Abstract
Here we report the draft genome sequence of bacterial strain CR71, consisting of a single chromosome with 5,914,775 base pairs (bp), 34.7% G + C content, and 5733 protein-coding genes. Phylogenetic analysis indicates that the CR71 strain is affiliated with Bacillus thuringiensis species, with an average nucleotide identity > 96% and genome to genome distance > 70%. The genome of B. thuringiensis strain CR71 contains genes potentially involved in a wide variety of both plant pathogen-antagonistic and plant-growth-promoting activities, such as biofilm production; acetoin, butanediol, and indoleacetic acid (IAA) synthesis; production of quorum-sensing molecules; synthesis of toxins and lytic enzymes; and promotion of tolerance to oxidative, metal, and salt stress. Additionally, antiSMASH analysis revealed a potential synthesis of siderophores and peptide antibiotics. To confirm the in silico data, strain CR71 was inoculated into cucumber plants (Cucumis sativus L.) in a field trial, in which we observed an increase in stem thickness, as well as shoot fresh weight and length. Importantly, compared to un-inoculated control plants, plants inoculated with strain CR71 increased the size/weight ratio of cucumber fruits (34.99%), biovolume index (16.8%), and total fruit yield (34.97%). In conclusion, genome analysis of strain CR71 confirmed multifactorial plant-beneficial mechanisms and the potential of CR71 as an agricultural bio-inoculant.
Keywords: Bacterial endophyte, Field trial, Plant growth-promoting bacteria, Bioinoculant
Productivity of agricultural crops is primarily determined by biological and environmental factors (Choudhary et al. 2016; Meena et al. 2017). Biotic factors may include phytopathogens that plants have to combat, while environmental conditions may include salinity, drought, or shortage of soil nutrients (Santoyo et al. 2017). To face these issues, producers have become increasingly dependent on agrochemicals, including nitrogen fertilisers and chemicals to prevent plant diseases caused by microorganisms. However, such practices have led to soil, water, and air pollution, with high risks to human health (Sharma and Singhvi 2017).
Among the alternatives to reduce the use of chemical products on agricultural systems is the use of plant-growth-promoting bacteria (PGPB), mainly of rhizospheric or endophytic origin (Santoyo et al. 2016). PGPB can stimulate plant growth through direct or indirect mechanisms (Glick 2012; Olanrewaju et al. 2017). Direct mechanisms include functions that facilitate the availability and uptake of nutrients for the plants or that modulate their hormones. Indirect mechanisms include plant protection against phytopathogens, acting as biocontrol agents. Some microbial compounds, such as siderophores, volatiles such as the N,N-dimethylhexadecylamine (DMHDA), and the 1-aminocyclopropane-1-carboxylate (ACC) deaminase, directly stimulate plant growth and at the same time inhibit the growth of fungal pathogens (Glick 2012; Santoyo et al. 2019; Orozco-Mosqueda et al. 2020). Research in the last few years has revealed that PGPB of various genera are the main component of bioinoculants acting as biopesticides and biofertilisers (Abbey et al. 2019). Unfortunately, not all PGPB are efficient under all environmental conditions or commercialised in all regions (Bashan et al. 2014).
The genus Bacillus encompasses a large group of genetically diverse, Gram-positive, spore-forming bacteria belonging to the phylum Firmicutes (Vos et al. 2011). Bacillus are characterised by their versatile habitats, occupying an immense variety of environments (Ceuppens et al. 2013; Villarreal-Delgado et al. 2018). Members of this genus possess direct and indirect mechanisms of plant growth promotion and have been widely reported as PGPB (Santoyo et al. 2012; Tiwari et al. 2019). In our previous report, we showed that strain CR71 deployed direct and indirect mechanisms to promote plant growth. CR71 demonstrated antifungal activity against the Botrytis cinerea through the emission of volatile compounds such as dimethyl disulfide. Interestingly, co-inoculation with another strain of Bacillus promoted stem and root growth, as well as increasing chlorophyll content in tomato plants (Rojas-Solís et al. 2018).
