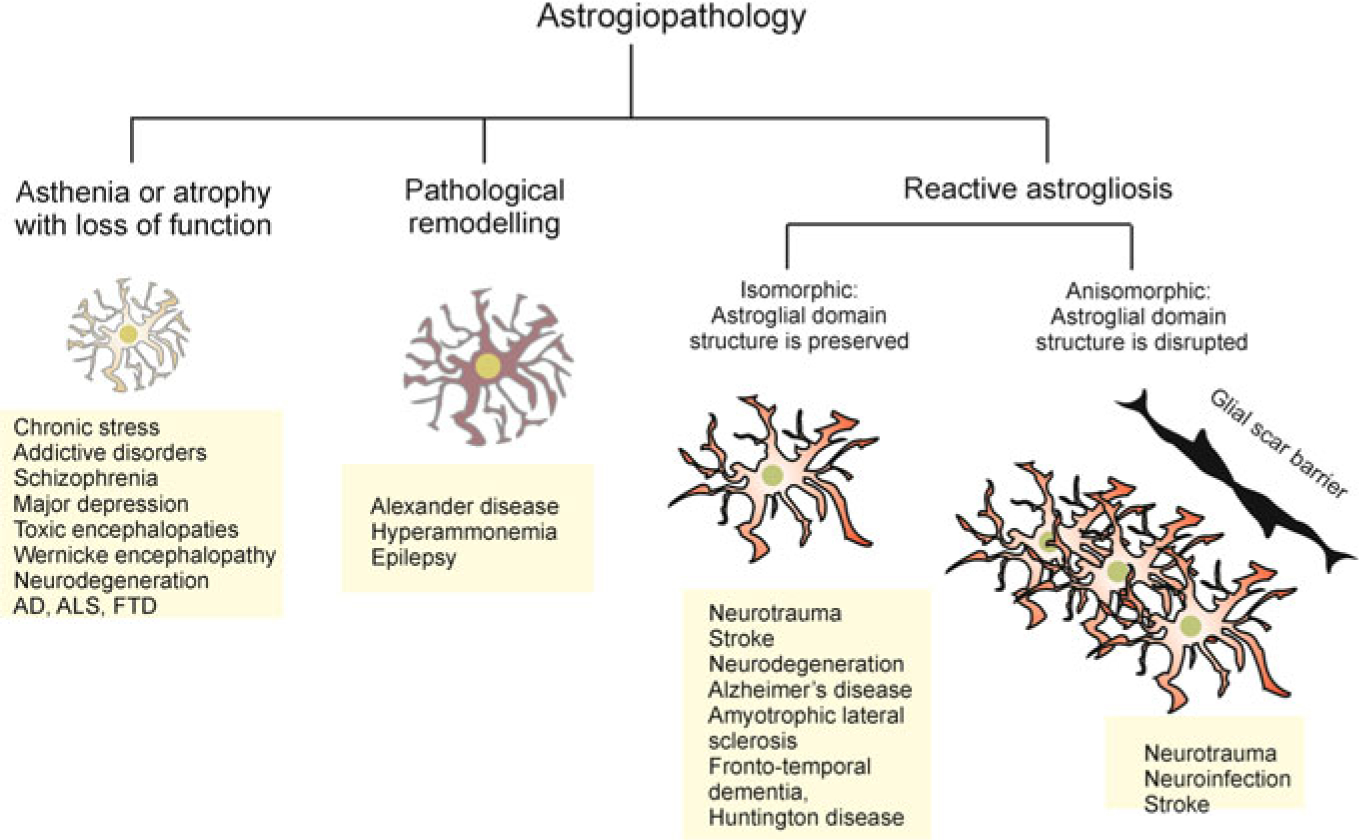
Principles of astrogliopathology. Astrocytes undergo several types of morpho-functional changes in the brain pathology (see text for details). AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; FTD, fronto-temporal dementia
7.2.1. Reactive Astrogliosis
Reactive astrogliosis is observed in many neurological disorders. Until very recently, astroglial reactivity was considered the sole manifestation of astrogliopathology. From histopathological point of view, astroglial reactivity is characterised by mor phological hypertrophy and up-regulation of two major cytoskeletal intermediate filaments/proteins, glial fibrillary acidic protein (GFAP) and vimentin [95, 155, 191]. Reactive astrocytes undergo a variety of substantial modifications, showing multiple phenotypes with both neuroprotective and neurotoxic features. These phenotypes arguably are disease-specific, although they all can share some common properties [120, 121, 156]. Transcriptomes of reactive astrocytes in ischemia and endotoxin activation, for example, show significant differences [233].
Conceptually, reactive astrogliosis represents an evolutionary conserved (the first manifestations of astroglial reactivity are observed in many invertebrates including annelids and insects) defensive reprogramming of astroglia aimed at: (i) increased neuroprotection and trophic support of the nervous tissue; (ii) isolation of the lesioned area; (iii) reconstruction of the compromised blood–brain barrier; and (iv) facilitating the post-lesion regeneration of the nervous tissue [7, 156, 191]. The astrogliotic programme, therefore, has a high degree of flexibility and tailors functional and biochemical reprogramming of astrocytes to the nature and strength of the insult. Even within the same lesioned regions, astrocytes demonstrate a degree of heterogeneity in expression of transcription factors, inflammatory agents and signalling molecules [78, 92].
The initiation of astrogliosis is regulated mainly by damage-associated molecular patterns (DAMPs) or pathogen-associated molecular patterns (PAMPs). The DAMPs are endogenous molecules released from damaged or dying cells (ATP being the most prominent example), blood-borne factors that infiltrate brain parenchyma, etc. The PAMPs are exogenous molecules associated with infectious invaders such as bacteria or viruses; they mostly act through Toll-like receptors (TLRs) widely expressed in astrocytes [99, 203]. Astroglial cells express a wide range of receptors to both DAMPs and PAMPs: P2X7 purinoceptors, TLRs, nucleotide-binding oligomerisation domains (NOD)-like receptors (NLRs), double-stranded RNA-dependent protein kinase, scavenger receptors, mannose receptor and receptors for complement components and mediators, such as CXCL10, CCL2, interleukin-6 and B-cell-activating factor of the tumour necrosis factor (TNF) family [70]. Often, exposure of astrocytes to DAMPs and PAMPs evokes cytosolic Ca2+ increases due to its release from the endoplasmic reticulum (ER) intracellular store. These Ca2+ signals are critical for instigating the astrogliotic programme. For instance, genetic deletion of predominant astroglial Ca2+ release channel of the ER, inositol 1,4,5-triphospate receptor type 2, suppresses astrogliotic response [101]. Similarly, pharmacological inhibition of Ca2+ release from the ER restrains astroglial reactivity triggered by amyloid-β [2]. Stimulation of astrocytes with ATP (a classical DAMP) not only triggers Ca2+ signalling [34, 211] but also induces formation of inflammasomes comprised of the NLR protein-1 or −2 LR, the adaptor protein apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC) and caspase-1. Activation of these inflammasomes leads to the processing of inflammatory caspase-1 and interleukin-1β (IL-1β) [137].
Reactive astrogliosis is classified according to the morphological properties and severity (Fig. 7.1). From the morphological perspective, astrogliosis is divided into isomorphic and anisomorphic astrogliosis. The isomorphic astrogliosis preserves astroglial territorial domains and it is fully reversible, whereas anisomorphic astrogliosis proceeds with violation of territorial domains, cell migration and territorial overlap, formation of astroglial palisades and ultimately the scar formation [156]. According to the severity, astrogliosis is classified into (i) mild to moderate astrogliosis; (ii) severe diffuse astrogliosis; and (iii) severe astrogliosis with compact scar formation [190, 191]. Fundamentally, astrogliosis provides for defence of the nervous tissue; it increases neuroprotection and is ultimately important for post-lesion regeneration. Even scar formation carries clear definitive function isolating the damaged area of the CNS and saving the whole at the expense of its part [156, 210]. Suppression of reactive astrogliosis usually exacerbates the course of pathology [156]. Inhibition of astroglial reactivity enlarges the size of the traumatic lesions and aggravates neurological deficit [148]. Deletion of GFAP and vimentin, both being critical for the execution of astrogliotic programme, facilitates the development of ischaemic infarcts [116] and exacerbates post-traumatic synaptic loss [154]. Ablation of astroglial reactivity increased the accumulation of β-amyloid and reduced microglial association with senile plaques in the animal model of the Alzheimer’s disease [157]. Nonetheless, in conditions of prolonged stress or severe damage, reactive astrocytes may acquire neurotoxic potential and astrogliosis as a process can become maladaptive [155].
7.2.2. Astroglial Atrophy
Astrodegeneration is a widespread class of astrocytopathy, which is represented by morphological atrophy, increased astroglial death and hence decrease in astroglial density and an impairment of homeostatic functions. Astrodegeneration has been observed in various types of neuropathologies [90, 212, 221]. Astrodegeneration is particularly prominent in major psychiatric diseases. For instance, schizophrenia, major depressive disorder, Wernicke–Korsakoff encephalopathy, and addictive disorders are all accompanied with a reduction in the packing density of astrocytes and a failure of their homeostatic cascades, the latter most notably associated with glutamate homeostasis and glutamate–glutamine shuttle, which are both impaired [50, 53, 54, 134, 163, 165, 178, 218]. Aberrant astroglial glutamate transport and catabolism are arguably responsible for abnormal neurotransmission as well as for excitotoxic neuronal death, both resulting in psychotic symptoms. In amyotrophic lateral sclerosis, insufficient astroglial glutamate clearance from the extracellular space instigates excitotoxic death of large motor neurones [177, 207], whereas in Alzheimer’s disease, reduced astroglial synaptic coverage contributes to early synaptic extinction and cognitive deficiency [215].
7.2.3. Pathological Remodelling of Astrocytes
Pathological remodelling of astrocytes covers abnormalities associated with an acquisition of abnormal molecular cascades or functional properties, which drive pathology [74, 156]. Pathological remodelling of astroglia contributes to various leukodys-trophies, most notably to Alexander disease, megalencephalic leukoencephalopathy with subcortical cysts or vanishing white matter syndrome, in all of which the astrocytopathy initiates lesions of the white matter [113]. In Alexander disease, astroglial expression of sporadically mutated GFAP gene results in early and severe leukomalacia [131]. Pathological remodelling in astroglia has been also described in mesial temporal lobe epilepsy, in which astrocytes acquire aberrant morphology, reduce gap junctional coupling and down-regulate expression of Kir4.1 channels; all these changes compromise K+ homeostasis thus contributing to the initiation of seizures [19].
7.3. Astrogliopathology in Neurological Diseases
7.3.1. Neurotrauma
Traumatic injury of the brain and of the spinal cord are classified according to their nature (penetrating wounds or concussions; the later when occurring in the cervical spinal cord is known medically as cervical cord neurapraxia), their severity (mild, moderate or severe), volume (focal or diffuse), outcome (death, vegetative state, severe disability, moderate disability and good recovery) and anatomical localisation. According to its very nature, a traumatic injury to the CNS has a complex pathophysiology associated not only with direct damage to neural cells, but also with a damage to the whole organ with destruction of the blood–brain barrier and blood vessels, ischaemic insults, opening the way for secondary infection, etc. Neurotrauma predominantly triggers astrogliotic response; reactive phenotypes, however, very much depend on the pathological context [32, 33] with the severity of the damage and its anatomical localisation affecting astroglial activation.
In the healthy brain, astrocytes form numerous barriers with blood vessels and with cerebrospinal fluid; endfeet of astroglial cells together with the parenchymal basement membrane create glia limitans, which physically separates the brain parenchyma from blood vessels, perivascular spaces and the meninges. In response to a neurotrauma, an astrogliotic scar barrier is formed that delineates and isolates the areas of a focal damage from the healthy brain. Suppression of astrogliosis with consequent malformation of an astroglial scar markedly exacerbates tissue damage and neurological deficit [189]. The heterogeneity of reactive astroglial phenotypes very much depends on the distance to the lesion core. Close to the lesion astrocytes lose their domains, their processes overlap and the astroglial palisades are formed, reflecting anisomorphic astrogliosis. Astrocytes gather around the damaged sites and form the scar [32]. Astrocytes distant to the lesion core undergo isomorphic gliosis; they become hypertrophic and arguably neuroprotective. Contribution of astrocytes to tissue pathology in neurotrauma is multifaceted. Besides forming a protective scar astrocytes regulate inflammatory response, provide for homeostatic protection of the nervous tissue through the removal of extracellular glutamate, buffering K+ or releasing scavengers of reactive oxygen species and regulate post-traumatic remodelling of synaptic networks. Reactive astrocytes are indispensable in remodelling as suppression of astrogliosis down-regulates post-traumatic regeneration of synaptic connectivity and neuronal networks [7].
