Abstract
Autophagy is an important component of the innate immune system in mammals. Low levels of basic autophagy are sustained in normal cells, to help with the clearance of aging organelles and misfolded proteins, thus maintaining their structural and functional stability. However, when cells are faced with challenges, such as starvation or pathogenic infection, their level of autophagy increases significantly. Salmonella is a facultative intracellular pathogen, which imposes an economic burden on the poultry farming industry and human public health. Previous studies have shown that Salmonella can induce the autophagy of cells following invasion, which to a certain extent helps to protect the cells from bacterial colonization. This review summarizes the latest research in the field of Salmonella-induced autophagy, including: (i) the autophagy induction and escape mechanisms employed by Salmonella during the infection of host cells; (ii) the effect of autophagy on intracellular Salmonella; (iii) the important autophagy adaptors that recognize intracellular Salmonella in host cells; and (iv) the effect of autophagy-modulating drugs on Salmonella infection.
Keywords: Salmonella, autophagy, xenophagy, autophagy adaptor, drug regulation
Introduction
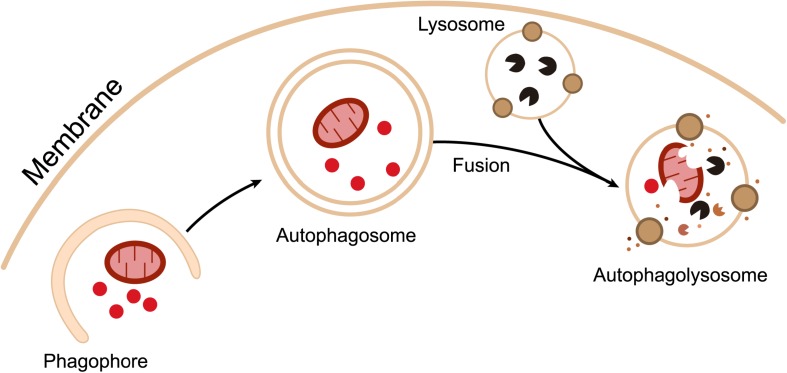
The concept of autophagy was first proposed by de Duve in 1963, to describe the process that transports cytoplasmic protein aggregates or damaged organelles to the lysosome for degradation and recycling (Yang and Klionsky, 2010). It is an evolutionarily conserved essential intracellular eukaryotic cellular process, mediated by monolayer or bilayer-containing membrane vesicles called “autophagosomes,” Generally, autophagy can be subdivided into three types according to the distinct delivering processes of substrates to lysosomes: macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA) (Xie and Klionsky, 2007). Macroautophagy uses “phagophore,” a horseshoe-shaped double-membraned structure, to envelop the captured cargoes and finally completely swallow them, forming a double-membraned autophagosome. By contrast, microautophagy and CMA usually form monolayer autophagosomes through direct or unfolded chaperone proteins mediated uptake of cargoes at the lysosome surface. Although the autophagosomes themselves are not degradative by nature, the subsequent fusion of autophagosomes with lysosomes provides a closed environment for degradation (Casanova, 2017). The term “autophagic flux” is used to describe the dynamic process of autophagosome synthesis, delivery of autophagic substrates to the lysosome, and degradation of autophagic substrates inside the lysosome (Figure 1; Mizushima et al., 2010). In the last 20 years, over 30 autophagy-related (Atg) proteins have been identified to involve in autophagic flux (Xie and Klionsky, 2007; Casanova, 2017). Autophagy plays an important role in maintaining protein metabolism and the stability of the cellular environment. Any part of autophagy flux being blocked will lead to autophagy dysfunction and corresponding consequences. Cellular autophagic activity is usually low under homeostatic conditions, but can be markedly up-regulated by numerous stimuli, such as nutrient starvation, hypoxia, energy loss, or microbial infection (Mizushima et al., 2010). Autophagy can be non-selective or selective. Non-selective autophagy displays no specificity toward the cargo, randomly capturing cytoplasmic components for degradation. Selective autophagy specifically degrades various cargoes, including organelle-specific autophagy (mitophagy, pexophagy, reticulophagy, etc.) and xenophagy (which is the degradation of microorganisms) (Cui et al., 2019).
FIGURE 1.
Outline of autophagic flux. Intracellular components are surrounded by cup-shaped double-membraned structures called “phagophores.” Phagophores gradually extend and eventually completely engulf the captured substrates to form double-membraned “autophagosomes.” Autophagosomes then fuse with lysosomes to form “autophagolysosomes,” where degradation occurs.
Xenophagy serves as an important innate immune mechanism for the intracellular clearance of pathogenic bacteria. Many invasive bacterial pathogens, such as Salmonella, Shigella, Listeria, Legionella, Mycobacterium, Franciscella, and Group A Streptococcus induce autophagy (Casanova, 2017). However, some pathogens have evolved complex escape mechanisms that either inhibit autophagy entirely or evade recognition and capture via autophagy mechanisms (Huang and Brumell, 2014; Sorbara and Girardin, 2015; Kohler and Roy, 2017). In fact, studies of Listeria, Shigella, Mycobacterium, and Salmonella have shown that xenophagy plays an important role in limiting intracellular bacterial replication (Cemma and Brumell, 2012). Of these, Salmonella autophagy currently represents the hottest research topic in the field of xenophagy. This was demonstrated by results from a PubMed search for papers, containing the words “autophagy” within the title/abstract and “bacteria” within the main body of text, published prior to September 2019. In total, we found 356 research papers, of which 86 were related to Salmonella autophagy, accounting for roughly 1/4 of all manuscripts. Therefore, this article will summarize the recent research progress in the area of Salmonella autophagy.
Manipulation of Host Cell-Mediated Autophagy by Salmonella
Pathogenic Salmonella harbors two type III secretion systems (T3SS1 and T3SS2), which are encoded by two different Salmonella pathogenicity islands; SPI-1 and SPI-2, respectively (Hansen-Wester and Hensel, 2001). These T3SSs are needle-like protein complexes that penetrate host cell membranes and inject various bacterial effectors into the cytoplasm (Xie and Klionsky, 2007). T3SS1 expression is essential for bacterial host cell invasion, while T3SS2 expression is induced only after entering host cells, and is required for intracellular bacterial survival (Figueira and Holden, 2003; Figueira et al., 2013).
T3SS1 and T3SS2 mediate the translocation of a large number of bacterial effectors. After invasion, some of these T3SS effectors can hijack the host cell endocytic pathway and convert the phagosomes into monolayer vacuoles, known as “Salmonella-containing vacuoles” (SCVs), which are well suited to the survival and replication of Salmonella (Steele-Mortimer, 2008). A recent study has shown that the expression of T3SS1 and its effectors were significantly down-regulated 1–2 h post-invasion, while the expression of T3SS2 increased with time, accompanied by the maturation of SCVs. The expression of these two groups of effectors therefore plays a synergistic role in the modification and maturation of SCVs during the infection of epithelial cells with Salmonella (Hautefort et al., 2008).
Salmonella Induces Autophagy
Salmonella invades host epithelial cells actively and partially triggers autophagy via the T3SS1-mediated disruption of membrane integrity and the subsequent translocation of effector proteins into cell. Pores formed by T3SS1 result in the leakage of amino acids from the cell, which leads to acute starvation stress. Amino acid starvation inhibits the activity of mTORC1 through a complex signaling cascade, activates the EIF2AK4/GCN2-EIF2S1/eIF2α/ATF3 pathway, and ultimately induces autophagy (Tattoli et al., 2012a, b).
