Abstract
As the nervous system evolved from the diffused to centralised form, the neurones were joined by the appearance of the supportive cells, the neuroglia. Arguably, these non-neuronal cells evolve into a more diversified cell family than the neurones are. The first ancestral neuroglia appeared in flatworms being mesenchymal in origin. In the nematode C. elegans proto-astrocytes/supportive glia of ectodermal origin emerged, albeit the ensheathment of axons by glial cells occurred later in prawns. The multilayered myelin occurred by convergent evolution of oligodendrocytes and Schwann cells in vertebrates above the jawless fishes. Nutritive partitioning of the brain from the rest of the body appeared in insects when the hemolymph-brain barrier, a predecessor of the blood-brain barrier was formed. The defensive cellular mechanism required specialisation of bona fide immune cells, microglia, a process that occurred in the nervous system of leeches, bivalves, snails, insects and above. In ascending phylogeny, new type of glial cells, such as scaffolding radial glia, appeared and as the bran sizes enlarged, the glia to neurone ratio increased. Humans possess some unique glial cells not seen in other animals.
Keywords: Astrocytes, Blood/haemolymph-brain barrier, Brain size, Complexity of glia, Glia to neuron ratio, Microglia, Myelination, Oligodendrocytes, Radial glia
2.1. Evolution of the Nervous System
Several taxonomy charts are currently in use. Here we use the system that classifies all living forms into Superkingdoms or Empires of Prokaryota and Eukaryota (Fig. 2.1). The Empire of Prokaryota comprises a single Kingdom of Bacteria, whereas the Empire of Eukaryota includes the Kingdoms of Protozoa, Animalia, Fungi, Plantae and Chromista [17, 18]. The nervous system is the sole prerogative of the Kingdom of Animalia, which is represented by radially symmetrical Cnidaria and Ctenophora, and Bilateralia. The bilateralia are further subclassified into Protostomia and Deuterostomia, the latter including Echinodermata, Hemichordata and, finally, Chordata to which vertebrates belong.
Fig. 2.1.
Tree of life and evolution of the nervous system and of neuroglia. Adapted from Verkhratsky and Butt [143]
Arguably, the most ancient nervous systems appeared in Ctenophora (comb jellies) and Cnidarians (hydras and sea jellies). This was represented by the so-called diffuse nervous system, formed by homogeneously distributed network of neurones connected with their processes [45]. The principal cells of the diffuse nervous system are multipolar and unipolar neurones, organised in several semi-independent networks connected through chemical synapses that mainly used peptides as neurotransmitters. The neurones remain the sole elements in the diffuse nervous system with no evidence of (and obviously no need for) any kind of specialised supportive cells. Of note, the primordial neurones evolved from epithelial cells [58], which expressed ion channels and transporters (that formed basis for electrical excitability) and the secretory vesicular system that was the morphological substrate for chemical neurotransmission [123].
Further evolution of the nervous system saw the change from diffuse nervous system to the centralised nervous system. At that stage the first conglomerates of neuronal somatas forming ganglia had emerged. The tendency to concentrate nervous elements was observed already in some Cnidarian polyps where neural networks appear denser around the oral opening. The first true centralised nervous systems, however, appear in early Bilateralia, in flatworms, earthworms and roundworms. For example, in roundworms the centralised nervous system is composed of several ganglia surrounding the oral orifice. In more advanced protostomes (e.g., insects and crustacea), the centralisation advanced further, with the polyganglionic brain. In vertebrates the new brain organisations in layers have appeared and progressed. The centralisation of the nervous system also signalled the appearance of supportive neural cells, the neuroglia.
Characterisation and morphological analysis of glial cells in early invertebrates and in phylogenetically lower taxa have been very much hampered by the absence of specific markers similar to those found for higher species. The main criteria for identification of glia is their close association with and coverage of neuronal elements, these being fundamental features of supportive cells. It is most probable that neuroglia has evolved several times in different species. Despite very similar functions, the appearance of glial cells is rather different between non-vertebrates and vertebrates, for example. Even genes responsible for glial differentiation can be distinct. In the insects, for example, development of glia is controlled by the gene glial cells missing (gsm—[60, 63]). In contrast, the gsm homologue gene is not even expressed in the CNS of mammals [69].
The very first glial cells provided for support of neuronal cell bodies within the ganglia (primeval astrocytes) as well as support of axons (primeval oligodendrocytes/Schwann cells). Another important function of primordial glia was formation of peripheral sensory organs, or sensilla. The glia-like cells are found in Acoelomorpha, the primitive flatworms, which are generally considered to be the earliest (or one of the earliest) Bilateralia. Electron microscopy of the brains of Symsagittifera roscoffensis, Convoluta psammophila, Amphiscolops sp. and Otocelis rubropunctata (free-living Acoela worms) characterised non-neuronal cells with electron-dense cell bodies in which nuclei occupy most of the cytosol, and lamellar processes extend into neuropil and surround groups of neurites [10, 11]. More advanced neuroglia is present in the nematode Caenorhabditis elegans. For example, the sheath glia of the cephalic sensilla in C. elegans possess some anatomical and functional characteristics that parallel those of astrocyte and oligodendrocyte lineages in the mammalian nervous system [133]. In Platyzoa (another member of early Bilateralia), glial cells have been found in polyclad flatworms and in some (but not in all) triclad planaria, whereas neuroglia seem to be absent in Rotifera (wheel animals) and in many platyhelminthes (for example, in tubellarian flatworms, Catenulida or Macrostomida). Neuroglia are generally present throughout Ecdysozoa and Lophotrochozoa, being well developed in molluscs, in Annelida, and even more developed and rather diverse in Arthropoda. In Deuterostomes the organisation of the brain as well as newly emerged spinal cord has changed form polyganglia to the layers, as a result of the appearance of the radial glia, a new type of neuroglia, which provided both for neurogenesis and migration of neuronal precursors to their appropriate layers. In early Chordata, the radial glial cells predominate and are present throughout life, while the parenchyma is quite under-developed. An increase in brain thickness instigated the emergence of parenchymal glia that diversified and became responsible for major homeostatic tasks in the CNS of mammals. The radial glial, however, remain active only in the prenatal period and largely disappear after birth. Below, we shall provide an account of the evolution of the main types of neuroglia.
2.2. Neuroglia in Invertebrates
2.2.1. Primitive Glia of Flatworm
The flatworms are the most primitive bilateralia with clearly formed centralised nervous system with the ‘brain’ represented by cerebral ganglia. The cerebral ganglia of at least two flatworms, Fasciola hepatica and Notoplana acticola, apparently contain some type of supportive cells that might be considered the ancestral neuroglia [135, 136]. These primitive glia, defined as mesenchymal cells, have long processes emanating from the cell body; some of these processes encircle the cerebral ganglion, some invaginate into giant nerve cells processes, whereas some other send processes into the ganglion and enclose clusters of neuronal processes. The ‘glia-like’ mesenchymal cells most likely originate from parenchymal cells of the worm and undergo morphological specialisation after contacting the nervous elements. Some of these cells contain glycogen and may act as a source of energy substrates [139].
2.2.2. Complex Neuroglia of the Earthworm
In the earthworm Eisenia fetida several types of glia have been characterised according to the morphology and localisation of the supportive cells. These neuroglia types were classified as neurilemmal-, subneurilemmal-, supporting-nutrifying-and periaxonal sheath-forming glial cells [26]. The neurilemmal glia are elongated with long processes. Subneurilemmal glia are small spindle-like cells with few processes. Supporting-nutrifying glial cells are positive for glial fibrillary acidic protein (GFAP, a well-accepted marker for mammalian astrocytes) and appear as brushes on the surface of the neuronal perikarya. Sheath-forming glial cells are found around giant axons [26]. The membranes of glial cells form close contacts with neuronal membranes, these possibly being involved in gliato-neurone transport. Glial cells in the earthworm contain multiple intracellular vesicular structures with diameters between 200 and 400 nm, some with dense cores, perhaps indicating glial secretion.
2.2.3. Proto-astrocytes in Caenorhabditis Elegans
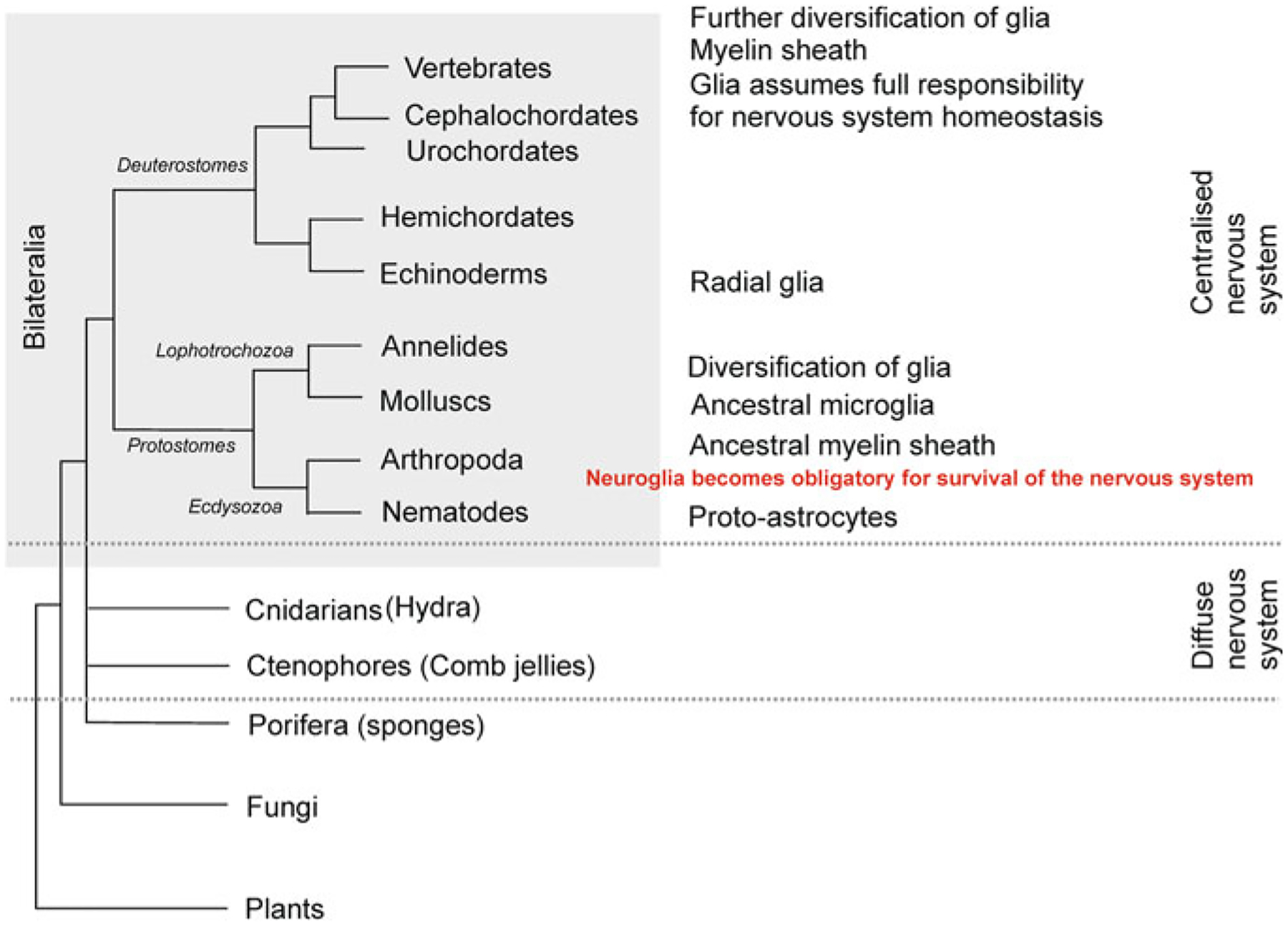
The nervous system of C. elegans has been precisely mapped with a wealth of details and meticulously categorised with structural information allowing identification of each neural cell [147]. The neuroglia of C. elegans were described and characterised based on light and electron microscopy (none of these glial cells express markers of mammalian glia), with these cells being defined as neural in origin lacking morphological characteristics of neurones, for example pre-synaptic structures [138, 147]. The nervous system of C. elegans contains 302 neurones, 50 supportive (glial) cells derived from the ectoderm and six supportive cells of the mesodermal origin [101, 134, 147]. The central nervous system of C. elegans comprises the nerve ring located in the frontal part of the body. The nerve ring receives processes of peripheral sensory neurones and also contains cephalic and motor neurones, axons of which convey efferent signals through the ventral and dorsal nerve cords.
Most of the neuroglial cells (that is 46 cells out of 56) of C. elegans are associated with the sensory system (Fig. 2.2). These cells are classified into 26 socket cells and 20 sheath cells that (together with neuronal processes) form sensory organs known as sensilla [112]. Four glial cells of the ectodermal origin known as cephalic sheath (CEPsh) cells are localised in the nerve ring (Fig. 2.2 and 2.3). The anterior processes of CEPsh cells cover cephalic neuronal dendrites and form sensilla in the lips of the animal. Posterior processes of CEPsh cell with lamellar morphology ensheath the nerve ring and send processes to the neuropil, where they contact and possibly enwrap synapses [101, 134]. The CEPsh cells control ion homeostasis in perisynaptic regions and are involved in neuronal development and morphogenesis. Complete ablation of glia in of C. elegans results in complex morphological, developmental, sensory and behavioural deficits, although it does not affect survival of the worm [4].
Fig. 2.2.
Glial cells in Caenorhabditis elegans. The “brain” of C. elegance is represented by the nerve ring. Most of the glial cells are part of sensory organs known as sensilla. Each sensilla has two glial cells: the sheath cell and socket cell. In the anterior part there are 4 CEP (cephalic) glial cells that ensheath nerve ring. The nerve ring also has 6 GLR glial cells which establish gap junctional contacts between ring motor neurones (RME) and muscle cells
Fig. 2.3.
