Abstract
The health of western honey bee (Apis mellifera) colonies is challenged by the parasitic mite Varroa destructor and the numerous harmful pathogens it vectors. Selective breeding for the naturally occurring social immune trait “hygienic behavior” has emerged as one sustainable approach to reducing the mites’ impact on honey bees. To expand our understanding of hygienic triggers and improve hygienic selection tools, we tested the hypothesis that the cuticular compounds (Z)-10-tritriacontene and (Z)-6-pentadecene, previously associated with unhealthy honey bee brood and/or brood targeted for hygiene, are triggers of honey bee hygienic behavior independent of brood health. In support of our hypothesis, application of synthetic (Z)-10-tritriacontene and (Z)-6-pentadecene onto brood and brood cell caps significantly increased hygienic behavior compared to application of similarly structured hydrocarbon controls (Z)-16-dotriacontene and (Z)-7-pentadecene. Furthermore, we demonstrate a significant positive correlation between colony-level hygienic responses to (Z)-10-tritriacontene and the traditional freeze-killed brood assay for selection of hygienic honey bee stocks. These results confirm biological activity of (Z)-6-pentadecene and reveal (Z)-10-tritriacontene as a novel hygiene trigger. They also support development of improved tools for honey bee colony monitoring and hygienic selection, and thus may accelerate development of honey bee stocks with greater resistance to Varroa and associated pathogens.
Subject terms: Biochemistry, Biological techniques, Chemical biology, Ecology
Introduction
As with other social insects, honey bees are highly susceptible to the horizontal spread of infectious diseases due to close contact, high coefficients of relatedness, and high frequency of social interactions among individuals within a colony1–3. Movement of parasites between bees within a colony can facilitate the spread of honey bee diseases2 because ectoparasites such as the mite Varroa destructor, hereafter Varroa, vector numerous honey bee pathogens4,5. In addition, Varroa can amplify viral loads6–8, alter viral strain diversity8, and affect virus virulence9,10. In the western honey bee (Apis mellifera), the spread and proliferation of harmful pathogens such as Deformed Wing Virus (DWV) is especially problematic considering honey bees have fewer immune-related genes than non-social insects11.
Combined with susceptibility to and spread of honey bee parasites and pathogens12–14, numerous anthropogenic threats such as pesticide exposure and poor nutrition have led to a shortage of colonies relative to an increasing global demand for crop pollinators15–18. Following pollinator population trends in much of the Northern Hemisphere19–21, managed honey bee colonies in the United States have declined more than 50% over the last six decades, from around 5.9 million colonies in 1947 to around 2.7 million colonies in 201522,23. Early declines were likely due to reduced public demand for honey after the end of World War II, while the spread of pests and pathogens are responsible for more recent spikes in annual colony losses22. Each year between 2012 and 2016, the average total annual colony losses in the United States exceeded 34%, and overwintering loss rates were consistently higher than levels deemed acceptable by beekeepers23–26. Among multiple, interacting risks to honey bee health, Varroa are considered the most severe threat to modern apiculture because their recent and rapid global expansion has resulted in increased beekeeping costs and decreased honey bee health, leading to decreases in the number of active beekeepers27 and colony overwintering survival21,28.
Despite substantial evidence of a need to control Varroa, no satisfactory solution has been discovered29. Miticides used to reduce Varroa populations are harmful to bees and are only temporarily effective due to mites’ rapid evolution of resistance27,30. Alternative control strategies, such as physical mite removal and use of essential oils, have many limitations that compromise efficacy, including increased labor for beekeepers (and thus lack of adoption), sensitivity to fluctuations in ambient temperature, and minimal differences between lethal doses for mites and honey bees29,31. Thus, one of the most promising strategies to combat Varroa is the selective breeding of disease-resistant honey bees, including Minnesota Hygienic (HYG) and Varroa Sensitive Hygienic (VSH) stocks.
Hygienic behavior is the detection, uncapping, and removal of diseased or parasite-infested brood from the colony32,33, and is one of the resistance mechanisms of Varroa’s original host, the eastern honey bee Apis cerana34. It also occurs naturally in A. mellifera at a low frequency and is most commonly observed in worker bees aged 15 to 20 days35. HYG bees are selected based on their hygienic removal of freeze-killed brood36, whereas VSH bees are selected based on their apparent suppression of mite reproduction37. Although both of these stocks have reduced pathogen loads compared to unselected (UNS) colonies32,37,38, the process of selecting for suppression of mite reproduction is time-consuming and other interventions, including miticides, are still needed to control severe mite infestations in HYG colonies39,40. Thus, there is need for an optimized assay that is rapid, user-friendly, and specific to honey bee pests and pathogens.
Insect cuticles are coated with various lipids, including hydrocarbons, wax esters, glycerides, free fatty acids, sterols, aldehydes, and alcohols41,42. These compounds reduce water loss and facilitate inter- and intraspecific communication43. In honey bees, cuticular compounds are typically dominated by hydrocarbons, specifically alkanes, alkenes, and methylalkanes44–46. Previous studies have provided evidence of significant effects of DWV and Varroa infestation on the composition of cuticular hydrocarbons in honey bee brood47–50 and have linked such quantitative changes to hygienic removal49,50. Specifically, parasitized and DWV-infected brood have been associated with higher proportions of several unsaturated hydrocarbons including pentadecene, hentriacontene, and tritriacontene47,49–52, and specific isomers of these alkenes, including (Z)-6-pentadecene (Z6-C15), (Z)-8-hentriacontene (Z8-C31), and (Z)-10-tritriacontene (Z10-C33; subsequent abbreviations conform to this pattern), have been associated with brood targeted with hygienic behavior49,50. However, with one notable exception49, current evidence for relationships between brood hydrocarbons and honey bee hygiene is either correlative or chemically undefined (i.e., cell treatment with brood extracts), suggesting the need for analysis of the effects of direct application of individual chemicals of interest to honey bee brood cells.
In this study we investigated hygienic effects of two hydrocarbons previously associated with stressed honey bee brood and hygienic behavior. Based on work by Nazzi et al.49 and our own work50, we tested the hypothesis that Z6-C15 and Z10-C33 are triggers of honey bee hygienic behavior, independent of brood health. These two hydrocarbons were selected based on their associations with honey bee stressors (parasites and viruses) and hygiene in previous studies as described above49,50. Additionally, these chemicals were chosen based on their distinct volatilities in typical colony conditions, as high and low volatility may be important for attracting hygienic workers to a general area, and pinpointing problematic cells, respectively53. We predicted that honey bee hygienic responses to Z10-C33 and Z6-C15 application to honey bee brood and brood cell caps would 1) be greater than hygienic responses toward hydrocarbon controls of similar structure, 2) exhibit a dose-response relationship, and 3) be positively correlated with honey bee responses in the freeze-killed brood (FKB) assay.