The molecular and physiological mechanisms by which Bacillus microorganisms exert these beneficial effects are not completely understood (Pérez-García et al. 2011). Without doubt, these microorganisms may have additional beneficial applications awaiting discovery.
In this paper, the draft genome sequence of CR71 is reported, highlighting genes that may contribute to its adaptation as a PGPB, alone and in interaction with other beneficial bacteria. Additionally, a field trial was performed to validate in silico results, which demonstrated increased growth and fruit productivity of cucumber plants exerted by the beneficial inoculating effect of strain CR71.
The strain CR71 was originally designated Stenotrophomonas maltophilia after part of the 16S ribosomal RNA gene was sequenced (Rojas-Solís et al. 2018). With the complete genome of strain CR71, as well as new genomic comparison tools and additional phylogenetic markers, strain CR71 was assigned to a different species (Rojas-Solis et al. 2020). A single colony of strain CR71 was cultured in nutritive agar (BD Bioxon) at 30 °C overnight, streaked out, and maintained at 4 °C. Genomic DNA was extracted using SDS/proteinase K and precipitating polysaccharides in the presence of high salt (Mahuku 2004), then further purified with Wizard® Genomic DNA Purification Kit (Promega), following the manufacturer’s instructions. The quality and quantity of the final DNA sample were evaluated by agarose gel electrophoresis and NanoDrop (Thermo Scientific). The genomic DNA of CR71 was sequenced by Mr. DNA (Texas, USA, https://www.mrdnalab.com/) using Illumina Hi-Seq technologies, with a coverage of 58×. Quality control of the raw reads was carried out by FastQC analysis, version 0.11.5 (Andrews 2010). Trimmomatic version 0.32 (Bolger et al. 2014) was used to remove adapter sequences and bases of low quality. The assembly was performed with contigs obtained through the PATRIC (https://www.patricbrc.org/) genome service and using SPAdes assembler version 3.10.0 (Bankevich et al. 2012). The draft genome of CR71 was built by re-ordering the sequences according to the reference chromosome replicon of Bacillus thuringiensis YBT1518 (NCBI project accession: CP005935). PLACNETw revealed the absence of plasmids in the CR71 genome (Vielva et al. 2017). In total, 96 contigs were obtained, and the quality of the assembly was evaluated with Quast (https://quast.sourceforge.net/quast). The draft genome consisted of a single chromosome (Fig. 1) with 5,914,775 bp containing 5733 protein-coding genes, 100 RNA genes, 12 rRNAs, 83 tRNA genes, 5 ncRNAs, and 294 pseudogenes (Table 1).
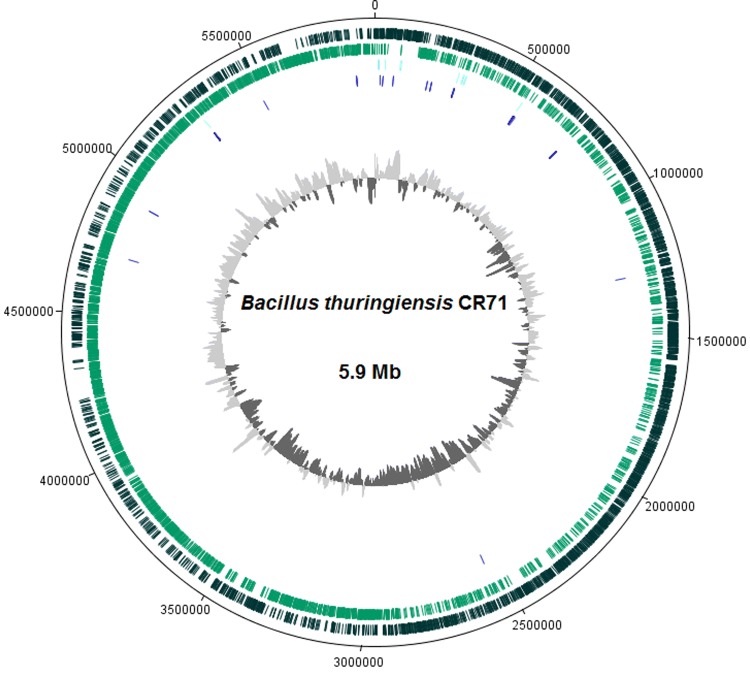
Fig. 1.
