Abstract
Tissue turnover, especially in the skin, is altered in systemic sclerosis (SSc), leading to tissue accumulation. The objective was to examine type III, IV, and VI collagens turnovers in SSc and investigate longitudinal alterations in relation to modified Rodnan Skin Score (mRSS). We included patients fulfilling the 2013 ACR/EULAR criteria for SSc (limited cutaneous [lcSSc, n = 20], diffuse cutaneous SSc [dcSSc, n = 23]) and healthy controls (HC, n = 10). Biomarkers of type III, IV, and VI collagens formation (PRO-C3, PRO-C4, PRO-C6) and degradation (C3M, C4M, C6M) were measured in serum. The fibrotic index of the individual collagens (FICol) were calculated. The fibrotic index of type III and VI collagens (FICol3 and FICol6) were increased in dcSSc compared to lcSSc (FICol3: 1.4 vs. 0.8, P = 0.0001; FICol6: 1.2 vs. 0.9, P = 0.03). The fibrotic index of type IV collagen (FICol4) was not different between the groups but was 1.5 times higher than HC (HC: 6.9, lcSSc 10.4, dcSSc: 10.5). Both FICol3 and FICol6 correlated with mRSS with rho’s of 0.59 (P < 0.0001) and 0.35 (P = 0.04). Furthermore, FICol3 steadily decrease over the disease course. Examining collagen turnover and specific collagens could be beneficial in following patients’ skin fibrosis and possibly identifying progressors.
Subject terms: Biomarkers, Skin diseases
Introduction
Systemic sclerosis (SSc) is a dynamic disease in which the literature suggests that microvascular damage leads to damage to endothelial cells resulting in fibrosis by activation of fibroblasts1,2. Fibrosis is both seen around the blood vessels and in the deeper interstitial matrix of the affected tissues. The majority of SSc patients have skin fibrosis, whereas some have fibrosis of various internal organs. The extent of skin fibrosis is used to divide patients into two subgroups; limited cutaneous SSc (lcSSc) and diffuse cutaneous SSc (dcSSc)3.
Fibrosis is the result of a tilted remodelling of the extracellular matrix (ECM). Fibroblasts, together with other cells, are responsible for maintaining the ECM by ensuring that damaged proteins, such as collagens, are degraded and replaced by new collagens. If fibroblasts are continuously activated, they will differentiate into myofibroblasts and produce more ECM proteins than needed resulting in fibrosis.
Collagens are the most abundant protein group in the body, where different collagens have different roles and locations. The synthesis of collagen is known to be upregulated in SSc, and the focus of the literature has primarily been on the two main collagens found in skin; type I and III collagens. These are usually found in the interstitial matrix, where they act as the “skeleton” of tissue as the skin. Other collagens as type VI collagen is furthermore found in the interstitial matrix of the skin where it is implicated in matrix organization and fibrillogenesis of collagens4. Type VI collagen gene and protein levels have been shown upregulated in SSc5,6. The ECM composition of the basement membrane around blood vessels differs from the interstitial matrix. The basement membrane mainly consists of type IV collagen and laminin compared to the type I and III collagens-rich interstitial membrane. Type VI collagen is primarily found in the intersection between the basement membrane and interstitial matrix and throughout both sections. Knowledge of different collagens can help understand the location of the fibrosis and target specific types of fibrosis.
The study aimed to examine collagen turnover in SSc patients by investigating the level of formation and degradation of ECM metabolites in blood. Furthermore, we studied the change in collagen turnover over time and in relation to the modified Rodnan Skin Score (mRSS).
Patients and Methods
Study population
In this cross-sectional and longitudinal pilot study, we included 43 SSc patients fulfilling the 2013 ACR/EULAR criteria7. Twenty patients had lcSSc and 23 patients fulfilled the criteria for dcSSc3. All patients had multiple visits to the department of rheumatology, Skåne university hospital-Lund (3–11 visits) where blood samples and clinical features were obtained8. Non-medicated asymptomatic controls (n = 10) were included. Serum samples were collected and stored at −80 °C until measured. The study was approved by the Swedish Ethical Review Authority in Lund (approval number Dnr 590/2008) and carried out following the Declaration of Helsinki. All participants had given written, informed consent.
Serological protein fingerprint biomarkers for ECM turnover
ECM turnover was examined by assessing biomarkers of type III, IV, and VI collagens formation (PRO-C39, PRO-C410, and PRO-C611, respectively) and degradation (C3M12, C4M13 and C6M14, respectively) (produced by Nordic Bioscience). Validated competitive ELISAs assessed analytes according to the manufacture’s protocols. Measurements below the lower limit of detection were assigned the value of the lower limit of detection.