Results
Hygienic response to treatment of pupae with Z10-C33
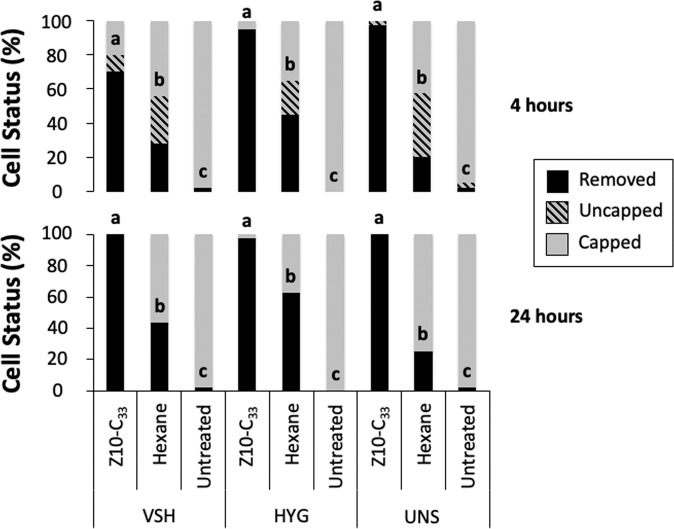
Treatment of pupae with 1 μL of 1% Z10-C33 in hexane had a significant effect on hygienic response in VSH (X2 = 64.25, d.f. = 2, p < 0.001), HYG (X2 = 75.80, d.f. = 2, p < 0.001), and UNS (X2 = 72.97, d.f. = 2, p < 0.001) colonies at 4 h, and in VSH (X2 = 96.76, d.f. = 2, p < 0.001), HYG (X2 = 78.42, d.f. = 2, p < 0.001), and UNS (X2 = 85.32, d.f. = 2, p < 0.001), colonies at 24 h (Fig. 1). Bonferroni-corrected post-hoc analyses indicated that Z10-C33, hexane, and control treatments were significantly different from one another in each colony and at each time point. Lower numbers of manipulated (removed or uncapped) cells at 24 h than 4 h provides evidence of recapping (Fig. 1).
Figure 1.
Hygienic responses to pupae treated with 1 μL of 1% Z10-C33 in hexane, or 1 μL hexane, or left as untreated controls, in three colonies from different breeding backgrounds. Letters indicate significant differences in cell status between treatments for each colony. Compared to either control (hexane or untreated), significantly more cells treated with Z10-C33 were uncapped and the pupae removed, at both 4 and 24 h post treatment, in all three colonies tested. Hexane treatment also elicited significantly greater hygienic responses than untreated controls at both time points and in all three colonies.
Development after treatment of pupae with Z10-C33
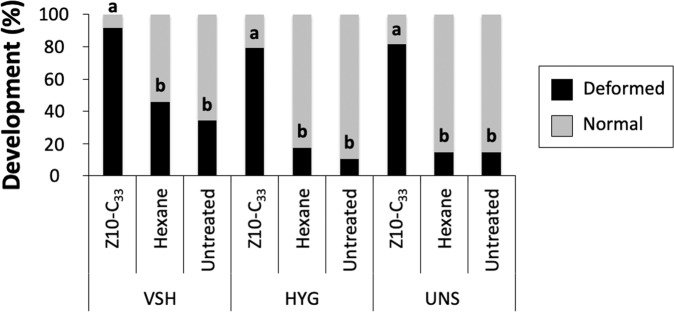
Direct treatment of pupae with 1 μL of 1% Z10-C33 in hexane had a significant effect on development in brood from VSH (X2 = 26.13, d.f. = 2, p < 0.001), HYG (X2 = 36.48, d.f. = 2, p < 0.001), and UNS (X2 = 34.31, d.f. = 2, p < 0.001) colonies (Fig. 2). Post-hoc analyses indicated that for VSH, HYG, and UNS colonies, abnormal development was significantly higher for Z10-C33-treated pupae than for either the hexane or untreated control treatments (Fig. 2).
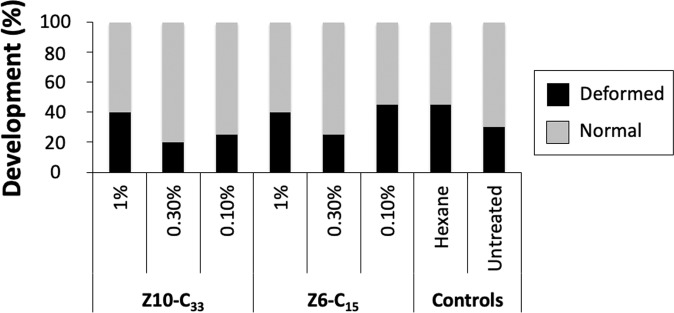
Figure 2.
Developmental status of in vitro reared pupae from three colonies with different breeding backgrounds treated with 1 μL of 1% Z10-C33 in hexane, or 1 μL hexane, or left untreated as controls. Letters indicate significant differences in developmental status between treatments for each colony. Treatment had a significant effect on the number of deformed bees at the time of expected adult emergence in VSH, HYG, and UNS colonies. Compared to either control, significantly more pupae treated with Z10-C33 exhibited deformities at the time of expected adult emergence in all three colonies tested.
Hygienic response to treatment of wax caps with hydrocarbons
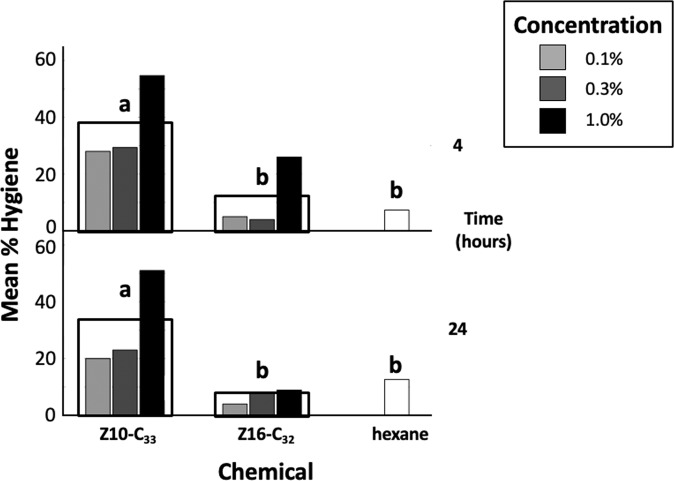
Z10-C33 application to cell caps compared to control applications (Fig. 3), had a significant effect on hygienic behavior at both 4 h (F1,10 = 9.34, p = 0.01) and 24 h (F1,10 = 30.42, p < 0.001). Post-hoc tests indicated that hygienic response to Z10-C33 was significantly higher than to Z16-C32 and hexane at 4 h (p = 0.03 and p = 0.04, respectively), and 24 h (p < 0.001 and p = 0.007, respectively). There were no significant differences between effects of Z16-C32 and hexane at 4 h (p > 0.99) or 24 h (p > 0.99). The effect of chemical concentration on hygienic removal was marginally significant at 4 h (F2,10 = 3.68, p = 0.06) and statistically significant at 24 h (F2,10 = 10.90, p = 0.003). At 24 h, the highest hydrocarbon concentration (1%) elicited significantly greater hygienic response than lower concentrations (p = 0.01, p = 0.003, and 0.007 for 0.3%, 0.1%, and 0% (hexane), respectively), but the effects on hygiene were not different between the two lower concentrations (p > 0.99 for each comparison). Interaction effects between chemical type and concentration were not significant at either 4 or 24 h (F2,10 = 0.038, p = 0.96 and F2,10 = 2.87, p = 0.10, respectively). Lower numbers of manipulated cells at 24 h than 4 h provides evidence of recapping.
Figure 3.