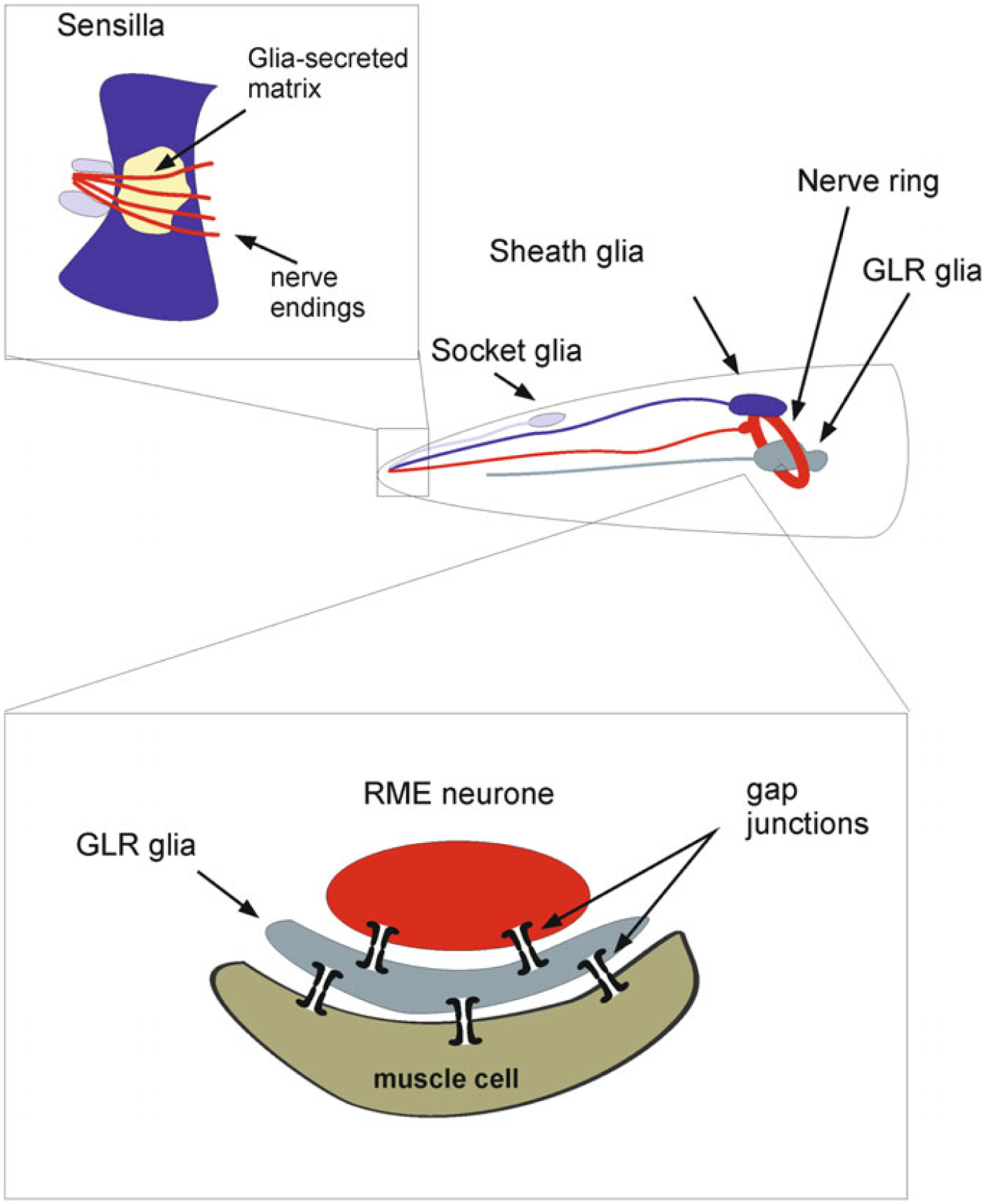
The CEPsh glia. a A cartoon of an adult worm showing the four CEPsh glial cells (green) positioned in the anterior of the worm (inset). The CEPsh cell bodies with their velate processes are positioned around the central nerve ring (red) which they enwrap along with the proximal section of the ventral nerve cord. Additionally, each CEPsh glial cell possesses a long anterior process, emanating to the anterior sensory tip, which closely interacts with the dendritic extension of a nearby CEP neuron (blue). Arrows indicate the dorsal (red arrow) and ventral (orange arrow) side of the worm. b A confocal image showing green fluorescent protein expression driven by the hlh-17 promoter to visualize the four CEPsh glial cells (worm strain VPR839). The anterior (head) of a juvenile (larval stage 4) worm is shown; the worm is turned ~45° from “upright” such that all four CEP sheath cells are visible. The sheath portion of the cells that form a tube around the dendritic endings of the CEP neurones are seen at the left of the image. The dorsal (red arrow) and ventral (orange arrow) CEPsh cell bodies are seen. The thin sheet-like extensions that surround and invade the nerve ring are seen in the rightmost part of the image. Scale bar, 20 μm. Image adapted from [134]
The glia of C. elegans have numerous differences from neuroglial cells of higher animals. Porto-astrocytes of the worm do not express GFAP; their physiology has both neuronal and non-neuronal properties—for example, Ca2+ signals in C. elegans glial are generated by Ca2+ entry through voltage-gated channels, while functional intracellular Ca2+ stores appear to be rudimentary or absent (Fig. 2.4, [133]). Glial cells of the worms, of course, do not form the glia limitans barrier because of the absence of the circulatory system. Main homeostatic functions of C. elegans glial cells similarly remain unknown; arguably, they may include K+ buffering and neurotransmitter catabolism [134]. At the same time several genetic pathways controlling development and differentiation of glia are shared between the worm and mammals. For example, the transcription factor LIN-26 contributes to glial cell development and ablation of the lin-26 gene may turn glial cells into neurones [75]. Another gene expressed in worms, the hlh-17 gene (the promotor of which was used to generate markers for CEPsh glia [86]) has homology to the mammalian regulator of glial development Olig2 [101, 154].
Fig. 2.4.
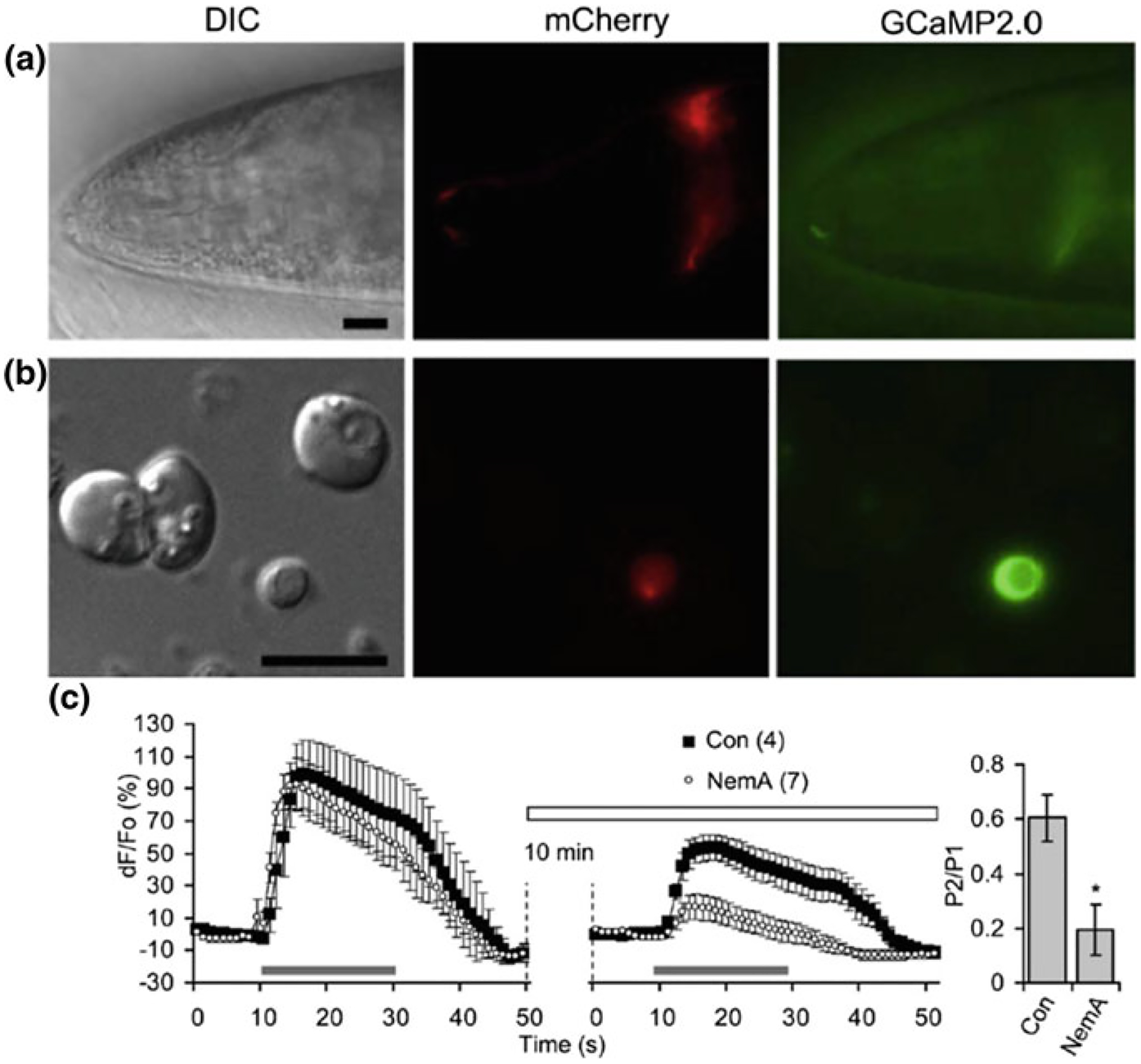
L-type voltage-gated Ca2+channels (VGCCs) play a role in depolarization-induced intracellular Ca2+ elevations in CEPsh glial cells. a The hlh-17 promoter can be used to drive expression of a red fluorescent protein marker (red, mCherry) in the CEPsh glia along with a fluorescent-protein based Ca2+ sensor (green, GCaMP2.0). DIC, differential interference contrast. An anterior portion of an L4 stage worm (VPR108 strain) is shown. b CEPsh glial cells in mixed culture prepared from embryos can be identified based on their mCherry/GCaMP2.0 expression. c Time-lapse of GCaMP2.0 fluorescence emission from CEPsh glial cells. Paired-pulse application of a depolarization stimulus, high extracellular potassium (HiK+, 100 mM; horizontal bar), to CEPsh glial cells results in an elevation of intracellular Ca2+ levels (black squares). Nemadipine-A (NemA), a pharmacological L-type VGCC blocker, can be used to test the channels present in glial cells in culture; Con, sham stimulated control. (right, bar graph). Ratio of the peak Ca2+ level in response to the second HiK+ application (P2) over the first application (P1). *Indicates a significant difference. Adapted from [133]
Neuroglia in C. elegans is responsible for the following functions: (i) establishment of the location of neuronal structures; (ii) regulation of size and morphology of sensory endings; (iii) creation of a barrier that bundles and separates neuronal elements from other cells; and (iv) modulation of neuronal activity. Conceptually, these functions are similar to the functions of glia in higher invertebrates and in vertebrates. Glial cells in C. elegans support development of the nervous system. In particular, glial cells modulate sensory activity by controlling the development of cellular compartments surrounding sensory cilia [114]. The sensory organs of the worm are singular aspects of the nematode nervous system, and their correct development is impaired in the absence of glia; moreover, development of sensory structures requires the expression of gene sets both in neurones and in glia [102]. Factors released by glia control sensory dendrites attachment during migration of neurones in development [53].
The four CEPsh glial cells (see above and Figs. 2.2, 2.3) differentially express netrin (UNC-6). Two ventral CEPsh cells express netrin, which regulates axon guidance, while the dorsal pair of CEPsh glia lacks the expression of this protein [52, 146, 154, 52, 146, 154].
There are some hints indicating that the CEPsh cells modulate dopamine-dependent behaviours in the worm, including feeding pattern and certain forms of learning [41]. Disruption of the hlh-17 gene, expressed in CEPsh cells affected the egg-laying behaviour, instigated deficits in feeding behaviour and plasticity, and disrupted gustatory associative learning. The CEPsh glia are closely associated with four CEP neurones, which mediate the aforementioned behaviours through release and up-take of dopamine, and hence CEPsh glia seem to modulate neuronal function [41, 134].
The C. elegans is also in possession of a rather unique class of glial cells, which connect neurones with myocytes. These so-called GLR cells are of the mesodermal origin [1]. The GLR cells are integral part of the nervous system of the C.elegans; these cells are involved into the development of the nerve ring and the muscle-based feeding organ of the worm known as pharynx. The most idiosyncratic feature of GLR cells is that the cells are being sandwiched between and connected to both neurones and muscle cells in the head by gap junctions. This arrangement arguably represents a signalling circuit for producing specialised fine motor movements of the frontal part of the worm during foraging [120, 148]. The GLR-neuronal circuit contains both coincidence detection and shunting activity that is based on gap junction intercellular communication [115]. Gap junctional connectivity between glial cells and neurones are not limited to invertebrates only; there are some indications of this form of neuronal-glial communications in higher animals [113] as well as in developing nervous system of the mammals [95, 105]. The special set of cells connecting neurones and muscle cells is present in vertebrates including mammals; these are the telocytes initially discovered by Santiago Ramón y Cajal in the gut as interstitial cells of Cajal at the beginning of the twentieth century; the telocytes are found now in various tissues [113].