7.4. Infectious Diseases
7.4.1. Infection of Nervous Tissue
Infections of the CNS caused by bacteria, viruses, fungi and parasites are classified into meningitis, encephalitis or brain abscess. Not every infectious agent can invade the CNS. Rather, only certain neurotropic viruses, bacteria, fungi and parasites can penetrate into the brain and the spinal cord with relative ease. Furthermore, most of the pathogens are effectively stopped by the brain barriers [110]. Infectious agents may cross the blood–brain barrier using the paracellular route, via transcytotic mechanism, inside entering monocytes (the Trojan horse hypothesis) as well as by other mechanisms such as, for example, hijacking of β-adrenergic receptors as shown for Neisseria meningitidis (meningococcus) [47].
Neuroglial contribution to the infectious lesions of the CNS is of fundamental importance. Neuroprotective activation of astrocytes and microglia to a large extent defines the spread of infection through the nervous tissue and hence determines the outcome of the disease. The glial response, in turn, depends on the nature of an infectious agent. For example, contact of astrocytes with the Gram-positive bacteria such as Pneumococcus or Staphylococcus triggers rapid astrogliotic activation with marked cellular hypertrophy and up-regulation of GFAP expression [96] accompanied with synthesis and secretion of pro-inflammatory agents such as TNF-α, ILs and macrophage inflammatory protein 1α [122]. Activation of astrocytes by infectious agents (see for example [70, 197, 228]) is mediated mainly through pattern recognition receptors (PRRs), which are represented by TLR 2, 3, 4, 9 [65], NLRs, retinoic acid-inducible gene (RIG)-like receptors (RLRs) and cytokine receptors [108]. The NOD2 receptor, operational in astrocytes, recognises a minimal motif present in all bacterial peptidoglycans and it is required for astroglial reactive reprogramming in response to N. meningitidis and Borrelia burgdorferi [43].
Activation of astrocytes is also linked to TLR receptors [67]. Distinct TLR subtypes recognise and respond to different PAMPs. Lipopolysaccharide (LPS), for example, signals through TLR4; TLR3 is activated by double-stranded RNA; peptidoglycans interacts with TLR2, while TLR9 senses CpG DNA [40]. Activated TLRs interact with adaptor proteins myeloid differentiation factor 88 (MyD88) and/or a TIR-containing adaptor molecule, Toll/interferon-1 receptor domain-containing adaptor inducing interferon-γ (TRIF), which acts as a part of relevant signalling cascade [40]. Bacterial infection of the nervous tissue down-regulates expression of connexins hence decreasing gap junctional connectivity of astroglial syncytia [66]. Direct interaction of several bacteria such as Streptococcus pneumonia, B. burgdorferi and N. meningitides triggers astroglial reactivity as well as increases the production of pro-inflammatory cytokines and chemokines such as IL-6, TNF-α, IL-8, CXCL-1 and CXCL-10 [240]. Besides activation, astrocytes may undergo pathological remodelling and act as a reservoir for infection. Furthermore, astrocytes can promote apoptotic death of their uninfected neighbours through gap junction route [68, 240]. Astroglial reactivity, that includes overexpression of GFAP, keeps infectious process at bay. Indeed, genetic deletion of GFAP associated with suppressed astrogliosis, significantly exacerbated the neurological damage induced by intra-brain injection of S. aureus [197].
Astrocytes are fundamental players not only in bacterial but also in viral infections of the CNS. First and foremost, astrocytes can be directly infected by a virus. For example, astrocytes accumulate human immunodeficiency virus-1 (HIV-1) in a cluster of differentiation 81 (CD81)-lined vesicles. Inside these vesicles, the virus is protected from degradation [83]. The very same vesicles contributed to the secondary trans-infection of T-cells [83]. In the dementia caused by HIV brain infection, astrocytes undergo both reactive remodelling and astroglial degeneration and astroglial death. These processes may reduce homeostatic support and hence contribute to cognitive deficit [46]. The astroglial infection with HIV-1 (similarly to bacterial infection) decreased expression of connexins and syncytial connectivity [150]. In a similar manner, astrogliotic response is mounted in response to infection with the herpes simplex virus 1 (HSV1). Here, activation of astrocytes is mediated by TLR3 and it is neuroprotective. Deletion of TLR3 suppressed astrogliosis and exacerbated HSV pathology in mice [168] as well as in humans [91]. Infection with cytomegalovirus (CMV) was associated with astroglial homeostatic failure. The CMV infected astrocytes showed decreased released of thrombospondins and deficient glutamate uptake, possibly linked to an increased excitotoxicity [235, 236]. Neurotropic viruses of the family of Flaviviridaem represented by Zika virus and tick-borne encephalitis virus (TBEV) invade astrocytes by endocytosis [159, 240]. Astroglial infection with TBEV does not visibly affect their survival or function, and it is generally believed that astroglial cells act as a reservoir for this type of virus [240]. Astrocytes also represent the cellular target for some protozoan parasite, most notably for Toxoplasma gondii. Astrocytes infected with T. gondii undergo biochemical remodelling associated with up-regulated synthesis of kynurenic acid that in turn may be linked to some forms of schizophrenia, which will be discussed in appropriate section below. In addition, infection of astrocytes with this protozoan results in the loss of gap junctions [37].
7.4.2. Systemic Infections and the Brain: Sepsis-Associated Encephalopathy
Systemic inflammation frequently accompanies various infectious and non-infectious diseases including degenerative and metabolic disorders. This systemic inflammation often is manifested in the form of sepsis. Sepsis (and in particular abdominal sepsis) is frequently accompanied with an acute brain dysfunction, generally defined as sepsis-associated encephalopathy or SAE [187]. From the clinical perspective, the SAE is regarded as a sign of the severity of a septic state, which potentially worsens the prognosis [158]. The SAE is defined as a clinical syndrome associated with the general brain dysfunction that develops in sepsis in the absence of primary infection of the nervous tissue. The histopathological signs of the SAE include infarctions, petechial and small focal haemorrhages, septicembolic abscesses and septicopyemic microabscesses, disseminated intravascular coagulation (DIC) syndrome with fibrinous microthrombi, multifocal necrotising leukoencephalopathy, necrotic or apoptotic neuronal death, perivascular and cytotoxic oedema, damage of the blood–brain barrier and reactive neuroinflammation [89, 186, 187]. Sepsis is often associated with the formation of abscesses and microabscesses in the brain parenchyma, which can be regarded as directly associated with the SAE. The SAE, especially at the early stages is often associated with ‘sickness behaviour’, the syndrome accompanying system inflammation. The symptomatology of sickness behaviour syndrome includes anxiety, anorexia, anhedonia, depression, cognitive changes, including decreased concentration, learning and memory [56].
At the neurochemical level, the leading pathological changes in an SAE are represented by aberrant neurotransmission, which is responsible for cognitive and psychotic symptoms. Substantial changes in expression of main neurotransmitter receptors including receptors for γ-aminobutyric acid (GABA), serotonin, dopamine and noradrenaline have been observed in the brain in systemic infections [100, 208]. Changes in neurotransmitter homeostasis in sepsis are arguably related to alterations of amino acids levels in the blood characterised by a decrease in branched chain amino acids together with relative increase in aromatic amino acids [15]. In addition, compromised brain barriers allow a substantial influx of aromatic amino acids, such as tyrosine, phenylalanine and tryptophan, which may act as false neurotransmitters and alter biosynthesis of true neurotransmitters (e.g., dopamine, noradrenaline and serotonin—[188]).
Astrocytes, endfeet of which form glia limitans and hence can be considered as the parenchymal portion of brain barriers (the blood–brain and the blood–cerebrospinal fluid), define, to a very large extent, the resistance of the nervous tissue to the systemic inflammation. Intimate contacts of astrocytes with all other cellular elements of the nervous tissue allow them to regulate the relationship between the CNS and systemic physiology and pathology. In the context of SAE, astroglial reactivity is the principal mechanism that limits the propagation of pathological agents through the nervous tissue [41, 193]. Inhibition of astrogliotic response compromises astroglial barrier function and aggravates encephalopathy in the context of systemic inflammation or infectious lesion to the brain. For example, in transgenic mice with deleted gene for GFAP (this intervention suppresses astrogliosis), brain abscesses caused by Staphylococcus aureus or Toxoplasma gondii were much larger. Lesions become poorly demarcated, bacterial penetration significantly increased, neuronal death was much exacerbated and severe brain oedema developed [197]. Suppression of astroglial reactivity by activation of NF-κB signalling cascade in retinal ischemia or in spinal cord injury, is associated with an increased neuronal damage [27, 63]. Finally, inability of astrocytes to acquire reactive phenotype results in swelling, cytotoxic oedema and spread of damage in infectious abscesses [187].
Astrocytes contribute to the pathology of the blood–brain barrier, which is classified as disruptive and non-disruptive alterations, with both variants present in systemic inflammation [187]. The non-disruptive BBB pathology develops at the molecular and cellular levels when BBB permeability is affected following up- or down-regulation of receptors and transporters expressed in endothelial cells and astrocytes [209]. Disruptive BBB alterations develop through anatomical changes, which include degradation of glycocalyx, a loss of integrity of tight junctions, mitochondrial damage, appearance of fenestrae between endothelial cells, endothelial cells death, collapse of glia limitans and astrocytopathy. Disruption of BBB in systemic inflammation is mediated by blood-derived metalloproteinases, prostanoids, nitric oxide and reactive oxygen species [38, 136]. The switch between non-disruptive and disruptive BBB pathology depends on the severity of systemic inflammation. At the early stages of SAE the non-disruptive changes prevail, whereas in severe sepsis, both non-disruptive and disruptive changes occur [209].
7.5. Toxic Damage of the CNS
7.5.1. Heavy Metal Toxic Encephalopathies
Heavy metals, which cause severe brain damage with cognitive deficits, target primarily astrocytes. This is because heavy metals (such as manganese, lead, aluminium or mercury, in the form of methylmercury) mainly accumulate into astrocytes through different plasmalemmal transporters. In general, heavy metals down-regulate astroglial expression of glutamate transporters which decrease glutamate clearance and trigger excitotoxicity [198, 199, 217, 232].
Poisoning by methylmercury is known as Minamata disease named after the city of Minamata in Japan where the disease was first described [129]. The symptoms of Minamata disease include visual abnormalities, sensory lesions, cerebellar ataxia, hearing loss, weakness, tremor and cognitive decline. Methylmercury primarily accumulates in astroglia, where it inhibits glutamate and cystine uptake [5]. Suppression of glutamate uptake instigates exocytotic neuronal death, whereas inhibition of cystine transport limits astroglial synthesis of glutathione hence reducing astroglial capacity to counteract the accumulation of reactive oxygen species; both these processes contribute to neurotoxicity and neuronal death [57, 232].
Exposure to toxic concentrations of lead similarly causes neurodegeneration. Lead primarily accumulates in astroglia, where it down-regulates expression of EAAT-2 glutamate transporter, increases astroglial production of vascular endothelial growth factor, and impairs astroglia-associated water homeostasis by increasing the water permeability of aquaporin 4 [85]. Arguably, these mechanisms contribute to cytotoxic and vascular brain oedema observed in patients with lead poisoning.
Aluminium toxic encephalopathy is manifested by cognitive impairments, speech alterations, seizures and flapping wrist tremor (asterixis). Treatment of cultured astrocytes with aluminium led to swelling, destruction of the cytoskeleton, reduction in gap junctional connectivity, inhibition of glutamate uptake and increased astroglial apoptosis. Loss of astroglial glutamate uptake triggered neuronal death in neuronal–glial co-cultures [198, 199].