Hygienic responses at 4 and 24 h post treatment of brood cell caps with 0.1%, 0.3%, or 1% Z10-C33 in hexane, 0.1%, 0.3%, or 1% Z16-C32 in hexane, or hexane. Hollow rectangles represent mean hygienic response over the three concentrations tested. Letters indicate significant differences in mean hygienic responses between treatment groups for all concentrations tested. Compared to either control, a significantly greater hygienic response was elicited by Z10-C33 at both 4 and 24 h after treatment.
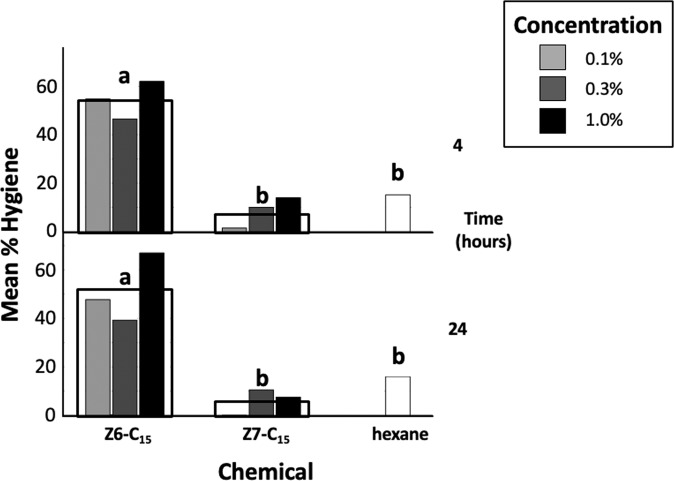
Z6-C15 applied to cell caps significantly affected hygienic behavior at both 4 h (F1,16 = 44.89, p < 0.001) and 24 h (F1,16 = 20.04, p < 0.001) compared to the Z7-C15 control (Fig. 4). In post-hoc tests, the effect of Z6-C15 on removal was significantly different from that of Z7-C15 and hexane at 4 h (p < 0.001 and p = 0.004, respectively), and 24 h (p = 0.001 and p = 0.01, respectively). There were no significant differences between effects of Z7-C15 and hexane at 4 h (p > 0.99) or 24 h (p > 0.99). The effect of chemical concentration on hygiene was not significant at 4 h (F2,16 = 0.49, p = 0.62) or 24 h (F2,16 = 0.72, p = 0.50). Interaction effects between chemical type and concentration were not significant at either 4 or 24 h (F2,16 = 0.28, p = 0.76 and F2,16 = 0.78, p = 0.47, respectively). Lower numbers of manipulated cells at 24 h than 4 h provides evidence of recapping.
Figure 4.
Hygienic responses at 4 and 24 h after treatment of brood cell caps with 0.1%, 0.3%, or 1% Z6-C15 in hexane, 0.1%, 0.3%, or 1% Z7-C15 in hexane, or hexane. Hollow rectangles represent mean hygienic response over the three concentrations tested. Letters indicate significant differences in mean hygienic responses between treatment groups for all concentrations tested. Compared to either control, Z6-C15 elicited significantly greater hygienic responses at both 4 and 24 h after treatment.
Effects of wax cap treatment on development
Treatment of wax caps with Z10-C33 and Z6-C15 had no significant effect on pupal development (X2 = 6.06, d.f. = 7, p = 0.53) (Fig. 5).
Figure 5.
Developmental status of in vitro reared pupae from cells whose caps were treated with hexane solutions of Z10-C33 or Z6-C15, or hexane, or that remained untreated as controls. Treatment had no significant effect on the number of deformed bees at the time of expected adult emergence.
Comparison of freeze-killed brood (FKB) assay and Z10-C33-treatment assay
A significant positive correlation (r = 0.70, n = 10, p = 0.01) was found between Z10-C33 and FKB assays tested in 10 colonies, with a greater range in responses in the FKB assay (Fig. 6).
Figure 6.
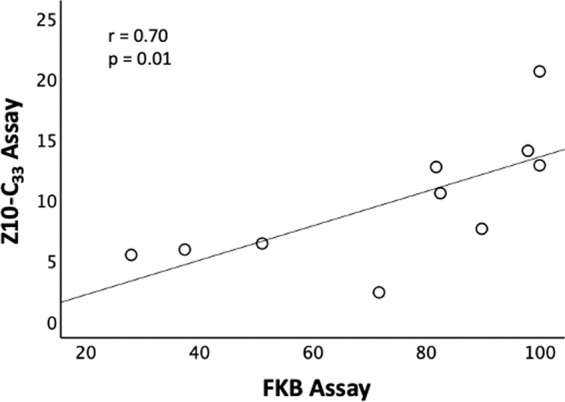
Correlation between scores for the FKB Assay (freeze-killed brood assay, scored for complete removal after 24 h) and Z10-C33 Assay (scored as uncapping or removal after 24 h) for 10 colonies of different origins. A significant positive correlation between colony responses to the two assays was observed.
Discussion
Two major types of chemical cues and signals can guide honey bee workers to perform hygienic behavior. First, compounds not naturally found on honey bee brood may be perceived as “foreign” cues by workers, and could elicit some form of uncapping and/or removal behavior when detected in brood cells or on brood cell caps. This form of chemosensory processing is similar to the recognition of foreign invaders in social insect colonies41,54–56. Hygienic behavior may also be stimulated by intraspecific signals (pheromones) through changes in amounts and ratios of naturally occurring native chemicals. This form of chemosensory processing is similar to the recognition of nestmates vs. conspecific non-nestmates in social insect colonies54,57–59. Notably, cuticular hydrocarbons play important functions in both intra- and interspecific recognition in social insects60–62. Our and previous results indicate that the hydrocarbons Z10-C33 and Z6-C15, when applied to brood or capped brood cells, induce hygienic behavior. In contrast, the structurally similar hydrocarbons Z16-C32 and Z7-C15, which have not been associated with honey bee brood, elicited significantly less hygienic behavior. Thus, the chemical trigger for hygienic behavior is more similar to intraspecific communication than to a general recognition of a foreign stimulus. Furthermore, we show that hygienic responses to Z10-C33 and Z6-C15 may be useful as indicators of hygienic behavior at the colony level. Though previous studies have involved application of synthetic brood chemicals directly to pupae49 and extracts of brood signals to brood cell caps50, to our knowledge, this is the first study to apply synthetic versions of honey bee brood compounds to brood cell caps to induce hygienic behavior. Therefore, results from this study not only confirm the biological activity of two cuticular hydrocarbons in triggering hygienic behavior towards otherwise healthy brood, but may also be useful in the development of improved tools for hygienic selection of pest- and disease-resistant honey bees, although further research related to quantitative and interactive effects of chemicals on hygienic behavior are needed.
Both Z10-C33 and C15 (isomers unidentified) occur naturally on honey bee cuticles, are found in higher quantities on Varroa-infested brood, and have been linked to hygienic behavior47,49,50. The experimental results support our hypothesis that Z10-C33 and Z6-C15 are triggers of honey bee hygienic behavior independent of brood health. Direct treatment of pupae with Z10-C33, and to a lesser extent hexane, elicited hygienic behavior in VSH, HYG, and UNS colonies. While these results are similar to those previously reported for Z6-C15-treated brood49, in vitro rearing indicated that increased hygienic behavior might have been partially due to a detrimental effect of direct exposure of brood to Z10-C33.