Genome map of Bacillus thuringiensis CR71. The figure illustrates from the outside towards the inner part: CDS of the forward strain (dark green), CDS of the reverse strain (light green), rRNA (light blue), tRNA (dark blue) and the GC content (light and dark grey). This figure was generated using DNA Plotter program
Table 1.
Overview of genomic features of Bacillus thuringiensis CR71
| Attribute | Value |
|---|---|
| Genome size (bp) | 5,914,775 |
| % GC | 34.7 |
| Number of contigs | 96 |
| Number of subsystems found in RAST | 483 |
| N50 (bp) | 406,943 |
| L50 | 6 |
| Sequencing technology | Illumina |
| Annotation pipeline | NCBI prokaryotic genome annotation pipeline and RAST |
| Genes (total) | 6127 |
| CDS (total) | 6027 |
| CDS (coding) | 5733 |
| Genes (RNA) | 100 |
| rRNAs | 11, 1 (5S, 16S) |
| Complete rRNAs | 11, 1 (5S, 16S) |
| tRNAs | 83 |
| ncRNAs | 5 |
| Pseudogenes | 294 |
| Gene bank accession number | CP031748 |
To assign the taxonomic position of strain CR71, a random 16S rRNA gene sequence was chosen and used as a BLAST homology search template, revealing high identity with the type strain B. thuringiensis ATCC10792T in the GenBank database. Other type Bacillus strains were then also compared to CR71 at the genome level by employing average nucleotide identity (ANI > 95–96%), using OrthoANI algorithm (Yoon et al. 2017) and GGDC (genome-to-genome distance calculator) 2.1 via BLAST (Meier et al. 2013). This comparison was based on cut-off values for species delimitation established for the 16S rRNA gene (> 98.7%) (Chun et al. 2018). Based on pre-established cut-off values for species delimitation for ANI > 95–96% (Varghese et al. 2015) and GGDC > 70% (Espariz et al. 2016), strain CR71 was found to be affiliated with Bacillus thuringiensis (Table 2). Phylogenetic analysis using the gene pycA further confirmed the close taxonomic position of strain CR71 with Bacillus thuringiensis (Fig. 2). The pycA-based phylogenetic tree was constructed using MEGA 7 (Kumar et al. 2016) via the maximum likelihood method, with a bootstrap support of 1000 replications. Additional phylogenetic analysis with 16S rDNA, gyrA, gyrB, and ccpA gene markers showed similar topology results (data not shown).
Table 2.
OGRIs values obtained from the comparison of CR71 and closely related species
| Species/strain | 16S ≥ 98.7% |
ANI ≥ 96% |
GGDC ≥ 70% |
|---|---|---|---|
| Bacillus thuringiensis ATCC 10792T | 100 | 96.23 | 76.69 |
| Bacillus albus N35-10-2T | 100 | 91.04 | 6.28 |
| Bacillus luti TD41T | 100 | 91.12 | 5.68 |
| Bacillus mobilis 0711P9-1T | 100 | 91.06 | 5.62 |