The main symptom of acute manganese neurotoxicity is an acute psychosis, whereas chronic manganese poisoning leads to parkinsonism. Astrocytes possess the high capacity manganese transport system; treatment of primary cultured astrocytes with manganese suppresses glutamate uptake and promotes apoptosis [57].
7.5.2. Hyperammonemia and Hepatic Encephalopathy
Increase in blood ammonium accompanies several diseases. The most frequent cause of hyperammonemia is, however, associated with an acute or chronic liver failure (the liver being the main organ for ammonia clearance). Hyperammonemia affects the brain and triggers a condition generally known as hepatic encephalopathy, manifested by cognitive and behavioural impairment; symptoms include confusion, forgetfulness, irritability and alterations of consciousness, such as lethargy and somnolence. Severe hyperammonemia provokes brain oedema, coma and death [31, 35, 73]. In the CNS ammonia is detoxified by glutamine synthetase localised exclusively in astrocytes; this enzyme catabolises ammonium reaction with glutamate and produces glutamine [3, 143, 175]. This reaction is central for glutamate-glutamine shuttle; it also fixes ammonium, which is liberated during physiological neuronal activity [126]. Ammonium overload occludes this pathway and blocks glutamine synthetase hence causing major disturbances of glutamatergic and GABAergic (as glutamate is the precursor to GABA) neurotransmission, which underlie all the symptoms outlined above [31, 35].
Hyperammonemia also affects homeostatic astroglial functions. Exposure of astrocytes to ammonium results in a down-regulation of expression of inward rectifying Kir4.1 channels, an event mediated through astrocytic NMDA receptors by a yet uncharacterised mechanism. Decrease in the density of Kir4.1 channels, in turn, affects astroglial K+ buffering which may impair neuronal excitability [144, 166]. Exposure of astrocytes to ammonium also produces aberrant Ca2+ signalling by increasing expression of Ca2+-permeable TRPC1 channels, up-regulating expression of Cav1.2 voltage-gated Ca2+ channels and facilitating Ca2+ release from the intercellular stores [86, 119, 223]. Increased Ca2+ load of astroglial cytosol, in turn, triggers the exocytotic secretion of glutamate which further exacerbates excitotoxic damage of the nervous tissue [82, 139]. Finally, increased ammonium compromises astroglial transport of Na+ and H+ which contributes to aberrant pH regulation in the CNS [105, 106]. All these molecular changes result in impaired synaptic transmission, synaptic plasticity and cognitive capabilities [45].
7.6. Astrogliopathology in Stroke
A disruption of the blood flow results either from a blood vessel rupture (that causes a haemorrhage), or by a restriction of blood supply to the brain or parts of the brain, because of a vascular occlusion (thrombosis or embolism), or to a systemic decrease in blood supply (resulting, for example, from a heart failure). This status is generally referred to as brain ischaemia. As a consequence, brain ischaemia can be either global, or focal, the latter corresponding to a stroke.
Astrogliopathology in stroke is complex and multifaceted, with astrocytes being both neuroprotective and neurotoxic [81, 239]. Focal ischaemia results in the infarction of nervous tissue creating a zone of pan-necrosis or an infarction core. At this core, all cells, neurones, glia and other non-neuronal cells undergo rapid necrosis. The size of the core is determined by anatomical location and duration of the ischemic attack. Quite frequently the focal ischemia is transient, as the blood flow can be restored when the vessel blockage is removed. In this case, restored blood flow results in reperfusion of the damaged area, which itself is potentially damaging because of the production of reactive oxygen species and secondary ion imbalances.The infarction core is surrounded by the ischemic penumbra, which contains viable cells, although with compromised metabolism and function. The infarction core is formed rapidly, within minutes to hours after initiation of the stroke. This is followed by a much slower process of expansion of the infarction zone through the penumbra, which develops over many hours and days. The final neurological deficit is often defined by the limits of the infarction expansion, which in turn depends on astroglial response.
Astrocytes support neurones in the ischaemic penumbra through several homeostatic pathways. First and foremost, astrocytes maintain homeostasis of glutamate in the ischaemic zones. They also feed neurones with metabolic substrates such as lactate. Energising astroglial mitochondria, for example, increase neuroprotection in the ischaemic context [180]. Taming glutamate excitotoxicity, which always follows stroke, almost solely falls onto astroglial cells. Down-regulation of expression of the astroglial glutamate transporter GLT-1/EAAT1 with siRNA increases the size of the infarct [167], whereas targeted overexpression of GLT-1 in astrocytes limits the infarction volume and alleviates neurological deficit [88]. Similarly, stimulation of glutamate uptake with pharmacological agents such as tamoxifen or riluzole decreased infarction volume in animal models [227, 238]. Of note, astroglial glutamate transporters are Na+ dependent, and hence maintenance of Na+ transmembrane gradients is critical for glutamate clearance [109]. Another important component of astroglia-dependent neuroprotection in the ischaemic penumbra is associated with antioxidant defence. Astrocytes are critical for both glutathione and ascorbic acid systems, which are the most powerful scavengers of reactive oxygen species [61, 62, 125]. Progression of cell death through the ischaemic penumbra is mediated by spreading depolarisation, which stresses astroglial ionostatic capacity. Furthermore, astrocytes may propagate death signal, triggering distant neuronal death [115, 142].
An important component of astroglial response to stroke is associated with reactive astrogliosis. Ischaemic damage to the brain tissue rapidly instigates astroglial activation through the release of DAMPs; the severity of astrogliotic remodelling and reactive phenotypes depend on the distance to the ischemic core [33]. Astrocytes close to the ischaemic core undergo anisomorphic gliosis, form astroglial palisades and produce astroglial scar that limits the damage to the nervous tissue. In parallel, distantly to the core astrocytes undergo isomorphic, neuroprotective astrogliotic remodelling, which is critically important for post-lesion regeneration The main outcome of astrogliosis in the immediate vicinity of the necrotic area is the formation of an astroglial scar, whereas more peripheral reactive astrocytes are important for post-lesion regeneration [81].
7.7. Metabolic Disorders
7.7.1. Congenital Glutamine Deficiency with Glutamine Synthetase Mutations
Congenital glutamine synthetase deficiency, a rather rare recessive inborn disease, results from mutations to the gene GLUL that encodes astroglia-specific glutamine synthetase, thus, this disorder can be considered as a specific astrogliopathy. This disease is characterised by pronounced malformation of the brain with severe white matter deficiency and abnormal gyri formation. Functionally, this deficiency is manifested as epileptic encephalopathy. The deficit in glutamine synthetase in the liver promotes chronic hyperammonemia. In addition, levels of glutamine are reduced in the brain as well as in other organism fluids. The disease results in prenatal malformation of various organs and is generally incompatible with life. Most of the infants die shortly after birth. The leading pathophysiological mechanism is associated with impaired ability of astrocytes to produce glutamine, which affects excitatory and inhibitory transmission; in addition, deficient glutamine synthetase cannot properly detoxify ammonium [195].
7.7.2. Pyruvate Carboxylase Deficiency
Pyruvate carboxylase is an enzyme of gluconeogenesis and it also contributes to anaplerotic metabolic pathways (i.e. producing intermediates for metabolic chains such as the Krebs cycle). In the CNS, pyruvate carboxylase is predominantly expressed in astrocytes. Pyruvate carboxylase deficiency is an autosomal recessive disease associated with impaired metabolism. The symptoms include retardation of mental development, recurrent seizures and metabolic acidosis [127]. There are three clinically distinct forms: type A, or the infantile form, in which children die in the early years; type B, or the severe neonatal form, with many neurological signs including pyramidal symptoms, in which babies die within 3 months after birth; type C or the benign form, which is characterised by mild neurological developmental deficits. The cellular pathogenesis remains largely unknown, but it is probably linked to reduced astroglial homeostatic function, such as glutamate buffering and regulation of angiogenesis [57]
7.7.3. Niemann–Pick Type C Disease
Niemann–Pick disease type C is a progressive neurodegenerative disease associated with hepatosplenomegaly. It is characterised as an autosomal recessive lysosomal storage disease, which results from loss-of-function mutations of genes encoding NPC-1 or NPC-2 proteins [176]. These proteins are localised in astroglial perisynaptic processes and may be involved in the regulation of cholesterol transport and, hence, synaptogenesis or synaptic maintenance [153]. Astroglia-specific genetic deletion of Npc1 from mice resulted in reduced neuronal cholesterol, which was associated with decreased neuronal and glial death and three times increase in the life span [237]. There is also evidence of a possible contribution for NPC-1 protein in calcium homeostasis and signalling.
7.7.4. Aceruloplasminemia
The enzyme ceruloplasmin (also known as ferroxidase) is a part of iron metabolism. In the CNS this enzyme is expressed almost exclusively in perivascular astrocytes. Ceruloplasmin is an important component of protection of the nervous tissue against iron-associated lipid peroxidation and formation of hydroxyl radicals. Mutation of the ceruloplasmin gene with loss-of-function causes the autosomal recessive disease known as aceruloplasminemia, which can be defined as an inherited neurodegenerative disorder with systemic iron-overload syndrome [138]. This disease is characterised by primary lesions to astrocytes, which affects their morphology and results in an appearance of foamy spheroid bodies at the vascular endfeet [147]. Aceruloplasminemia is also associated with neuronal death and the appearance of iron deposition.
7.8. Alexander Disease
Alexander disease (AxD), named after William Stewart Alexander, a neuropathologist who described it for the first time [4], is a rare, chronic and usually fatal neurodegenerative disorder. Clinically, AxD may be defined as a severe leukodystrophy; pathophysiologically, it is a primary genetic astrogliopathology [131]. The AxD results from a dominant gain-of-function mutation of the gene encoding GFAP. This leads to astroglial pathology that, in turn, results in a severe damage to the developing white matter. The histopathological hallmark of AxD is an accumulation of protein aggregates, known as Rosenthal fibres, around astroglial nuclei and endfeet [131]. AxD is subclassified into: (i) Type I, characterised with an early onset and severe mental and physical disabilities, megalencephaly, seizures, spasticity, difficulty speaking and swallowing, and (ii) Type II, with a later onset and somewhat different and milder clinical manifestations with normal development and head size, with rare occurrence of seizures, but with ataxia, visual and autonomic abnormalities, troubles in sleeping patterns, hyperreflexia, difficulty speaking and swallowing [160].
Astrocytes in AxD demonstrate reactive morphology. These glial cells also remodel their biochemistry and secretome. In particular, astrocytes start to release pro-inflammatory factors TNF-α and IL-1β. In addition, astrocytes in AxD have reduced expression of glutamate plasmalemmal transporters, decreased activity of proteasomes, increased autophagy and increased activity of stress-activated protein kinase/c-Jun N-terminal kinase (JNK) pathway [131]. Multiple mechanisms by which pathological mutation of GFAP affects cellular functions have been considered. These include: (i) mutated GFAP through positive feedback loop inhibits proteasome function which activates JNK, and activated JNK directly further inhibits proteasome [204]; (ii) mutated GFAP inactivates one or more proteins by degradation of the Rosenthal fibres, where fragments of the small stress proteins, HSP27, αB crystalline, the 20S proteasome subunit, p-JNK, p62 and plectin, have been detected [131]. So far the AxD remains incurable, although several therapeutic strategies aimed at reducing GFAP expression are in development.
7.9. Neurodevelopmental Disorders
7.9.1. Autism Spectrum Disorders (ASD)
The class of autistics spectrum disorders (ASD) embraces numerous pathological conditions of heterogeneous clinical presentation and pathophysiology. They all, however, are manifested by deficits in social interactions and restrictive patterns of behaviours. Some of the autistic diseases are associated with intellectual deficits [162]. The underlying mechanism of ASDs is most likely associated with malformation of neuronal networks and aberrant neurotransmission in embryonic development caused by environmental and/or intrinsic factors [42, 80, 130, 174]. Formation of neuronal ensembles, synaptogenesis and synaptic elimination all critically depend on the performance of the astroglial cradle, which controls birth, life and death of synapses [213]. Astrocytes are responsible for neuroprotection and detoxification of harmful agents, including reactive oxygen species (through secreting antioxidants such as glutathione and ascorbic acid [30, 229]). Astrocytes tame excitotoxicity through glutamate uptake and they also control neurotransmitters catabolism and supply of neurotransmitter precursors [214]. In parallel, astrocytes are the main target for neurotoxic factors, such as heavy metals, which are linked to the aetiology of ASD [234]. Astrogliopathology in ASD has not been investigated in great details; there are some indications for astrogliosis [234], increased expression of connexin 43 and decreased expression of aquaporin 4 [71].