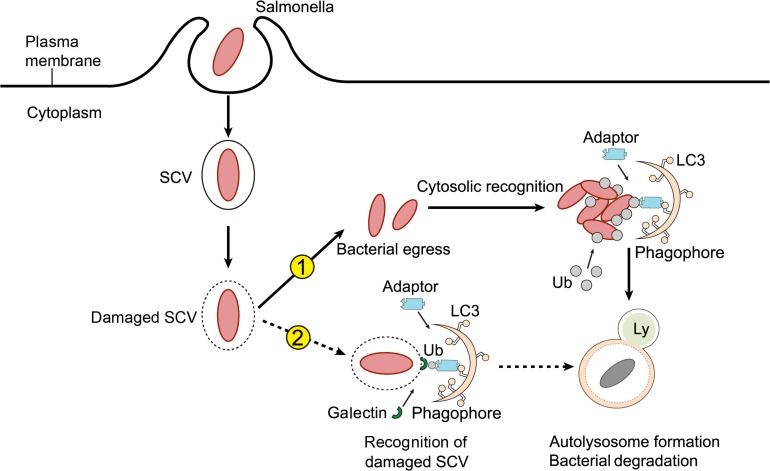
Salmonella can induce autophagy via two pathways (Figure 2). The first involves the recognition of intracellular Salmonella by ubiquitin. As mentioned above, intracellular Salmonella mainly resides in SCVs, surviving and replicating in these membrane-bound compartments (Malik-Kale et al., 2012). However, a small number of Salmonella can penetrate the SCV monolayer through the use of their needle-like T3SS1, and escape from the SCV into the cytoplasm to achieve high rates of replication. High copy numbers of intracellular Salmonella are quickly recognized by the ubiquitination system of host cells, resulting in the formation of an intensive ubiquitin chain layer surrounding the bacteria. Ubiquitinated Salmonella is recognized by autophagy adaptors such as NDP52, OPTN, and p62. These adaptors bind to the ubiquitinated bacteria via their ubiquitin binding domain and interact with the autophagic membrane anchoring protein LC3 to direct the bacteria to primary autophagosomes (Mostowy et al., 2011). The second strategy that Salmonella employs to induce autophagy involves the recognition of destroyed SCVs resulting from the pore-forming properties of T3SS1. In this scenario, Salmonella need not be in a planktonic state for autophagic capture and degradation (Birmingham and Brumell, 2006). As previously mentioned, the expression of T3SS1 continues for 1–2 h after bacterial internalization. In the meantime, T3SS1 mediates SCV membrane destruction in the same way that it breaks down the cell membrane (Kreibich et al., 2015). If the damage is sufficiently substantial, the SCV lumen will become accessible to the cytoplasmic ubiquitin ligases, labeling Salmonella by ubiquitination (Perrin et al., 2004). In addition to ubiquitination, SCV damage is also recognized by galectin-8, a cytoplasmic galactose lectin. Galectin-8 serves as a monitor for endolysosomal integrity by recognizing β-galactoside, which is normally present on the inner surface of SCVs. SCVs identified by galectin-8, in turn, recruit autophagy adaptors to the SCV membrane, thereby attaching damaged SCVs to primary autophagosomes (Thurston et al., 2012).
FIGURE 2.
How Salmonella induces autophagy. After internalization by host cells, Salmonella resides within a modified phagosomal compartment called the Salmonella-containing vacuole (SCV). While the needle-like Type III secretion system (T3SS-1) of Salmonella can cause damage to the SCV, resulting in one of two consequences for the pathogen. Firstly, if the T3SS-mediated damage is sufficient, the SCV can rupture, allowing bacteria to enter the cytoplasm. Cytosolic Salmonella are rapidly tagged with ubiquitin (Ub) and marked for autophagy by adaptor molecules. Autophagy adaptors interact with Atg8 family members, such as LC3, to promote autophagosome biogenesis. Secondly, Salmonella residing in damaged SCVs can also be targeted for autophagy. In this case, modified carbohydrate structures on the SCV recruit galectins, adaptors, and ubiquitin to the membrane, which tag these compartments for autophagic elimination.
The Principal Effectors of Salmonella-Specific Autophagy Induction
As mentioned above, Salmonella can induce host cell-mediated autophagy. We will now discuss in more detail which Salmonella effector proteins are involved in autophagy induction. To date, studies have found that Salmonella cytolethal distending toxin B (cdtB), asparaginase, SipB virulence protein, and β-barrel outer membrane protein (β-OMP) are involved in the induction of Salmonella autophagy (Table 1).
TABLE 1.
Mechanisms used by Salmonella to induce or escape autophagy.
| Bacterium | Bacterial factors | Model systems | Refs |
| Inducing autophagy: | |||
| S. Javiana | Cytolethal distending toxin B (CdtB) | J774A.1 | 25 |
| S. Typhimurium | Type II L-asparaginase | T cells | 26 |
| S. Typhimurium | SipD | Bone marrow-derived primary macrophages | 29 |
| S. Typhimurium | β-barrel outer membrane protein (β-OMP) | THP1 and Caco-2/TC7 cells | 30 |
| Escaping autophagy: | |||
| S. Typhimurium | SsrB, ssaV | Bone marrow-derived primary macrophages | 33 |
| S. Typhimurium | SseG, SseF | HEK293T, HeLa, THP-1, RAW264.7, mice | 36 |
| S. Typhimurium | SseL | HeLa, RAW264.7, bone marrow-derived primary macrophages | 38 |
| S. Typhimurium | PR ST98 plasmid | THP-1, J774A.1, embryonic fibroblasts | 40–43 |
| S. Typhimurium | SpvB | J774A.1, HeLa, mice, zebrafish | 46–48 |
| S. Typhimurium | reactive persulfides | RAW264.7, bone marrow-derived primary macrophages | 52 |
| S. Typhimurium | – | FAK–/– peritoneal macrophages, mice lacking macrophage-specific FAK | 54 |
The cytolethal distending toxin (CDT), a type of toxin which plays an important role in bacterial pathogenesis, was initially found to be produced by Escherichia coli (E. coli), and later shown to be a product of many types of Gram-negative bacteria. CDT mimics the role of DNA enzymes and damages macrophage DNA, thus causing cell cycle arrest and macrophage swelling and apoptosis (Hassane et al., 2003). CDT consists of three polypeptides: cdtA, cdtB, and cdtC. Of these, cdtA and cdtC transport cdtB into host cells, while cdtB mediates cellular chromosomal DNA degradation (Lara-Tejero and Galan, 2001). Salmonella expresses cdtB, but not cdtA and cdtC. Williams et al. (2015) found that macrophages infected with the Salmonella Javiana (SJ) mutant strain lacking cdtB, displayed lower levels of LC3 and autophagy-related gene expression than cells infected with the wild-type strain, suggesting that cdtB was involved in the SJ-mediated autophagy induction in host cells.
L-asparaginase (L-ASNase) is a hydrolase that converts L-asparagine to L-aspartic acid and ammonia. L-ASNase has anti-tumor activity and is widely used in the fields of medicine and nutrition. Torres et al. (2016) found that Salmonella can produce a type II L-ASNase, which can hydrolyze the L-asparagine required for T cell activation, thereby inhibiting mTOR signaling and activating autophagy.
The simplest virulence strategy employed by Salmonella is its ability to penetrate the intestinal mucosal barrier. Salmonella invasive protein D (SipD) is an effector protein located within SPI-1. SipD is secreted primary by T3SS-1 and binds to the top of the T3SS-1 structure. During the invasion stage of infection, Salmonella makes contact with the endothelial cell membrane via the effector SipD (Lunelli et al., 2011; Rathinavelan et al., 2014). Hernandez et al. (2003) showed that the infection of macrophages by SipD-deficient Salmonella did not result in autophagy, in contrast to wild type Salmonella.
Gram-negative bacteria and the mitochondrial outer membrane possess a unique β-barrel tertiary structure, which can serve as a pathogen-associated molecular pattern (PAMP) for innate immune system recognition. Chaudhary et al. (2018) found that the β-OMP purified from Salmonella, induced endosomal acidification, LC3B lipidation, and p62 degradation, in addition to reducing mTORC2 and Akt phosphorylation, suggesting that β-OMP is also an important effector protein of Salmonella-induced host cell-mediated autophagy.