The turnover (fibrotic index) was calculated to examine collagen deposition. It was calculated by dividing the collagen formation with the degradation biomarker.
x indicating the collagen number.
Statistics
Summary statistics were used to examine the baseline demographics at their first visit. Data are depicted as mean and standard deviation (SD) or numbers and percent (%). As the biomarkers were not normally distributed, non-parametric tests were used. The difference in biomarker levels between lcSSc and dcSSc was tested by the Mann-Whitney U test and correlations were evaluated by spearman’s correlation. Duration and SSc specific differences in the levels of the measurements (mRSS or biomarkers) were analysed using Generalized Additive Mixed Models15. We used a quasi-poisson distribution as a model of the probability distribution of mRSS measurements, and a gamma distribution as a model of the probability distribution of biomarker measurements. To account for the repeated measurements within patients we used an exchangeable correlation structure. The expected levels of the measurements were smoothed using thin plate splines with automatic smoothing selection16.
For each measurement we consider three nested model
Base model: Smooth effect of duration, no SSc-form differences
General model: Separate smooth effects duration age by the two SSc-forms. The base and general models were compared by likelihood-ratio tests to assess the hypothesis of no SSc-form differences in the level and shape of the timecourse of the measurements.
The biomarker levels were displayed as mean. P-values ≤ 0.05 were considered statistically significant. Graphical illustrations and Mann-Whitney U test were performed using GraphPad Prism version 8, spearman’s correlation was analysed by MedCalc statistical software version 18.11.6, and the analyses and graphics of Generalized Additive Mixed Models were performed in R version 3.6.117.
Results
The patients were subdivided based on the extent of skin involvement and the demographic data analysed (Table 1). The lcSSc subgroup had longer disease duration and lower mRSS, as expected. Otherwise, the two subgroups were similar regarding age, BMI, and the patients being predominately females.
Table 1.
Baseline characteristics of limited and diffuse cutaneous SSc and healthy controls.
| Healthy controls (n = 10) | Limited cutaneous SSc (n = 20) | Diffuse cutaneous SSc (n = 23) | |
|---|---|---|---|
| Age, years | 50 (9) | 54 (14) | 53 (16) |
| Female, % | 7 (70) | 16 (80) | 18 (78) |
| BMI, kg/m2 | 24.4 (4.7) | 24.8 (4.1) | |
| Disease duration, months | 32.3 (41.0) | 13.5 (14.9) | |
| MRSS | 4.3 (3.1) | 20.3 (11.7) | |
| Organ involvement | |||
| Pulmonary fibrosis | 11 (55) | 9 (39) | |
| Treatments | |||
| Calcium antagonist, % | 9 (45) | 8 (35) | |
| ACE inhibitor, % | 1 (5) | 3 (13) | |
| Iloprost, % | 0 (0) | 1 (4) | |
| Prednisolone, % | 3 (15) | 6 (26) | |
| Mycophenolate mophetil, % | 1 (5) | 0 (0) | |
| Azathioprine, % | 1 (5) | 0 (0) | |
| Methotrexate, % | 1 (5) | 0 (0) | |
BMI: Body mass index. mRSS: Modified Rodnan skin score. Data are shown as numbers (percent) or mean (standard deviation).