2.3. Homeostatic Proto-astrocytes in Annelida
The nervous system of the medicinal leech Hirudo medicinalis includes the anterior and posterior brains and the chain of 21 ganglia that are positioned in between (Fig. 2.5a). The anterior brain is made of six ganglia fused into two neuronal masses, while seven fused ganglia in the tail form the posterior brain. Somatic ganglia innervate corresponding segments of the leech body [22, 30]. Every ganglion contains ~400 neurones (with exception of the 5th and 6th ganglia innervating the reproductive system, which have about 700 neurones) and ten glial cells. These glial cells are (Fig. 2.5b): (i) two connective glial cells, which ensheath axons; (ii) six packet cells covering neuronal cell bodies and (iii) two giant glial cells [30]. Glial cells of the leech nervous system are interconnected by gap junctions assembled from innexins [37, 65, 79] thus creating a panglial syncytium. The nervous system of the leech additionally contains some amount of microglial cells that can be activated in response to lesions [76]. Within the ganglia the homeostatic support is maintained by packet glia and giant glial cells which in this function resemble mammalian astrocytes. The packet glial cells buffer extracellular K+, especially at high extracellular K+ concentrations [97, 124]. The giant glial cells have somata of 80–100 μm in diameter; these somata are localised in the centre of the ganglion. The processes of giant glial cells are 300–350 μm long; they extend through the entire neuropil and contact neuronal dendrites [93]. The neuropil is partitioned by these processes into several functional domains. In addition, glial processes sometimes invaginate into neuronal somata creating a structure described as ‘trophospongium’ [59]. The membrane of giant glial cells is highly permeable to K+, which underlies hyperpolarised resting potential of about−75 mV. Giant glial cells express multiple types of neurotransmitter receptors including ionotropic glutamate, acetylcholine and serotonin receptors as well as metabotropic receptors to glutamate, serotonin, myomodulin and possibly P2Y-like purinoceptors and A1-like adenosine receptors [30, 92]. Giant glial cells regulate many homeostatic responses, including regulation of pH involving plasmalemmal Na+–HCO3− co-transporter, Na+–H+ and Cl−–HCO3− exchangers [28, 31, 32]. They also regulate neurotransmitters uptake and catabolism through plasmalemmal Na+-dependent glutamate and Na+-dependent choline transporters [33, 56, 149]. Giant glial cells respond to neuronal activity and to evoked behaviours by changes in membrane potential [29] and by generation of cytosolic Ca2+ signals that occur in both somata and processes [34]. In contrast to mammalian glia, and rather similar to C. elegans CEPsh glial cells, the main source of Ca2+ signal generation in leech glia is associated with the opening of plasmalemmal Ca2+ channels. Termination of Ca2+ signal is mediated by plasmalemmal Ca2+ pump and Na+/Ca2+ exchanger. The intracellular Ca2+ stores seem to play a minor role in Ca2+ dynamics of leech glia [34, 80].
Fig. 2.5.
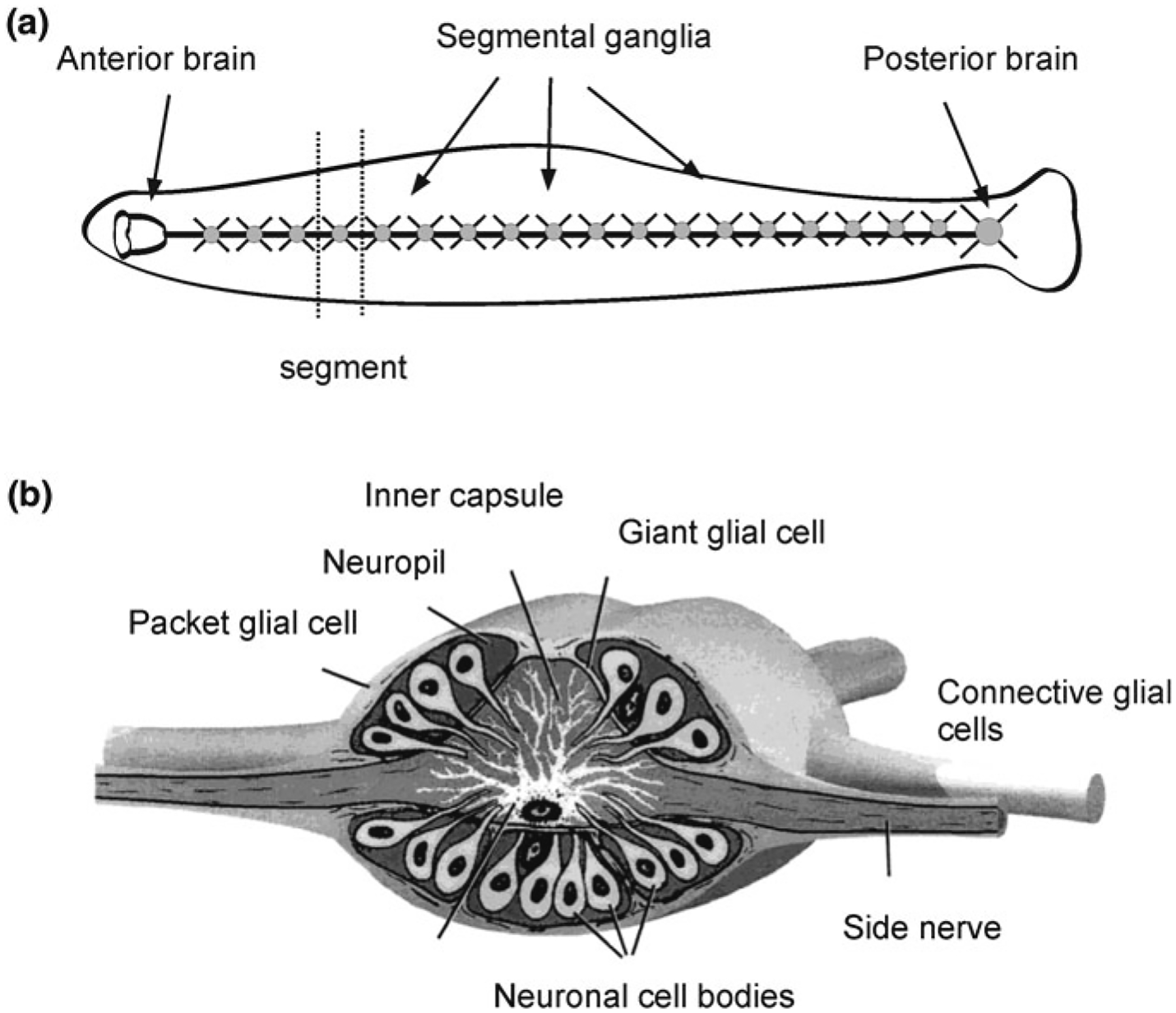
Neuroglia in medicinal leech Hirudo medicinalis. a General structure of the nervous system. b Structure of a segmental ganglion, which contains three types of glial cells: the giant glial cell; packet glial cells and connective glial cells. Adapted from Verkhratsky and Butt [143]
2.4. Proto-astrocytes in Insects
The brain of Drosophila contains ~90,000 cells of which 10% belong to neuroglia; some glial cells also exist in the peripheral nervous system. Neuroglia of Drosophila are classified (Fig. 2.6) into the following classes [39, 43, 49, 108]: (i) Wrapping glia of the peripheral nervous system; (ii) Surface glia, which make the brain-hemolymph barrier, is further subclassified into perineural glia (small cells lying on the ganglionic surface) and subperineural or basal glia (which are large sheet-like cells connected with septate junctions that form the barrier); (iii) Cortex glia that contact neuronal cells somata in the CNS, with each glial cell establishing contacts with many neurones; (iv) Neuropil glia, which are located in the neuropil and cover axons and synapses. The neuropil glia are further subdivided into ensheathing or fibrous and astrocyte-like glia, which forms a perisynaptic glial cover. Finally, (v) Tract glial cells cover axonal tracts connecting different neuropils. The cell mapping using the glia-specific GAL4-UAS system derived numerical calculation of various glial cell types in the Drosophila CNS. The perineural glia accounted for ~17%, subperineural—2%, cortex glia—20%, astrocyte-like glia—34% and ensheathing glia for 27% of total glial cells [70].
Fig. 2.6.
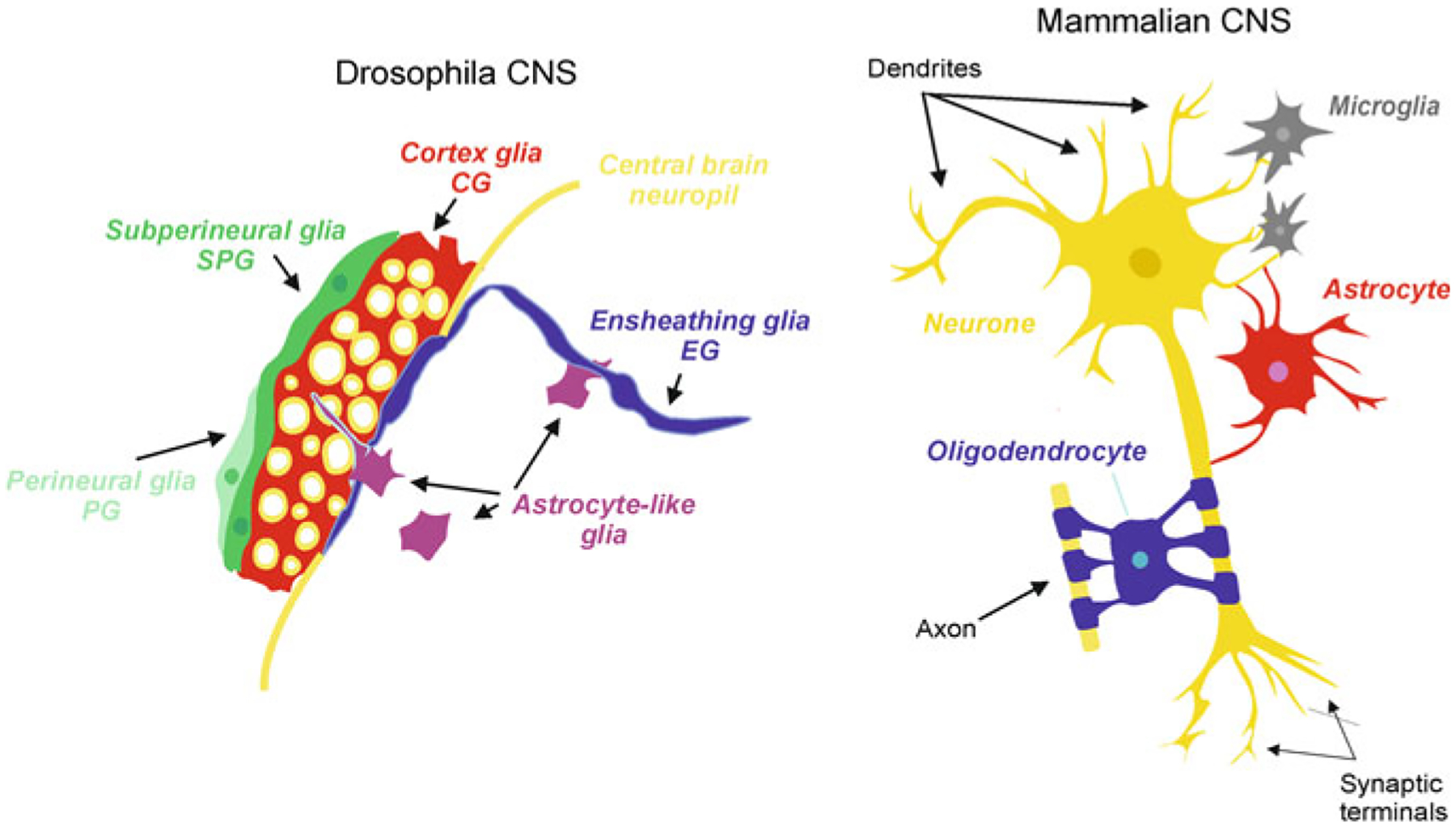
Neuroglia in Drosophila and mammals
The cells of the above classes ii, iii and iv (i.e. surface glia, cortex glia and neuropil glia) are homeostatic cells being thus similar to astrocytes in mammals; the degree of morphological specialisation is, however, greater with specialised cell types responsible for distinct set of functions. A layer of perineural glia covers and delineates the brain as an organ. This coverage is provided by ~100 perineural glial cells, which create a physical barrier of the CNS. Perineural glia and primary surface glia of the ventral nerve cord are derived from embryonic neuro-glioblasts. Both types of primary glial cells increase in size, but not in number, during the larval stage. These cells are retained throughout metamorphosis and become the functional adult glia [35]. Primary perineural glia are mitotically active in the larva, undergo a late phase of proliferation during late larval stage, and differentiate into the optic lobe wrapping glia and then into optic lobe distal satellite glia [104].
Immediately beneath the perineural glial layer, the subperineural glia forms the hemolymph-brain barrier that controls exchange of substances between the CNS and the rest of the body [3, 131]. The subperineural glial cells have a flat morphology and are connected with innexin-based septate junctions, which seal the barrier. Molecular components of septate junctions include Neurexin IV, Gliotactin, Neuroglian and both of α and β subunits of Na+/K+ ATPase [8, 44, 132]. Loss of function of subperineural glia results in aberrant physiological properties such as the alternation in the permeability of extracellular dextrans [132] and reversed polarity of the electroretinogram [150, 151]. The G-protein coupled receptors moody expressed in the surface glia is essential for proper barrier formation. Glial cells in moody locus mutant flies exhibit disruptive actin cytoskeleton, which leads to reduced number of septate junctions and a leaky and malfunctional brain-hemolymph barrier [6, 50, 126]. Mutations in the ATP-binding cassette (ABC) transporter gene mdr65 expressed in surface glia alters the passage of substrates and increases the sensitivity of the haemolymph-brain barrier to toxic pharmaceuticals, thus playing a role in neuroprotection [85]. The glial barrier in the insects is functionally analogous to the endothelial blood-brain barrier in vertebrates. This glial barrier in Drosophila is particularly important in guarding the CNS against substantial fluctuations in systemic K+ that occur after feeding [39].