The Escape Mechanism Employed by Salmonella to Evade Host Cell Autophagy
It is well known that autophagy, as a catabolic pathway for the degradation of dysfunctional cellular proteins, organelles, and pathogenic microorganisms, plays an important role in host defense, and is an integral part of innate and adaptive immune responses. Salmonella, as a typical facultative intracellular bacterium, can induce the autophagy of host cells following infection, and maintain normal physiological functions by degrading pathogens through autophagy. On the other hand, Salmonella has evolved a unique escape mechanism to evade autophagy, which can interfere with the process of autophagy-mediated pathogen degradation and clearance, allowing Salmonella to persist inside host cells. Salmonella-induced autophagy is in fact temporary, occurring mainly in the early stages of infection. It has been demonstrated that, in cultured epithelial cells, autophagy peaks 1 h following infection initiation and lasts for only 3 h post-infection (Tattoli et al., 2012b), coinciding with the time period involved in the up- or down-regulation of the two T3SSs. Down-regulation of T3SS1 and up-regulation of T3SS2 may lead to the normalization of plasma membrane integrity and amino acid levels, thus inhibiting autophagy. Previous studies have also confirmed that the induction of T3SS2 and its effectors plays a crucial role in the mechanism employed by Salmonella to escape phagocytosis. In addition, Salmonella virulence plasmids and genes, as well as bacterial sulfur metabolites, are also involved in its evasion of autophagy.
SsrB, a member of the NarL/FixJ subfamily of DNA-binding response regulators, specifically regulates the majority of the SPI-2-encoded virulence factors, and is mainly expressed after SCV acidification (Walthers et al., 2007). SsaV is also a member of T3SS2 that injects SPI-2 virulence factors into host cells (Grant et al., 2012). Ganesan et al. (2017) found that the Salmonella virulence factors SsrB and SsaV disrupted Sirt1/LKB1/AMPK signaling, thus activating mTOR and evading autophagy; it was demonstrated for the first time that the Sirt1/LKB1/AMPK complex was the target of effectors encoded by Salmonella SPI-2. Salmonella therefore manipulates mTOR activity through the actions of the virulence factors SsrB and SsaV, thus evading the defense mechanism of autophagic host cells.
SseG and SseF are two effector proteins encoded by SPI-2 that play a crucial role in the process of bacterial infection and the maintenance of SCV membrane integrity (Kuhle and Hensel, 2002; Deiwick et al., 2006). Feng et al. (2018) found that the SseF and SseG secreted by Salmonella Typhimurium could impair the recruitment and activation of ULK1 by blocking Rab1A activity in host cells. This resulted in a reduction in PI3P production, ultimately impeding autophagosome formation in host cells, and thereby establishing a replicative niche for the bacteria in the cytoplasm. Furthermore, the survival and growth rates of SseF- or SseG-deficient Salmonella strains were reduced both in vitro and in vivo, but could be restored to wild type levels by depleting Rab1A. These findings suggest that the virulence factors SseG and SseF can help Salmonella evade autophagy and the host defense system via the inactivation of GTPase Rab1A.
SseL is another SPI-2 T3SS-encoded effector protein, and an important Salmonella virulence factor, which regulates the host’s inflammatory response by deubiquitization (Le Negrate et al., 2008). As mentioned previously, bacterial infection leads to the formation of ubiquitin aggregates in cells, which are recognized by autophagy adaptors before entering the autophagic degradation pathway. Mesquita et al. (2012) found that the ubiquitination markers of Salmonella-infected cells could be counteracted by the deubiquitinase SseL, thereby impeding their autophagic degradation and favoring intracellular bacterial replication. The results showed that SseL was also important in enabling Salmonella to escape autophagic degradation. However, there are some differences in the genetic sequences encoding SseL between Salmonella Typhimurium and Salmonella Enteritidis, implying that the functions of SseL may also vary between different Salmonella strains and even different serotypes of Salmonella Enteritidis.
The pR (ST98) plasmid is a conjugative transfer plasmid that mediates the drug resistance and virulence of Salmonella Typhi. pR (ST98) has a molecular weight of 98.6 × 106 Da (about 159 kb), and was first identified and reported by Huang et al. (2005). Subsequent studies in their laboratory have shown that mutant Salmonella, harboring a pR (ST98) plasmid deletion, inhibited autophagy in infected macrophages (He et al., 2012; Chu et al., 2014; Wu et al., 2014) and mouse embryonic fibroblasts (Lv et al., 2012), while enhancing Salmonella proliferation within cells and consequently promoting cell death. These results demonstrate that the pR (ST98) plasmid enables Salmonella to escape autophagy.
The Salmonella plasmid virulence (spv) locus is a highly conserved sequence commonly carried by all pathogenic Salmonella. It can increase the growth of Salmonella in extraintestinal tissue cells, and is associated with bacterial serum resistance, adhesion, and colonization (Kuźmińska-Bajor et al., 2012). The spv region contains five genes: spvR, spvA, spvB, spvC, and spvD. Of these, spvB is a structural gene, which has a great influence on bacterial virulence (Guiney and Fierer, 2011). It has been found that spvB can depolymerize actin, inhibit autophagy in human epithelial HeLa cells, macrophage-like J774A.1 cells, and BALB/c mice, and aggravate the host’s inflammatory damage to Salmonella infection (Chu et al., 2016). Moreover, in the zebrafish model infected with Salmonella, it was also confirmed that Salmonella spvB could promote bacterial survival and intestinal damage by inhibiting autophagy (Li et al., 2016; Wu et al., 2016). Recent work using the Raw264.7 mouse macrophage cell line and zebrafish larvae as models, also showed that the Salmonella spv locus could inhibit the type I interferon response and the chemotaxis of neutrophils by suppressing autophagy (Wang et al., 2019). In conclusion, these data indicate that spvB is an important virulence factor employed by Salmonella to escape autophagy.
In addition, Salmonella and other pathogenic bacteria produce reactive persulfides, such as cysteine persulfate (CysSSH) and glutathione persulfate (GSSH), during their metabolism of sulfur (Ida et al., 2014; Akaike et al., 2017). It has been shown that the reactive persulfides produced by Salmonella can metabolize 8-nitro-cGMP to regulate macrophage autophagy (Shahzada et al., 2018). 8-nitro-cGMP signaling is necessary for infected macrophages to clear intracellular Salmonella by autophagy, suggesting that bacteria-derived reactive persulfides can also help Salmonella escape from autophagic degradation (Owen et al., 2007).
Although autophagic escape mechanisms are predominantly orchestrated by bacterial factors, the focal adhesion kinase (FAK), a non-receptor tyrosine kinase known for its role in adhesion-mediated signal transduction in a variety of cell types (Owen et al., 2014), was also found to be implicated. FAK is recruited to the SCV surface via the Salmonella SPI-2 system, leading to the amplification of signals through the Akt-mTOR axis, thus inhibiting autophagy and promoting the survival of bacteria within macrophages. Indeed, FAK–/– macrophages display a weaker Akt/mTOR signal, resulting in the improved autophagic capture of bacteria, and ultimately reducing overall bacterial survival. In vivo, knocking out FAK in macrophages also results in the rapid elimination of Salmonella from various tissues. Collectively, these results suggest that FAK represents a means for Salmonella to escape autophagy through T3SS2, thereby promoting its viability in cells (Owen et al., 2014).
The Effect of Autophagy on Intracellular Salmonella
At present, the consensus regarding the effect of autophagy on intracellular Salmonella is that autophagy helps cells to remove invasive intracellular bacteria. However, in recent years, it has been reported that Salmonella can also exploit autophagy to promote bacterial replication.