Collagen turnover in SSc subgroups
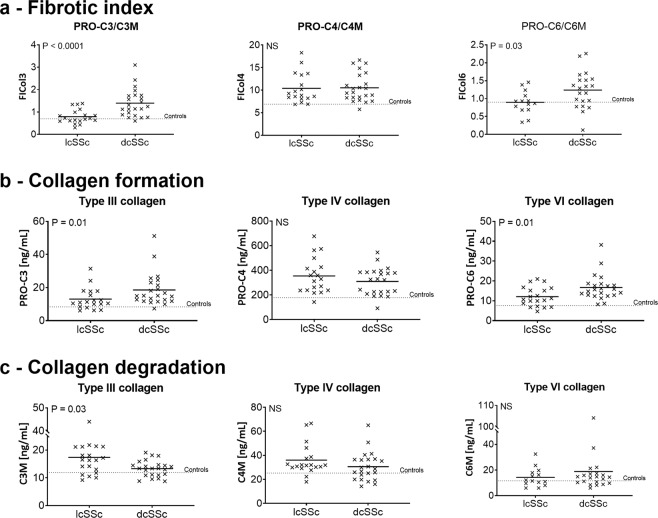
Collagen turnover was examined at the first time point to examine levels in the two subgroups of SSc. The turnover of type III (FICol3) and VI (FICol6) collagens were increased in dcSSc compared to lcSSc (FICol3; lcSSc (mean): 0.8, dcSSc (mean): 1.4, P = 0.0001, FICol6; lcSSc: 0.9, dcSSc: 1.2, P = 0.03; Fig. 1). For both collagens, the level in lcSSc was similar to healthy controls. Type IV collagen turnover (FICol4) was not different between the two SSc subgroups (lcSSc: 10.4, dcSSc: 10.5). However, it was approximately 1.5 times higher than in the healthy controls. The collagen turnover is a product of formation and degradation. Formation and degradation were also examined separately. Type III and VI collagens formation were significantly higher in dcSSc compared to lcSSc (PRO-C3; lcSSc: 31.4 ng/ml, dcSSc: 51.2 ng/ml, P = 0.01, PRO-C6; lcSSc: 4.7 ng/ml, dcSSc: 8.2 ng/ml, P = 0.1). Type III collagen degradation was significantly increased in lcSSc compared to dcSSc (lcSSc: 17.4 ng/ml, dcSSc: 13.3 ng/ml, P = 0.03) and type VI collagen degradation was (lcSSc: 14.3 ng/ml, dcSSc: 18.8 ng/ml) was not different in the two SSc subsets. Type IV collagen formation (lcSSc: 354.4 ng/ml, dcSSc: 310.3 ng/ml,) showed a tendency for a small increase in lcSSc compared to dcSSc and the same was seen in type IV collagen degradation (lcSSc: 36.1 ng/ml, dcSSc: 30.6 ng/ml).
Figure 1.
Fibrotic index of type III, IV, and VI collagens in limited and diffuse SSc. (a) cross-sectional view of the fibrotic index of type III collagen. (b) Cross-sectional view of the fibrotic index of type IV collagen. (c) Cross-sectional view of the fibrotic index of type VI collagen. Data are shown as mean. Data were analysed by the Mann-Whitney U test. *P < 0.05, **P < 0.01, ***P < 0.001.
Type III and VI collagens correlates with mRSS
The levels of formation, degradation and turnover of type III, IV and VI collagens correlated with mRSS at first time point. Type III collagen formation (PRO-C3) and turnover (FICol3) showed the best correlations with Rho’s of 0.53 (P = 0.0003) and 0.59 (P < 0.0001), respectively. Type VI collagen formation and turnover followed with Rho’s of 0.40 (P = 0.008) and 0.35 (P = 0.04), respectively. Type IV collagen formation and turnover did not correlate with mRSS (PRO-C4: Rho = −0.07, FICol4: Rho = 0.17). Collagen degradation biomarkers did not correlate with mRSS (C3M: Rho = −0.16, C4M: Rho = −0.14, C6M: Rho = 0.04).
Type III collagen changes over time
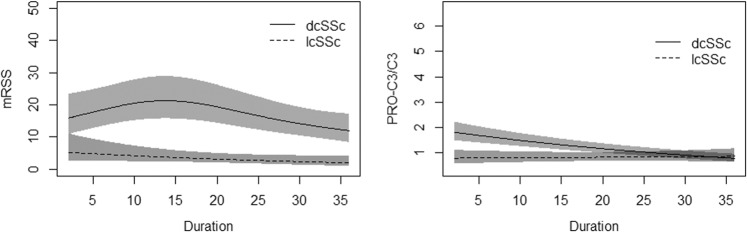
Type III and VI collagens formation and turnover correlated moderately with mRSS at a cross-sectional measure, and we were therefore interested in the changes over time. Serum samples at different time points were collected for each patient. However, patients did not have the same time points. MRSS was higher in dcSSc compared to lcSSc, especially at the beginning of the disease (Fig. 2). It reached a peak between 10 and 20 months where after it decreased. Type III collagen turnover furthermore observed a difference between lcSSc and dcSSc over time. DcSSc had high levels of type III collagen turnover at the beginning, which steadily fell over the disease duration.
Figure 2.
Modified Rodnan skin score and the fibrotic index of type III collagen in lcSSc and dcSSc. Data are shown as mean. Data were analysed by Generalized Additive Mixed Models15.
Discussion
In this study, we examined the blood levels of formation, degradation, and turnover of type III, IV, and VI collagens metabolites and their relation to mRSS. We found that collagen turnover was better at separating SSc subsets than single collagen biomarkers and that type III and VI collagens, but not type IV collagen, correlated with mRSS. From these data, we argue that it is crucial to analyse collagen turnover and individual collagen types as type III, IV, and VI collagens.