The specialised type of parenchymal glia, known as cortex glia covers neuronal somata; these cells are very much different from mammalian CNS glia in that a single cell can provide coverage for many neuronal cell bodies. The cortex glial cells also make contact with the hemolymph-brain barrier and provide trophic support to neurones. The cortical glial cells generate activity-dependent intracellular Ca2+ oscillations and regulate seizure susceptibility [88]. Similarly to the perineural glia, primary cortex glial cells enter a phase of proliferation during the late larval period, likely forming the secondary population of adult cortical glia. Glial cells in the insect CNS also plaster tracheoles, which deliver oxygen to the nerve tissue [111].
The neuropil glia in Drosophila are represented by the ensheathing glia and the astrocyte-like glia, both of which are critical elements for the neuropil homeostasis, synaptogenesis and synaptic transmission. Physical contacts between neuropil glia and axons as well as with axon fascicles allow these glial cells to function in a variety of synaptic contexts to regulate neuronal activity and survival. The flat ensheathing glial cells line the borders of the neuropil thus segregating it from the cortex. In addition, ensheathing glia enwrap glomeruli in the antennae lobe. The astroglia-like cells are morphologically similar to parenchymal astrocytes in vertebrates with elaborated arborisation; these cells extend processes into the neuropil and provide for synaptic coverage [36]. Astrocyte-like glia express plasmalemmal amino acid transporters to regulate uptake and release of neurotransmitters to modulate synaptic activity. The ensheathing glia express the engulfment receptor Draper and dCed-6, which control clearing axonal debris due to injury in adult brains. The astrocyte-like glia, also express Draper, which regulates pruning of axons during the development [137] Both types of neuropil glia, the ensheathing glia and astrocyte-like glia share the same origin being the progenies of the embryonic lateral glioblast. The lateral glioblast-generated primary glia undergo several rounds of mitotic divisions to produce a cluster of cells that differentiate into the ensheathing glia and astrocyte-like glia. The primary glial cells are subject to programmed cell death and are not retained into adulthood [9]. Instead, a secondary wave of proliferation from larval neuro-glioblasts is responsible for generating adult ensheathing and astrocyte-like glia [104].
Neuroglial cells in Drosophila exhibit classical intracellular ionic excitability, which contributes to neuronal-glial interactions. Different types of insect glia demonstrate spontaneous and evoked Ca2+ signalling mediated by both intracellular Ca2+ release (mostly in soma) and plasmalemmal Ca2+ entry [88]. Mutation in glial Na+/Ca2+-K+ exchanger (NCKX), which arguably mediates plasmalemmal Ca2+ flux in cortical glia, results in seizures [88]. The astrocyte-like glial cells in Drosophila demonstrate prominent Ca2+ oscillations, seen as fast fluctuations of intracellular Ca2+ in processes. Acute induction of Ca2+ influx into these astroglia-like cells triggered rapid behavioural paralysis and suppressed neuronal activity [156].
Homeostatic functions of glial cells in Drosophila CNS include regulation of ionic balance and control over clearance, recycling and metabolism of neurotransmitters. In the retina, for example, glial cells are providing for recycling of the principal neurotransmitter histamine. Histamine, released from photoreceptors, is accumulated by glial cells, processes of which enwrap relevant synapses [87]. After entering the glial cytoplasm histamine is converted into β-alanyl-histamine (also known as carcinine) by Ebony (N-β-alanyl-biogenic amine synthetase)-catalysed reaction [13]. This carcinine is then shuttled back to photoreceptors, which is mediated by a plasmalemmal carT (in humans—organic anion transporter family Slc22a) transporter [130]. In the photoreceptor cell, carcinine is hydrolysed to histamine by Tan (acyltransferase) protein [140]. Mutations in the components of this histamine/carcinine shuttle impair vision of the fly Drosophila [19, 157]. Genetic alterations of glial cells in Drosophila glia which interfere with vesicle trafficking (by specific expression of temperature-sensitive dynamin) and ionic transport (by glia-specific expression of bacterial Na+ channel) alter circadian rhythm [96].
Another important function of homeostatic neuroglia lies in regulation of neurotransmitters turnover and catabolism. For example, Drosophila neuropil glia express plasmalemmal transporters for excitatory amino acid transporters dEAAT1and dEAAT2 [66, 127], as well as glutamine synthetase [27], all these being key components of glutamate-glutamine shuttle. The plasmalemmal glutamate transporters are preferentially localised at glial perisynaptic processes [121]; decreased expression of these transporters triggers neurotoxicity, degeneration of the neuropil and premature death [78]. In addition, Drosophila glia regulate the homeostasis of inhibitory neurotransmitter γ-aminobutyric acid (GABA) through activity of relevant transporters [103]. The glia-specific cascades regulating glutamate transport are involved in control of sexual behaviour and courtship of the flies. These cascades are represented by glial cystine-glutamate transporter, which controls ambient glutamate concentration and therefore affects the strength of glutamatergic transmission. Loss of function mutation of these transporters (observed in a mutant known as a genderblind, gb) results in ‘homosexual’ courting [47].
The neuroglia in insects is fundamental for metabolic support of neurones and for neuroprotection. In the retina of the honeybee, glial cells supply photoreceptors with alanine, which subsequently is converted to pyruvate for use in the Krebs cycle and production of energy [141]. Targeted ablation of glial cells in the fly instigates extensive neuronal death [12]. Similarly, neuronal loss and progressive neurodegeneration are observed in mutants with aberrant or non-functional glia (mutants designated as drop dead, swiss cheese and repo [14, 71, 150]). Insect neuroglia provides for nervous tissue defence through reactive gliosis and phagocytosis activated in response to lesions [72, 81].
Neuroglia in Drosophila form the neurogenic niche, a specialised anatomical location where stem cells reside, proliferate and differentiate [20, 90]. The cortex glial cells in particular regulate neuroblast proliferation; the neuroblasts establish a specific adhesive contact with cortex glia, this process involving phosphoinositide 3-kinase (PI3-kinase) and DE-cadherin. The cortical glia also regulate neuroblast proliferation through secretion of nutritional signal molecules, such as insulin-like peptides (ILPs) or the glycoprotein encoded by anachronism locus [38, 128, 129]. Cortex glia also produce and secrete ILPs in response to nutrition; these peptides activate the PI3 K/AKT signalling in neuroblasts, thus stimulating neuroblast growth and proliferation. The insulin/insulin-like growth factor (IGF) receptor pathway is necessary for neuroblasts to exit quiescence [20]. Likewise, a fat-body-derived signal required for neuroblast activation is linked to rapamycin (TOR) signalling cascade [128]. Another type of glia, the optic lobe-associated cortex glia, promote neuroepithelial proliferation and neuroblast formation by activating epidermal growth factor receptor. Drosophila microRNA mir-8 (the homolog of vertebrate miR-200 family) is expressed in a subpopulation of optic-lobe-associated cortex glia processes, which ensheath the neuroepithelium. In the absence of glial mir-8, excess proliferation and ectopic neuroblast transition were detected, suggesting that optic-lobe-associated cortex glia use signalling via mir-8 to communicate with the neuroepithelium. The optic-lobe-associated miR-8-positive cortex glia thus acts as a niche component that contributes signals for the growth and morphogenesis of the neuroepithelium [89]. Taken together, these findings suggest that glia are indispensable components of the neurogenic niche in insects; glial cells regulate formation, proliferation and differentiation of neuroblasts.
Drosophila glia also positively regulates and promotes synaptogenesis as well as synaptic maturation through activation of a homologue of the Gabapentin receptor α2δ1 [73].
The glia-regulated signalling cascade involving the peripheral glia expressing Eiger, the first invertebrate tumour necrosis factor (TNF) superfamily ligand [61], and the motorneurone-specific Drosophila TNF-α receptor (TNFR) Wengen [64], regulates neuromuscular junction synaptogenesis. This glia-initiated TNF signalling depends on caspase and mitochondria to regulate neuromuscular junction degeneration, further demonstrating the importance of glia in regulating neuromuscular junction synaptogenesis [67].
2.5. Astrocytes in Chordata and Low Vertebrates
In the CNS of Chordata, Hemichordata and Echinodermata, the main (and often the only) type of neuroglia is represented by radial glial cells. The radial glia, although occurring at some developmental stages in the insects and being identified in some protostomes (for example, in Annelida and Scalidophora [54]), is mainly associated with vertebrates [116]. In the Echinodermata (sea urchin, star fishes or sea cucumber), radial glial cells are the only glia in the CNS. These radial glial cells produce and secrete the Reissner’s substance [84, 145], which mainly contains the glycoprotein known as SCO-spondin, that acts as cell adhesion modulator [46]. This Reissner’s substance has been identified in radial glia throughout Chordata from cephalochordates to Homo sapiens. Glia of Echinodermata have a characteristic radial morphology with elongated shape; these cells have long processes penetrating the whole thickness of the neural parenchyma, and orienting perpendicularly to the surface of the neuroepithelium and high level of expression of intermediate filaments in the cytoplasm [83, 84].
Radial glia represent the main type of neuroglia in the CNS of many early vertebrates, which are almost completely devoid of other types of parenchymal glia. This in particular is characteristic for the brains with thin parenchyma. In Elasmobranchii (chondrichthyan/cartilaginous fish, such as sharks and rays), the brains are sub-classified into the type I, or ‘laminar’ brain (with thin brain wall and large ventricles) and the type II or ‘elaborate’ brain (with thicker parenchyma and smaller ventricles) [2, 16]. In type I brains radial glia predominate, whereas type II brains contain numerous well-developed parenchymal glia resembling astrocytes [2]. Emergence of parenchymal astrocytes in elaborate brains probably reflects an increased homeostatic challenge of the enlarged nervous tissue that cannot be met by radial glia. This constrains homeostatic capabilities of the radial glia and hence prompts an increase in numbers and complexity of parenchymal astrocytes [118].
Similarly radial glia are well developed in bony fish, with teleosts (e.g., zebra fish) being a particular example. The radial glia of the zebrafish, extend their processes through the entire thickness of the brain from the ependymal cells of the ventricles to the pial surface. In these radial glial cells high expression of GFAP has been detected; in addition, these cells possess glutamine synthetase contributing to glutamate homeostasis, and express aquaporin-4 contributing to water homeostasis, [48]). Zebrafish does not possess parenchymal glia (i.e., astrocyte-like cells) and hence radial glial cells are specially important for the responses of zebrafish nervous tissue to injury. Brain lesions in the teleost do not instigate astrogliosis. The stab wounds are closed in several days without formation of the scar; because of rapid increase in the neurogenesis, that generates new cells to fill up the wound [7]. Of note the blood-brain barrier in teleosts is formed by endothelial cells lining brain capillaries, which is similar to all higher vertebrates.
2.6. Astrocytes in Higher Vertebrates and Hominids
An increased complexity of the brain, which developed in parallel with increased intellectual power and increased homeostatic and energy demands stipulated high diversification of neuroglia in mammals [99, 100, 117, 119]. Glial cells became heterogeneous in their form and function. This evolutionary advance in astroglial complexity is specifically prominent in the brains of higher primates and humans [99, 144].
The number of glial cells varies substantially between different species and the GNR does not simply increase with increasing brain size (Fig. 2.7). Albeit already discussed in Chap. 1, we here revisit the glial-to-neurone ratio (GNR) in the nervous system. In invertebrates, it varies between 0.001 and 0.1 (56 glia per 302 neurones in C. elegans [101]; 10 glial cells per 400–700 neurones in every ganglion of the leech [30]; ~9000 glial cells per 90,000 neurones in the central nervous system (CNS) of Drosophila [39, 70]). There are exceptions though: the buccal ganglia of the great ramshorn snail Planorbis corneus contains 298 neurones and 391 glial cells with GNR of 1.3 [110]. In vertebrates the GNR is about 0.3–0.4 in rodents, ~1.1 in cat, ~1.2 in horse, 0.5–1.0 in rhesus monkey, 2.2 in Göttingen minipig, ~1.5 in humans, and as high as 4–8 in elephants and the fin whale [21, 40, 51, 62, 77, 107]. The largest absolute number of glial cells has been identified in the neocortex of whales [40, 31, 35]; for example the neocortex of the long-finned pilot whale (Globicephala melas) contains ~ 37.2 × 109 neurones and 127 109 glial cells with GNR of 3.4 [91]. In the human brain, the total number of glia is more or less the same as number of neurones (about ~80 billion of neurones and ~60 billion of glia) with remarkable regional differences. Of note, the more primitive parts of the human brain have a higher GNR of 7–10 in the brainstem, or even more according to some studies [106]. The GNR of ~5 was determined for the spinal cord of cynomolgus monkey and GNR of almost 7 for spinal cord in humans [5]. These trends argue against the concept that a high GNR reflects evolutionary advance and increased intelligence. Nonetheless, it is important to be aware that evolution brought with it substantial changes in the morphology and complexity of astroglia in the human cortex, which also contains several highly specialised types of glial cell which are absent in the brains of lesser vertebrates.
Fig. 2.7.