Autophagy Promotes the Elimination of Salmonella From Host Cells
In 2006, Birmingham et al. observed that some intracellular Salmonella are recognized by autophagy under in vitro infection conditions, and confirmed that autophagic recognition restricted the replication of intracellular Salmonella. To further delineate the role of autophagy in limiting the growth of intracellular Salmonella, Birmingham and his colleagues utilized Atg5–/– mouse embryonic fibroblasts (MEFs) infected with Salmonella Typhimurium to measure the intracellular growth of Salmonella. The results showed that, compared with wild type MEFs, the growth of bacteria in Atg5–/–MEF cells increased. These results suggest that the autophagy induced by Salmonella infection enables host cells to restrict the growth of bacteria and protect their cytoplasm from bacterial colonization (Birmingham et al., 2006). Since then, other studies have strengthened this concept. Jia et al. (2009) studied the effect of autophagy gene inactivation on Salmonella infection in two model organisms, Cryptorhabditis elegans and Reticulium discoides. The results showed that genetic inactivation of the autophagy pathway in both organisms resulted in increased bacterial intracellular replication and decreased animal lifespan, suggesting that autophagy plays an important role in the host defense against intracellular Salmonella in vivo. Conway et al. (2013) generated mice whose epithelial cells were deficient in the autophagy-implicated protein Atg16L1. After the mice were infected with Salmonella Typhimurium, the autophagy level, immune function, and bacterial clearance ability of the cecum and small intestine tissues were analyzed. The results showed that Salmonella infection increased autophagy within the intestinal epithelium in control mice but made no difference to the level of autophagy observed in the epithelial cells of ATG16L1-deficient animals. Furthermore, mice deficient in ATG16L1 displayed abnormal immune function and decreased bacterial clearance ability, compared to the control mice. It was suggested that the autophagy performed by intestinal epithelial cells helps mice to eliminate infected bacteria and prevent systemic infection. Curt et al. (2014) used the C. elegans model to demonstrate that inhibition of the autophagy gene bec1 in intestinal epithelial cells made the organism more susceptible to Salmonella infection, suggesting that autophagy promoted the elimination of Salmonella by intestinal epithelial cells. ATP6V0D2 is a critical component mediating autophagosome-lysosome fusion in macrophage cells. Atp6v0d2-deficient macrophage cells exhibited decreased levels of Salmonella Typhimurium elimination, while Atp6v0d2-knockout mice became more susceptible to Salmonella Typhimurium-induced death (Xia et al., 2019). Raw264.7 cells pretreated with the autophagy inhibitors 5Z-7-oxozeaenol or compound C, prior to infection with Salmonella Typhimurium, also showed accelerated intracellular Salmonella replication (Liu et al., 2019).
Cell Autophagy Facilitates Salmonella Survival in Host Cells
As an important component of the host’s defense system, autophagy is recognized for its role in degrading Salmonella and limiting its replication and proliferation. Contrary to this concept, however, autophagy has recently been reported to facilitate the replication of Salmonella in HeLa cells. Yu et al. (2014) investigated the density of intracellular Salmonella and found that the Salmonella targeted by autophagy mainly resided in the cytosol and replicated rapidly; while, the bacteria localized to intact SCVs were not associated with autophagosomes and replicated slowly. In addition, depletion of autophagy mediators by specific siRNAs (p62/LC3/Atg5/Atg16L1/TBK1) significantly inhibited the proliferation of Salmonella in the cytoplasm of HeLa cells. High rates of autophagy-targeted Salmonella replication in the cytoplasm eventually lead to increased cell exfoliation, promoting the spread of Salmonella to adjacent cells. Researchers speculate that the variation in results could be attributed to the off-target effects of high-dose siRNAs used by other groups or the incomplete dissociation of membrane-associated Salmonella when quantifying intracellular bacterial numbers. The cell type and time point chosen for the assessment of bacterial replication may also have an impact (Yu et al., 2014). Interestingly, prior to their report, it was also shown that knocking out Rab1, a GTPase required for Salmonella autophagy, reduced the rate of Salmonella replication in HeLa cells (Huang et al., 2011). Although the authors initially speculated that this result was due to the inhibition of bacterial replication in SCVs, it was later confirmed by live imaging that the replicative niche for Salmonella was predominantly the cytoplasm (Malik-Kale et al., 2012). Therefore, the inhibition of bacterial replication caused by the deletion of Rab1 can also be attributed to the inhibition of autophagy, thus reducing Salmonella replication in the cytoplasm.
Adaptors of Salmonella Autophagy
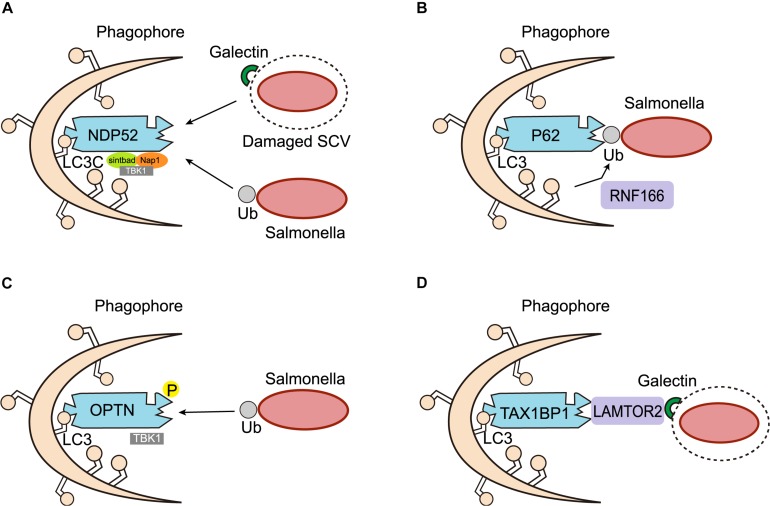
In accordance with the autophagy of other bacterial species, intracellular Salmonella labeled with E3 ubiquitin ligase also needs to be recognized by autophagy adaptors in order to be targeted for autophagic degradation. Autophagy adaptors are proteins that simultaneously interact with specific substrates and components of the autophagy machinery, and serve to link substrate degradation to newly formed autophagosomes. Autophagy adaptors generally possess several protein-protein interaction domains, such as the LC3 interaction region (LIR) for LC3 binding, and the ubiquitin-associated (UBA) domain for interacting with ubiquitin-coated substrates. Researchers have found four main autophagy adaptors implicated in Salmonella autophagy, namely NDP52, p62, OPTN, and TAX1BP1 (Figure 3).
FIGURE 3.
Adaptors of Salmonella autophagy. (A) NDP52 functions as a scaffold for the assembly of a TBK1-Sintbad-Nap1 signaling complex, transports Salmonella marked by ubiquitin (Ub) or damaged SCV membranes marked by galectin-8 into the autophagic pathway through an interaction with LC3C. (B) P62 serves as an autophagy adaptor, was recruited to ubiquitinated Salmonella targeted by autophagy. The ring finger protein 166 (RNF166) is vital for the recruitment of the autophagy adaptor p62 to ubiquitinated Salmonella. (C) OPTN recognizes ubiquitinated Salmonella and targets them for autophagy. Phosphorylation (P) of OPTN by TBK1 promotes its interaction with LC3. (D) TAX1BP1, a close homolog of NDP52, also acts as an autophagy adaptor in Salmonella autophagy. In response to membrane damage caused by bacteria, LAMTOR2 is recruited to the damaged membrane and associates with galectins. LAMTOR2 then recruits TAX1BP1 to promote the formation of autophagy.