7.9.2. Down Syndrome
Down syndrome (DS), which is linked to the trisomy of chromosome 21, is characterised by mental retardation. In DS, the density of astrocytes is significantly reduced in the cortex [102] with decreased ability to properly support synaptogenesis and neuronal maturation [44].
7.9.3. Fragile X Syndrome
Expression of Fragile X mental retardation protein (FXMRP) causes a specific form of a neurodevelopmental disease manifested in ASD symptoms and mental disability, Fragile X syndrome that is also known as Martin–Bell syndrome or Escalante’s syndrome [107]. Expression of FXMRP in astrocytes weakens their homeostatic function and neuroprotection in the in vitro experiments. Co-culturing healthy neurones with astrocytes harbouring FXMRP leads to abnormal neuronal dendritic morphology and reduced synaptic connectivity. In contrast, co-culturing FXMRP expressing neurones with healthy astrocytes prevents the development of abnormal dendritic morphology [97, 98].
7.9.4. Costello Syndrome
Costello syndrome (named so after its discoverer Jack Costello [48]) belongs to the family of the so-called RASopathies (where RAS stands for rat sarcoma) characterised by aberrant Ras signalling [205]. In this pathology, astroglial cells expressing a mutated HRAS (Harvey rat sarcoma viral oncogene homolog) gene demonstrate hyperactive Ras signalling, which accelerates differentiation and maturation of astrocytes, and leads to astroglial hypertrophy. This is also associated with pathological extracellular matrix and abnormal formation of neuronal networks that in turn causes cognitive and behavioural abnormalities [112].
7.10. Major Neuropsychiatric Diseases
7.10.1. Schizophrenia
In schizophrenia the wide spectrum of astroglial abnormalities is present. Conceptually, schizophrenia is associated with astroglial asthenia, atrophy, loss of homeostatic capabilities and arguably pathological remodelling, while reactive changes are not characteristic. Decrease in astroglial numbers, as well as dystrophic or swollen astroglial profiles, appear in various brain regions, including cortical and hippocampal structures [69, 163, 181, 226]. Astrocytes derived from human induced pluripotent stem cells obtained from schizophrenic patients and injected into mice, demonstrated atrophic morphology and loss of homeostatic functions [230].
Astrocytes in schizophrenia are characterised by a significant down-regulation of expression of several astroglia-specific molecules such as deiodinase type II, aquaporin-4, S100β, glutamine synthetase, plasmalemmal glutamate transporters and thrombospondin. These changes were the most prominent in the deep layers of the anterior cingulate gyrus, suggesting that a subset of astrocytes localised to specific cortical layers can be affected [231]. In the prefrontal cortex and hippocampus, a decrease in the expression of EAAT1/2 plasmalemmal glutamate transporters has been detected [16, 17, 146, 185], which may be linked to abnormalities in glutamatergic transmission. Genetic deletion of EAAT1 glutamate transporter promoted appearance of schizophrenia-like phenotypes manifested by locomotor hyperactivity and abnormal social behaviour [103, 104]. Astrocytes from rodent phencyclidine model of schizophrenia demonstrated a decrease in the expression of plasmalemmal cystine–glutamate exchanger Sxc− [12], which modulates extrasynaptic concentration of glutamate and contributes to the biosynthesis of glutathione. Astrocytes may promote aberrant neurotransmission through synthesis and release of kynurenic acid that acts as an endogenous inhibitor of the NMDA receptor glycine binding site; kynurenic acid also blocks acetylcholine nicotinic receptors. The astroglial production of kynurenic acid is significantly up-regulated following brain infection with Toxoplasma gondii, which increases the risk of schizophrenia [183].
7.10.2. Mood Disorders
Astrogliopathology seems to be rather prominent in mood disorders [165, 178, 218]. The total number of glial cells and of astrocytes, in particular, is decreased in the orbitofrontal area and anterior cingulate, prefrontal, entorhinal and subgenual cortices, as well as the amygdala of the brains obtained from patients with major depression or bipolar disorder. [26, 49, 50, 149, 164]. In animals subjected to chronic stress, which instigates depressive phenotypes, GFAP expression and number of GFAP positive cells were reduced [28, 55]. Similarly, in models of attention deficit disorder and depression other astroglial markers, including aquaporin 4, astroglial connexins, astroglial plasmalemmal glutamate transporters and glutamine synthetase were all down-regulated [14, 21, 184].
Ablation of astrocytes in the medial prefrontal cortex of mice with the neuroglial toxin L-α-aminoadipic acid triggered an emergence of a depressive phenotype similar to that induced by chronic stress [13]. Exposure to chronic stress led to a down-regulation of astroglial expression of connexin 43 along with the reduction of gap junctional coupling in astrocytic syncytia. Pharmacological inhibition of astroglial connexon-based channels in the prefrontal cortex induced depressive behaviour manifested by anhedonia [201]. A similar phenotype was observed after inhibition of astroglial plasmalemmal glutamate transporters [18]. Chronic treatment with antidepressants directly affected astroglia, by increasing expression of a variety of receptors and transporters responsible for CNS homeostasis and limiting glutamate release [53, 60, 123, 171]. In conclusion, mood disorders are associated with astroglial degen eration and astroglial asthenia, which compromise brain homeostatic reserve and arguably synaptic transmission.
7.10.3. Addictive Disorders
Various nosological forms of addictive disorders are associated with astrogliopathies. Post-mortem analysis of the human brain samples revealed both astroglial reactivity with astroglial degeneration, and astroglial cell death with astroglial atrophy [9, 36, 72, 134, 145, 200, 225]. In the animal models of addiction with cocaine, methamphetamine and morphine, astroglial activation and increase in GFAP expression have been identified [25, 76, 84, 194]. In contrast, in the model of chronic alcoholism a decrease in GFAP expression and morphological atrophy of astrocytes were detected [75, 172]. In post-mortem tissues isolated from alcoholic sufferers, both hypertrophic GFAP positive astrocytes as well as areas with decreased GFAP expression and decreased density of astrocytes were described [52, 133].
The number of astrocytes is decreased in the prefrontal cortex of alcoholics [135]. A similar decrease in astroglial density and GFAP expression was detected in the prelimbic cortex of ethanol-preferring chronically alcoholic rats [133]. Additionally, a decrease in astrocyte density was observed in response to acute binge drinking in male (but not female) adult rats [111]. Ablation of astroglia with L-α-aminoadipic acid or uncoupling astroglial syncytia using a pharmacological inhibitor of connexin channels in the prefrontal cortex increases alcohol preference [132].
Addictive disorders are linked to astroglial plasmalemmal glutamate transport. Expression of EAAT2 as well as Sxc− glutamate transporters is decreased in the context of alcoholism. Incidentally, total extracellular glutamate increases most likely due to an imbalance between glutamate uptake (EAAT2) and release (Sxc−) [141, 169, 170]. Increase in the expression of EAAT2 by treatment with β-lactam antibiotic ceftriaxone decreased alcohol dependence [161, 179].
7.11. Epilepsy
In epilepsy, astrocytes undergo substantial pathological remodelling, which greatly affects their homeostatic capabilities and is linked to pathophysiology of this disease. In particular, the epileptic astroglial phenotype includes changes (mutations and/or expression levels) in ion channels, receptors and transporters [19, 196]. Abnormal electrophysiological characteristics have been observed in astrocytes isolated from patients with mesial temporal lobe epilepsy and associated sclerosis. These astrocytes, in addition, have severe impairment of intercellular coupling [19]. Astrocytes in sclerotic tissue up-regulated the expression of GFAP, suggesting thus their activation. Decrease in K+ buffering seems to be the dominant feature of astroglial remodelling in epileptic brains, which results in an increase of extracellular K+ concentration [124, 140]. Such an increase in extracellular K+ can be sufficient to instigate seizures [206]. Abnormal astroglial K+ buffering, at least in part, is linked to a significant down-regulation of inward rectifier Kir4.1 channels. Here, decreases in Kir4.1 current density and protein content have been found in astrocytes from the human sclerotic CA1 hippocampal area [24, 93, 94]. Genetic deletion of KCNJ10 gene encoding Kir4.1 channel specifically from astroglia resulted in impaired K+ buffering, depolarisation of astrocytes, motor impairments and early death [59]. Other studies confirmed this finding by demonstrating that deletion of Kir4.1 channels induces epileptiform symptoms in animals [196]. Mutations of KCNJ10 gene in humans are associated with the development of SeSAME syndrome (also called EAST syndrome), an autosomal recessive disorder characterised by epilepsy, ataxia, sensorineural deafness, wasting renal tubulopathy, mental retardation and electrolyte imbalance [22, 182]. Whether the modifications of Na+/K+ ATPase (NKA), another critical component of astroglial K+ buffering (NKA is primarily responsible for K+ uptake, whereas Kir4.1 channels for K+ release and shuttling back to neurones [29, 114]) contribute to SeSAME, it remains to be explored. One of the forms of migraine, the familial hemiplegic migraine type 2, is however associated with loss-of-function mutation of astroglial specific α2 subunits of NKA [39]. Considering fundamental similarities of pathogenesis of migraine and epilepsy we may expect some abnormalities of astroglial NKA in the later pathology.
Epileptic astrocytes also demonstrate compromised glutamate uptake and homeostasis [51]. Deletion of the astroglial EAAT2 glutamate transporter results in an epileptiform phenotype with lethal spontaneous seizures, increased susceptibility to acute cortical injury and seizures after administration of sub-convulsive doses of pentylenetetrazole [202]. Similarly, seizures and epileptiform phenotype were triggered by pharmacological inhibition of EAAT by intracerebroventricular injections of DL-threo-beta-benzyloxyaspartate [58]. Down-regulation of glutamine synthetase was also linked to epilepsy through affecting inhibition in neuronal networks [151]. Animals subjected to long-lasting pharmacological blockade of glutamine synthetase demonstrated seizures [20, 224], whereas levels of glutamine synthetase were found to be significantly decreased in the human hippocampus and amygdala of patients with temporal lobe epilepsy [64]. Finally, loss-of-function mutations of glutamine synthetase induced severe seizures [87]. Astrocytes can also contribute to pathogenesis of epilepsy through anomalous adenosine homeostasis, resulting from modified expression of the astroglia-specific adenosine kinase, which is the key enzyme for adenosine turnover in the CNS [10, 23]. Expression of adenosine kinase is high in tissues from subjects with pharmacologically refractory temporal lobe epilepsy [10, 11, 128]. Increase in expression and activity of adenosine kinase diminishes the availability of adenosine, thus increasing neuronal network excitability and increasing probability of seizures [117, 118].
7.12. Epilogue
Since the inception of neurobiology, we have had a conceptual roller coaster ride in regards to the role of neuroglia in pathology of the brain. Two centuries ago our founding fathers of gliology had a clear vision on the active role of glia, i.e. glia is more than putty and has prominent roles in pathophysiology of the brain. Awkwardly, the twentieth century brought a different view where starring role has been solely played by neurones. This dominant neurono-centric approach has been challenged by the resurgence of neurogliopathology in the past 20 years. While we here presented the astrocyte-centric view of the brain pathology, we surely support the notion that it is the interaction between neurones and glia that underlies physiology and pathology of the brain. These two major cellular constituents interact, so that perturbing one will affect the other. Thus, only intellectually acceptable approaches to grapple with the management of the brain diseases will be those that have gestalt assets.
Acknowledgments
VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Oxford Road, Manchester M13 9PT, UK; Center for Basic and Translational Neuroscience, Faculty of Health and Medical Sciences, University of Copenhagen, 2200 Copenhagen, Denmark; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, 48011, Bilbao, Spain.