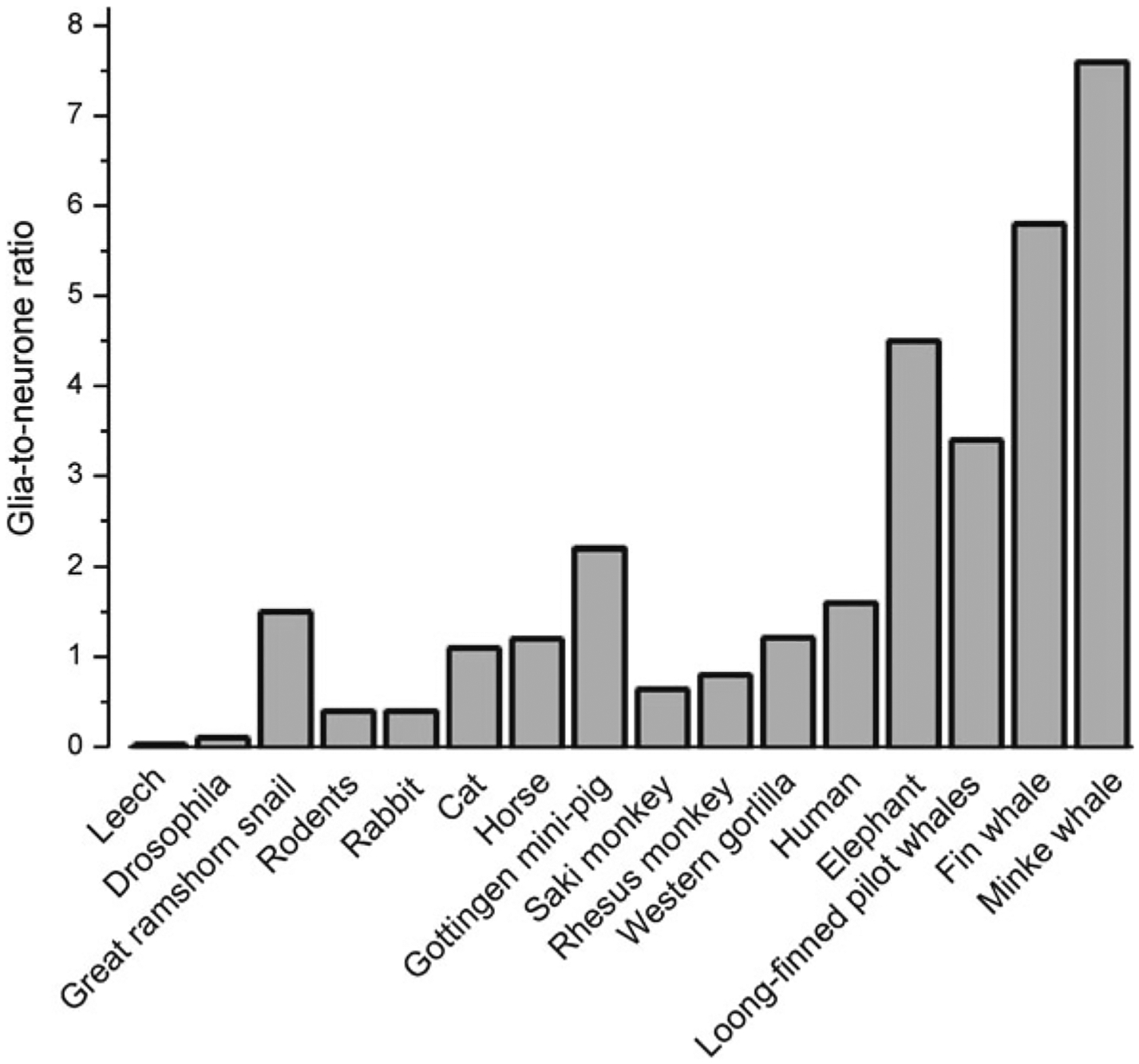
Phylogenetical advance of neuroglia. Glia-to-neurone ratio in the nervous system of invertebrates and in the cortex of vertebrates. Glia-to-neurone ratio is generally increased in phylogeny; more or less this ratio linearly follows an increase in the size of the brain
Human astrocytes are much larger and far more complex than astroglial cells in, for example, rodent brain (Fig. 2.8). In the human brain the average diameter of the domain belonging to a human protoplasmic astrocyte is ~2.5 times larger than the domain formed by a rat astrocyte (142 vs. 56 μm). The volume of the human protoplasmic astrocyte domain was ~16.5 times larger than that of the corresponding domain in a rat brain. Human protoplasmic astrocytes have ~10 times more primary processes, and correspondingly much more complex processes arborisation than rodent astroglia. Similarly, fibrous astrocytes, localised in the white matter are ~2.2 times larger in humans when compared to rodents. Due to this increased complexity human protoplasmic astrocytes enwrap ~2 millions of synapses localised in their territorial domains, whereas in rodents single astrocyte covers only 20,000–120,000 synaptic contacts [15, 99].
Fig. 2.8.
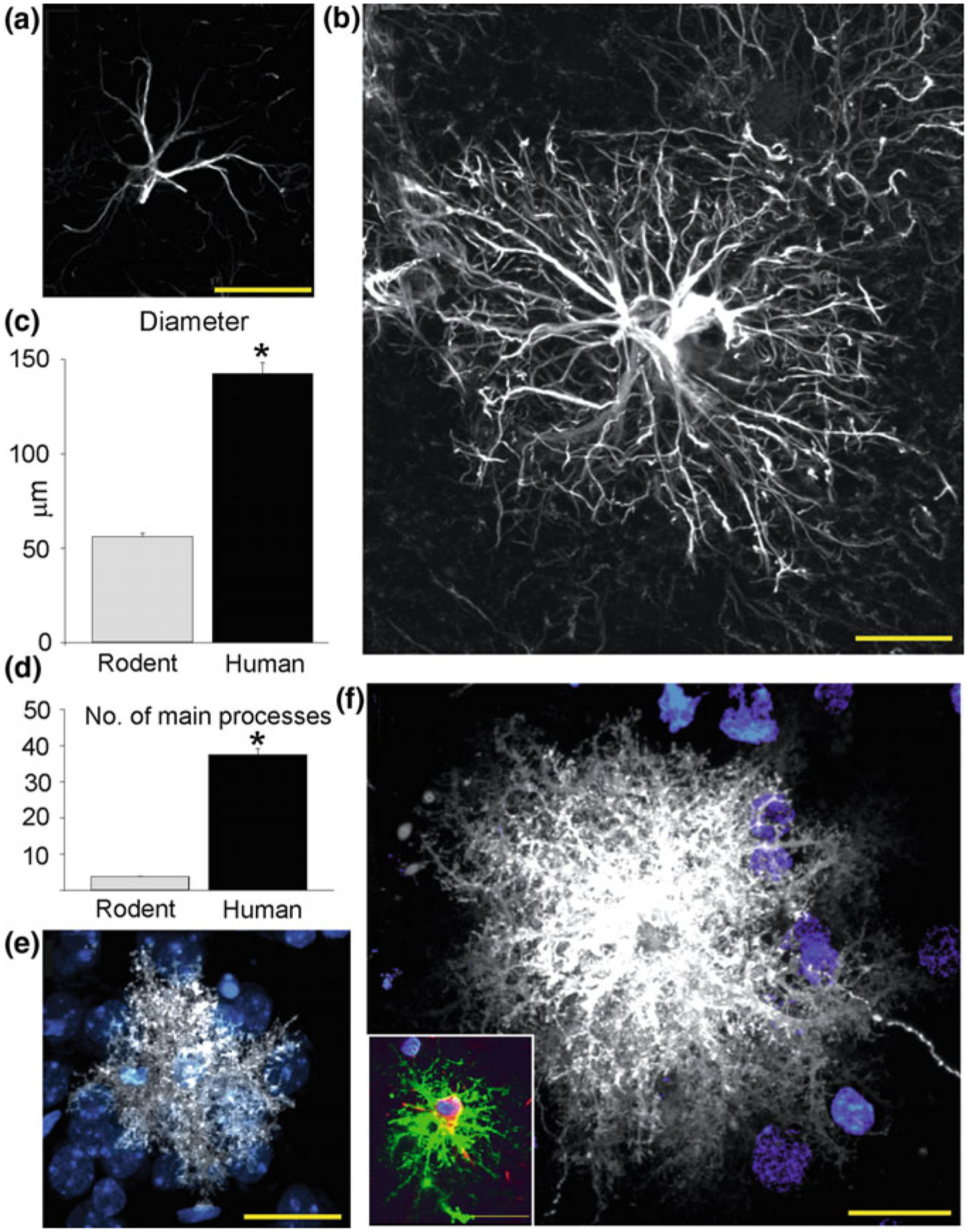
Comparison of rodent and human protoplasmic astrocytes. a Typical mouse protoplasmic astrocyte. GFAP, White. Scale bar, 20 μm. b Typical human protoplasmic astrocyte at the same scale. Scale bar, 20 μm. c, d Human protoplasmic astrocytes are 2.55-fold larger and have 10-fold more main GFAP processes than mouse astrocytes (human, n = 50 cells from 7 patients; mouse, n = 65 cells from 6 mice; mean ± SEM; *p < 0.005, t test). e Mouse protoplasmic astrocyte diolistically labelled with DiI (white) and sytox (blue) revealing the full structure of the astrocyte including its numerous fine processes. Scale bar, 20 μm. f Human astrocyte demonstrates the highly complicated network of fine process that defines the human protoplasmic astrocyte. Scale bar, 20 μm. Inset, Human protoplasmic astrocyte diolistically labelled as well as immunolabelled for GFAP (green) demonstrating colocalisation. Scale bar, 20 μm. Reproduced, with permission from [99, 144]
Special subpopulations of astrocytes are found in the brains of higher primates and humans (Fig. 2.9). The first type of these specialised astroglial cells is known as interlaminar astrocytes [23–25]. The somata of these interlaminar astrocytes are localised in the layer I of the cortex, which has low density of neuronal cell bodies and high density of synaptic connections. Somata of interlaminar astrocytes are rather small not exceeding 10 μm, from these somata emanate several short and one or two very long processes. These long processes, which can be as long as 1 mm penetrate through the cortex, and terminate in layers III and IV. At the tips of these long processes special structures known as the ‘terminal mass’ or ‘end bulb’ have been detected. These terminal masses often contain mitochondria. Physiological properties of interlaminar astrocytes remain completely unknown. It has been suggested that they may form astroglial component of neuronal columns that are regarded as cortical functional units. It has been further speculated that interlaminar astrocytes may contribute to long distance signalling and integration within cortical columns.
Fig. 2.9.
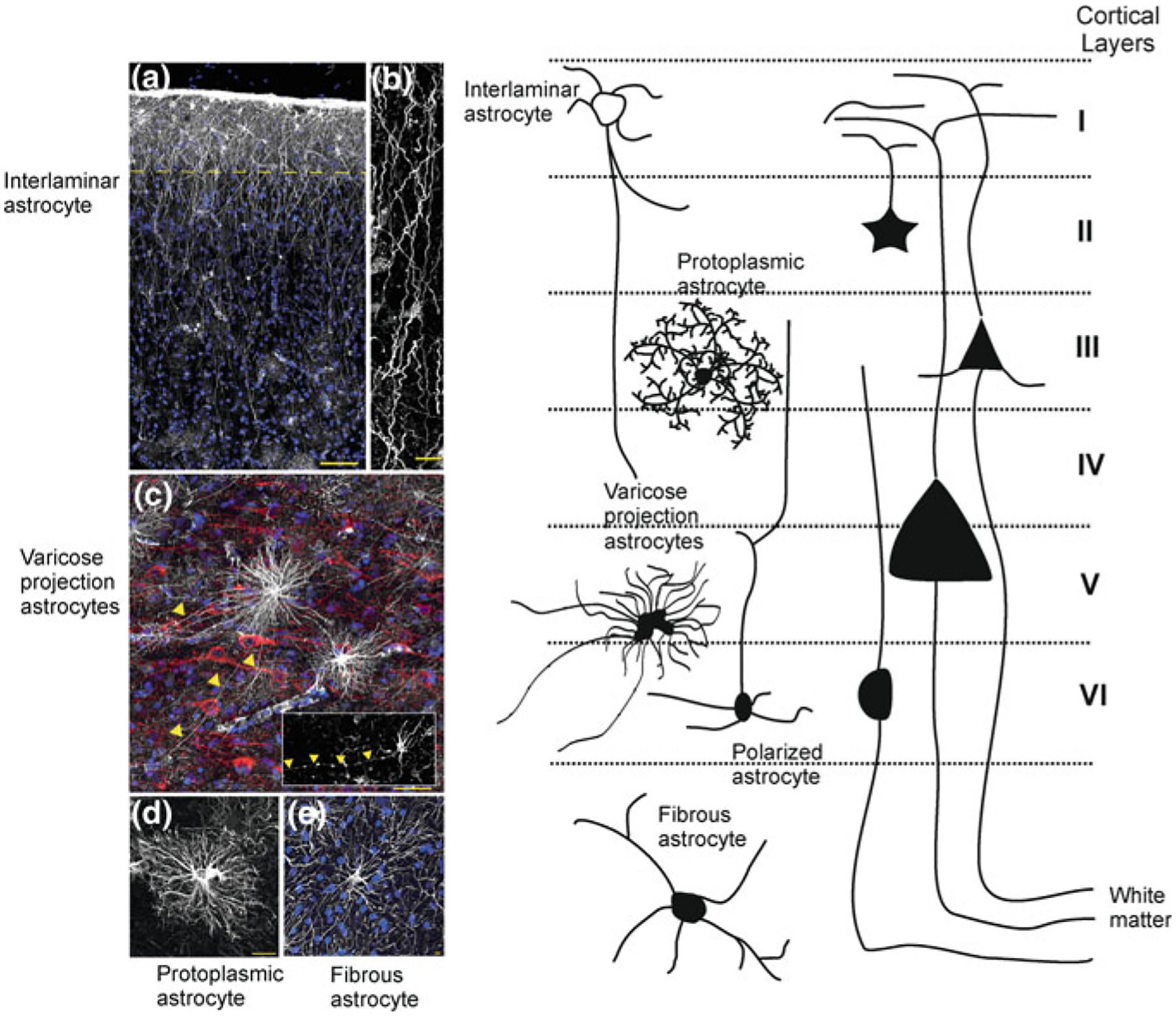
Morphological heterogeneity and subtypes of astrocytes in the cortex of higher primates. a Pial surface and layers 1–2 of human cortex. GFAP staining in white; DAPI, in blue. Scale bar, 100 μm. Yellow line indicates border between layer I and II. b Interlaminar astrocyte processes. Scale bar, 10 μm. c Varicose projection astrocytes reside in layers V and VI and extend long processes characterized by evenly spaced varicosities. Inset: Varicose projection astrocyte from chimpanzee cortex. Yellow arrowheads indicate varicose projections. Scale bar, 50 μm. d Typical human protoplasmic astrocyte. Scale bar, 20 μm. e Human fibrous astrocytes in white matter. Scale bar, 10 μm. (modified with permission from [98]. Left panel schematically shows different astrocytes and their relatuons to cortical layers. Adapted from [143]
The second type of specialised human astrocytes is represented by polarised astrocytes, represented by unior bipolar cells, somata of which are in cortical layers V and VI close to the white matter. These cells possess one or two very long (up to 1 mm) and very thin (2–3 μm in diameter) processes that end in the neuropil. In addition these processes have numerous varicosities with yet unknown functions [99, 144].