This study analysed SSc patients according to their skin involvement, i.e., lcSSc and dcSSc. The dcSSc patients are known to have increased fibrosis, not only of the skin but also internal organs. This fits with our data showing increased collagen production in this patient subset. The dcSSc patients had increased type III and VI collagens turnover compared to lcSSc patients. We have previously examined the formation and degradation of collagens and seen that especially collagen formation is upregulated in SSc6,18. Increased skin thickness is likely due to increased collagen production. Both type III and VI collagens turnover correlated moderately to strongly with mRSS. The correlation supports the hypothesis that type III and VI collagens is related to skin fibrosis. The formation biomarkers of type III and VI collagens were also elevated in dcSSc patients and correlated moderately with mRSS. However, with increased formation, there might also be increased degradation, i.e., an overall higher turnover. We have previously shown that degradation biomarkers can be elevated in dcSSc patients6,19. We, therefore, argue that the collagen turnover gives a more accurate picture of the collagen deposition.
Type III and VI collagens are known to be in the interstitial matrix throughout the dermis20. Type IV collagen is found in the basement membrane around blood vessels and in the dermo-epidermal junction21. Contrary to type III and VI collagens, type IV collagen did not differ between lcSSc and dcSSc; it was elevated in both subgroups compared to controls. This indicates that type IV collagen turnover is overall elevated in SSc patients but not related to subtypes. Furthermore, type IV collagen turnover did not correlate with mRSS. A hallmark of SSc is vascular damage, especially microangiopathy22. Type IV collagen remodelling is affected by constant vascular damage. As vascular damage is found in both lcSSc and dcSSc, it might explain the increase of type IV collagen turnover in both lcSSc and dcSSc compared to controls. Type IV collagen has previously been shown upregulated in SSc by Motegi and colleagues23. They found that type IV collagen was elevated in patients with three years of disease duration or less. This corresponds well with our findings as our average disease duration was below three years. We have previously shown that type IV collagen formation and degradation were elevated in dcSSc patients compared to controls, especially in early SSc (<2 years of disease duration)6. Overall, these three studies show that there is an increased amount of type IV collagen with SSc and that its potential and meaning should be further examined.
Longitudinal samples showed a trend for type III collagen turnover to change over time in dcSSc. This indicates that type III collagen changes throughout the disease and could offer insight into the pathogenesis. It was increased in the first 20 months of the disease, similar to mRSS. However, instead of having a peak, the levels steadily fell over the disease course. Type III collagen turnover, therefore, shows that serologically measured biomarkers could help understand the changes in disease over time. These data are preliminary and needs to be validated.
The limitations of this study include that different treatments may have affected biomarker levels. Nevertheless, analysis of type III collagen turnover mirrored the widely used clinical readout mRSS throughout the disease.
In conclusion, type III and VI collagens are correlated with mRSS and elevated in dcSSc patients. Type III collagen furthermore changes over time, indicating that especially type III collagen turnover can be used as a biomarker of skin fibrosis. Type IV collagen turnover is not related to mRSS but elevated in SSc patients, suggesting that it might be useful for vascular involvement and not skin fibrosis. Collagen turnover might have additional power compared to only looking at formation or degradation biomarkers. This opens the field for following patients with serological biomarkers.
Acknowledgements
A great thank you goes to Peder Frederiksen for assisting with statistics guidance and calculations.