| Bacillus cereus ATCC 14579T | 99.93 | 95.96 | 69.28 |
| Bacillus toyonensis BCT-7112T | 99.93 | 91.5 | 7.9 |
| Bacillus wiedmannii FSL W8-0169T | 99.93 | 91.5 | 7.25 |
| Bacillus tropicus N24T | 99.93 | 91.6 | 7.74 |
| Bacillus proteolyticus TD42T | 99.86 | 89.69 | 2.28 |
| Bacillus paranthracis Mn5T | 99.86 | 91.37 | 6.78 |
| Bacillus pacificus EB422T | 99.86 | 91.26 | 6.55 |
| Bacillus nitratireducens 4049T | 99.86 | 89.8 | 2.26 |
| Bacillus paramycoides NH24A2T | 99.79 | 88.92 | 1.24 |
| Bacillus anthracis Ames | 99.79 | 91.3 | 6.59 |
| Bacillus mycoides DSM 2048T | 99.5 | 89.49 | 1.78 |
| Bacillus pseudomycoides DSM 12442T | 99.5 | 82.51 | 0.03 |
| Bacillus bingmayongensis FJAT-13831T | 99.08 | 82.66 | 0.03 |
| Bacillus gaemokensis KCTC 13318T | 99.01 | 82.36 | 0.02 |
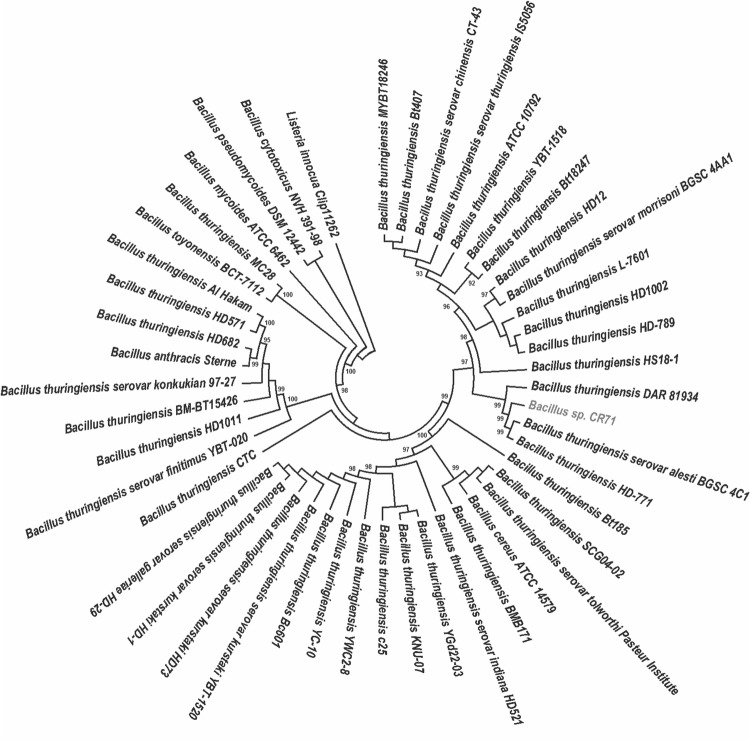
Fig. 2.
Phylogenetic relationships of CR71 with closely related members of the Bacillus cereus group. The phylogenetic tree was constructed using the pycA gene of the B. cereus group members and the pycA gene of Listeria innocua Clip11262 used as an outgroup. The tree was built with MEGA 7 using the Maximum Likelihood method and bootstrap support of 1000 replications
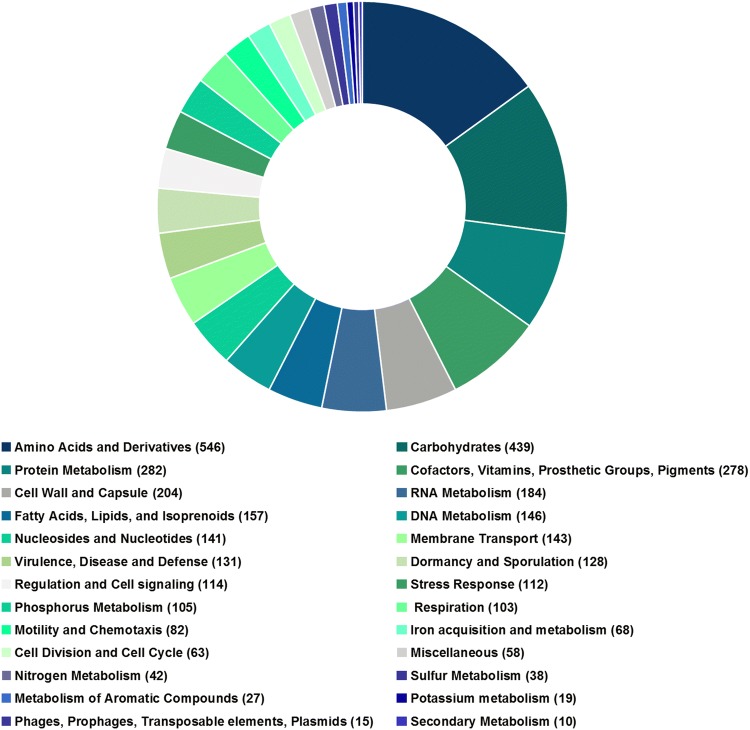
The assembled genome was annotated with the Rapid Annotation using Subsystem Technology (RAST) server (https://rast.theseed.org/FIG/rast.cgi), which assign functions to genes into subsystems by homology. Determination of genes with beneficial functions was established by BGC (biosynthetic gene clusters) predicted by antiSMASH 4.0 (Blin et al. 2017), and manually inspected from the annotations generated in RAST. Functional genomic analysis was performed using the RAST server (https://rast.nmpdr.org) (Aziz et al., 2008), and in particular the RASTtk pipeline. RAST revealed a total of 5969 coding DNA sequences (CDS), of which only 42% were categorised into subsystems or functional categories (Fig. 3). The majority of subsystems were categorised into amino acids and derivatives (546), carbohydrates (439), protein metabolism (282), cofactors and vitamins (278), dormancy and sporulation (128), stress response (112), phosphorous metabolism (105), motility and chemotaxis (82), and iron acquisition and metabolism (68), among others.
Fig. 3.
Subsystems category distribution found in the genome of strain CR71. The major part of the subsystems was categorised into amino acids and derivatives, carbohydrates, protein metabolism, cofactors and vitamins, dormancy and sporulation, stress response, phosphorous metabolism, motility and chemotaxis, iron acquisition and metabolism