Hygienic behavior has been likened to programmed cell death63 or “social apoptosis”64, with the idea that removal of unhealthy individuals may improve overall colony health. Although the effect of Z10-C33 on brood susceptibility at quantities naturally produced by brood was not tested here, the considerable detrimental effect of Z10-C33 on pupal development combined with the lack of a hexane effect suggests that brood susceptibility to natural hygienic signals could play a role in hygiene at the colony level. It is unclear whether the hygienic signal could represent a coopted apoptosis mechanism64 but this idea is consistent with recent findings that brood signaling differs by honey bee stock50, and that high brood susceptibility at the individual level may confer resistance to Varroa at the colony level64. Indeed, further studies regarding relationships among honey bee stock, quantitative brood signaling, and brood susceptibility are needed to clarify whether developmental interference caused by naturally produced brood signals plays a role in the apoptotic induction of hygienic behavior.
Because we were primarily interested in the function of applicable chemical signals (independent of induced mortality and/or developmental abnormalities), we shifted to treating brood cell caps rather than pupae directly. We found clear evidence that the hydrocarbons Z10-C33 and Z6-C15, previously associated with Varroa and DWV-stressed brood, when applied to brood cell caps, elicited hygienic behavior. This effect was specific to these particular compounds because the structurally similar chemicals Z16-C32 and Z7-C15, which have not previously been associated with honey bee brood, did not have a comparable effect. Because development of brood under Z10-C33 and Z6-C15 treated caps was not different from that of brood under hexane treated and untreated caps, we conclude that the hygienic behavior observed was not a result of brood abnormality related to cap treatment. Differential responses to Z10-C33 and Z6-C15 compared with structurally similar hydrocarbon controls may be related to two chemosensory mechanisms. First, control hydrocarbons may be detected but ignored as irrelevant. Some, albeit low, responses to high concentrations of control hydrocarbons support this idea. Second, honey bees may possess receptors specifically tuned to Z10-C33 and Z6-C15, whereas they may be functionally anosmic to the structurally related but “non-natural” Z16-C32 and Z7-C15. Nazzi et al.49 tested honey bee responses to brood treated with Z6-, Z7-, and Z8-C15 isomers, and found Z6-C15 to be the most effective at triggering honey bee hygienic behavior. Additional structure-activity studies that expand analyses of bioactive natural hydrocarbons to multiple colonies could improve the conclusiveness of these findings, and provide critical data to discriminate between these two hypotheses.
Finally, we identified a significant positive correlation between hygienic responses to Z10-C33 and to FKB across 10 honey bee colonies. This suggests that hygienic response to Z10-C33 may be related to hygienic response to the similarly non-volatile necromone oleic acid, previously associated with FKB53. While our findings suggest that chemical hygiene triggers may be useful in measuring colony hygiene level, colony response 24 h after treatment with Z10-C33 was relatively low and had a smaller range than response to FKB. Accordingly, and because natural signals likely involve mixtures rather than isolated compounds50, further studies involving shorter assay times that prevent misclassification of recapped cells, higher chemical concentrations, additional compounds, and mixtures of chemical stimuli should be conducted to optimize development of an improved assay for measuring hygienic behavior specific to Varroa and brood diseases.
Together, our findings provide support for the hypothesis that Z10-C33 and Z6-C15 are triggers of honey bee hygienic behavior independent of brood health. From a practical viewpoint, our results showed that synthetically produced compounds can be applied to capped brood cells to elicit honey bee hygienic behavior. This approach could be developed as a tool for evaluation of honey bee hygiene at the colony level. Such an assay may be useful for the improvement of selective breeding, because colony responses to actual brood stress signals may rely on different mechanisms or olfactory sensitivities than selection based on brood killed by freezing in the FKB assay65–67. Thus, semiochemical assays may be better suited to distinguish colonies with enhanced disease- and pest-resistance, facilitating honey bee management decisions such as which colonies may benefit most from chemical treatment to manage Varroa, or which breeders provide the most hygienic queens. A semiochemical hygiene assay may also be more rapid, and more beekeeper-friendly than current selection methods which require killing, infestation, and/or meticulous inspection of brood. Consequently, testing and breeding for hygienic behavior may become more widespread. Improved honey bee breeding and management decisions have potential to sustainably improve honey bee health, reducing the risks associated with many current Varroa management practices, such as evolution of pest and pathogen resistance, and contamination of commercial honey and beeswax. Thus, although further development is needed, our findings suggest that the exploitation of intrinsic signals associated with honey bee health may provide new tools and strategies of benefit to queen breeders, commercial beekeepers, farmers, and consumers.
Methods
A recent study linked Z10-C33 to Varroa-infested, DWV-infected, and hygiene-targeted honey bee brood50. Given the structural similarity of Z10-C33 to Z6-C15, previously linked to hygienic removal49, we decided to investigate the effectiveness of Z10-C33 and Z6-C15 in triggering hygienic behavior. For this purpose, Varroa-Sensitive Hygienic (VSH), Minnesota Hygienic (HYG), and unselected control (UNS) honey bee colonies were established at the University of North Carolina at Greensboro apiary in the Spring of 2017. The VSH queen was sourced from the USDA-ARS Honey Bee Breeding Laboratory in Baton Rouge, where queens are selected based on suppression of mite reproduction. The HYG queen was sourced from the well-established breeder Jeff Hull (Minnesota) and was selected based on removal of>95% freeze-killed brood. The UNS queen was sourced from Triad Bee Supply which obtains their queens from Gardner Apiaries in Baxley, Georgia. For each experimental hydrocarbon, a control hydrocarbon of similar size and structure, but not known to be a component of the honey bee’s cuticular hydrocarbons, was also tested. Hydrocarbons (Z10-C33, Z16-C32, Z6-C15, and Z7-C15) were synthesized by Z-selective Wittig reactions between the appropriate aldehydes and phosphonium salts, or by Z-selective olefin metathesis reactions. Crude products were purified in two steps, by flash vacuum chromatography on silica gel, eluting with hexanes, followed by recrystallization from acetone at ~4 °C for longer chain compounds, or ~−20 °C for shorter chain compounds. Synthesis is described in further detail, below. Dilutions in hexane of 0.1%, 0.3%, and 1.0% (wt/vol) were prepared for each hydrocarbon. The lowest dilution is equivalent to that previously reported49, and higher dilutions were chosen to approximate dose effects on a logarithmic scale. All sample collections and analyses were conducted at the University of North Carolina at Greensboro.
Synthesis of alkenes tested in bioassays
Synthesis of Z6-C15
A solution of 1-decyne (5.52 g, 40 mmol) and ~50 mg triphenylmethane indicator in dry THF under Ar was cooled to ~−15 °C in an ice/acetone bath, and butyllithium (2.6 M in hexanes) was added dropwise until the solution turned pink. An additional 15.4 ml of butyllithium solution (40 mmol) was then added over 30 min, and the resulting solution was warmed to room temperature and stirred 1 h. Powdered NaI (0.6 g, 4 mmol) was then added, followed by dropwise addition of bromopentane (3.93 g, 26 mmol). The mixture was heated to reflux and stirred 22 h, then cooled and quenched with 1 M aqueous NH4Cl solution, and extracted with hexane. The hexane layer was washed with saturated NaHCO3 and brine, then dried and concentrated. The residue was purified by Kugelrohr distillation, taking a forerun of the excess 1-decyne (oven temp <40 °C, 0.05 mm Hg), then changing the collection bulb and collecting the desired product (2.8 g, bp~60 °C, 0.05 mm Hg).