Margaret S. Ho, School of Life Science and Technology, ShanghaiTech University, 201210 Shanghai, China
Nina Vardjan, Laboratory of Neuroendocrinology-Molecular Cell Physiology, Faculty of Medicine, Institute of Pathophysiology, University of Ljubljana, Ljubljana, Slovenia; Celica BIOMEDICAL, Ljubljana, Slovenia.
Robert Zorec, Laboratory of Neuroendocrinology-Molecular Cell Physiology, Faculty of Medicine, Institute of Pathophysiology, University of Ljubljana, Ljubljana, Slovenia; Celica BIOMEDICAL, Ljubljana, Slovenia.
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA.
References
- 1.Achucarro N (1910) Some pathological findings in the neuroglia and in the ganglion cells of the cortex in senile conditions. Bull Gov Hosp Insane 2:81–90 [Google Scholar]
- 2.Alberdi E, Wyssenbach A, Alberdi M, Sanchez-Gomez MV, Cavaliere F, Rodriguez JJ, Verkhratsky A, Matute C (2013) Ca2+-dependent endoplasmic reticulum stress correlates with astrogliosis in oligomeric amyloid beta-treated astrocytes and in a model of Alzheimer’s disease. Aging Cell 12:292–302 [DOI] [PubMed] [Google Scholar]
- 3.Albrecht J, Zielinska M, Norenberg MD (2010) Glutamine as a mediator of ammonia neurotoxicity: A critical appraisal. Biochem Pharmacol 80:1303–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander WS (1949) Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. Brain 72:373–381 [DOI] [PubMed] [Google Scholar]
- 5.Allen JW, Shanker G, Aschner M (2001) Methylmercury inhibits the in vitro uptake of the glutathione precursor, cystine, in astrocytes, but not in neurons. Brain Res 894:131–140 [DOI] [PubMed] [Google Scholar]
- 6.Alzheimer A (1910) Beiträge zur Kenntnis der pathologischen Neuroglia und ihrer Beziehungen zu den Abbauvorgängen im Nervengewebe In: Nissl F, Alzheimer A (eds) Histologische und histopathologische Arbeiten über die Grosshirnrinde mit besonderer Berücksichtigung der pathologischen Anatomie der Geisteskrankheiten. Jena, Gustav Fischer, pp 401–562 [Google Scholar]
- 7.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andriezen WL (1893) The neuroglia elements of the brain. Brit Med J 2:227–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong V, Reichel CM, Doti JF, Crawford CA, McDougall SA (2004) Repeated amphetamine treatment causes a persistent elevation of glial fibrillary acidic protein in the caudate-putamen. Eur J Pharmacol 488:111–115 [DOI] [PubMed] [Google Scholar]
- 10.Aronica E, Sandau US, Iyer A, Boison D (2013) Glial adenosine kinase–a neuropathological marker of the epileptic brain. Neurochem Int 63:688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA (2011) Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia 52:1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I (2008) Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology 33:1760–1772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banasr M, Duman RS (2008) Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry 64:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barley K, Dracheva S, Byne W (2009) Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res 112:54–64 [DOI] [PubMed] [Google Scholar]
- 15.Basler T, Meier-Hellmann A, Bredle D, Reinhart K (2002) Amino acid imbalance early in septic encephalopathy. Intensive Care Med 28:293–298 [DOI] [PubMed] [Google Scholar]
- 16.Bauer D, Gupta D, Harotunian V, Meador-Woodruff JH, McCullumsmith RE (2008) Abnormal expression of glutamate transporter and transporter interacting molecules in prefrontal cortex in elderly patients with schizophrenia. Schizophr Res 104:108–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE (2010) Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res 117:92–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bechtholt-Gompf AJ, Walther HV, Adams MA, Carlezon WA Jr, Ongur D, Cohen BM (2010) Blockade of astrocytic glutamate uptake in rats induces signs of anhedonia and impaired spatial memory. Neuropsychopharmacology 35:2049–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedner P, Dupper A, Huttmann K, Muller J, Herde MK, Dublin P, Deshpande T, Schramm J, Haussler U, Haas CA, Henneberger C, Theis M, Steinhauser C (2015) Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138:1208–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benedetti B, Matyash V, Kettenmann H (2011) Astrocytes control GABAergic inhibition of neurons in the mouse barrel cortex. J Physiol 589:1159–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, Schatzberg AF, Myers RM, Akil H, Watson SJ (2011) Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry 16:634–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bockenhauer D, Feather S, Stanescu HC, Bandulik S, Zdebik AA, Reichold M, Tobin J, Lieberer E, Sterner C, Landoure G, Arora R, Sirimanna T, Thompson D, Cross JH, van’t Hoff W, Al Masri O, Tullus K, Yeung S, Anikster Y, Klootwijk E, Hubank M, Dillon MJ, Heitzmann D, Arcos-Burgos M, Knepper MA, Dobbie A, Gahl WA, Warth R, Sheridan E, Kleta R (2009) Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med 360, 1960–1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boison D, Aronica E (2015) Comorbidities in neurology: is adenosine the common link? Neuropharmacology 97:18–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bordey A, Sontheimer H (1998) Properties of human glial cells associated with epileptic seizure foci. Epilepsy Res 32:286–303 [DOI] [PubMed] [Google Scholar]
- 25.Bowers MS, Kalivas PW (2003) Forebrain astroglial plasticity is induced following withdrawal from repeated cocaine administration. Eur J Neurosci 17:1273–1278 [DOI] [PubMed] [Google Scholar]
- 26.Bowley MP, Drevets WC, Ongur D, Price JL (2002) Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry 52:404–412 [DOI] [PubMed] [Google Scholar]
- 27.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR (2005) Inhibition of astroglial nuclear factor κB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braun K, Antemano R, Helmeke C, Buchner M, Poeggel G (2009) Juvenile separation stress induces rapid region- and layer-specific changes in S100ss- and glial fibrillary acidic protein-immunoreactivity in astrocytes of the rodent medial prefrontal cortex. Neuroscience 160:629–638 [DOI] [PubMed] [Google Scholar]
- 29.Breslin K, Wade JJ, Wong-Lin K, Harkin J, Flanagan B, Van Zalinge H, Hall S, Walker M, Verkhratsky A, McDaid L (2018) Potassium and sodium microdomains in thin astroglial processes: A computational model study. PLoS Comput Biol 14:e1006151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bridges RJ, Natale NR, Patel SA (2012) System xc− cystine/glutamate antiporter: an update on molecular pharmacology and roles within the CNS. Br J Pharmacol 165:20–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ (2010) Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics 7:452–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burda JE, Bernstein AM, Sofroniew MV (2016) Astrocyte roles in traumatic brain injury. Exp Neurol 275(Pt 3):305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burda JE, Sofroniew MV (2014) Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 81:229–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnstock G, Fredholm BB, Verkhratsky A (2011) Adenosine and ATP receptors in the brain. Curr Top Med Chem 11:973–1011 [DOI] [PubMed] [Google Scholar]
- 35.Butterworth RF (2011) Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology 53:1372–1376 [DOI] [PubMed] [Google Scholar]
- 36.Büttner A, Weis S (2006) Neuropathological alterations in drug abusers. The involvement of neurons, glial and vascular systems. Forensic Sci Med Pathol 2:115–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Campos de Carvalho AC, Roy C, Hertzberg EL, Tanowitz HB, Kessler JA, Weiss LM, Wittner M, Dermietzel R, Gao Y, Spray DC (1998) Gap junction disappearance in astrocytes and leptomeningeal cells as a consequence of protozoan infection. Brain Res 790:304–314 [DOI] [PubMed] [Google Scholar]
- 38.Candelario-Jalil E, Taheri S, Yang Y, Sood R, Grossetete M, Estrada EY, Fiebich BL, Rosenberg GA (2007) Cyclooxygenase inhibition limits blood-brain barrier disruption following intracerebral injection of tumor necrosis factor-alpha in the rat. J Pharmacol Exp Ther 323:488–498 [DOI] [PubMed] [Google Scholar]
- 39.Capuani C, Melone M, Tottene A, Bragina L, Crivellaro G, Santello M, Casari G, Conti F, Pietrobon D (2016) Defective glutamate and K+ clearance by cortical astrocytes in familial hemiplegic migraine type 2. EMBO Mol Med 8:967–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carpentier PA, Duncan DS, Miller SD (2008) Glial toll-like receptor signaling in central nervous system infection and autoimmunity. Brain Behav Immun 22:140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cekanaviciute E, Buckwalter MS (2016) Astrocytes: integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics 13:685–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cellot G, Cherubini E (2014) GABAergic signaling as therapeutic target for autism spectrum disorders. Front Pediatr 2:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chauhan VS, Sterka DG Jr, Furr SR, Young AB, Marriott I (2009) NOD2 plays an important role in the inflammatory responses of microglia and astrocytes to bacterial CNS pathogens. Glia 57:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C, Jiang P, Xue H, Peterson SE, Tran HT, McCann AE, Parast MM, Li S, Pleasure DE, Laurent LC, Loring JF, Liu Y, Deng W (2014) Role of astroglia in Down’s syndrome revealed by patient-derived human-induced pluripotent stem cells. Nat Commun 5:4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chepkova AN, Sergeeva OA, Gorg B, Haas HL, Klocker N, Haussinger D (2017) Impaired novelty acquisition and synaptic plasticity in congenital hyperammonemia caused by hepatic glutamine synthetase deficiency. Sci Rep 7:40190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Churchill MJ, Wesselingh SL, Cowley D, Pardo CA, McArthur JC, Brew BJ, Gorry PR (2009) Extensive astrocyte infection is prominent in human immunodeficiency virus-associated dementia. Ann Neurol 66:253–258 [DOI] [PubMed] [Google Scholar]
- 47.Combes V, Guillemin GJ, Chan-Ling T, Hunt NH, Grau GE (2012) The crossroads of neuroinflammation in infectious diseases: endothelial cells and astrocytes. Trends Parasitol 28:311–319 [DOI] [PubMed] [Google Scholar]
- 48.Costello JM (1977) A new syndrome: mental subnormality and nasal papillomata. Aust Paediatr J 13:114–118 [DOI] [PubMed] [Google Scholar]
- 49.Cotter D, Mackay D, Chana G, Beasley C, Landau S, Everall IP (2002) Reduced neuronal size and glial cell density in area 9 of the dorsolateral prefrontal cortex in subjects with major depressive disorder. Cereb Cortex 12:386–394 [DOI] [PubMed] [Google Scholar]
- 50.Cotter D, Mackay D, Landau S, Kerwin R, Everall I (2001) Reduced glial cell density and neuronal size in the anterior cingulate cortex in major depressive disorder. Arch Gen Psychiatry 58:545–553 [DOI] [PubMed] [Google Scholar]
- 51.Coulter DA, Eid T (2012) Astrocytic regulation of glutamate homeostasis in epilepsy. Glia 60:1215–1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cullen KM, Halliday GM (1994) Chronic alcoholics have substantial glial pathology in the forebrain and diencephalon. Alcohol Alcohol Suppl 2:253–257 [PubMed] [Google Scholar]
- 53.Czeh B, Di Benedetto B (2013) Antidepressants act directly on astrocytes: evidences and functional consequences. Eur Neuropsychopharmacol 23:171–185 [DOI] [PubMed] [Google Scholar]
- 54.Czeh B, Nagy SA (2018) Clinical findings documenting cellular and molecular abnormalities of glia in depressive disorders. Front Mol Neurosci 11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Czeh B, Simon M, Schmelting B, Hiemke C, Fuchs E (2006) Astroglial plasticity in the hippocampus is affected by chronic psychosocial stress and concomitant fluoxetine treatment. Neuropsychopharmacology 31:1616–1626 [DOI] [PubMed] [Google Scholar]
- 56.Dantzer R, Kelley KW (2007) Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun 21:153–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Keyser J, Mostert JP, Koch MW (2008) Dysfunctional astrocytes as key players in the pathogenesis of central nervous system disorders. J Neurol Sci 267:3–16 [DOI] [PubMed] [Google Scholar]