NDP52 is an important adaptor of selective autophagy. It maintains cellular homeostasis mainly through a process called “mitophagy,” which removes damaged mitochondria from cells (Heo et al., 2015). Several studies have also proven that NDP52 plays a crucial role in the process of xenophagy. For instance, various bacteria, such as Salmonella, Shigella flexneri, and Streptococcus pyogenes, have been shown to be targets of NDP52-mediated selective autophagy (von Muhlinen et al., 2010; Mostowy et al., 2011; Minowa-Nozawa et al., 2017). In the context of Salmonella autophagy, NDP52 acts as a bridging junction to specifically recognize ubiquitinated intracellular Salmonella. It was found that NDP52 selectively binds LC3C through its non-typical LIR and transports ubiquitinated Salmonella into the autophagic pathway. Cells lacking either NDP52 or LC3C therefore cannot protect their cytoplasm against Salmonella infection by antibacterial autophagy (von Muhlinen et al., 2012, 2013). Meanwhile, NDP52 also recruits TANK-binding kinase 1 (TBK1), an IKK family kinase that coexists with NDP52 on bacterial surfaces, by combining Nap1 and Sintbad, to interact with LC3. Deletion of NDP52 and TBK1, increases the proliferation of intracellular Salmonella, and causes the ubiquitin-modified bacteria to aggregate in ATG8/LC3(+) autophagosomes. These results suggest that NDP52 and the ubiquitin system can be used to activate autophagy and combat Salmonella colonization of the cytoplasm of human cells (Thurston et al., 2009; von Muhlinen et al., 2010). Although NDP52 is known to be important for the selective autophagic degradation of invasive pathogens, the mechanism underlying its recognition of ubiquitinated bacteria remains unclear. Through biochemical and structural analysis, Xie et al. (2015) found that the C-terminal cargo recognition region of NDP52 contains a typical C2H2-type zinc finger, which can specifically bind to cargo-covered ubiquitin or ubiquitin chains, revealing a new recognition and targeting mechanism of ubiquitin-coated substrates by the ubiquitin-binding autophagy adaptor NDP52 in selective autophagy. In addition to serving as an adaptor of xenophagy, targeting ubiquitinated bacteria for primary autophagy in vitro, NDP52 also promotes the maturation of autophagosomes containing Salmonella. These two unrelated roles depend on the different NDP52-binding domains and chaperone proteins, respectively. Specifically, NDP52 promotes autophagy maturation by interacting with LC3A, LC3B, and/or GABARAPL2 via a unique LIR and myosin VI, while the NDP52-mediated targeting of intracellular Salmonella for autophagy depends on its interaction with LC3C (Ivanov and Roy, 2009; Verlhac et al., 2015a, b,c). In addition to ubiquitin, galectin-8 binding to damaged SCV membranes can also recruit NDP52 to activate antimicrobial autophagy (Thurston et al., 2012; Li et al., 2013; Kwon and Song, 2018; Boyle et al., 2019; Ravenhill et al., 2019). However, the galectin-8-dependent recruitment of NDP52 to SCVs is only temporary, and therefore performs a secondary role to the ubiquitin-dependent recruitment of NPD52 (Thurston et al., 2012).
P62 is one of the earliest autophagy adaptors to have been identified and described in mammals. The role of p62 in the induction of xenophagy has mainly been investigated in the context of Salmonella infection. Researchers found that p62 was recruited to Salmonella targeted for autophagy, and that the recruitment of p62 was required for the efficient elimination of Salmonella by xenophagy (Zheng et al., 2009). Ishimura et al. (2014) also confirmed that once a cell was infected with Salmonella, p62 assembled on the pathogen and induced xenophagy. Regulators of p62 recruitment by Salmonella autophagy have also been reported. Heath et al. (2016) screened and identified a series of E3 ligases, of which, the ring finger protein 166 (RNF166) was found to be vital for the interaction with the autophagy network and the recruitment of the autophagy adaptor p62 to ubiquitinated Salmonella. Mechanistic studies have also demonstrated that RNF166 catalyzes the polyubiquitination of p62.
Optineurin (OPTN) is a 67 kDa intracellular protein distributed among different tissues, where it serves as an important autophagy adaptor involved in different types of autophagy (Wild et al., 2011; Heo et al., 2015; Richter et al., 2016; Slowicka and van Loo, 2018). Studies have proven that OPTN is necessary for limiting the proliferation of Salmonella following infection. Indeed, silencing OPTN weakens Salmonella autophagy, leading to an increase in intracellular bacterial replication (Wild et al., 2011). However, in contrast to other autophagy adaptors, the LIR of OPTN binds LC3 with very low affinity and requires input from TBK1. TBK1 can directly bind and phosphorylate OPTN on Ser177, enhancing its association with LC3, therefore allowing OPTN to recognize ubiquitin-coated intracellular microbes and target them for autophagy (Weidberg and Elazar, 2011; Rogov et al., 2013). To evaluate the in vivo importance of OPTN in inflammation and infection, Slowicka et al. (2016) generated OPTN-deficient mice, and found that they were more sensitive to Salmonella infection, demonstrating that OPTN in critical for bacterial clearance in vivo.
TAX1BP1, a close homolog of NDP52, acts as an autophagy adaptor mainly in xenophagy. The role of TAX1BP1 in xenophagy was first emphasized by a study that revealed its function in the eradication of Salmonella Typhimurium (Tumbarello et al., 2015). The clearance of intracellular Salmonella relies on the association between TAX1BP1 and motor myosin VI, which aids the fusion of autophagosomes with lysosomes. It has been demonstrated that TAX1BP1-deficiency increased the number of ubiquitin-labeled Salmonella, whereas the absence of myosin VI raised the number of ubiquitin- and LC3-positive Salmonella (Tumbarello et al., 2015). A recent study has also reported the involvement of TAX1BP1 in the clearance of Salmonella Typhimurium. The authors found that knocking out TAX1BP1 resulted in a decrease in the formation of autolysosomes and subsequent microbe elimination. In addition, they identified LAMTOR2 and LAMTOR1 as previously overlooked xenophagy regulators of TAX1BP1 in response to Salmonella infection, and confirmed that LAMTOR2 was recruited to damaged SCVs via LAMTOR1, an endosome-resident protein. LAMTOR2 was critical for the subsequent recruitment of TAX1BP1, thus accelerating the formation of autolysosomes during bacterial infection (Lin et al., 2019).
Although the above autophagic adaptors all target Salmonella for autophagy, it should be noted that these adaptors are independently recruited to bacteria-associated microdomains. A recent study has shown that p62 and NDP52 were recruited to Salmonella independently of each other, at two non-overlapping microdomains. Moreover, although antibacterial autophagy was impaired by the loss of either adaptor, there was no synergistic impairment of xenophagy when losing both adaptors simultaneously, suggesting that p62 and NDP52 may be implicated in the same pathway (Cemma et al., 2011).
Certain Drugs Regulate Xenophagy to Combat Salmonella Infection
Salmonella represents one of the major foodborne pathogens worldwide. As a common zoonotic pathogen, Salmonella has a serious impact on animal husbandry, human health, and food safety. However, effective methods for the elimination of Salmonella infection are still limited. The main prevention and control measures for Salmonella infection include antibiotic therapy, competitive inhibition, and vertical purification. However, with the exception of antibiotics, the majority of antibacterial methods remain ineffective. What is worse, antibiotic therapy is also confronted with problems such as the accumulation of drug residues, drug resistance, and poor intracellular bactericidal efficiency. Therefore, there is an urgent need to develop effective agents for the control of Salmonella infection.