The distilled product was flushed through a plug of silica gel with hexane and into a 200 ml round-bottomed flask with a magnetic stir bar. Lindlar catalyst (150 mg) and quinolone (1.5 ml) were added, and the flask was sealed and flushed with nitrogen, then hydrogen. With the sealed flask attached to a gas burette filled with hydrogen, stirring was then started, resulting in uptake of ~310 ml of hydrogen, at which point uptake virtually ceased. The flask was flushed with nitrogen, and the mixture was filtered through a plug of celite, rinsing well with hexane. The resulting solution was washed twice with 1 M HCl, then dried and concentrated. The residue was flushed through a pad of silica gel with hexane, then Kugelrohr distilled (2.82 g, bp~60 °C, 0.05 mm Hg). Because the resulting product was contaminated with about 4% of the alkyne starting material, a portion (1.2 g) was repurified by vacuum flash chromatography on silica gel in a 60 ml Buchner funnel. The silica was prewetted with hexane, then the impure alkene was loaded as a hexane solution, and the column was eluted with 5 ×30 ml hexane. Z6-C15 eluted cleanly in fractions 1 and 2, with the alkyne eluting in fractions 4 and 5. EI Mass spectrum (m/z, abundance): 210 (53, M+), 182 (3), 168 (1), 154 (2), 140 (4), 125 (15), 111 (41), 97 (79), 84 (42), 83 (90), 70 (69), 69 (100), 57 (59), 56 (59), 55 (91), 43 (42), 41 (56).
Synthesis of Z7-C15
Z7-C15 was made in analogous fashion from 1-octyne and 1-iodoheptane. EI Mass spectrum (m/z, abundance): 210 (41), 182 (3), 168 (1), 154 (2), 140 (4), 125 (13), 111 (38), 97 (76), 84 (43), 83 (89), 70 (69), 69 (100), 57 (59), 56 (63), 55 (94), 43 (63), 41 (64).
Synthesis of Z16-C32
1-Heptadecene (2.38 g, 10 mmol; GFS Chemicals, Powell OH) was flushed through a plug of silica gel with hexane, then concentrated and transferred to a dry 3-neck flask. The flask was flushed thoroughly with Ar while stirring and warming to 45 °C. Grubbs catalyst C675 (190 mg, 0.25 mmol; gift from Materia Inc., Pasadena CA) was added in one portion, and the mixture was stirred 3 h at 45 °C under a slow flush of nitrogen to remove the ethylene formed. The mixture was then cooled, diluted with hexane, and flushed through a plug of silica gel with hexane. The resulting semicrystalline residue was Kugelrohr distilled to 105 °C (0.05 mm Hg) to remove unreacted 1-heptadecene, and the residue was recrystallized from hexane at −20 °C. The resulting white crystals (1.04 g) were 99.6% chemically pure by GC. The Z/E ratio (>99% Z) was checked by epoxidation of a sample with meta-chloroperbenzoic acid in methylene chloride, followed by GC-MS; the trans-epoxide eluted before and was completed separated from the cis-epoxide. EI Mass spectrum (m/z, abundance): 448 (20), 420 (2), 376 (1), 362 (1), 334 (1), 320 (1), 306 (2), 292 (2), 278 (2), 264 (2), 250 (2), 236 (3), 222 (3), 210 (3), 196 (4), 181 (5), 167 (7), 153 (10), 139 (16), 125 (34), 111 (61), 97 (100), 85 (37), 83 (82), 71 (50), 69 (56), 57 (74), 55 (52), 43 (54), 41 (25).
Synthesis of Z10-C33
Triflic anhydride (2.2. ml, 12 mmol) was added dropwise to a slurry of docosanol (3.26 g, 10 mmol), pyridine (0.8 ml, 10 mmol), and ~50 mg dimethylaminopyridine catalyst in 50 ml methylene chloride, cooling as necessary to keep the reaction temperature <25 °C. When the addition was complete the mixture was stirred 1 h at room temperature, producing a pale brown, slightly cloudy solution. The solution was diluted with 100 ml hexane, and filtered through a pad of silica gel, rinsing the filter pad with 2:1 hexane in methylene chloride. The resulting clear solution was concentrated to a white solid which was taken up in ether and used immediately.
Butyllithium (2.24 M in hexanes) was added to an ice-bath cooled solution of 1-undecyne (2.28 g, 15 mmol) and ~50 mg triphenylmethane indicator in 50 ml dry THF under Ar until a pink color persisted (~7 ml, 15.7 mmol). The solution was stirred for 1 h, then the ether solution of the triflate was added dropwise at 0 °C, and the mixture was warmed to room temperature and stirred overnight. The reaction was then quenched with saturated aqueous NH4Cl, and extracted with hexane. The hexane layer was washed with brine, dried, and concentrated. The residue was flushed through a pad of silica gel with hexane, then Kugelrohr distilled (oven temp ~50 °C, 0.2 mm Hg) to remove excess 1-undecyne. The solid residue was then recrystallized from 50 ml acetone, warming to solubilize the product, then cooling to room temperature, yielding the alkyne product as a single peak (1.76 g), with additional impure alkyne in the filtrate.
The alkyne (1.7 g) was taken up in 30 ml hexane, and quinoline (0.75 ml) and Lindlar catalyst (75 mg) were added. The reaction flask was sealed and flushed sequentially with nitrogen, then hydrogen, then connected to a gas burette filled with hydrogen. The mixture was stirred until hydrogen uptake ceased. After flushing with nitrogen, the mixture was filtered through celite, most of the quinoline was removed under high vacuum, and the residue was recrystallized from 30 ml of hot acetone, after cooling to 4 °C. The resulting white solid was still contaminated with quinoline, and so a hexane solution was flushed through a plug of silica gel with hexane. After concentration, the residue was recrystallized again from acetone, yielding the alkene as a white solid (1.64 g, 98.7% pure by GC). EI Mass spectrum (m/z, abundance): 462 (12), 434 (1), 390 (1), 376 (1), 362 (1), 348 (1), 334 (1), 320 (1), 306 (1), 292 (1), 278 (1), 264 (2), 250 (2), 236 (1), 222 (2), 208 (3), 195 (3), 181 (4), 167 (6), 153 (9), 139 (14), 125 (30), 111 (54), 97 (97), 85 (37), 83 (84), 71 (54), 69 (69), 57 (100), 55 (74), 43 (90), 41 (43).
Hygienic response to treatment of pupae with Z10-C33
This experiment was conducted using one VSH, one HYG, and one UNS colony. To obtain a same-age cohort of honey bee brood, the locations of uncapped brood cells containing 5th instar larvae were marked using a permanent marker on transparent plastic sheets secured above experimental cells with thumbtacks. Combs containing experimental cells were placed back into the colony and recollected within 8 h. Cells capped within that time were marked for experimental use, and frames were returned to their respective colonies. On day 6 post-capping, experimental cells were opened by cutting and lifting one side of the cell cap with a razor blade. The pupa underneath received either no treatment, or treatment with either 1 μL of hexane or with 1 μL of 1.0% Z10-C33 in hexane. Cells were then resealed by gently pressing the cap against the cell wall with the side of a razor blade, and frames were returned to their respective colonies. Uncapping and removal of brood in experimental cells were recorded at 4 and 24 h after the frame was reintroduced. Sample sizes were 40 cells per treatment for UNS and HYG colonies, and 50 cells per treatment for the VSH colony.