- 58.Demarque M, Villeneuve N, Manent JB, Becq H, Represa A, Ben-Ari Y, Aniksztejn L (2004) Glutamate transporters prevent the generation of seizures in the developing rat neocortex. J Neurosci 24:3289–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Djukic B, Casper KB, Philpot BD, Chin LS, McCarthy KD (2007) Conditional knock-out of Kir4.1 leads to glial membrane depolarization, inhibition of potassium and glutamate uptake, and enhanced short-term synaptic potentiation. J Neurosci 27:11354–11365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dong L, Li B, Verkhratsky A, Peng L (2015) Cell type-specific in vivo expression of genes encoding signalling molecules in the brain in response to chronic mild stress and chronic treatment with fluoxetine. Psychopharmacology (Berl) [DOI] [PubMed] [Google Scholar]
- 61.Dringen R, Gutterer JM, Hirrlinger J (2000) Glutathione metabolism in brain metabolic interaction between astrocytes and neurons in the defense against reactive oxygen species. Eur J Biochem 267:4912–4916 [DOI] [PubMed] [Google Scholar]
- 62.Dringen R, Hirrlinger J (2003) Glutathione pathways in the brain. Biol Chem 384:505–516 [DOI] [PubMed] [Google Scholar]
- 63.Dvoriantchikova G, Barakat D, Brambilla R, Agudelo C, Hernandez E, Bethea JR, Shestopalov VI, Ivanov D (2009) Inactivation of astroglial NF-kappa B promotes survival of retinal neurons following ischemic injury. Eur J Neurosci 30:175–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eid T, Tu N, Lee TS, Lai JC (2013) Regulation of astrocyte glutamine synthetase in epilepsy. Neurochem Int 63:670–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El-Hage N, Podhaizer EM, Sturgill J, Hauser KF (2011) Toll-like receptor expression and activation in astroglia: differential regulation by HIV-1 Tat, gp120, and morphine. Immunol Invest 40:498–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esen N, Shuffield D, Syed MM, Kielian T (2007) Modulation of connexin expression and gap junction communication in astrocytes by the gram-positive bacterium S. aureus. Glia 55:104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Esen N, Tanga FY, DeLeo JA, Kielian T (2004) Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem 88:746–758 [DOI] [PubMed] [Google Scholar]
- 68.Eugenin EA, Berman JW (2007) Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. J Neurosci 27:12844–12850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Falkai P, Bogerts B (1986) Cell loss in the hippocampus of schizophrenics. Eur Arch Psychiatry Neurol Sci 236:154–161 [DOI] [PubMed] [Google Scholar]
- 70.Farina C, Aloisi F, Meinl E (2007) Astrocytes are active players in cerebral innate immunity. Trends Immunol 28:138–145 [DOI] [PubMed] [Google Scholar]
- 71.Fatemi SH, Folsom TD, Reutiman TJ, Lee S (2008) Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse 62:501–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S (2002) Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience 110:1–6 [DOI] [PubMed] [Google Scholar]
- 73.Felipo V (2013) Hepatic encephalopathy: effects of liver failure on brain function. Nat Rev Neurosci 14:851–858 [DOI] [PubMed] [Google Scholar]
- 74.Ferrer I (2018) Astrogliopathy in tauopathies. Neuroglia 1:126–150 [Google Scholar]
- 75.Franke H (1995) Influence of chronic alcohol treatment on the GFAP-immunoreactivity in astrocytes of the hippocampus in rats. Acta Histochem 97:263–271 [DOI] [PubMed] [Google Scholar]
- 76.Friend DM, Keefe KA (2013) Glial reactivity in resistance to methamphetamine-induced neurotoxicity. J Neurochem 125:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frommann C (1878) Untersuchungen über die Gewebsveränderungen bei der Multiplen Sklerose des Gehirns und Rückenmarks. Verlag von Gustav Fischer, Jena [Google Scholar]
- 78.Garcia AD, Petrova R, Eng L, Joyner AL (2010) Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci 30:13597–13608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giaume C, Kirchhoff F, Matute C, Reichenbach A, Verkhratsky A (2007) Glia: the fulcrum of brain diseases. Cell Death Differ 14:1324–1335 [DOI] [PubMed] [Google Scholar]
- 80.Giovedi S, Corradi A, Fassio A, Benfenati F (2014) Involvement of synaptic genes in the pathogenesis of autism spectrum disorders: the case of synapsins. Front Pediatr 2:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gleichman AJ, Carmichael ST (2014) Astrocytic therapies for neuronal repair in stroke. Neurosci Lett 565:47–52 [DOI] [PubMed] [Google Scholar]
- 82.Gorg B, Morwinsky A, Keitel V, Qvartskhava N, Schror K, Haussinger D (2010) Ammonia triggers exocytotic release of L-glutamate from cultured rat astrocytes. Glia 58:691–705 [DOI] [PubMed] [Google Scholar]
- 83.Gray LR, Turville SG, Hitchen TL, Cheng WJ, Ellett AM, Salimi H, Roche MJ, Wesselingh SL, Gorry PR, Churchill MJ (2014) HIV-1 entry and trans-infection of astrocytes involves CD81 vesicles. PLoS ONE 9:e90620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guilarte TR, Nihei MK, McGlothan JL, Howard AS (2003) Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neuroscience 122:499–513 [DOI] [PubMed] [Google Scholar]
- 85.Gunnarson E, Axehult G, Baturina G, Zelenin S, Zelenina M, Aperia A (2005) Lead induces increased water permeability in astrocytes expressing aquaporin 4. Neuroscience 136:105–114 [DOI] [PubMed] [Google Scholar]
- 86.Haack N, Dublin P, Rose CR (2014) Dysbalance of astrocyte calcium under hyperammonemic conditions. PLoS ONE 9:e105832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haberle J, Shahbeck N, Ibrahim K, Hoffmann GF, Ben-Omran T (2011) Natural course of glutamine synthetase deficiency in a 3 year old patient. Mol Genet Metab 103:89–91 [DOI] [PubMed] [Google Scholar]
- 88.Harvey BK, Airavaara M, Hinzman J, Wires EM, Chiocco MJ, Howard DB, Shen H, Gerhardt G, Hoffer BJ, Wang Y (2011) Targeted over-expression of glutamate transporter 1 (GLT-1) reduces ischemic brain injury in a rat model of stroke. PLoS ONE 6:e22135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heming N, Mazeraud A, Verdonk F, Bozza FA, Chretien F, Sharshar T (2017) Neuroanatomy of sepsis-associated encephalopathy. Crit Care 21:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Heneka MT, Rodriguez JJ, Verkhratsky A (2010) Neuroglia in neurodegeneration. Brain Res Rev 63:189–211 [DOI] [PubMed] [Google Scholar]
- 91.Herman M, Ciancanelli M, Ou YH, Lorenzo L, Klaudel-Dreszler M, Pauwels E, Sancho-Shimizu V, Perez de Diego R, Abhyankar A, Israelsson E, Guo Y, Cardon A, Rozenberg F, Lebon P, Tardieu M, Heropolitanska-Pliszka E, Chaussabel D, White MA, Abel L, Zhang SY, Casanova JL (2012) Heterozygous TBK1 mutations impair TLR3 immunity and underlie herpes simplex encephalitis of childhood. J Exp Med 209:1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heuser K, Nagelhus EA, Tauboll E, Indahl U, Berg PR, Lien S, Nakken S, Gjerstad L, Ottersen OP (2010) Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res 88:55–64 [DOI] [PubMed] [Google Scholar]
- 94.Hinterkeuser S, Schroder W, Hager G, Seifert G, Blumcke I, Elger CE, Schramm J, Steinhauser C (2000) Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci 12:2087–2096 [DOI] [PubMed] [Google Scholar]
- 95.Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130 [DOI] [PubMed] [Google Scholar]
- 96.Iovino F, Orihuela CJ, Moorlag HE, Molema G, Bijlsma JJ (2013) Interactions between blood-borne Streptococcus pneumoniae and the blood-brain barrier preceding meningitis. PLoS ONE 8:e68408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jacobs S, Doering LC (2010) Astrocytes prevent abnormal neuronal development in the fragile x mouse. J Neurosci 30:4508–4514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jacobs S, Nathwani M, Doering LC (2010) Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci 11:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J (2017) Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron 95:1246–1265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kadoi Y, Saito S (1996) An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit Care Med 24:298–305 [DOI] [PubMed] [Google Scholar]
- 101.Kanemaru K, Kubota J, Sekiya H, Hirose K, Okubo Y, Iino M (2013) Calcium-dependent N-cadherin up-regulation mediates reactive astrogliosis and neuroprotection after brain injury. Proc Natl Acad Sci U S A 110:11612–11617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karlsen AS, Pakkenberg B (2011) Total numbers of neurons and glial cells in cortex and basal ganglia of aged brains with Down syndrome–a stereological study. Cereb Cortex 21:2519–2524 [DOI] [PubMed] [Google Scholar]
- 103.Karlsson RM, Tanaka K, Heilig M, Holmes A (2008) Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry 64:810–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Karlsson RM, Tanaka K, Saksida LM, Bussey TJ, Heilig M, Holmes A (2009) Assessment of glutamate transporter GLAST (EAAT1)-deficient mice for phenotypes relevant to the negative and executive/cognitive symptoms of schizophrenia. Neuropsychopharmacology 34:1578–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kelly T, Kafitz KW, Roderigo C, Rose CR (2009) Ammonium-evoked alterations in intracellular sodium and pH reduce glial glutamate transport activity. Glia 57:921–934 [DOI] [PubMed] [Google Scholar]
- 106.Kelly T, Rose CR (2010) Ammonium influx pathways into astrocytes and neurones of hippocampal slices. J Neurochem 115:1123–1136 [DOI] [PubMed] [Google Scholar]
- 107.Kidd SA, Lachiewicz A, Barbouth D, Blitz RK, Delahunty C, McBrien D, Visootsak J, Berry-Kravis E (2014) Fragile X syndrome: a review of associated medical problems. Pediatrics 134:995–1005 [DOI] [PubMed] [Google Scholar]
- 108.Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW (2014) Pattern recognition receptors and central nervous system repair. Exp Neurol 258:5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirischuk S, Parpura V, Verkhratsky A (2012) Sodium dynamics: another key to astroglial excitability? Trends Neurosci 35:497–506 [DOI] [PubMed] [Google Scholar]
- 110.Klein RS, Hunter CA (2017) Protective and pathological immunity during central nervous system infections. Immunity 46:891–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koss WA, Sadowski RN, Sherrill LK, Gulley JM, Juraska JM (2012) Effects of ethanol during adolescence on the number of neurons and glia in the medial prefrontal cortex and basolateral amygdala of adult male and female rats. Brain Res 1466:24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krencik R, Hokanson KC, Narayan AR, Dvornik J, Rooney GE, Rauen KA, Weiss LA,Rowitch DH, Ullian EM (2015) Dysregulation of astrocyte extracellular signaling in Costello syndrome. Sci Transl Med 7:286ra266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanciotti A, Brignone MS, Bertini E, Petrucci TC, Aloisi F, Ambrosini E (2013) Astrocytes: emerging stars in leukodystrophy pathogenesis. Transl Neurosci 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Larsen BR, Assentoft M, Cotrina ML, Hua SZ, Nedergaard M, Kaila K, Voipio J, MacAulay N (2014) Contributions of the Na+/K+-ATPase, NKCC1, and Kir4.1 to hippocampal K+ clearance and volume responses. Glia 62:608–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lauritzen M, Dreier JP, Fabricius M, Hartings JA, Graf R, Strong AJ (2011) Clinical relevance of cortical spreading depression in neurological disorders: migraine, malignant stroke, subarachnoid and intracranial hemorrhage, and traumatic brain injury. J Cereb Blood Flow Metab 31:17–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M (2008) Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab 28:468–481 [DOI] [PubMed] [Google Scholar]
- 117.Li T, Lan JQ, Boison D (2008) Uncoupling of astrogliosis from epileptogenesis in adenosine kinase (ADK) transgenic mice. Neuron Glia Biol 4:91–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li T, Quan Lan J, Fredholm BB, Simon RP, Boison D (2007) Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biol 3:353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liang C, Du T, Zhou J, Verkhratsky A, Peng L (2014) Ammonium increases Ca2+ signalling and up-regulates expression of TRPC1 gene in astrocytes in primary cultures and in the in vivo brain. Neurochem Res 39:2127–2135 [DOI] [PubMed] [Google Scholar]