As a major constituent of innate immunity, xenophagy plays an important role in the body’s defense against foreign pathogenic bacteria. In recent years, accumulating studies have tried to find effective xenophagy-implicated agents to target Salmonella replication (Table 2). It is well documented that many phenolic compounds, such as Carvacrol (Gaio et al., 2017), Thymol (Marchese et al., 2016), Quercetin (Wang et al., 2018), Resveratrol (Hwang and Lim, 2015), Acacetin (Bi et al., 2016), and Epigallocatechin gallate (Nakasone et al., 2017) exhibit antibacterial activity. Recently, Al Azzaz et al. (2019) found that Resveratrol promotes the autophagy-dependent elimination of intracellular Salmonella both in vitro and in vivo. Ammanathan et al. (2019) found that Acacetin (5,7-dihydroxy-4-methoxyflavone), a potent xenophagy inducer, could enhance the host response against intracellular Salmonella. Moreover, biochanin A (BCA), a polyphenolic compound found in certain species of plants, has been proven to target Salmonella infection through AMPK/ULK1/mTOR-mediated autophagy, extracellular traps, and the reversal of SPI-1-dependent macrophage M2 polarization (Zhao et al., 2018). In addition, Huang (2016) found that 1,25-dihydroxyvitamin D3 may promote the autophagic removal of intracellular Salmonella and modulate inflammatory responses to prevent the host from the adverse effects of excessive inflammation. Kim et al. (2018) evaluated the antibacterial effect of bioprocessed (fermented) rice bran extract (BPRBE) against Salmonella Typhimurium infection, and investigated the mechanisms involved. The results showed that, BPRBE enhanced systemic and cell-autonomous antibacterial activities through the autophagic capture of Salmonella, resulting in increased fecal bacteria excretion and decreased bacterial colonization of internal organs. These findings confirm that BPRBE has in vivo antimicrobial activities and could be used as a functional antibiotic in the food production industry and medicine. Triclosan (TCS) is a broad-spectrum antibacterial agent widely used in personal care and household cleaning. Shi et al. (2016) investigated the antibacterial properties of TCS and demonstrated that it could indeed induce autophagy via an ERK-dependent pathway, thus enhancing the abilities of macrophages to kill intracellular bacteria. Chiu et al. (2009) synthesized and characterized a new small-molecule agent celled AR-12 and tested its effect on the survival of Salmonella Typhimurium in macrophages. The results showed that AR-12 induced autophagy in macrophages and potently inhibited the survival of intracellular Salmonella Typhimurium. The ectopic expression of constitutively-activated Akt1 in macrophages partially reversed the AR-12-mediated inhibition of bacterial survival. Furthermore, oral administration of AR-12 reduced hepatic and splenic bacterial burdens, and significantly prolonged the survival of mice infected with Salmonella Typhimurium. These findings show that AR-12 can inhibit invasive intracellular bacteria by regulating the level of autophagy, thus promoting the survival of host cells. AR-12 microcapsules, prepared using a novel degradable acetalated dextran (Ac-Dex) biopolymer, further enhanced the clearance of intracellular Salmonella and significantly reduced drug toxicity (Hoang et al., 2014). In order to identify small molecules capable of interfering with Salmonella survival or replication in macrophages, Nagy et al. (2019) screened the 14,400-compound Maybridge HitFinder v11 library in a high-content Screen for Anti-Infectives. They found that one small molecule, D61, reduced Salmonella load both in vitro and in vivo, through its autophagy-stimulating activity. Qiu et al. (2019) constructed and characterized a polymeric micelle, HAASD-Rapa micelle, which contains a hyaluronan-streptomycin conjugate with an autophagy activator, rapamycin. They found that the HAASD-Rapa micelle could facilitate the host’s intracellular Salmonella killing capacity by promoting streptomycin uptake and rapamycin-mediated autophagy activation. Their study also indicated that the combined antibiotic and rapamycin treatment may represent a promising strategy for the eradication of intracellular bacteria. In recent years, cumulative studies have attempted to develop new nanomaterials for the eradication of bacteria. As the single largest reservoir host for Salmonella, chickens were used as experimental models to investigate the antibacterial efficacy of iron oxide nanozyme (IONzyme) against Salmonella infection in our previous study (Shi et al., 2018). We found that IONzyme enhanced the generation of reactive oxygen species (ROS) to promote the bactericidal effects of acid autophagic vacuoles, thereby inhibiting the survival of invading intracellular Salmonella Enteritidis. Collectively, the above findings demonstrate that the search for xenophagy-regulating agents may represent a powerful strategy for combating Salmonella infection and maintaining intestinal homeostasis.
TABLE 2.
Drugs regulate xenophagy to combat Salmonella infection.
| Drugs | Model systems and results | Refs |
| Resveratrol | HCT116, HeLa, MEFs, RAW264.7 and THP-1 cells; Zebrafish. Stimulating autophagy and inducing intracellular Salmonella clearance. | 96 |
| Acacetin | RAW 264.7 and HeLa cells; mice. Restricting intracellular Salmonella replication by restoring TFEB-mediated xenophagy. | 97 |
| Biochanin A | HeLa, Raw264.7 and THP-1 cells; mice. Enhancing the defense against Salmonella infection through AMPK/ULK1/mTOR-mediated autophagy and extracellular traps and reversing SPI-1-dependent macrophage M2 Polarization. | 98 |
| 1,25-dihydroxy vitamin D3 | Caco-2 cells. Promoting the autophagic removal of intracellular Salmonella and modulating inflammatory responses to prevent the host from the adverse effects of excessive inflammation. | 99 |
| bioprocessed (fermented) rice bran extract | RAW264.7 cells; mice. Enhancing systemic and cell-autonomous antibacterial activities through the autophagic capture of Salmonella, resulting in increased fecal bacteria excretion and decreased bacterial colonization of internal organs. | 100 |
| Triclosan | HeLa and Raw264.7 cells. Inducing autophagy via an ERK-dependent pathway, thus enhancing the abilities of macrophages to kill intracellular Salmonella. | 101 |
| AR-12 | RAW264.7, J774.1 and THP-1 cells; mice. Inhibiting invasive intracellular Salmonella by regulating the level of autophagy, thus promoting the survival of host cells. | 102 |
| AR-12 microcapsules | RAW264.7 and primary human monocyte-derived primary macrophages. Enhancing the clearance of intracellular Salmonella and significantly reduced drug toxicity. | 103 |
| D61 | RAW 264.7, mice and human bone marrow-derived primary macrophages, and HeLa cells; mice. Reducing Salmonella load both in vitro and in vivo through its autophagy-stimulating activity | 104 |
| HAASD-Rapa micelles | RAW 264.7 and HK-2 cells. Facilitating the host’s intracellular Salmonella killing capacity by promoting streptomycin uptake and rapamycin-mediated autophagy activation | 105 |
| IONzyme | LMH cells; SPF chicken. Enhancing the generation of reactive oxygen species (ROS) to promote the bactericidal effects of acid autophagic vacuoles, thereby inhibiting the survival of invading intracellular Salmonella. | 106 |
Summary and Future Prospects
In summary, autophagy acts as double-edged sword in the context of intracellular Salmonella infection. On the one hand, autophagy is beneficial to the host through the elimination of intracellular Salmonella. While, on the other hand, it facilitates intracellular bacterial replication. The prevention and control of Salmonella infection has always been considered a major research focus for the scientific community. Using drugs to regulate Salmonella autophagy may therefore represent an effective alternative solution to the eradication of intracellular infection caused by this persistent foodborne pathogen.
Although our understanding of Salmonella autophagy is ever increasing, many questions remain to be answered. For example, what causes the two contradictory effects of autophagy on intracellular Salmonella? Are other, as-yet-unidentified virulent factors or intracellular signals involved in the autophagy of Salmonella? How do the different autophagic adaptors interact in the targeting of Salmonella for autophagy? Do different serotypes of Salmonella affect the autophagic process and the outcome of infection? What are the Salmonella-specific autophagy processes and signaling pathways participating in the immune response during infection?
In this era of increasing antibiotic resistance, further study of the mechanisms implicated in bacterial autophagy has important guiding significance in the discovery of novel strategies for the prevention and control of Salmonella infection.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by the National Key R&D Program of Intergovernmental Key Projects of China (Grant No: 2018YFE0101700), the National Natural Science Foundation of China (31972587 and 31702132), the Jiangsu Agricultural Industry Technology System (JATS[2019]380), and the Innovation Capacity Building Program of Jiangsu Province (BM2018026).