Effects of treatment of pupae with Z10-C33on development
This experiment was conducted using the same three VSH, HYG, and UNS colonies. As described above, capped cells containing brood 6-d post-capping were carefully opened, and the brood inside received either no treatment, or treatment with either 1 μL of hexane, or 1 μL of 1.0% Z10-C33 in hexane. Pupae were then removed from the brood comb using flexible-tipped forceps, and gently placed onto fan-folded filter paper in Petri dishes. Petri dishes were placed in an incubator maintained at 34 °C and 50% RH. Brood was examined for injury (dark pigmentation) after 48 h in the incubator, and any injured brood were discarded. On the day after expected emergence, brood were examined for normal development, defined by typical adult pigmentation and shape with proper wing development. Any deviation from this was considered “deformed” and used to calculate developmental success. Brood sample sizes were 35, 29, and 27 individuals per treatment for VSH, HYG, and UNS colonies, respectively.
Hygienic response to wax cap treatment
We tested effects on hygienic behavior of application of test compounds to wax caps of brood cells. Hygienic assays were conducted by applying hexane, 0.1%, 0.3%, or 1.0% dilutions of Z10-C33, Z6-C15, or appropriate controls (Z16-C32 and Z7-C15, respectively) in hexane to capped brood cells in a VSH colony. For each assay, 2 mL of solution were applied to a circular area of capped honey bee brood. Similar to the established freeze-killed brood assay68, the treated area was isolated using a piece of PVC pipe (7.5 cm inner diameter, approximately 8 cm long). Chemicals were applied using an H-100D Single Action airbrush and compressor (Paasche, Kenosha, WI), modified with glass bottles fitted with glass tubing for this application. For each assay, 2 mL of the solution were added to the bottle immediately before application to wax caps. Thus, 0.1%, 0.3%, and 1.0% solutions deposited approximately 45, 136, and 453 µg hydrocarbon/cm2 of capped honey bee brood, respectively. Capped cells were counted directly after treatment, and frames were returned to the colony. After 24 h, frames were recollected, and capped cells in the treated region were recounted. For comparisons of Z10-C33, Z16-C32, and hexane, sample sizes were 8, 6, and 3 replicates, respectively. For comparisons of Z6-C15, Z7-C15, and hexane, sample sizes were 12, 6, and 5 replicates, respectively. Because brood availability was limited, priority was given to replication of Z10-C33 and Z6-C15 assays, followed by assays of the structural controls Z16-C32 and Z7-C15. Assay scores were determined by dividing the total number of uncapped and removed cells after 24 h by the total number of capped cells in the circular assay area at the beginning of the assay.
Effects of wax cap treatment on development
Capped VSH brood cells in a 7.5 cm diameter circular area were left untreated (control) or treated with 2 mL of hexane, 0.1%, 0.3%, or 1.0% Z10-C33 or Z6-C15 solutions in hexane with the airbrush, as described above. After 30 min, white-eye pupae (aged 5-6 days post-capping) were removed from the brood comb using flexible-tipped forceps, and gently placed onto filter paper in Petri dishes held in an incubator (34 °C, 50% RH). Brood was examined for injury (dark pigmentation) after 48 h in the incubator, and any injured brood were discarded. On the day after expected emergence, brood were examined for normal development, as described above. Any deviation from this was considered “deformed” and the proportion of successful development calculated. Brood sample size was 40 pupae per treatment.
Comparison of freeze-killed brood (FKB) and Z10-C33-treatment assays
Hygienic responses to Z10-C33 wax cap treatment and FKB assays were compared in ten colonies, including 2 HYG, 2 VSH, and 6 UNS. For the chemical assay, 1.0% Z10-C33 was applied to wax caps as described above, and any uncapping or removal of a cell after 24 h was counted as a cell targeted by hygienic behavior. The percentage of such cells among all initially capped cells was calculated for the assay score, as above. For FKB assays, a 7.5 cm diameter PVC tube was placed on a section of capped pupae aged 3-10 d post-capping, and brood in the assay area were frozen using liquid nitrogen. Frames were returned to their colony of origin, and after 24 h, all cells within the test area that still contained any pupae were counted and recorded, according to standard practice68. Assay scores were determined by dividing the total number of cells containing any pupae after 24 h by the total number of capped cells in the circular assay area at the beginning of the assay and subtracting this number from 1.
Statistical analyses
Pearson’s Chi-square analysis with Bonferroni correction was used to test effects of pupal treatment on hygienic responses in VSH, HYG, and UNS colonies. All analyses were comparisons of manipulated (uncapped or removed) versus non-manipulated (capped) cells. Pearson’s Chi-square analysis with Bonferroni correction was also used to test effects of pupal treatment on the numbers of successfully and unsuccessfully developing VSH, HYG, and UNS brood. Two-way ANOVAs with Bonferroni-corrected post-hoc comparisons were used to test effects of chemical type and chemical concentration on hygienic response to wax cap treatments. Pearson’s Chi-square analysis with Bonferroni correction was used to test effects of wax cap treatment on the development of VSH, HYG, and UNS brood. A Pearson’s correlation coefficient was calculated to test for a positive correlation between hygienic responses to Z10-C33 and FKB assays across colonies, and a one-tailed p-value is reported. Chi-square analyses were calculated based on raw data, while assay scores and related analyses were based on percentages. All statistics were performed using IBM SPSS Statistics, Version 25.
Acknowledgements
We thank Marla Spivak and Chris Reid for their respective contributions to the content and completion of this work. This work was funded by Project ApisM, the North Carolina Biotechnology Center (2016-TEG-1503), and the United States Department of Agriculture (National Institute for Food and Agriculture, grant number 2017-68004-26321).