Predicted production of metabolites was detected with antiSMASH (Table 3). Sixteen gene clusters for the synthesis of secondary metabolites were detected, including five groups for bacteriocins. These five groups corresponded to lanthipeptides, eight non-ribosomal peptides (Nrps), a ladderane, a terpene, and an unidentified cluster. The lanthipeptide is a secondary metabolite of ribosomal origin, first identified as thusin in B. thuringiensis BGSC 4BT1 (Xin et al. 2016). In addition, two siderophores, petrobactin and bacillibactin, were identified in the CR71 genome (Table 3). Moreover, the NCBI Prokaryotic Genome Annotation Pipeline revealed genes for the synthesis of biofilm/indole acetic acid/volatiles, oxidative/metal/salt stress tolerance, and quorum-sensing metabolites in the genome of CR71.
Table 3.
Secondary metabolites predicted in Bacillus thuringiensis CR71 by antiSMASH
| Cluster | Length | Most similar known cluster | Similarity | BGC-ID |
|---|---|---|---|---|
| Bacteriocin | 9220 | – | – | – |
| Bacteriocin | 9629 | – | – | – |
| Bacteriocin | 12,948 | – | – | – |
| Betalactone | 25,238 | Fengycin | 40% | BGC0001095 |
| Ladderane | 65,212 | S-layer glycan | 26% | BGC0000794 |
| Lanthipeptide | 45,187 | Thusin | 100% | BGC0001391 |
| Lassopeptide | 22,251 | – | – | – |
| Nrps | 64,975 | – | – | – |
| Nrps | 57,965 | Polyoxypeptin | 5% | BGC0001036 |
| Nrps | 48,938 | Bacillibactin | 46% | BGC0000309 |
| Nrps | 46,710 | – | – | – |
| Nrps | 44,376 | – | – | – |
| Nrps | 43,420 | – | – | – |
| Nrps | 56,313 | – | – | – |
| Siderophore | 13,707 | Petrobactin | 100% | BGC0000942 |
| Terpene | 21,853 | Molybdenum cofactor | 17% | BGC0000916 |
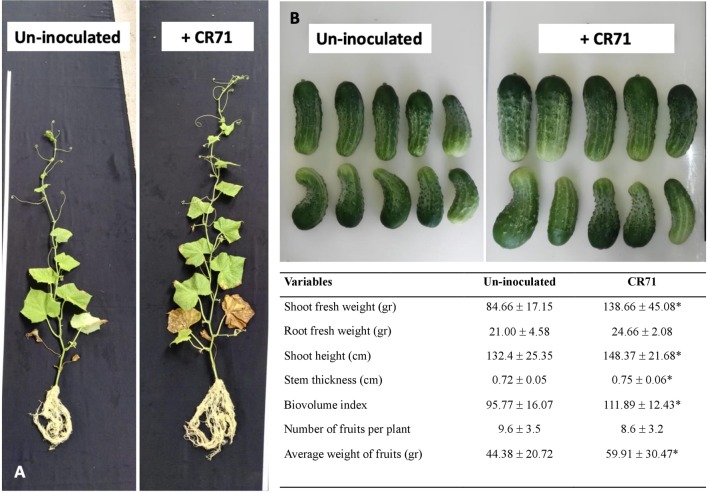
The plant-growth-promotion capacities of strain CR71 have been determined previously (Rojas-Solís et al. 2018); however, in this work we further evaluate this capacity and fruit yield in inoculated cucumber plants in a field experiment. The field trial was conducted from August to November in 2019 in Álvaro Obregón (19°48′00″ N 101°01′59″ O) in Michoacán State, México. Cucumber (Cucumis sativus L. cv. Calypso) seeds were germinated in pots and subsequently planted in the experimental soil. Soil was a sandy loam type, with 67.48% sand, 13.60% clay, 18.92% silt, and a near-neutral pH of 7.25. Soil fertility was as follows: 2.30% organic matter, 63.28 ppm inorganic nitrogen, 594 ppm potassium, 2,970.2 ppm calcium, 228.5 ppm magnesium, 129.5 ppm sodium, 140.5 ppm iron, 4.5 ppm zinc, 5.7 ppm manganese, and 4.2 ppm copper. Soil analyses were carried out at INIFAP-Celaya, Gto. The experimental design