References
- Akaike T., Ida T., Wei F. Y., Nishida M., Kumagai Y., Alam M. M., et al. (2017). Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat. Commun. 8:1177. 10.1038/s41467-017-01311-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Azzaz J., Rieu A., Aires V., Delmas D., Chluba J., Winckler P., et al. (2019). Resveratrol-induced xenophagy promotes intracellular bacteria clearance in intestinal epithelial cells and macrophages. Front. Immunol. 9:3149. 10.3389/fimmu.2018.03149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammanathan V., Mishra P., Chavalmane A. K., Muthusamy S., Jadhav V., Siddamadappa C., et al. (2019). Restriction of intracellular Salmonella replication by restoring TFEB-mediated xenophagy. Autophagy. 10.1080/15548627.2019.1689770 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi C. W., Dong X. Y., Zhong X. B., Cai H. J., Wang D. C., Wang L. (2016). Acacetin protects mice from Staphylococcus aureus bloodstream infection by inhibiting the activity of Sortase A. Molecules 21:1285. 10.3390/molecules21101285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham C. L., Brumell J. H. (2006). Autophagy recognizes intracellular Salmonella enterica serovar Typhimurium in damaged vacuoles. Autophagy 2 156–158. 10.4161/auto.2825 [DOI] [PubMed] [Google Scholar]
- Birmingham C. L., Smith A. C., Bakowski M. A., Yoshimori T., Brumell J. H. (2006). Autophagy controls Salmonella infection in response to damage to the Salmonella-containing vacuole. J. Biol. Chem. 281 11374–11383. 10.1074/jbc.M509157200 [DOI] [PubMed] [Google Scholar]
- Boyle K. B., Ravenhill B. J., Randow F. (2019). CALCOCO2/NDP52 initiates selective autophagy through recruitment of ULK and TBK1 kinase complexes. Autophagy 15 1655–1656. 10.1080/15548627.2019.1628548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. E. (2017). Bacterial autophagy: offense and defense at the host-pathogen interface. Cell. Mol. Gastroenterol. Hepatol. 4 237–243. 10.1016/j.jcmgh.2017.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cemma M., Brumell J. H. (2012). Interactions of pathogenic bacteria with autophagy systems. Curr. Biol. 22 R540–R545. 10.1016/j.cub.2012.06.001 [DOI] [PubMed] [Google Scholar]
- Cemma M., Kim P. K., Brumell J. H. (2011). The ubiquitin-binding adaptor proteins p62/SQSTM1 and NDP52 are recruited independently to bacteria-associated microdomains to target Salmonella to the autophagy pathway. Autophagy 7 341–345. 10.4161/auto.7.3.14046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary A., Kamischke C., Leite M., Altura M. A., Kinman L., Kulasekara H., et al. (2018). β-Barrel outer membrane proteins suppress mTORC2 activation and induce autophagic responses. Sci. Signal. 11:eaat7493. 10.1126/scisignal.aat7493 [DOI] [PubMed] [Google Scholar]
- Chiu H. C., Kulp S. K., Soni S., Wang D., Gunn J. S., Schlesinger L. S., et al. (2009). Eradication of intracellular Salmonella enterica serovar Typhimurium with a small-molecule, host cell-directed agent. Antimicrob. Agents Chemother. 53 5236–5244. 10.1128/AAC.00555-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. Y., Gao S., Wang T., Yan J., Xu G. M., Li Y. Y., et al. (2016). A novel contribution of spvB to pathogenesis of Salmonella Typhimurium by inhibiting autophagy in host cells. Oncotarget 7 8295–8309. 10.18632/oncotarget.6989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y. Y., Yang Y. R., Li Y. Y., Ye Y., Yan J., Wang T., et al. (2014). A Salmonella enterica conjugative plasmid impairs autophagic flux in infected macrophages. Microbes Infect. 16 553–561. 10.1016/j.micinf.2014.05.001 [DOI] [PubMed] [Google Scholar]
- Conway K. L., Kuballa P., Song J. H., Patel K. K., Castoreno A. B., Yilmaz O. H., et al. (2013). Atg16L1 is required for autophagy in intestinal epithelial cells and protection of mice from Salmonella infection. Gastroenterology 145 1347–1357. 10.1053/j.gastro.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L. F., Zhao H., Yin Y. J., Liang C., Mao X. L., Liu Y. Z., et al. (2019). Function of Atg11 in non-selective autophagy and selective autophagy of Candida albicans. Biochem. Biophys. Res. Commun. 516 1152–1158. 10.1016/j.bbrc.2019.06.148 [DOI] [PubMed] [Google Scholar]
- Curt A., Zhang J. L., Minnerly J., Jia K. L. (2014). Intestinal autophagy activity is essential for host defense against Salmonella Typhimurium infection in Caenorhabditis elegans. Dev. Comp. Immunol. 45 214–218. 10.1016/j.dci.2014.03.009 [DOI] [PubMed] [Google Scholar]
- Deiwick J., Salcedo S. P., Boucrot E., Gilliland S. M., Henry T., Petermann N., et al. (2006). The translocated Salmonella effector proteins SseF and SseG interact and are required to establish an intracellular replication niche. Infect. Immun. 74 6965–6972. 10.1128/IAI.00648-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Z. Z., Jiang A. J., Mao A. W., Feng Y. H., Wang W. N., Li J. J., et al. (2018). The Salmonella effectors SseF and SseG inhibit Rab1A-mediated autophagy to facilitate intracellular bacterial survival and replication. J. Biol. Chem. 293 9662–9673. 10.1074/jbc.M117.811737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueira R., Holden D. W. (2003). Functions of the Salmonella pathogenicity island 2 (SPI-2) type III secretion system effectors. Microbiology 5 501–511. 10.1099/mic.0.058115-0 [DOI] [PubMed] [Google Scholar]
- Figueira R., Watson K. G., Holden D. W., Helaine S. (2013). Identification of Salmonella pathogenicity island-2 type III secretion system effectors involved in intramacrophage replication of S. enterica serovar Typhimurium: implications for rational vaccine design. mBio 4:e00065-13. 10.1128/mBio.00065-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaio V., Lima C. A., Oliveira F., França Â., Cerca N. (2017). Carvacrol is highly disruptive against coagulase-negative staphylococci in in vitro biofilms. Future Microbiol. 12 1487–1496. 10.2217/fmb-2017-0122 [DOI] [PubMed] [Google Scholar]
- Ganesan R., Hos N. J., Gutierrez S., Fischer J., Stepek J. M., Daglidu E., et al. (2017). Salmonella Typhimurium disrupts Sirt1/AMPK checkpoint control of mTOR to impair autophagy. PLoS Pathog. 13:e1006227. 10.1371/journal.ppat.1006227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. J., Morgan F. J., McKinley T. J., Foster G. L., Maskell D. J., Mastroeni P. (2012). Attenuated Salmonella Typhimurium lacking the pathogenicity island-2 type 3 secretion system grow to high bacterial numbers inside phagocytes in mice. PLoS Pathog. 8:e1003070. 10.1371/journal.ppat.1003070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Fierer J. (2011). The role of the spv genes in Salmonella pathogenesis. Front. Microbiol. 2:129. 10.3389/fmicb.2011.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen-Wester I., Hensel M. (2001). Salmonella pathogenicity islands encoding type III secretion systems. Microbes Infect. 3 549–559. 10.1111/cmi.12939 [DOI] [PubMed] [Google Scholar]
- Hassane D. C., Lee R. B., Pickett C. L. (2003). Campylobacter Jejuni cytolethal distending toxin promotes DNA repair responses in normal human cells. Infect. Immun. 71 541–545. 10.1128/IAI.71.1.541-545.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hautefort I., Thompson A., Eriksson-Ygberg S., Parker M. L., Lucchini S., Danino V., et al. (2008). During infection of epithelial cells Salmonella enterica serovar Typhimurium undergoes a time-dependent transcriptional adaptation that results in simultaneous expression of three type 3 secretion systems. Cell. Microbiol. 10 958–984. 10.1111/j.1462-5822.2007.01099.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- He P. Y., Wu S. Y., Chu Y. Y., Yang Y. R., Li Y. Y., Huang R. (2012). Salmonella enterica serovar Typhi plasmid pR ST98 enhances intracellular bacterial growth and S. Typhi-induced macrophage cell death by suppressing autophagy. Braz. J. Infect. Dis. 16 262–266. 10.1590/S1413-86702012000300008 [DOI] [PubMed] [Google Scholar]