Author contributions
P.J. was the main author of the manuscript. P.J. carried out the measurements, data analysis, and statistical analysis in discussion with A.B., R.H., A.S.S, and D.M.W. R.H. and D.M.W. provided the samples and patient information. A.B., R.H., A.S.S., and D.M.W. critically revised the manuscript and approved the final manuscript.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
P.J., A.B., and A.S.S are full-time employees at Nordic Bioscience. Nordic Bioscience is a privately-owned; small-medium size enterprise partly focused on the development of biomarkers for rheumatic and fibrotic diseases. None of the authors received fees, bonuses, or other benefits for the work described in the manuscript. All the other authors declare no conflict of interest. The authors have no other relevant affiliation or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allanore, Y. et al. Systemic sclerosis [review]. Nat. Rev. Dis. Prim. 1 (2015). [DOI] [PubMed]
- 2.Ciechomska M, van Laar J, O’Reilly S. Current frontiers in systemic sclerosis pathogenesis. Exp. Dermatol. 2015;24:401–406. doi: 10.1111/exd.12673. [DOI] [PubMed] [Google Scholar]
- 3.LeRoy EC, et al. Scleroderma (systemic sclerosis): Classification, subsets and pathogenesis [editorial] J. Rheumatol. 1988;15:202–205. [PubMed] [Google Scholar]
- 4.Theocharidis G, et al. Type VI Collagen Regulates Dermal Matrix Assembly and Fibroblast Motility. J. Invest. Dermatol. 2016;136:74–83. doi: 10.1038/JID.2015.352. [DOI] [PubMed] [Google Scholar]
- 5.Peltonen J, Kahari L, Uitto J, Jimenez SA. Increased expression of type VI collagen genes in systemic sclerosis. Arthritis Rheum. 1990;33:1829–1835. doi: 10.1002/art.1780331211. [DOI] [PubMed] [Google Scholar]
- 6.Juhl P, et al. Serum biomarkers of collagen turnover as potential diagnostic tools in diffuse systemic sclerosis: A cross-sectional study. PlosOne. 2018;Dec:1–12. doi: 10.1371/journal.pone.0207324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Hoogen F, et al. 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013;72:1747–1755. doi: 10.1136/annrheumdis-2013-204424. [DOI] [PubMed] [Google Scholar]
- 8.Wuttge, D. M. et al. Serum IL-15 in patients with early systemic sclerosis: A potential novel marker of lung disease. Arthritis Res. Ther. 9, (2007). [DOI] [PMC free article] [PubMed]
- 9.Nielsen MJ, et al. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013;5:303–315. [PMC free article] [PubMed] [Google Scholar]
- 10.Leeming DJ, Karsdal MA, Rasmussen LM, Scholze A, Tepel M. Association of systemic collagen type IV formation with survival among patients undergoing hemodialysis. PLoS One. 2013;8:e71050. doi: 10.1371/journal.pone.0071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S, et al. Collagen type III and VI turnover in response to long-term immobilization. PLoS One. 2015;10:e0144525. doi: 10.1371/journal.pone.0144525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barascuk N, et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clin. Biochem. 2010;43:899–904. doi: 10.1016/j.clinbiochem.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Sand JM, et al. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis – validation of two novel biomarker assays. PLoS One. 2013;8:e84934. doi: 10.1371/journal.pone.0084934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veidal SS, et al. MMP mediated degradation of type VI collagen is highly associated with liver Fibrosis - Identification and validation of a novel biochemical marker assay. PLoS One. 2011;6:e24753. doi: 10.1371/journal.pone.0024753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wood, S. Generalized Additive Models: An Introduction with R (2nd edition). Chapman and Hall/CRC. (2017).
- 16.Wood SN. Stable and efficient multiple smoothing parameter estimation for generalized additive models. J. Am. Stat. Assoc. 2004;99:673–686. doi: 10.1198/016214504000000980. [DOI] [Google Scholar]
- 17.Team, R. core. R: A language and environment for statistical computing. R Foundation for Statistical Computing. (2019).
- 18.Juhl, P. et al. Association of Metabolites Reflecting Type III and VI Collagen Formation with Modified Rodnan Skin Score in Systemic Sclerosis – a Cross-sectional Study. Biomarkers 1–24, 10.1080/1354750X.2019.1587509 (2019). [DOI] [PubMed]
- 19.Siebuhr AS, et al. Citrullinated vimentin and biglycan protein fingerprints as candidate serological biomarkers for disease activity in systemic sclerosis: A pilot study. Biomarkers. 2019;24:249–254. doi: 10.1080/1354750X.2018.1548032. [DOI] [PubMed] [Google Scholar]
- 20.Uitto, J., Olsen, D. R. & Fazio, M. J. Extracellular matrix of the skin: 50 years of progress. J. Invest. Dermatol. 92, (1989). [DOI] [PubMed]
- 21.Velez AMA, Howard MS. Collagen IV in normal and in disease process. N. Am. J. Med. Sci. 2012;4:1–8. doi: 10.4103/1947-2714.92892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastiani M, et al. Measuring microangiopathy abnormalities in systemic sclerosis patients: The role of capillaroscopy-based scoring models. Am. J. Med. Sci. 2014;348:331–336. doi: 10.1097/MAJ.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 23.Motegi SI, Sekiguchi A, Fujiwara C, Toki S, Ishikawa O. Possible association of elevated serum collagen type IV level with skin sclerosis in systemic sclerosis. J. Dermatol. 2017;44:167–172. doi: 10.1111/1346-8138.13564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.