included two treatment groups: un-inoculated plants (n = 24) and plants inoculated with CR71 (n = 24), for a total of 48 experimental units. Three inoculations with 3 ml of the CR71 inoculum (cell density approximately 108 CFU mL−1) were applied directly to soil, 5 cm from the stems of each of the 24 treated plants at day 0, 15, and 30. Another set of 24 cucumber plants were irrigated with only sterilised water and used as controls (un-inoculated). After three months, plant parameters were measured and indicated that the inoculation of CR71 significantly increased the stem thickness and shoot fresh weight and length (Fig. 4). Additionally, plants inoculated with strain CR71 produced cucumber fruits with increased weight (34.99%) and biovolume index (16.8%). Importantly, CR71 increased the total fruit yield in inoculated plants, producing a total of 2516 g versus the 1863 g produced by un-inoculated cucumber plants, an increase of 34.97%. The results of this field experiment support the discovery of multiple PGP mechanisms found in the genome of B. thuringiensis strain CR71.
Fig. 4.
Composite figure of cucumber plants (Cucumis sativus L.) and harvested fruits in a field trial. Panel a shows un-inoculated (control) and inoculated plants with Bacillus thuringiensis CR71. Panel b shows representative cucumber fruits grown from un-inoculated and inoculated with strain CR71. The table shows the variables analyzed in the field experiment with cucumber (Cucumis sativus L.) plants, either un-inoculated (control) or inoculated with strain CR71. Values shown are the mean of 24 independent replicates with ± standard errors values. Values represent the mean ± standard error for 24 biological replicates, asterisks after standard error indicate significant differences, following a Student’s t test (p ≤ 0.05)
In conclusion, the results obtained in the present study revealed that B. thuringiensis CR71 has the necessary genetic machinery to stimulate plant growth through potential direct and indirect mechanisms, including synthesis of secondary metabolites and IAA production. These genes make it an interesting candidate for agricultural application. In addition, the discovery of genes involved in resistance to metals, oxidative stress, and soil salinity (Rojas-Solis et al. 2020) opens the door to explore novel roles of strain CR71 for growing plants under stressful conditions.
Accession number
This Whole Genome Shotgun sequence project has been deposited into GenBank under the accession number CP031748.1. The BioSample (SAMN09425887) and BioProject (PRJNA476111) numbers are also available.