Author contributions
K.W., J.M., C.S. and O.R. designed the experiments, J.M. synthesized the hydrocarbons, and K.W. carried out the experiments. K.W. and O.R. analyzed and interpreted the data. K.W. wrote the initial manuscript, and all coauthors contributed to the final manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cremer S, Armitage SA, Schmid-Hempel P. Social immunity. Current Biology. 2007;17:R693–R702. doi: 10.1016/j.cub.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Evans JD, Schwarz RS. Bees brought to their knees: microbes affecting honey bee health. Trends in Microbiology. 2011;19:614–620. doi: 10.1016/j.tim.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Möckel N, Gisder S, Genersch E. Horizontal transmission of deformed wing virus: pathological consequences in adult bees (Apis mellifera) depend on the transmission route. Journal of General Virology. 2011;92:370–377. doi: 10.1099/vir.0.025940-0. [DOI] [PubMed] [Google Scholar]
- 4.Bowen-Walker P, Martin S, Gunn A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni Oud. Journal of Invertebrate Pathology. 1999;73:101–106. doi: 10.1006/jipa.1998.4807. [DOI] [PubMed] [Google Scholar]
- 5.Mondet F, de Miranda JR, Kretzschmar A, Le Conte Y, Mercer AR. On the front line: quantitative virus dynamics in honeybee (Apis mellifera L.) colonies along a new expansion front of the parasite Varroa destructor. PLoS Pathogens. 2014;10:e1004323. doi: 10.1371/journal.ppat.1004323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X, Cox-Foster DL. Impact of an ectoparasite on the immunity and pathology of an invertebrate: evidence for host immunosuppression and viral amplification. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:7470–7475. doi: 10.1073/pnas.0501860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Annoscia D, et al. Haemolymph removal by Varroa mite destabilizes the dynamical interaction between immune effectors and virus in bees, as predicted by Volterra’s model. Proceedings of the Royal Society B: Biological Sciences. 2019;286:20190331. doi: 10.1098/rspb.2019.0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin SJ, et al. Global honey bee viral landscape altered by a parasitic mite. Science. 2012;336:1304–1306. doi: 10.1126/science.1220941. [DOI] [PubMed] [Google Scholar]
- 9.Ryabov EV, et al. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLOS Pathogens. 2014;10:e1004230. doi: 10.1371/journal.ppat.1004230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McMahon DP, et al. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proceedings of the Royal Society B: Biological Sciences. 2016;283:20160811. doi: 10.1098/rspb.2016.0811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barribeau SM, et al. A depauperate immune repertoire precedes evolution of sociality in bees. Genome Biology. 2015;16:83. doi: 10.1186/s13059-015-0628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson D, Trueman J. Varroa jacobsoni (Acari: Varroidae) is more than one species. Experimental & Applied Acarology. 2000;24:165–189. doi: 10.1023/A:1006456720416. [DOI] [PubMed] [Google Scholar]
- 13.Wenner A, Bushing W. Varroa mite spread in the United States. Bee Culture. 1996;124:341–343. [Google Scholar]
- 14.Wilfert L, et al. Deformed wing virus is a recent global epidemic in honeybees driven by Varroa mites. Science. 2016;351:594–597. doi: 10.1126/science.aac9976. [DOI] [PubMed] [Google Scholar]
- 15.Aizen MA, Garibaldi LA, Cunningham SA, Klein AM. Long-term global trends in crop yield and production reveal no current pollination shortage but increasing pollinator dependency. Current Biology. 2008;18:1572–1575. doi: 10.1016/j.cub.2008.08.066. [DOI] [PubMed] [Google Scholar]
- 16.Aizen MA, Harder LD. The global stock of domesticated honey bees is growing slower than agricultural demand for pollination. Current Biology. 2009;19:915–918. doi: 10.1016/j.cub.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 17.Holden C. Report warns of looming pollination crisis in North America. Science. 2006;314:397–397. doi: 10.1126/science.314.5798.397. [DOI] [PubMed] [Google Scholar]
- 18.Kearns, C. A., Inouye, D. W. & Waser, N. M. Endangered mutualisms: the conservation of plant-pollinator interactions. Annual Review of Ecology and Systematics, 83-112 (1998).
- 19.Meixner MD. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology. 2010;103:S80–S95. doi: 10.1016/j.jip.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Potts SG, et al. Global pollinator declines: trends, impacts and drivers. Trends in Ecology & Evolution. 2010;25:345–353. doi: 10.1016/j.tree.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Genersch E, et al. The German bee monitoring project: a long term study to understand periodically high winter losses of honey bee colonies. Apidologie. 2010;41:332–352. doi: 10.1051/apido/2010014. [DOI] [Google Scholar]
- 22.Smith KM, et al. Pathogens, pests, and economics: drivers of honey bee colony declines and losses. EcoHealth. 2013;10:434–445. doi: 10.1007/s10393-013-0870-2. [DOI] [PubMed] [Google Scholar]
- 23.Kulhanek K, et al. A national survey of managed honey bee 2015–2016 annual colony losses in the USA. Journal of Apicultural Research. 2017;56:328–340. doi: 10.1080/00218839.2017.1344496. [DOI] [Google Scholar]
- 24.Lee KV, et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie. 2015;46:292–305. doi: 10.1007/s13592-015-0356-z. [DOI] [Google Scholar]
- 25.Steinhauer NA, et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: results from the Bee Informed Partnership. Journal of Apicultural Research. 2014;53:1–18. doi: 10.3896/IBRA.1.53.1.01. [DOI] [Google Scholar]
- 26.Seitz N, et al. A national survey of managed honey bee 2014–2015 annual colony losses in the USA. Journal of Apicultural Research. 2015;54:292–304. doi: 10.1080/00218839.2016.1153294. [DOI] [Google Scholar]
- 27.Rosenkranz P, Aumeier P, Ziegelmann B. Biology and control of Varroa destructor. Journal of Invertebrate Pathology. 2010;103:S96–S119. doi: 10.1016/j.jip.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 28.vanEnglesdorp D, Hayes J, Jr., Underwood RM, Pettis J. A survey of honey bee colony losses in the US, Fall 2007 to Spring 2008. PloS one. 2008;3:e4071. doi: 10.1371/journal.pone.0004071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietemann V, et al. Varroa destructor: research avenues towards sustainable control. Journal of Apicultural Research. 2012;51:125–132. doi: 10.3896/IBRA.1.51.1.15. [DOI] [Google Scholar]
- 30.Sammataro D, Untalan P, Guerrero F, Finley J. The resistance of Varroa mites (Acari: Varroidae) to acaricides and the presence of esterase. International Journal of Acarology. 2005;31:67–74. doi: 10.1080/01647950508684419. [DOI] [Google Scholar]
- 31.Imdorf A, Charrière J-D, Kilchenmann V, Bogdanov S, Fluri P. Alternative strategy in central Europe for the control of Varroa destructor in honey bee colonies. Apiacta. 2003;38:258–285. [Google Scholar]
- 32.Spivak M, Reuter GS. Resistance to American foulbrood disease by honey bee colonies Apis mellifera bred for hygienic behavior. Apidologie. 2001;32:555–565. doi: 10.1051/apido:2001103. [DOI] [Google Scholar]
- 33.Wilson-Rich N, Spivak M, Fefferman NH, Starks PT. Genetic, individual, and group facilitation of disease resistance in insect societies. Annual Review of Entomology. 2009;54:405–423. doi: 10.1146/annurev.ento.53.103106.093301. [DOI] [PubMed] [Google Scholar]
- 34.Lin Z, et al. Go east for better honey bee health: Apis cerana is faster at hygienic behavior than A. mellifera. PloS one. 2016;11:e0162647. doi: 10.1371/journal.pone.0162647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spivak M, Masterman R, Ross R, Mesce KA. Hygienic behavior in the honey bee (Apis mellifera L.) and the modulatory role of octopamine. Journal of Neurobiology. 2003;55:341–354. doi: 10.1002/neu.10219. [DOI] [PubMed] [Google Scholar]
- 36.Spivak M. Honey bee hygienic behavior and defense against Varroa jacobsoni. Apidologie. 1996;27:245–260. doi: 10.1051/apido:19960407. [DOI] [Google Scholar]
- 37.Harris JW. Bees with Varroa sensitive hygiene preferentially remove mite infested pupae aged ≤ five days post capping. Journal of Apicultural Research. 2007;46:134–139. doi: 10.1080/00218839.2007.11101383. [DOI] [Google Scholar]
- 38.Harbo JR, Harris JW. Resistance to Varroa destructor (Mesostigmata: Varroidae) when mite-resistant queen honey bees (Hymenoptera: Apidae) were free-mated with unselected drones. Journal of Economic Entomology. 2001;94:1319–1323. doi: 10.1603/0022-0493-94.6.1319. [DOI] [PubMed] [Google Scholar]
- 39.Ibrahim A, Reuter GS, Spivak M. Field trial of honey bee colonies bred for mechanisms of resistance against Varroa destructor. Apidologie. 2007;38:67–76. doi: 10.1051/apido:2006065. [DOI] [Google Scholar]
- 40.Spivak M, Reuter GS. Varroa destructor infestation in untreated honey bee (Hymenoptera: Apidae) colonies selected for hygienic behavior. Journal of Economic Entomology. 2001;94:326–331. doi: 10.1603/0022-0493-94.2.326. [DOI] [PubMed] [Google Scholar]
- 41.Blomquist, G. J. & Bagnères, A.-G. Insect hydrocarbons: biology, biochemistry, and chemical ecology. (Cambridge University Press, 2010).