In contrast to neuroglia neurones did not increase their size that much. Similarly, the density of synaptic contacts in rodents and primates is similar (the mean density of synaptic contacts in the rodent brain is ~1397 millions/mm3, whereas in humans synaptic density in the cortex is around 1100 millions/mm3). Likewise, the number of synapses per neurone is not much different between primates and rodents. The average size of human neurones is ~1.5 times larger than in rodents. Thus, at least morphologically, evolution resulted in much more prominent changes in glia than in neurones, which most likely has important, although yet undetermined, significance.
2.7. Evolution of Myelination and Oligodendroglia
The emergence and evolution of myelination is linked to an increase in animal size that requires faster nerve conductance; increase in action potential propagation velocity can be achieved either through an increase in axon diameter, or through introduction of saltatory nerve impulse propagation. Increase in axon diameter reduces resistance of the axon proportionally to the square of diameter, with the conductance velocity being directly proportional to the square root of the axon diameter [57]. In the Loligo squid, for instance, thick axons of 0.5 mm in diameter sustain action potential propagation velocity of about 30 m/s. Large axons, however, present several problems to the complex nervous system. Firstly, the conduction through large axons is energetically costly, because Na+/K+ pump responsible for ion gradients maintenance consumes substantial amounts of ATP [82]. Secondly, large diameter axons are associated with prominent space constrains incompatible with a rather compact design of advanced CNS.
The saltatory propagation of action potential, which allows high speed nerve impulse conductance, solves both problems due to restriction of ion fluxes to small portions of axonal membrane. Namely, axons are covered with multiple layers of lipid membranes, interrupted by gaps known as nodes of Ranvier. The nodal membranes have high densities of voltage-gated Na+ and K+ channels responsible for action potential generation [94]. The lipid-rich membranous lamellae insulate parts of the axons in between the nodes, thus increasing axonal transverse resistance and reducing transverse capacitance.
The very first structures unsheathing the axons and allowing saltatory propagation of action potentials emerged early in evolution. Arguably, the first organisms in possession of this mechanism are prawns, which appeared in the Cambrian period (500–540 million years ago). At that time, the giant prawn, Anomalocaridids, was the sole and the most dangerous predator of the sea [142]. These predatory prawns had about 1 m in length, and they were endowed with large compound eyes. These eyes were exceptionally big (according to the fossil studies the visual surface was 22 mm long and 12 mm wide) being composed of tens of thousands of hexagonal ommatidial lenses ~70–110 μm in diameter [109]. Feeding thousands of axons into the CNS of these animals most likely required insulating ensheathing of axons.
Modern Crustacean (e.g., prawns, shrimps and crabs) retain this arrangements having elaborated axonal ensheathment. In the prawns of the genus Penaeus (such as Japanese tiger shrimp, or Chinese white shrimp), axons are surrounded by glial membranes and by a large submyelin space positioned between the axonal membrane and the first layer of glial membrane. The ion currents thus are trapped in this space as if the normal axon is surrounded by a giant axon (the submyelin space acts in essence as a low-resistance pathway), this topography allows for an unprecedented speed of action potential propagation of up to 210 m/s [74, 152, 153]. The submyelin spaces are tightly sealed at the nodes thus allowing the saltatory conductance. The node (which in invertebrates is called the ‘fenestration node’) diameter and internodal distance is proportional to the axon diameter and, in prawns, vary between 5 and 50 μm and 3 and 12 μm, respectively. The thickness of the glial membranous sheath is ~10 μm; it is comprised of 10–60 stacked membrane layers separated by 8–9 nm. Like in vertebrates, voltage-gated sodium channels in prawns are concentrated at the nodes where their density can reach thousands of channels/μm2. There is a fundamental difference between vertebrates and prawn axonal coverage. In vertebrates the single Schwann cell (peripheral nerves) or a process of an oligodendrocyte (CNS) spirals around the axon forming multiple membranous lamellae. In the prawns a single myelinating glial cell sends multiple processes forming multiple layers, with each process encircling the axon once, meeting itself on the opposite side in a seam [55]. Another difference is location of the nuclei of myelinating cell. In vertebrates it is located as a rule at the outer edge of myelin sheath, whereas in prawns the nuclei are randomly dispersed between membrane laminae [153].
Axons covered with multilayered glial membranes (although these glial cells do not produce myelin) are operative in some other invertebrates. In the earthworm Lumbricus terrestrils the central axons of 50–100 μm in diameter are enwrapped with 60–200 layers of cell membranes produced by many glial cells, nuclei of which are scattered along the axon [122]. In this structure the nodes are not clearly seen; nonetheless, the conductance velocity reaches ~20–45 m/s, which is higher compared to thicker giant axons of Loligo squid. The glial axonal coverage was also found in marine Annelida phoronids; in these animals axons are covered with 9–20 membranous layers [42]. Similar number of layers of glial membranes covers the axon of the aquatic sludge worm Branchiura sowerbyi [158].
Emergence of myelin is associated with relatively developed vertebrates. Compacted myelin sheaths are absent in lower vertebrates, such as hagfish and lampreys, and begun to develop in sharks and bony fish. There are some arguments indicating that the most ancient forms of myelin sheath emerged in placoderms (now extinct jawed armoured fish from the early Silurian period ~420 million years ago). These fish form the base for chondrichtyan and bony fishes. The fossil records indicate that the diameters of the foramina for oculomotor nerves in the jawed fish and in jawless Osteostraci fish (which do not have myelin) are the same (about 0.1 mm), whereas the length of nerve in placoderms was 10 times larger, that highlights the necessity for myelin to maintain the same speed of action potential-mediated signal transduction [155]. Further reasoning suggests the connection between appearance of the jaw in early Gnathostomata (jawed vertebrates, which embrace all higher vertebrates living today, including mammals) and myelination. By acquiring myelinated nerves, the jawed fishes arguably acquired better ability to hunt the prey, while keeping the axonal diameter the same or even smaller compared to their jawless predecessors [155].
Coverage of axons with glial membranes emerged in early evolutionary forms. In the beginning this coverage arguably supported axonal mechanical stability and provided energy support. At the same time glial membranous lamellae increased action potential propagation velocity, and once emerged, myelination provided obvious evolutionary advantages. One of the advantages was an increase in compactness of the nervous system and decrease in energy expenditure for restoring ion balances.
2.8. Evolution of Microglia
The evolutionary origins of microglia remain largely unexplored. It is possible to suggest, however, that appearance of innate immune and phagocytic cells in the nerve tissue coincided with the emergence of barriers separating early brains from the circulation. Such barriers restricted pathways for entry of immune/defence cells into the brain parenchyma, thus calling for a specialised intra-brain defence system. This problem was solved after immune cells found the way to migrate and retain in the nerve tissue; exposure to the specific neurochemical environment as well as epigenetic trends most likely stimulated acquisition of specific microglial phenotype. Evidence for phylogenetically early microglial cells is available for Annelida (leech), Mollusca (Bivalvia and snails) and some Arthropoda (insects) (see [68] for detailed review).
The nervous system of medicinal leech contains substantial numbers of microglial cells. The microglia of the leech is represented by small spindle-like shape cells. Insults to the leech nervous system trigger microglial activation; these cells migrate to the site of the insult and become phagocytes. The weak silver carbonate staining (a classical microglial staining technique developed by Pío del Río Hortega) is generally used to visualise activated microglia in leech. In response to infectious attack the leech microglial cells were found to produce and secrete antimicrobial peptides [125].
Well developed microglia were also found in nerve ganglia of molluscs. In the marine bivalve Mytilus edulis microglial cells can be activated and their migration can be instigated in response to various molecular signals including nitric oxide, opioids, cannabinoids and cytokines. Similarly, migrating microglia were observed in the snail Planorbarius corneus and in the insect Leucophaea maderae. The microglial cells (morphologically distinguished by phagocytic inclusions) of snail Planorbis corneus, were mainly concentrated in the neuropil of nerve ganglia while mechanical lesion increased the number of these phagocytic cells [110].
2.9. Conclusions
Most ancient glial cells developed as a supportive element of the sensory organs. The centralisation and increase in complexity of the nervous system created high demand for homeostatic support which was met by diversification of glial cells. Such a diversification resulted in multiple phenotypes in invertebrates, which are in possession of very specialised glial cells such as giant glial cells in the leech or cortex glia in Drosophila. These glial cells of invertebrates have performed many supportive functions from regulation of ion and neurotransmitter homeostasis to metabolic support and regulation of neuronal development. In the invertebrates glial cells formed brain to body barriers which stipulated the emergence of specialised immune and defence cells known as microglia. Increase in complexity of brain connectome and increase in axonal density were factors defining evolutionary benefits of the myelin sheath and development of myelinating cells. The evolution of myelination formed the basis for increased complexity of the nervous system that relies on interneuronal connections. In early Chordata radial glia become the main sub-type which was instrumental in formation of the layered brain. Subsequent increase in brain thickness promoted evolution of homeostatic astroglia. Finally, in the brain of primates and especially in the brain of humans, astrocytes become exceedingly complex and new types of astroglial cells involved in interlayer communication/integration have evolved.
Acknowledgments
VP’s work is supported by a grant from the National Institute of General Medical Sciences of the National Institutes of Health (R01GM123971). VP is an Honorary Professor at University of Rijeka, Croatia.
Contributor Information
Alexei Verkhratsky, Faculty of Biology, Medicine and Health, The University of Manchester, Manchester M13 9PT, UK; Faculty of Health and Medical Sciences, Center for Basic and Translational Neuroscience, University of Copenhagen, 2200 Copenhagen, Denmark; Achucarro Center for Neuroscience, IKERBASQUE, Basque Foundation for Science, 48011 Bilbao, Spain.
Margaret S. Ho, School of Life Science and Technology, Shanghai Tech University, #230 Haike Road, Shanghai 201210, China
Vladimir Parpura, Department of Neurobiology, The University of Alabama at Birmingham, Birmingham, AL, USA; University of Rijeka, Rijeka, Croatia.