Acknowledgements
This study was funded by Consejo Nacional de Ciencia y Tecnología (Grant no. A1-S-15956)
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain original research involving human participants or animals.
References
- Abbey L, Abbey J, Leke-Aladekoba A, Iheshiulo EMA, Ijenyo M. Biopesticides and biofertilizers: types, production, benefits, and utilization. Byproducts from agriculture and fisheries: adding value for food, feed, pharma, and fuels. Hoboken: Wiley; 2019. [Google Scholar]
- Andrews S (2010) FastQC: a quality control tool for high throughput sequence data. https://www.bioinformatics.babraham.ac.uk/projects/fastqc/.
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Meyer F, et al. The RAST server: rapid annotations using subsystems technology. BMC Genom. 2008;9:1–14. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashan Y, de Bashan LE, Prabhu SR, Hernandez JP. Advances in plant growth-promoting bacterial inoculant technology: formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- Blin K, Wolf T, Chevrette MG, Lu X, Schwalen CJ, Kautsar SA, Dickschat JS. AntiSMASH 4.0—improvements in chemistry prediction and gene cluster boundary identification. Nucleic Acids Res. 2017;45:W36–W40. doi: 10.1093/nar/gkx319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens S, Boon N, Uyttendaele M. Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles. FEMS Microbiol Ecol. 2013;84:433–450. doi: 10.1111/1574-6941.12110. [DOI] [PubMed] [Google Scholar]
- Choudhary DK, Kasotia A, Jain S, Vaishnav A, Kumari S, Sharma KP, Varma A. Bacterial-mediated tolerance and resistance to plants under abiotic and biotic stresses. J Plant Growth Regul. 2016;35:276–300. doi: 10.1007/s00284-019-01817-2. [DOI] [Google Scholar]
- Chun J, Oren A, Ventosa A, Christensen H, Arahal DR, da Costa MS, Trujillo ME. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int J Syst Evol Microbiol. 2018;68:461–466. doi: 10.1099/ijsem.0.002516. [DOI] [PubMed] [Google Scholar]
- Espariz M, Zuljan FA, Esteban L, Magni C. Taxonomic identity resolution of highly phylogenetically related strains and selection of phylogenetic markers by using genome-scale methods: the Bacillus pumilus group case. PLoS ONE. 2016;11:1–17. doi: 10.1371/journal.pone.0163098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica. 2012;2012:1–8. doi: 10.6064/2012/963401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahuku GS. A simple extraction method suitable for PCR-based analysis of plant, fungal, and bacterial DNA. Plant Mol Biol Rep. 2004;22:73–74. doi: 10.1007/BF02773351. [DOI] [Google Scholar]
- Meena KK, Sorty AM, Bitla UM, Choudhary K, Gupta P, Pareek A, Singh HB. Abiotic stress responses and microbe-mediated mitigation in plants: the omics strategies. Front Plant Sci. 2017;8:1–16. doi: 10.3389/fpls.2017.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier J, Auch A, Klenk H, Göker M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013;14:1–14. doi: 10.1186/1471-2105-14-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju OS, Glick BR, Babalola OO. Mechanisms of action of plant growth promoting bacteria. World J Microbiol Biotechnol. 2017;33:1–15. doi: 10.1007/s11274-017-2364-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Mosqueda MC, Glick BR, Santoyo G. ACC deaminase in plant growth-promoting bacteria (PGPB): an efficient mechanism to counter salt stress in crops. Microbiol Res. 2020 doi: 10.1016/j.micres.2020.126439. [DOI] [PubMed] [Google Scholar]