- 42.Lockey KH. Insect cuticular lipids. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1985;81:263–273. doi: 10.1016/0305-0491(85)90311-6. [DOI] [Google Scholar]
- 43.Blomquist, G. J. & Vogt, R. G. Insect pheromone biochemistry and molecular biology: The biosynthesis and detection of pheromones and plant volatiles. (Elsevier Academic Press, 2003).
- 44.Annoscia D, Del Piccolo F, Nazzi F. How does the mite Varroa destructor kill the honeybee Apis mellifera? Alteration of cuticular hydrcarbons and water loss in infested honeybees. Journal of Insect Physiology. 2012;58:1548–1555. doi: 10.1016/j.jinsphys.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 45.Aumeier P, Rosenkranz P, Francke W. Cuticular volatiles, attractivity of worker larvae and invasion of brood cells by Varroa mites. A comparison of Africanized and European honey bees. Chemoecology. 2002;12:65–75. doi: 10.1007/s00049-002-8328-y. [DOI] [Google Scholar]
- 46.Francis B, Blanton W, Nunamaker R. Extractable surface hydrocarbons of workers and drones of the genus Apis. Journal of Apicultural Research. 1985;24:13–26. doi: 10.1080/00218839.1985.11100644. [DOI] [Google Scholar]
- 47.Salvy M, et al. Modifications of the cuticular hydrocarbon profile of Apis mellifera worker bees in the presence of the ectoparasitic mite Varroa jacobsoni in brood cells. Parasitology. 2001;122:145–159. doi: 10.1017/S0031182001007181. [DOI] [PubMed] [Google Scholar]
- 48.Schöning C, et al. Evidence for damage-dependent hygienic behaviour towards Varroa destructor-parasitised brood in the western honey bee, Apis mellifera. Journal of Experimental Biology. 2012;215:264–271. doi: 10.1242/jeb.062562. [DOI] [PubMed] [Google Scholar]
- 49.Nazzi F, Della Vedova G, D’Agaro M. A semiochemical from brood cells infested by Varroa destructor triggers hygienic behaviour in Apis mellifera. Apidologie. 2004;35:65–70. doi: 10.1051/apido:2003065. [DOI] [Google Scholar]
- 50.Wagoner K, Spivak M, Hefetz A, Reams T, Rueppell O. Stock-specific chemical brood signals are induced by Varroa and Deformed Wing Virus, and elicit hygienic response in the honey bee. Scientific Reports. 2019;9:8753. doi: 10.1038/s41598-019-45008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nation J, Sanford M, Milne K. Cuticular hydrocarbons from Varroa jacobsoni. Experimental & Applied Acarology. 1992;16:331–344. doi: 10.1007/BF01218575. [DOI] [Google Scholar]
- 52.Nazzi F, Milani N, Della Vedova G. (Z)-8-Heptadecene from infested cells reduces the reproduction of Varroa destructor under laboratory conditions. Journal of Chemical Ecology. 2002;28:2181–2190. doi: 10.1023/A:1021041130593. [DOI] [PubMed] [Google Scholar]
- 53.McAfee A, et al. A death pheromone, oleic acid, triggers hygienic behavior in honey bees (Apis mellifera L.) Scientific Reports. 2018;8:5719. doi: 10.1038/s41598-018-24054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hefetz A. The evolution of hydrocarbon pheromone parsimony in ants (Hymenoptera: Formicidae)—interplay of colony odor uniformity and odor idiosyncrasy. Myrmecological News. 2007;10:59–68. [Google Scholar]
- 55.Errard C, et al. Early learning of volatile chemical cues leads to interspecific recognition between two ant species. Insectes Sociaux. 2008;55:115–122. doi: 10.1007/s00040-008-0979-4. [DOI] [Google Scholar]
- 56.Lucas C, Pho D, Jallon J, Fresneau D. Role of cuticular hydrocarbons in the chemical recognition between ant species in the Pachycondyla villosa species complex. Journal of Insect Physiology. 2005;51:1148–1157. doi: 10.1016/j.jinsphys.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 57.Dani FR, Jones GR, Destri S, Spencer SH, Turillazzi S. Deciphering the recognition signature within the cuticular chemical profile of paper wasps. Animal Behaviour. 2001;62:165–171. doi: 10.1006/anbe.2001.1714. [DOI] [Google Scholar]
- 58.Nascimento D, Nascimento F. Acceptance threshold hypothesis is supported by chemical similarity of cuticular hydrocarbons in a stingless bee, Melipona asilvai. Journal of Chemical Ecology. 2012;38:1432–1440. doi: 10.1007/s10886-012-0194-7. [DOI] [PubMed] [Google Scholar]
- 59.LeConte Y, Hefetz A. Primer pheromones in social hymenoptera. Annual Review of Entomology. 2008;53:523–542. doi: 10.1146/annurev.ento.52.110405.091434. [DOI] [PubMed] [Google Scholar]
- 60.Howard RW, Blomquist GJ. Chemical ecology and biochemistry of insect hydrocarbons. Annual Review of Entomology. 1982;27:149–172. doi: 10.1146/annurev.en.27.010182.001053. [DOI] [PubMed] [Google Scholar]
- 61.Howard, R. W. Cuticular hydrocarbons and chemical communication. (University of Nebraska Press, 1993).
- 62.Lockey KH. Lipids of the insect cuticle: origin, composition and function. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1988;89:595–645. doi: 10.1016/0305-0491(88)90305-7. [DOI] [Google Scholar]
- 63.Mondet, F. et al. Specific cues associated with honey bee social defence against Varroa destructor infested brood. Scientific Reports6 (2016). [DOI] [PMC free article] [PubMed]
- 64.Page P, et al. Social apoptosis in honey bee superorganisms. Scientific Reports. 2016;6:27210. doi: 10.1038/srep27210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chakroborty NK, Bienefeld K, Menzel R. Odor learning and odor discrimination of bees selected for enhanced hygienic behavior. Apidologie. 2015;46:499–514. doi: 10.1007/s13592-014-0342-x. [DOI] [Google Scholar]
- 66.Châline N, Sandoz J-C, Martin SJ, Ratnieks FL, Jones GR. Learning and discrimination of individual cuticular hydrocarbons by honeybees (Apis mellifera) Chemical Senses. 2005;30:327–335. doi: 10.1093/chemse/bji027. [DOI] [PubMed] [Google Scholar]
- 67.Hu H, et al. Proteome analysis of the hemolymph, mushroom body, and antenna provides novel insight into honeybee resistance against Varroa infestation. Journal of Proteome Research. 2016;15:2841–2854. doi: 10.1021/acs.jproteome.6b00423. [DOI] [PubMed] [Google Scholar]
- 68.Reuter G, Spivak M. A simple assay for honey bee hygienic behavior. Bee Culture. 1998;126:23–25. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon request.