References
- 1.Altun ZF, Chen B, Wang ZW, Hall DH (2009) High resolution map of Caenorhabditis elegans gap junction proteins. Dev Dyn 238:1936–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ari C, Kalman M (2008) Evolutionary changes of astroglia in Elasmobranchii comparing to amniotes: a study based on three immunohistochemical markers (GFAP, S-100, and glutamine synthetase). Brain Behav Evol 71:305–324 [DOI] [PubMed] [Google Scholar]
- 3.Awasaki T, Lai SL, Ito K, Lee T (2008) Organization and postembryonic development of glial cells in the adult central brain of Drosophila. J Neurosci 28:13742–13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacaj T, Tevlin M, Lu Y, Shaham S (2008) Glia are essential for sensory organ function in C. elegans. Science 322:744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahney J, von Bartheld CS (2018) The cellular composition and glia-neuron ratio in the spinal cord of a human and a nonhuman primate: comparison with other species and brain regions. Anat Rec (Hoboken) 301:697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bainton RJ, Tsai LT, Schwabe T, DeSalvo M, Gaul U, Heberlein U (2005) Moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 123:145–156 [DOI] [PubMed] [Google Scholar]
- 7.Baumgart EV, Barbosa JS, Bally-Cuif L, Gotz M, Ninkovic J (2010) Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60:343–357 [DOI] [PubMed] [Google Scholar]
- 8.Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, ChiquetEhrismann R, Prokop A, Bellen HJ (1996) A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell 87:1059–1068 [DOI] [PubMed] [Google Scholar]
- 9.Beckervordersandforth RM, Rickert C, Altenhein B, Technau GM (2008) Subtypes of glial cells in the Drosophila embryonic ventral nerve cord as related to lineage and gene expression. Mech Dev 125:542–557 [DOI] [PubMed] [Google Scholar]
- 10.Bedini C, Lanfranchi A (1991) The central and peripheral nervous system of Acoela (plathelminthes). An electron microscopical study. Acta Zoologica (Stockholm) 72:101–106 [Google Scholar]
- 11.Bery A, Cardona A, Martinez P, Hartenstein V (2010) Structure of the central nervous system of a juvenile acoel, Symsagittifera roscoffensis. Dev Genes Evol 220:61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth GE, Kinrade EF, Hidalgo A (2000) Glia maintain follower neuron survival during Drosophila CNS development. Development 127:237–244 [DOI] [PubMed] [Google Scholar]
- 13.Borycz J, Borycz JA, Loubani M, Meinertzhagen IA (2002) tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci 22:10549–10557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchanan RL, Benzer S (1993) Defective glia in the Drosophila brain degeneration mutant drop-dead. Neuron 10:839–850 [DOI] [PubMed] [Google Scholar]
- 15.Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler AB, Hodos W (2005) Vertebrate neuroanatomy: evolution and adaptation, 2nd edn. Wiley, New York [Google Scholar]
- 17.Cavalier-Smith T (1998) A revised six-kingdom system of life. Biol Rev Camb Philos Soc 73:203–266 [DOI] [PubMed] [Google Scholar]
- 18.Cavalier-Smith T (2009) Megaphylogeny, cell body plans, adaptive zones: causes and timing of eukaryote basal radiations. J Eukaryot Microbiol 56:26–33 [DOI] [PubMed] [Google Scholar]
- 19.Chaturvedi R, Reddig K, Li HS (2014) Long-distance mechanism of neurotransmitter recycling mediated by glial network facilitates visual function in Drosophila. Proc Natl Acad Sci USA 111:2812–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chell JM, Brand AH (2010) Nutrition-responsive glia control exit of neural stem cells from quiescence. Cell 143:1161–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christensen JR, Larsen KB, Lisanby SH, Scalia J, Arango V, Dwork AJ, Pakkenberg B (2007) Neocortical and hippocampal neuron and glial cell numbers in the rhesus monkey. Anat Rec (Hoboken) 290:330–340 [DOI] [PubMed] [Google Scholar]
- 22.Coggeshall RE, Fawcett DW (1964) The fine structure of the central nervous system of the leech, Hirudo Medicinalis. J Neurophysiol 27:229–289 [DOI] [PubMed] [Google Scholar]
- 23.Colombo JA, Reisin HD (2004) Interlaminar astroglia of the cerebral cortex: a marker of the primate brain. Brain Res 1006:126–131 [DOI] [PubMed] [Google Scholar]
- 24.Colombo JA, Sherwood CC, Hof PR (2004) Interlaminar astroglial processes in the cerebral cortex of great apes. Anat Embryol (Berl) 208:215–218 [DOI] [PubMed] [Google Scholar]
- 25.Colombo JA, Yanez A, Puissant V, Lipina S (1995) Long, interlaminar astroglial cell processes in the cortex of adult monkeys. J Neurosci Res 40:551–556 [DOI] [PubMed] [Google Scholar]
- 26.Csoknya M, Dénes V, Wilhelm M (2012) Glial cells in the central nervous system of earthworm, Eisenia fetida. Acta Biol Hung 63(Suppl. 1):114–128 [DOI] [PubMed] [Google Scholar]
- 27.De Pinto V, Caggese C, Prezioso G, Ritossa F (1987) Purification of the glutamine synthetase II isozyme of Drosophila melanogaster and structural and functional comparison of glutamine synthetases I and II. Biochem Genet 25:821–836 [DOI] [PubMed] [Google Scholar]
- 28.Deitmer JW (1991) Electrogenic sodium-dependent bicarbonate secretion by glial cells of the leech central nervous system. J Gen Physiol 98:637–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deitmer JW, Kristan WB Jr (1999) Glial responses during evoked behaviors in the leech. Glia 26:186–189 [DOI] [PubMed] [Google Scholar]
- 30.Deitmer JW, Rose CR, Munsch T, Schmidt J, Nett W, Schneider HP, Lohr C (1999) Leech giant glial cell: functional role in a simple nervous system. Glia 28:175–182 [PubMed] [Google Scholar]
- 31.Deitmer JW, Schlue WR (1987) The regulation of intracellular pH by identified glial cells and neurones in the central nervous system of the leech. J Physiol 388:261–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deitmer JW, Schlue WR (1989) An inwardly directed electrogenic sodium-bicarbonate co-transport in leech glial cells. J Physiol 411:179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deitmer JW, Schneider HP (1997) Intracellular acidification of the leech giant glial cell evoked by glutamate and aspartate. Glia 19:111–122 [PubMed] [Google Scholar]
- 34.Deitmer JW, Verkhratsky AJ, Lohr C (1998) Calcium signalling in glial cells. Cell Calcium 24:405–416 [DOI] [PubMed] [Google Scholar]
- 35.DeSalvo MK, Mayer N, Mayer F, Bainton RJ (2011) Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila. Glia 59:1322–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doherty J, Logan MA, Tasdemir OE, Freeman MR (2009) Ensheathing glia function as phagocytes in the adult Drosophila brain. J Neurosci 29:4768–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dykes IM, Freeman FM, Bacon JP, Davies JA (2004) Molecular basis of gap junctional communication in the CNS of the leech Hirudo medicinalis. J Neurosci 24:886–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebens AJ, Garren H, Cheyette BN, Zipursky SL (1993) The Drosophila anachronism locus: a glycoprotein secreted by glia inhibits neuroblast proliferation. Cell 74:15–27 [DOI] [PubMed] [Google Scholar]
- 39.Edwards TN, Meinertzhagen IA (2010) The functional organisation of glia in the adult brain of Drosophila and other insects. Prog Neurobiol 90:471–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eriksen N, Pakkenberg B (2007) Total neocortical cell number in the mysticete brain. Anat Rec (Hoboken) 290:83–95 [DOI] [PubMed] [Google Scholar]
- 41.Felton CM, Johnson CM (2011) Modulation of dopamine-dependent behaviors by the Caenorhabditis elegans Olig homolog HLH-17. J Neurosci Res 89:1627–1636 [DOI] [PubMed] [Google Scholar]
- 42.Fernandez I, Pardos F, Benito J, Roldan C (1996) Ultrastructural observations on the phoronid nervous system. J Morphol 230:265–281 [DOI] [PubMed] [Google Scholar]
- 43.Freeman MR, Doherty J (2006) Glial cell biology in Drosophila and vertebrates. Trends Neurosci 29:82–90 [DOI] [PubMed] [Google Scholar]
- 44.Genova JL, Fehon RG (2003) Neuroglian, Gliotactin, and the Na+/K+ATPase are essential for septate junction function in Drosophila. J Cell Biol 161:979–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghysen A (2003) The origin and evolution of the nervous system. Int J Dev Biol 47:555–562 [PubMed] [Google Scholar]
- 46.Gobron S, Monnerie H, Meiniel R, Creveaux I, Lehmann W, Lamalle D, Dastugue B, Meiniel A (1996) SCO-spondin: a new member of the thrombospondin family secreted by the sub-commissural organ is a candidate in the modulation of neuronal aggregation. J Cell Sci 109(Pt 5):1053–1061 [DOI] [PubMed] [Google Scholar]
- 47.Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE (2008) A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci 11:54–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grupp L, Wolburg H, Mack AF (2010) Astroglial structures in the zebrafish brain. J Comp Neurol 518:4277–4287 [DOI] [PubMed] [Google Scholar]
- 49.Hartenstein V (2011) Morphological diversity and development of glia in Drosophila. Glia 59:1237–1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatan M, Shinder V, Israeli D, Schnorrer F, Volk T (2011) The Drosophila blood brain barrier is maintained by GPCR-dependent dynamic actin structures. J Cell Biol 192:307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hawkins A, Olszewski J (1957) Glia/nerve cell index for cortex of the whale. Science 126:76–77 [DOI] [PubMed] [Google Scholar]
- 52.Hedgecock EM, Culotti JG, Hall DH (1990) The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron 4:61–85 [DOI] [PubMed] [Google Scholar]
- 53.Heiman MG, Shaham S (2009) DEX-1 and DYF-7 establish sensory dendrite length by anchoring dendritic tips during cell migration. Cell 137:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Helm C, Karl A, Beckers P, Kaul-Strehlow S, Ulbricht E, Kourtesis I, Kuhrt H, Hausen H, Bartolomaeus T, Reichenbach A, Bleidorn C (2017) Early evolution of radial glial cells in Bilateria. Proc Biol Sci 284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heuser JE, Doggenweiler CF (1966) The fine structural organization of nerve fibers, sheaths, and glial cells in the prawn, Palaemonetes vulgaris. J Cell Biol 30:381–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirth IC, Deitmer JW (2006) 5-Hydroxytryptamine-mediated increase in glutamate uptake by the leech giant glial cell. Glia 54:786–794 [DOI] [PubMed] [Google Scholar]
- 57.Hodgkin AL (1954) A note on conduction velocity. J Physiol 125:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holland ND (2003) Early central nervous system evolution: an era of skin brains? Nat Rev Neurosci 4:617–627 [DOI] [PubMed] [Google Scholar]
- 59.Holmgren E (1901) Beiträge zur Morphologie der Zelle: I. Nervenzellen. Anat Hefte 18:267–326 [Google Scholar]
- 60.Hosoya T, Takizawa K, Nitta K, Hotta Y (1995) glial cells missing: a binary switch between neuronal and glial determination in Drosophila. Cell 82:1025–1036 [DOI] [PubMed] [Google Scholar]
- 61.Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M (2002) Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J 21:3009–3018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jelsing J, Nielsen R, Olsen AK, Grand N, Hemmingsen R, Pakkenberg B (2006) The postnatal development of neocortical neurons and glial cells in the Gottingen minipig and the domestic pig brain. J Exp Biol 209:1454–1462 [DOI] [PubMed] [Google Scholar]
- 63.Jones BW, Fetter RD, Tear G, Goodman CS (1995) glial cells missing: a genetic switch that controls glial versus neuronal fate. Cell 82:1013–1023 [DOI] [PubMed] [Google Scholar]
- 64.Kanda H, Igaki T, Kanuka H, Yagi T, Miura M (2002) Wengen, a member of the Drosophila tumor necrosis factor receptor superfamily, is required for Eiger signaling. J Biol Chem 277:28372–28375 [DOI] [PubMed] [Google Scholar]
- 65.Kandarian B, Sethi J, Wu A, Baker M, Yazdani N, Kym E, Sanchez A, Edsall L, Gaasterland T, Macagno E (2012) The medicinal leech genome encodes 21 innexin genes: different combinations are expressed by identified central neurons. Dev Genes Evol 222:29–44 [DOI] [PubMed] [Google Scholar]
- 66.Kawano T, Takuwa K, Kuniyoshi H, Juni N, Nakajima T, Yamamoto D, Kimura Y (1999) Cloning and characterization of a Drosophila melanogaster cDNA encoding a glutamate transporter. Biosci Biotechnol Biochem 63:2042–2044 [DOI] [PubMed] [Google Scholar]
- 67.Keller LC, Cheng L, Locke CJ, Muller M, Fetter RD, Davis GW (2011) Glial-derived prodegenerative signaling in the Drosophila neuromuscular system. Neuron 72:760–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kettenmann H, Hanisch UK, Noda M, Verkhratsky A (2011) Physiology of microglia. Physiol Rev 91:461–553 [DOI] [PubMed] [Google Scholar]
- 69.Kim J, Jones BW, Zock C, Chen Z, Wang H, Goodman CS, Anderson DJ (1998) Isolation and characterization of mammalian homologs of the Drosophila gene glial cells missing. Proc Natl Acad Sci USA 95:12364–12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kremer MC, Jung C, Batelli S, Rubin GM, Gaul U (2017) The glia of the adult Drosophila nervous system. Glia 65:606–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kretzschmar D, Hasan G, Sharma S, Heisenberg M, Benzer S (1997) The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J Neurosci 17:7425–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kurant E (2011) Keeping the CNS clear: glial phagocytic functions in Drosophila. Glia 59:1304–1311 [DOI] [PubMed] [Google Scholar]
- 73.Kurshan PT, Oztan A, Schwarz TL (2009) Presynaptic alpha2delta-3 is required for synaptic morphogenesis independent of its Ca2+-channel functions. Nat Neurosci 12:1415–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusano K (1966) Electrical activity and structural correlates of giant nerve fibers in Kuruma shrimp (Penaeus japonicus). J Cell Physiol 68:361–383 [Google Scholar]
- 75.Labouesse M, Sookhareea S, Horvitz HR (1994) The Caenorhabditis elegans gene lin-26 is required to specify the fates of hypodermal cells and encodes a presumptive zinc-finger transcription factor. Development 120:2359–2368 [DOI] [PubMed] [Google Scholar]
- 76.Le Marrec-Croq F, Drago F, Vizioli J, Sautiere PE, Lefebvre C (2013) The leech nervous system: a valuable model to study the microglia involvement in regenerative processes. Clin Dev Immunol 2013:274019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lidow MS, Song ZM (2001) Primates exposed to cocaine in utero display reduced density and number of cerebral cortical neurons. J Comp Neurol 435:263–275 [DOI] [PubMed] [Google Scholar]
- 78.Lievens JC, Rival T, Iche M, Chneiweiss H, Birman S (2005) Expanded polyglutamine peptides disrupt EGF receptor signaling and glutamate transporter expression in Drosophila. Hum Mol Genet 14:713–724 [DOI] [PubMed] [Google Scholar]