- 120.Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967 [DOI] [PubMed] [Google Scholar]
- 121.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC, Wilton DK, Frouin A, Napier BA, Panicker N, Kumar M, Buckwalter MS, Rowitch DH, Dawson VL, Dawson TM, Stevens B, Barres BA (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541:481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liu X, Chauhan VS, Young AB, Marriott I (2010) NOD2 mediates inflammatory responses of primary murine glia to Streptococcus pneumoniae. Glia 58:839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu Z, Song D, Yan E, Verkhratsky A, Peng L (2015) Chronic treatment with anti-bipolar drugs suppresses glutamate release from astroglial cultures. Amino Acids 47:1045–1051 [DOI] [PubMed] [Google Scholar]
- 124.Lothman EW, Somjen GG (1976) Functions of primary afferents and responses of extracellular K+ during spinal epileptiform seizures. Electroencephalogr Clin Neurophysiol 41:253–267 [DOI] [PubMed] [Google Scholar]
- 125.Makar TK, Nedergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJ (1994) Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem 62:45–53 [DOI] [PubMed] [Google Scholar]
- 126.Marcaggi P, Coles JA (2001) Ammonium in nervous tissue: transport across cell membranes, fluxes from neurons to glial cells, and role in signalling. Prog Neurobiol 64:157–183 [DOI] [PubMed] [Google Scholar]
- 127.Marin-Valencia I, Roe CR, Pascual JM (2010). Pyruvate carboxylase deficiency: mechanisms, mimics and anaplerosis. Mol Genet Metab 1:9–17 [DOI] [PubMed] [Google Scholar]
- 128.Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D (2011) A ketogenic diet suppresses seizures in mice through adenosine A(1) receptors. J Clin Invest 121:2679–2683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mc AD, Araki S (1958) Minamata disease: an unusual neurological disorder caused by contaminated fish. Lancet 2:629–631 [DOI] [PubMed] [Google Scholar]
- 130.McGinnis WR (2004) Oxidative stress in autism. Altern Ther Health Med 10:22–36; quiz 37, 92 [PubMed] [Google Scholar]
- 131.Messing A, Brenner M, Feany MB, Nedergaard M, Goldman JE (2012) Alexander disease. J Neurosci 32:5017–5023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Miguel-Hidalgo J, Shoyama Y, Wanzo V (2009) Infusion of gliotoxins or a gap junction blocker in the prelimbic cortex increases alcohol preference in Wistar rats. J Psychopharmacol 23:550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miguel-Hidalgo JJ (2005) Lower packing density of glial fibrillary acidic protein-immunoreactive astrocytes in the prelimbic cortex of alcohol-naive and alcohol-drinking alcohol-preferring rats as compared with alcohol-nonpreferring and Wistar rats. Alcohol Clin Exp Res 29:766–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Miguel-Hidalgo JJ (2009) The role of glial cells in drug abuse. Curr Drug Abuse Rev 2:76–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Miguel-Hidalgo JJ, Overholser JC, Meltzer HY, Stockmeier CA, Rajkowska G (2006) Reduced glial and neuronal packing density in the orbitofrontal cortex in alcohol dependence and its relationship with suicide and duration of alcohol dependence. Alcohol Clin Exp Res 30:1845–1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Minami T, Okazaki J, Kawabata A, Kawaki H, Okazaki Y, Tohno Y (1998) Roles of nitric oxide and prostaglandins in the increased permeability of the blood-brain barrier caused by lipopolysaccharide. Environ Toxicol Pharmacol 5:35–41 [DOI] [PubMed] [Google Scholar]
- 137.Minkiewicz J, de Rivero Vaccari JP, Keane RW (2013) Human astrocytes express a novel NLRP2 inflammasome. Glia 61:1113–1121 [DOI] [PubMed] [Google Scholar]
- 138.Miyajima H (2003) Aceruloplasminemia, an iron metabolic disorder. Neuropathology 23:345–350 [DOI] [PubMed] [Google Scholar]
- 139.Montana V, Verkhratsky A, Parpura V (2014) Pathological role for exocytotic glutamate release from astrocytes in hepatic encephalopathy. Curr Neuropharmacol 12:324–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Moody WJ, Futamachi KJ, Prince DA (1974) Extracellular potassium activity during epileptogenesis. Exp Neurol 42:248–263 [DOI] [PubMed] [Google Scholar]
- 141.Moussawi K, Riegel A, Nair S, Kalivas PW (2011) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nedergaard M (1996). Spreading depression as a contributor to ischemic brain damage. Adv Neurol 71:75–83; discussion 83–74 [PubMed] [Google Scholar]
- 143.Norenberg MD (1987) The role of astrocytes in hepatic encephalopathy. Neurochem Pathol 6:13–33 [DOI] [PubMed] [Google Scholar]
- 144.Obara-Michlewska M, Ruszkiewicz J, Zielinska M, Verkhratsky A, Albrecht J (2014) Astroglial NMDA receptors inhibit expression of K4.1 channels in glutamate-overexposed astrocytes in vitro and in the brain of rats with acute liver failure. Neurochem Int [DOI] [PubMed] [Google Scholar]
- 145.Oehmichen M, Meissner C, Reiter A, Birkholz M (1996) Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol 91:642–646 [DOI] [PubMed] [Google Scholar]
- 146.Ohnuma T, Tessler S, Arai H, Faull RL, McKenna PJ, Emson PC (2000) Gene expression of metabotropic glutamate receptor 5 and excitatory amino acid transporter 2 in the schizophrenic hippocampus. Brain Res Mol Brain Res 85:24–31 [DOI] [PubMed] [Google Scholar]
- 147.Oide T, Yoshida K, Kaneko K, Ohta M, Arima K (2006) Iron overload and antioxidative role of perivascular astrocytes in aceruloplasminemia. Neuropathol Appl Neurobiol 32:170–176 [DOI] [PubMed] [Google Scholar]
- 148.Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T, Ishii K, Yamane J, Yoshimura A, Iwamoto Y, Toyama Y, Okano H (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834 [DOI] [PubMed] [Google Scholar]
- 149.Ongur D, Drevets WC, Price JL (1998) Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A 95:13290–13295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Orellana JA, Saez JC, Bennett MV, Berman JW, Morgello S, Eugenin EA (2014) HIV increases the release of dickkopf-1 protein from human astrocytes by a Cx43 hemichannel-dependent mechanism. J Neurochem 128:752–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ortinski PI, Dong J, Mungenast A, Yue C, Takano H, Watson DJ, Haydon PG, Coulter DA (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A (2012) Glial cells in (patho)physiology. J Neurochem 121:4–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Patel SC, Suresh S, Kumar U, Hu CY, Cooney A, Blanchette-Mackie EJ, Neufeld EB, Patel RC, Brady RO, Patel YC, Pentchev PG, Ong WY (1999) Localization of Niemann-Pick C1 protein in astrocytes: implications for neuronal degeneration in Niemann-Pick type C disease. Proc Natl Acad Sci U S A 96:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J (1999) Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol 145:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Pekny M, Pekna M (2014) Astrocyte reactivity and reactive astrogliosis: costs and benefits. Physiol Rev 94:1077–1098 [DOI] [PubMed] [Google Scholar]
- 156.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131:323–345 [DOI] [PubMed] [Google Scholar]
- 157.Pekny M, Wilhelmsson U, Pekna M (2014) The dual role of astrocyte activation and reactive gliosis. Neurosci Lett 565:30–38 [DOI] [PubMed] [Google Scholar]
- 158.Piva S, McCreadie VA, Latronico N, Name J (2015) Neuroinflammation in sepsis: sepsis associated delirium. Cardiovasc Hematol Disord Drug Targ 1:10–18 [DOI] [PubMed] [Google Scholar]
- 159.Potokar M, Korva M, Jorgacevski J, Avsic-Zupanc T, Zorec R (2014) Tick-borne encephalitis virus infects rat astrocytes but does not affect their viability. PLoS ONE 9:e86219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Prust M, Wang J, Morizono H, Messing A, Brenner M, Gordon E, Hartka T, Sokohl A, Schiffmann R, Gordish-Dressman H, Albin R, Amartino H, Brockman K, Dinopoulos A, Dotti MT, Fain D, Fernandez R, Ferreira J, Fleming J, Gill D, Griebel M, Heilstedt H, Kaplan P, Lewis D, Nakagawa M, Pedersen R, Reddy A, Sawaishi Y, Schneider M, Sherr E, Takiyama Y, Wakabayashi K, Gorospe JR, Vanderver A (2011) GFAP mutations, age at onset, and clinical subtypes in Alexander disease. Neurology 77:1287–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Qrunfleh AM, Alazizi A, Sari Y (2013) Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol 27:541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Quaak I, Brouns MR, Van de Bor M (2013) The dynamics of autism spectrum disorders: how neurotoxic compounds and neurotransmitters interact. Int J Environ Res Public Health 10:3384–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rajkowska G, Miguel-Hidalgo JJ, Makkos Z, Meltzer H, Overholser J, Stockmeier C (2002) Layer-specific reductions in GFAP-reactive astroglia in the dorsolateral prefrontal cortex in schizophrenia. Schizophr Res 57:127–138 [DOI] [PubMed] [Google Scholar]
- 164.Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA (1999) Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098 [DOI] [PubMed] [Google Scholar]
- 165.Rajkowska G, Stockmeier CA (2013) Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets 14:1225–1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rangroo Thrane V, Thrane AS, Wang F, Cotrina ML, Smith NA, Chen M, Xu Q, Kang N, Fujita T, Nagelhus EA, Nedergaard M (2013) Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med 19:1643–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rao VL, Dogan A, Todd KG, Bowen KK, Kim BT, Rothstein JD, Dempsey RJ (2001) Anti-sense knockdown of the glial glutamate transporter GLT-1, but not the neuronal glutamate transporter EAAC1, exacerbates transient focal cerebral ischemia-induced neuronal damage in rat brain. J Neurosci 21:1876–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reinert LS, Harder L, Holm CK, Iversen MB, Horan KA, Dagnaes-Hansen F, Ulhoi BP, Holm TH, Mogensen TH, Owens T, Nyengaard JR, Thomsen AR, Paludan SR (2012) TLR3 deficiency renders astrocytes permissive to herpes simplex virus infection and facilitates establishment of CNS infection in mice. J Clin Invest 122:1368–1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Reissner KJ, Kalivas PW (2010) Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 21:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Reissner KJ, Kalivas PW (2014) Emerging roles for glial pathology in addiction In: Parpura V, Verkhratsky A (eds) Pathological potential of neuroglia: possible new targets for medical intervention. Sprinegr, New York, Heidelberg, Dordrecht, London, pp 397–418 [Google Scholar]
- 171.Ren J, Song D, Bai Q, Verkhratsky A, Peng L (2015) Fluoxetine induces alkalinization of astroglial cytosol through stimulation of sodium-hydrogen exchanger 1: dissection of intracellular signaling pathways. Front Cell Neurosci 9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rintala J, Jaatinen P, Kiianmaa K, Riikonen J, Kemppainen O, Sarviharju M, Hervonen A (2001) Dose-dependent decrease in glial fibrillary acidic protein-immunoreactivity in rat cerebellum after lifelong ethanol consumption. Alcohol 23:1–8 [DOI] [PubMed] [Google Scholar]
- 173.Rodriguez JJ, Butt AM, Gardenal E, Parpura V, Verkhratsky A (2016) Complex and differential glial responses in Alzheimer’s disease and ageing. Curr Alzheimer Res 13:343–358 [DOI] [PubMed] [Google Scholar]
- 174.Rojas DC (2014) The role of glutamate and its receptors in autism and the use of glutamate receptor antagonists in treatment. J Neural Transm 121:891–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rose CF, Verkhratsky A, Parpura V (2013) Astrocyte glutamine synthetase: pivotal in health and disease. Biochem Soc Trans 41:1518–1524 [DOI] [PubMed] [Google Scholar]
- 176.Rosenbaum AI, Maxfield FR (2011) Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem 116:789–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Rossi D, Brambilla L, Valori CF, Roncoroni C, Crugnola A, Yokota T, Bredesen DE, Volterra A (2008) Focal degeneration of astrocytes in amyotrophic lateral sclerosis. Cell Death Differ 15:1691–1700 [DOI] [PubMed] [Google Scholar]