- Pérez-García A, Romero D, de Vicente A. Plant protection and growth stimulation by microorganisms: biotechnological applications of Bacilli in agriculture. Curr Opin Biotechnol. 2011;22:187–193. doi: 10.1016/j.copbio.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Rojas-Solís D, Zetter-Salmón E, Contreras-Pérez M, del Carmen R-G, Macías-Rodríguez L, Santoyo G. Pseudomonas stutzeri E25 and Stenotrophomonas maltophilia CR71 endophytes produce antifungal volatile organic compounds and exhibit additive plant growth-promoting effects. Biocatal Agric Biotechnol. 2018;13:46–52. doi: 10.1016/j.bcab.2017.11.007. [DOI] [Google Scholar]
- Rojas-Solis D, Vences-Guzmán MÁ, Sohlenkamp C, Santoyo G. Antifungal and plant growth–promoting bacillus under saline stress modify their membrane composition. J Soil Sci Plant Nutr. 2020 doi: 10.1007/s42729-020-00246-6. [DOI] [Google Scholar]
- Santoyo G, Orozco-Mosqueda MC, Govindappa M. Mechanisms of biocontrol and plant growth-promoting activity in soil bacterial species of Bacillus and Pseudomonas: a review. Bio-con Sci Technol. 2012;22:855–872. doi: 10.1080/09583157.2012.694413. [DOI] [Google Scholar]
- Santoyo G, Moreno-Hagelsieb G, Orozco-Mosqueda MC, Glick BR. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. doi: 10.1016/j.micres.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Santoyo G, Pacheco CH, Salmerón JH, León RH. The role of abiotic factors modulating the plant-microbe-soil interactions: toward sustainable agriculture. A review. Span J Agric Res. 2017;15:1–11. doi: 10.5424/sjar/2017151-9990. [DOI] [Google Scholar]
- Santoyo G, Equihua A, Flores A, Sepulveda E, Valencia-Cantero E, Sanchez-Yañez JM, de los Santos-Villalobos S. Plant growth promotion by ACC deaminase-producing bacilli under salt stress conditions. In: Islam M, Rahman M, Pandey P, Boehme M, Haesaert G, editors. Bacilli and agrobziotechnology: phytostimulation and biocontrol. Cham: Springer; 2019. pp. 81–95. [Google Scholar]
- Sharma N, Singhvi R. Effects of chemical fertilizers and pesticides on human health and environment: a review. IJEAB. 2017;10:675–679. doi: 10.5958/2230-732X.2017.00083.3. [DOI] [Google Scholar]
- Tiwari S, Prasad V, Lata C. New and future developments in microbial biotechnology and bioengineering. Amsterdam: Elsevier; 2019. Bacillus: plant growth promoting bacteria for sustainable agriculture and environment. [Google Scholar]
- Varghese NJ, Mukherjee S, Ivanova N, Konstantinidis KT, Mavrommatis K, Kyrpides NC, Pati A. Microbial species delineation using whole genome sequences. Nucleic Acids Res. 2015;43:6761–6771. doi: 10.1093/nar/gkv657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielva L, de Toro M, Lanza VF, de la Cruz PLACNETw: a web-based tool for plasmid reconstruction from bacterial genomes. Bioinformatics. 2017;33:3796–3798. doi: 10.1093/bioinformatics/btx462. [DOI] [PubMed] [Google Scholar]
- Villarreal-Delgado MF, Villa-Rodríguez ED, Cira-Chávez LA, Estrada-Alvarado MI, Parra-Cota FI, Santos-Villalobos S. The genus Bacillus as a biological control agent and its implications in the agri-cultural biosecurity. Revista Mexicana de Fitopatología. 2018;36(1):95–130. [Google Scholar]
- Vos P, Garrity G, Jones D, Krieg NR, Ludwig W, Rainey FA, Whitman WB. Bergey's manual of systematic bacteriology: the firmicutes. Berlin: Springer Science & Business Media; 2011. [Google Scholar]
- Xin B, Zheng J, Liu H, Li J, Ruan L, Peng D, Sun M. Thusin, a novel two-component lantibiotic with potent antimicrobial activity against several Gram-positive pathogens. Front Microbiol. 2016;7:1–10. doi: 10.3389/fmicb.2016.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SH, Ha SM, Kwon S, Lim J, Kim Y, Seo H, Chun J. Introducing EzBioCloud: a taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int J Syst Evol Microbiol. 2017;67:1613–1617. doi: 10.1099/ijsem.0.001755. [DOI] [PMC free article] [PubMed] [Google Scholar]