- 79.Lohr C, Deitmer JW (1997) Structural and physiological properties of leech giant glial cells as studied by confocal microscopy. Exp Biol Online 2:8 [Google Scholar]
- 80.Lohr C, Deitmer JW (2006) Calcium signaling in invertebrate glial cells. Glia 54:642–649 [DOI] [PubMed] [Google Scholar]
- 81.MacDonald JM, Beach MG, Porpiglia E, Sheehan AE, Watts RJ, Freeman MR (2006) The Drosophila cell corpse engulfment receptor Draper mediates glial clearance of severed axons. Neuron 50:869–881 [DOI] [PubMed] [Google Scholar]
- 82.Magistretti PJ (2009) Neuroscience. Low-cost travel in neurons. Science 325:1349–1351 [DOI] [PubMed] [Google Scholar]
- 83.Mashanov VS, Zueva OR, Garcia-Arraras JE (2010) Organization of glial cells in the adult sea cucumber central nervous system. Glia 58:1581–1593 [DOI] [PubMed] [Google Scholar]
- 84.Mashanov VS, Zueva OR, Heinzeller T, Aschauer B, Naumann WW, Grondona JM, Cifuentes M, Garcia-Arraras JE (2009) The central nervous system of sea cucumbers (Echinodermata: Holothuroidea) shows positive immunostaining for a chordate glial secretion. Front Zool 6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mayer F, Mayer N, Chinn L, Pinsonneault RL, Kroetz D, Bainton RJ (2009) Evolutionary conservation of vertebrate blood-brain barrier chemoprotective mechanisms in Drosophila. J Neurosci 29:3538–3550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McMiller TL, Johnson CM (2005) Molecular characterization of HLH-17, a C. elegans bHLH protein required for normal larval development. Gene 356:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meinertzhagen IA, O’Neil SD (1991) Synaptic organization of columnar elements in the lamina of the wild type in Drosophila melanogaster. J Comp Neurol 305:232–263 [DOI] [PubMed] [Google Scholar]
- 88.Melom JE, Littleton JT (2013) Mutation of a NCKX eliminates glial microdomain calcium oscillations and enhances seizure susceptibility. J Neurosci 33:1169–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morante J, Vallejo DM, Desplan C, Dominguez M (2013) Conserved miR-8/miR-200 defines a glial niche that controls neuroepithelial expansion and neuroblast transition. Dev Cell 27:174–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morrison SJ, Spradling AC (2008) Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132:598–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mortensen HS, Pakkenberg B, Dam M, Dietz R, Sonne C, Mikkelsen B, Eriksen N (2014) Quantitative relationships in delphinid neocortex. Front Neuroanat 8:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Muller M, Henrich A, Klockenhoff J, Dierkes PW, Schlue WR (2000) Effects of ATP and derivatives on neuropile glial cells of the leech central nervous system. Glia 29:191–201 [DOI] [PubMed] [Google Scholar]
- 93.Munsch T, Deitmer JW (1992) Calcium transients in identified leech glial cells in situ evoked by high potassium concentrations and 5-hydroxytryptamine. J Exp Biol 167:251–265 [DOI] [PubMed] [Google Scholar]
- 94.Nave KA, Werner HB (2014) Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol 30:503–533 [DOI] [PubMed] [Google Scholar]
- 95.Nedergaard M (1994) Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263:1768–1771 [DOI] [PubMed] [Google Scholar]
- 96.Ng FS, Tangredi MM, Jackson FR (2011) Glial cells physiologically modulate clock neurons and circadian behavior in a calcium-dependent manner. Curr Biol 21:625–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nicholls JG, Kuffler SW (1964) Extracellular space as a pathway for exchange between blood and neurons in the central nervous system of the leech: ionic composition of glial cells and neurons. J Neurophysiol 27:645–671 [DOI] [PubMed] [Google Scholar]
- 98.Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Oberheim NA, Wang X, Goldman S, Nedergaard M (2006) Astrocytic complexity distinguishes the human brain. Trends Neurosci 29:547–553 [DOI] [PubMed] [Google Scholar]
- 101.Oikonomou G, Shaham S (2011) The glia of Caenorhabditis elegans. Glia 59:1253–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Oikonomou G, Shaham S (2012) On the morphogenesis of glial compartments in the sensory organs of Caenorhabditis elegans. Worm 1:51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oland LA, Gibson NJ, Tolbert LP (2010) Localization of a GABA transporter to glial cells in the developing and adult olfactory pathway of the moth Manduca sexta. J Comp Neurol 518:815–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omoto JJ, Lovick JK, Hartenstein V (2016) Origins of glial cell populations in the insect nervous system. Curr Opin Insect Sci 18:96–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pakhotin P, Verkhratsky A (2005) Electrical synapses between Bergmann glial cells and Purkinje neurones in rat cerebellar slices. Mol Cell Neurosci 28:79–84 [DOI] [PubMed] [Google Scholar]
- 106.Pakkenberg B, Gundersen HJ (1988) Total number of neurons and glial cells in human brain nuclei estimated by the disector and the fractionator. J Microsc 150:1–20 [DOI] [PubMed] [Google Scholar]
- 107.Pakkenberg B, Gundersen HJ (1997) Neocortical neuron number in humans: effect of sex and age. J Comp Neurol 384:312–320 [PubMed] [Google Scholar]
- 108.Parker RJ, Auld VJ (2006) Roles of glia in the Drosophila nervous system. Semin Cell Dev Biol 17:66–77 [DOI] [PubMed] [Google Scholar]
- 109.Paterson JR, Garcia-Bellido DC, Lee MS, Brock GA, Jago JB, Edgecombe GD (2011) Acute vision in the giant Cambrian predator Anomalocaris and the origin of compound eyes. Nature 480:237–240 [DOI] [PubMed] [Google Scholar]
- 110.Pentreath VW, Radojcic T, Seal LH, Winstanley EK (1985) The glial cells and glia-neuron relations in the buccal ganglia of Planorbis corneus (L.): cytological, qualitative and quantitative changes during growth and ageing. Philos Trans R Soc Lond B Biol Sci 307:399–455 [DOI] [PubMed] [Google Scholar]
- 111.Pereanu W, Spindler S, Cruz L, Hartenstein V (2007) Tracheal development in the Drosophila brain is constrained by glial cells. Dev Biol 302:169–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perens EA, Shaham S (2005) C. elegans daf-6 encodes a patched-related protein required for lumen formation. Dev Cell 8:893–906 [DOI] [PubMed] [Google Scholar]
- 113.Popescu LM, Faussone-Pellegrini MS (2010) TELOCYTES—a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med 14:729–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Procko C, Shaham S (2010) Assisted morphogenesis: glial control of dendrite shapes. Curr Opin Cell Biol 22:560–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rabinowitch I, Chatzigeorgiou M, Schafer WR (2013) A gap junction circuit enhances processing of coincident mechanosensory inputs. Curr Biol 23:963–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rakic P (2003) Elusive radial glial cells: historical and evolutionary perspective. Glia 43:19–32 [DOI] [PubMed] [Google Scholar]
- 117.Reichenbach A (1989) Glia:neuron index: review and hypothesis to account for different values in various mammals. Glia 2:71–77 [DOI] [PubMed] [Google Scholar]
- 118.Reichenbach A, Neumann M, Bruckner G (1987) Cell length to diameter relation of rat fetal radial glia–does impaired K+ transport capacity of long thin cells cause their perinatal transformation into multipolar astrocytes? Neurosci Lett 73:95–100 [DOI] [PubMed] [Google Scholar]
- 119.Reichenbach A, Pannicke T (2008) Neuroscience. A new glance at glia. Science 322:693–694 [DOI] [PubMed] [Google Scholar]
- 120.Ringstad N, Abe N, Horvitz HR (2009) Ligand-gated chloride channels are receptors for biogenic amines in C. elegans. Science 325:96–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rival T, Soustelle L, Strambi C, Besson MT, Iche M, Birman S (2004) Decreasing glutamate buffering capacity triggers oxidative stress and neuropil degeneration in the Drosophila brain. Curr Biol 14:599–605 [DOI] [PubMed] [Google Scholar]
- 122.Roots BI, Lane NJ (1983) Myelinating glia of earthworm giant axons: thermally induced intramembranous changes. Tissue Cell 15:695–709 [DOI] [PubMed] [Google Scholar]
- 123.Ryan TJ, Grant SG (2009) The origin and evolution of synapses. Nat Rev Neurosci 10:701–712 [DOI] [PubMed] [Google Scholar]
- 124.Saubermann AJ, Castiglia CM, Foster MC (1992) Preferential uptake of rubidium from extracellular space by glial cells compared to neurons in leech ganglia. Brain Res 577:64–72 [DOI] [PubMed] [Google Scholar]
- 125.Schikorski D, Cuvillier-Hot V, Leippe M, Boidin-Wichlacz C, Slomianny C, Macagno E, Salzet M, Tasiemski A (2008) Microbial challenge promotes the regenerative process of the injured central nervous system of the medicinal leech by inducing the synthesis of antimicrobial peptides in neurons and microglia. J Immunol 181:1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Schwabe T, Bainton RJ, Fetter RD, Heberlein U, Gaul U (2005) GPCR signaling is required for blood-brain barrier formation in drosophila. Cell 123:133–144 [DOI] [PubMed] [Google Scholar]
- 127.Seal RP, Daniels GM, Wolfgang WJ, Forte MA, Amara SG (1998) Identification and characterization of a cDNA encoding a neuronal glutamate transporter from Drosophila melanogaster. Recept Channels 6:51–64 [PubMed] [Google Scholar]
- 128.Sousa-Nunes R, Yee LL, Gould AP (2011) Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature 471:508–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Speder P, Liu J, Brand AH (2011) Nutrient control of neural stem cells. Curr Opin Cell Biol 23:724–729 [DOI] [PubMed] [Google Scholar]
- 130.Stenesen D, Moehlman AT, Kramer H (2015) The carcinine transporter CarT is required in Drosophila photoreceptor neurons to sustain histamine recycling. Elife 4:e10972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stork T, Bernardos R, Freeman MR (2012) Analysis of glial cell development and function in Drosophila. Cold Spring Harb Protoc 2012:1–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Stork T, Engelen D, Krudewig A, Silies M, Bainton RJ, Klambt C (2008) Organization and function of the blood-brain barrier in Drosophila. J Neurosci 28:587–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Stout RF Jr, Parpura V (2011) Voltage-gated calcium channel types in cultured C. elegans CEPsh glial cells. Cell Calcium 50:98–108 [DOI] [PubMed] [Google Scholar]
- 134.Stout RF Jr, Verkhratsky A, Parpura V (2014) Caenorhabditis elegans glia modulate neuronal activity and behavior. Front Cell Neurosci 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sukhdeo SC, Sukhdeo MVK (1994) Mesehnchyme cells in Fasciola hepatica (Platyhelmintes): Primive glia? Tissue Cell 26:123–131 [DOI] [PubMed] [Google Scholar]
- 136.Sukhdeo SC, Sukhdeo MVK, Mettrick DF (1988) Neurocytology of the cerebral ganglion of Fasciola hepatica (Platyhelminthes). J Comp Neurol 278:337–343 [DOI] [PubMed] [Google Scholar]
- 137.Tasdemir-Yilmaz OE, Freeman MR (2014) Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev 28:20–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thomas JH (1994) The mind of a worm. Science 264:1698–1699 [DOI] [PubMed] [Google Scholar]
- 139.Threadgold LT, Arme C (1974) Electron microscope studies of Fasciola hepatica. Expr Parasitol 35:389–405 [DOI] [PubMed] [Google Scholar]
- 140.True JR, Yeh SD, Hovemann BT, Kemme T, Meinertzhagen IA, Edwards TN, Liou SR, Han Q, Li J (2005) Drosophila tan encodes a novel hydrolase required in pigmentation and vision. PLoS Genet 1:e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tsacopoulos M, Veuthey AL, Saravelos SG, Perrottet P, Tsoupras G (1994) Glial cells transform glucose to alanine, which fuels the neurons in the honeybee retina. J Neurosci 14:1339–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Van Roy P, Briggs DE (2011) A giant Ordovician anomalocaridid. Nature 473:510–513 [DOI] [PubMed] [Google Scholar]
- 143.Verkhratsky A, Butt AM (2013) Glial physiology and pathophysiology. Wiley-Blackwell, Chichester [Google Scholar]
- 144.Verkhratsky A, Nedergaard M (2018) Physiology of astroglia. Physiol Rev 98:239–389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Viehweger E, Robitail S, Rohon MA, Jacquemier M, Jouve JL, Bollini G, Simeoni MC (2008) Measuring quality of life in cerebral palsy children. Ann Readapt Med Phys 51:119–137 [DOI] [PubMed] [Google Scholar]
- 146.Wadsworth WG, Bhatt H, Hedgecock EM (1996) Neuroglia and pioneer neurons express UNC-6 to provide global and local netrin cues for guiding migrations in C. elegans. Neuron 16:35–46 [DOI] [PubMed] [Google Scholar]
- 147.Ward S, Thomson N, White JG, Brenner S (1975) Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans. J Comp Neurol 160:313–337 [DOI] [PubMed] [Google Scholar]
- 148.White JG, Southgate E, Thomson JN, Brenner S (1986) The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci 314:1–340 [DOI] [PubMed] [Google Scholar]
- 149.Wuttke WA, Pentreath VW (1990) Evidence for the uptake of neuronally derived choline by glial cells in the leech central nervous system. J Physiol 420:387–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xiong WC, Montell C (1995) Defective glia induce neuronal apoptosis in the repo visual system of Drosophila. Neuron 14:581–590 [DOI] [PubMed] [Google Scholar]
- 151.Xiong WC, Okano H, Patel NH, Blendy JA, Montell C (1994) repo encodes a glial-specific homeo domain protein required in the Drosophila nervous system. Genes Dev 8:981–994 [DOI] [PubMed] [Google Scholar]
- 152.Xu K, Terakawa S (1993) Saltatory conduction and a novel type of excitable fenestra in shrimp myelinated nerve fibers. Jpn J Physiol 43(Suppl 1):S285–293 [PubMed] [Google Scholar]
- 153.Xu K, Terakawa S (1999) Fenestration nodes and the wide submyelinic space form the basis for the unusually fast impulse conduction of shrimp myelinated axons. J Exp Biol 202:1979–1989 [DOI] [PubMed] [Google Scholar]
- 154.Yoshimura S, Murray JI, Lu Y, Waterston RH, Shaham S (2008) mls-2 and vab-3 Control glia development, hlh-17/Olig expression and glia-dependent neurite extension in C. elegans. Development 135:2263–2275 [DOI] [PubMed] [Google Scholar]
- 155.Zalc B, Goujet D, Colman D (2008) The origin of the myelination program in vertebrates. Curr Biol 18:R511–512 [DOI] [PubMed] [Google Scholar]
- 156.Zhang YV, Ormerod KG & Littleton JT. (2017). Astrocyte Ca2+ Influx Negatively Regulates Neuronal Activity. eNeuro 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ziegler AB, Brusselbach F, Hovemann BT (2013) Activity and coexpression of Drosophila black with ebony in fly optic lobes reveals putative cooperative tasks in vision that evade electroretinographic detection. J Comp Neurol 521:1207–1224 [DOI] [PubMed] [Google Scholar]
- 158.Zoran MJ, Drewes CD, Fourtner CR, Siegel AJ (1988) The lateral giant fibers of the tubificid worm, Branchiura sowerbyi: structural and functional asymmetry in a paired interneuronal system. J Comp Neurol 275:76–86 [DOI] [PubMed] [Google Scholar]