- 178.Sanacora G, Banasr M (2013) From pathophysiology to novel antidepressant drugs: glial contributions to the pathology and treatment of mood disorders. Biol Psychiatry 73:1172–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sari Y, Sreemantula SN, Lee MR, Choi DS (2013) Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci 51:779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Sayre NL, Chen Y, Sifuentes M, Stoveken B, Lechleiter JD (2014) Purinergic receptor stimulation decreases ischemic brain damage by energizing astrocyte mitochondria In: Parpura V, Verkhratsky A (eds) Glutamate and ATP at the interface of metabolism and signaling in the brain. Springer, Cham, Heidelberg, New York, Dordrecht, London, pp 121–150 [DOI] [PubMed] [Google Scholar]
- 181.Schmitt A, Steyskal C, Bernstein HG, Schneider-Axmann T, Parlapani E, Schaeffer EL, Gattaz WF, Bogerts B, Schmitz C, Falkai P (2009) Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol 117:395–407 [DOI] [PubMed] [Google Scholar]
- 182.Scholl UI, Choi M, Liu T, Ramaekers VT, Hausler MG, Grimmer J, Tobe SW, Farhi A, Nelson-Williams C, Lifton RP (2009) Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A 106:5842–5847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Schwarcz R, Hunter CA (2007) Toxoplasma gondii and schizophrenia: linkage through astrocyte-derived kynurenic acid? Schizophr Bull 33:652–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sequeira A, Mamdani F, Ernst C, Vawter MP, Bunney WE, Lebel V, Rehal S, Klempan T, Gratton A, Benkelfat C, Rouleau GA, Mechawar N, Turecki G (2009) Global brain gene expression analysis links glutamatergic and GABAergic alterations to suicide and major depression. PLoS ONE 4:e6585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shan D, Lucas EK, Drummond JB, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE (2013) Abnormal expression of glutamate transporters in temporal lobe areas in elderly patients with schizophrenia. Schizophr Res 144:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sharshar T, Annane D, de la Grandmaison GL, Brouland JP, Hopkinson NS, Francoise G (2004) The neuropathology of septic shock. Brain Pathol 14:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shulyatnikova T, Verkhratsky A (2019) Astroglia in sepsis associated encephalopathy. Neurochem Res. E-pub ahead of print. 10.1007/s11064-019-02743-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Skowronska M, Albrecht J (2012) Alterations of blood brain barrier function in hyperammonemia: an overview. Neurotox Res 21:236–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407 [DOI] [PubMed] [Google Scholar]
- 190.Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sofroniew MV (2014) Astrogliosis. Cold Spring Harb Perspect Biol 7:a020420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sofroniew MV (2014) Multiple roles for astrocytes as effectors of cytokines and inflammatory mediators. Neuroscientist 20:160–172 [DOI] [PubMed] [Google Scholar]
- 193.Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16:249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Song P, Zhao ZQ (2001) The involvement of glial cells in the development of morphine tolerance. Neurosci Res 39:281–286 [DOI] [PubMed] [Google Scholar]
- 195.Spodenkiewicz M, Diez-Fernandez C, Rufenacht V, Gemperle-Britschgi C, Haberle J (2016) Minireview on glutamine synthetase deficiency, an ultra-rare inborn error of amino acid biosynthesis. Biology (Basel) 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Steinhauser C, Grunnet M, Carmignoto G (2015) Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience [DOI] [PubMed] [Google Scholar]
- 197.Stenzel W, Soltek S, Schluter D, Deckert M (2004) The intermediate filament GFAP is important for the control of experimental murine Staphylococcus aureus-induced brain abscess and Toxoplasma encephalitis. J Neuropathol Exp Neurol 63:631–640 [DOI] [PubMed] [Google Scholar]
- 198.Struys-Ponsar C, Guillard O, van den Bosch de Aguilar P (2000) Effects of aluminum exposure on glutamate metabolism: a possible explanation for its toxicity. Exp Neurol 163:157–164 [DOI] [PubMed] [Google Scholar]
- 199.Suarez-Fernandez MB, Soldado AB, Sanz-Medel A, Vega JA, Novelli A, Fernandez-Sanchez MT (1999) Aluminum-induced degeneration of astrocytes occurs via apoptosis and results in neuronal death. Brain Res 835:125–136 [DOI] [PubMed] [Google Scholar]
- 200.Suarez I, Bodega G, Ramos JA, Fernandez-Ruiz JJ, Fernandez B (2000) Neuronal and astroglial response to pre- and perinatal exposure to delta-9-tetra-hydrocannabinol in the rat substantia nigra. Dev Neurosci 22:253–263 [DOI] [PubMed] [Google Scholar]
- 201.Sun JD, Liu Y, Yuan YH, Li J, Chen NH (2012) Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology 37:1305–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, Iwama H, Nishikawa T, Ichihara N, Kikuchi T, Okuyama S, Kawashima N, Hori S, Takimoto M, Wada K (1997) Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science 276:1699–1702 [DOI] [PubMed] [Google Scholar]
- 203.Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT (2012) PAMPs and DAMPs: signal 0 s that spur autophagy and immunity. Immunol Rev 249:158–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang G, Xu Z, Goldman JE (2006) Synergistic effects of the SAPK/JNK and the proteasome pathway on glial fibrillary acidic protein (GFAP) accumulation in Alexander disease. J Biol Chem 281:38634–38643 [DOI] [PubMed] [Google Scholar]
- 205.Tidyman WE, Rauen KA (2009) The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev 19:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Traynelis SF, Dingledine R (1988) Potassium-induced spontaneous electrographic seizures in the rat hippocampal slice. J Neurophysiol 59:259–276 [DOI] [PubMed] [Google Scholar]
- 207.Valori CF, Brambilla L, Martorana F, Rossi D (2014) The multifaceted role of glial cells in amyotrophic lateral sclerosis. Cell Mol Life Sci 71:287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.van Gool WA, van de Beek D, Eikelenboom P (2010) Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet 375:773–775 [DOI] [PubMed] [Google Scholar]
- 209.Varatharaj A, Galea I (2017) The blood-brain barrier in systemic inflammation. Brain Behav Immun 60:1–12 [DOI] [PubMed] [Google Scholar]
- 210.Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester [Google Scholar]
- 211.Verkhratsky A, Krishtal OA, Burnstock G (2009) Purinoceptors on neuroglia. Mol Neurobiol 39:190–208 [DOI] [PubMed] [Google Scholar]
- 212.Verkhratsky A, Marutle A, Rodriguez-Arellano JJ, Nordberg A (2015) Glial asthenia and functional paralysis: a new perspective on neurodegeneration and Alzheimer’s disease. Neuroscientist 21:552–568 [DOI] [PubMed] [Google Scholar]
- 213.Verkhratsky A, Nedergaard M (2014) Astroglial cradle in the life of the synapse. Philos Trans R Soc Lond B Biol Sci 369:20130595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Verkhratsky A, Olabarria M, Noristani HN, Yeh CY, Rodriguez JJ (2010) Astrocytes in Alzheimer’s disease. Neurotherapeutics 7:399–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Verkhratsky A, Parpura V (2016) Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis 85:254–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Verkhratsky A, Rodriguez JJ, Parpura V (2013) Astroglia in neurological diseases. Future Neurol 8:149–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Verkhratsky A, Rodriguez JJ, Steardo L (2014) Astrogliopathology: a central element of neuropsychiatric diseases? Neuroscientist 20:576–588 [DOI] [PubMed] [Google Scholar]
- 219.Verkhratsky A, Steardo L, Parpura V, Montana V (2016) Translational potential of astrocytes in brain disorders. Prog Neurobiol 144:188–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Verkhratsky A, Zorec R, Parpura V (2017) Stratification of astrocytes in healthy and diseased brain. Brain Pathol 27:629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Verkhratsky A, Zorec R, Rodriguez JJ, Parpura V (2017) Neuroglia: functional paralysis and reactivity in Alzheimer’s disease and other neurodegenerative pathologies. Adv Neurobiol 15:427–449 [DOI] [PubMed] [Google Scholar]
- 222.Virchow R (1858) Die Cellularpathologie in ihrer Begründung auf physiologische and pathologische Gewebelehre Zwanzig Vorlesungen gehalten während der Monate Februar, März und April 1858 im pathologischen Institut zu Berlin. August Hirschwald, Berlin [Google Scholar]
- 223.Wang F, Du T, Liang C, Verkhratsky A, Peng L (2015) Ammonium increases Ca2+ signalling and upregulates expression of Cav1.2 gene in astrocytes in primary cultures and in the in vivo brain. Acta Physiol (Oxf) 214:261–274 [DOI] [PubMed] [Google Scholar]
- 224.Wang Y, Zaveri HP, Lee TS, Eid T (2009) The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol 220:293–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Weber M, Scherf N, Kahl T, Braumann UD, Scheibe P, Kuska JP, Bayer R, Buttner A, Franke H (2013) Quantitative analysis of astrogliosis in drug-dependent humans. Brain Res 1500:72–87 [DOI] [PubMed] [Google Scholar]
- 226.Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH (2001) Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun 15:388–400 [DOI] [PubMed] [Google Scholar]
- 227.Weng YC, Kriz J (2007) Differential neuroprotective effects of a minocycline-based drug cocktail in transient and permanent focal cerebral ischemia. Exp Neurol 204:433–442 [DOI] [PubMed] [Google Scholar]
- 228.Wilson EH, Hunter CA (2004) The role of astrocytes in the immunopathogenesis of toxoplasmic encephalitis. Int J Parasitol 34:543–548 [DOI] [PubMed] [Google Scholar]
- 229.Wilson JX, Peters CE, Sitar SM, Daoust P, Gelb AW (2000) Glutamate stimulates ascorbate transport by astrocytes. Brain Res 858:61–66 [DOI] [PubMed] [Google Scholar]
- 230.Windrem MS, Osipovitch M, Liu Z, Bates J, Chandler-Militello D, Zou L, Munir J, Schanz S, McCoy K, Miller RH, Wang S, Nedergaard M, Findling RL, Tesar PJ, Goldman SA (2017) Human iPSC glial mouse chimeras reveal glial contributions to schizophrenia. Cell Stem Cell 21(195–208):e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Xia M, Abazyan S, Jouroukhin Y, Pletnikov M (2014) Behavioral sequelae of astrocyte dys-function: focus on animal models of schizophrenia. Schizophr Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Yin Z, Milatovic D, Aschner JL, Syversen T, Rocha JB, Souza DO, Sidoryk M, Albrecht J, Aschner M (2007) Methylmercury induces oxidative injury, alterations in permeability and glutamine transport in cultured astrocytes. Brain Res 1131:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012) Genomic analysis of reactive astrogliosis. J Neurosci 32:6391–6410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zeidan-Chulia F, Salmina AB, Malinovskaya NA, Noda M, Verkhratsky A, Moreira JC (2014) The glial perspective of autism spectrum disorders. Neurosci Biobehav Rev 38:160–172 [DOI] [PubMed] [Google Scholar]
- 235.Zhang L, Li L, Wang B, Qian DM, Song XM, Hu M (2013) Human cytomegalovirus infection modulates thrombospondins 1 and 2 in primary fetal astrocytes. NeuroReport 24:526–535 [DOI] [PubMed] [Google Scholar]
- 236.Zhang L, Li L, Wang B, Qian DM, Song XX, Hu M (2014) HCMV induces dysregulation of glutamate uptake and transporter expression in human fetal astrocytes. Neurochem Res 39:2407–2418 [DOI] [PubMed] [Google Scholar]
- 237.Zhang M, Strnatka D, Donohue C, Hallows JL, Vincent I, Erickson RP (2008) Astrocyte-only Npc1 reduces neuronal cholesterol and triples life span of Npc1−/− mice. J Neurosci Res 86:2848–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, Kimelberg HK (2005) Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol 196:41–46 [DOI] [PubMed] [Google Scholar]
- 239.Zhao Y, Rempe DA (2010) Targeting astrocytes for stroke therapy. Neurotherapeutics 7:439–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zorec R, Zupanc TA, Verkhratsky A (2019) Astrogliopathology in the infectious insults of the brain. Neurosci Lett 689:56–62 [DOI] [PubMed] [Google Scholar]