- Heath R. J., Goel G., Baxt L. A., Rush J. S., Mohanan V., Paulus G. L. C., et al. (2016). RNF166 determines recruitment of adaptor proteins during antibacterial autophagy. Cell Rep. 17 2183–2194. 10.1016/j.celrep.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo J. M., Ordureau A., Paulo J. A., Rinehart J., Harper J. W. (2015). The Pink1-Parkin mitochondrial ubiquitylation pathway drives a program of OPTN/NDP52 recruitment and TBK1 activation to promote mitophagy. Mol. Cell 60 7–20. 10.1016/j.molcel.2015.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez L. D., Pypaert M., Flavell R. A., Galán J. E. (2003). A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163 1123–1131. 10.1083/jcb.200309161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang K. V., Borteh H. M., Rajaram M. V., Peine K. J., Curry H., Collier M. A., et al. (2014). Acetalated dextran encapsulated AR-12 as a host-directed therapy to control Salmonella infection. Int. J. Pharm. 477 334–343. 10.1016/j.ijpharm.2014.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F. C. (2016). Vitamin D differentially regulates Salmonella-induced intestine epithelial autophagy and interleukin-1β expression. World J. Gastroenterol. 22 10353–10363. 10.3748/wjg.v22.i47.10353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Birmingham C. L., Shahnazari S., Shiu J., Zheng Y. Y. T., Smith A. C., et al. (2011). Antibacterial autophagy occurs at PI(3)P-enriched domains of the endoplasmic reticulum and requires Rab1 GTPase. Autophagy 7 17–26. 10.4161/auto.7.1.13840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Brumell J. H. (2014). Bacteria-autophagy interplay: a battle for survival. Nat. Rev. Microbiol. 12 101–114. 10.1038/nrmicro3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Wu S. Y., Zhang X. G., Zhang Y. Y. (2005). Molecular analysis and identification of virulence gene on pR(ST98) from multi-drug resistant Salmonella Typhi. Cell. Mol. Immunol. 2 136–140. [PubMed] [Google Scholar]
- Hwang D., Lim Y. H. (2015). Resveratrol antibacterial activity against Escherichia coli is mediated by Z-ring formation inhibition via suppression of FtsZ expression. Sci. Rep. 5:10029. 10.1038/srep10029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ida T., Sawa T., Ihara H., Tsuchiya Y., Watanabe Y., Kumagai Y., et al. (2014). Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc. Natl. Acad. Sci. U.S.A. 111 7606–7611. 10.1073/pnas.1321232111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimura R., Tanaka K., Komatsu M. (2014). Dissection of the role of p62/Sqstm1 in activation of Nrf2 during xenophagy. FEBS Lett. 588 822–828. 10.1016/j.febslet.2014.01.045 [DOI] [PubMed] [Google Scholar]
- Ivanov S., Roy C. R. (2009). NDP52: the missing link between ubiquitinated bacteria and autophagy. Nat. Immunol. 10 1137–1139. 10.1038/ni1109-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K. L., Thomas C., Akbar M., Sun Q., Adams-Huet B., Gilpin C., et al. (2009). Autophagy genes protect against Salmonella Typhimurium infection and mediate insulin signaling-regulated pathogen resistance. Proc. Natl. Acad. Sci. U.S.A. 106 14564–14569. 10.1073/pnas.0813319106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. P., Lee S. J., Nam S. H., Friedman M. (2018). The composition of a bioprocessed shiitake (Lentinus edodes) mushroom mycelia and rice bran formulation and its antimicrobial effects against Salmonella enterica subsp. enterica serovar Typhimurium strain SL1344 in macrophage cells and in mice. BMC Complement. Altern. Med. 18:322. 10.1186/s12906-018-2365-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler L. J., Roy C. R. (2017). Autophagic targeting and avoidance in intracellular bacterial infections. Curr. Opin. Microbiol. 35 36–41. 10.1016/j.mib.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich S., Emmenlauer M., Fredlund J., Münz C., Dehio C., Enninga J., et al. (2015). Autophagy proteins promote repair of endosomal membranes damaged by the Salmonella type three secretion system 1. Cell Host Microbe 18 527–537. 10.1016/j.chom.2015.10.015 [DOI] [PubMed] [Google Scholar]
- Kuhle V., Hensel M. (2002). SseF and SseG are translocated effectors of the type III secretion system of Salmonella pathogenicity island 2 that modulate aggregation of endosomal compartments. Cell. Microbiol. 4 813–824. 10.1046/j.1462-5822.2002.00234.x [DOI] [PubMed] [Google Scholar]
- Kuźmińska-Bajor M., Kuczkowski M., Grzymajło K., Wojciech Ł, Sabat M., Kisiela D., et al. (2012). Decreased colonization of chicks by Salmonella enterica serovar Gallinarum expressing mannose-sensitive FimH adhesin from Salmonella enterica serovar Enteritidis. Vet. Microbiol. 158 205–210. 10.1016/j.vetmic.2012.01.029 [DOI] [PubMed] [Google Scholar]
- Kwon D. H., Song H. K. (2018). A structural view of xenophagy, a battle between host and microbes. Mol. Cells 41 27–34. 10.14348/molcells.2018.2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara-Tejero M., Galan J. E. (2001). CdtA, cdtB, and cdtC form a tripartite complex that is required for cytolethal distending toxin activity. Infect. Immun. 69 4358–4365. 10.1128/IAI.69.7.4358-4365.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Negrate G., Faustin B., Welsh K., Loeffler M., Krajewska M., Hasegawa P., et al. (2008). Salmonella secreted factor L deubiquitinase of Salmonella Typhimurium inhibits NF-κB, suppresses I κBα ubiquitination and modulates innate immune responses. J. Immunol. 180 5045–5056. 10.4049/jimmunol.180.7.5045 [DOI] [PubMed] [Google Scholar]
- Li S., Wandel M. P., Li F. D., Liu Z. H., He C., Wu J. H., et al. (2013). Sterical hindrance promotes selectivity of the autophagy cargo receptor NDP52 for the danger receptor galectin-8 in antibacterial autophagy. Sci. Signal. 6:ra9. 10.1126/scisignal.2003730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Wang T., Gao S., Xu G. M., Niu H., Huang R., et al. (2016). Salmonella plasmid virulence gene spvB enhances bacterial virulence by inhibiting autophagy in a zebrafish infection model. Fish Shellfish Immunol. 49 252–259. 10.1016/j.fsi.2015.12.033 [DOI] [PubMed] [Google Scholar]
- Lin C. Y., Nozawa T., Minowa-Nozawa A., Toh H., Aikawa C., Nakagawa I., et al. (2019). LAMTOR2/LAMTOR1 complex is required for TAX1BP1-mediated xenophagy. Cell. Microbiol. 21:e12981. 10.1111/cmi.12981 [DOI] [PubMed] [Google Scholar]
- Liu W., Zhuang J., Jiang Y. Y., Sun J., Prinz R. A., Sun J., et al. (2019). Toll-like receptor signalling cross-activates the autophagic pathway to restrict Salmonella Typhimurium growth in macrophages. Cell. Microbiol. 21:e13095. 10.1111/cmi.13095 [DOI] [PubMed] [Google Scholar]
- Lunelli M., Hurwitz R., Lambers J., Kolbe M. (2011). Crystal structure of PrgI-SipD: insight into a secretion competent state of the type three secretion system needle tip and its interaction with host ligands. PLoS Pathog. 7:e1002163. 10.1371/journal.ppat.1002163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J., Wu S., Wei L., He P., Huang R. (2012). Salmonella enterica serovar Typhi plasmid pR ST98-mediated inhibition of autophagy promotes bacterial survival in infected fibroblasts. Indian J. Med. Microbiol. 30 423–430. 10.4103/0255-0857.103763 [DOI] [PubMed] [Google Scholar]
- Malik-Kale P., Winfree S., Steele-Mortimer O. (2012). The bimodal lifestyle of intracellular Salmonella in epithelial cells: replication in the cytosol obscures defects in vacuolar replication. PLoS One 7:e38732. 10.1371/journal.pone.0038732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese A., Orhan I. E., Daglia M., Barbieri R., Di L. A., Nabavi S. F., et al. (2016). Antibacterial and antifungal activities of thymol: a brief review of the literature. Food Chem. 210 402–414. 10.1016/j.foodchem.2016.04.111 [DOI] [PubMed] [Google Scholar]
- Mesquita F. S., Thomas M., Sachse M., Santos A. J. M., Figueira R., Holden D. W. (2012). The Salmonella deubiquitinase SseL inhibits selective autophagy of cytosolic aggregates. PLoS Pathog. 8:e1002743. 10.1371/journal.ppat.1002743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowa-Nozawa A., Nozawa T., Okamoto-Furuta K., Kohda H., Nakagawa I. (2017). Rab35 GTPase recruits NDP52 to autophagy targets. EMBO J. 36:3405 10.15252/embj.201798293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N., Yoshimori T., Levine B. (2010). Methods in mammalian autophagy research. Cell 140 313–326. 10.1016/j.cell.2010.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostowy S., Sancho-Shimizu V., Hamon M. A., Simeone R., Brosch R., Johansen T., et al. (2011). P62 and NDP52 proteins target intracytosolic Shigella and Listeria to different autophagy pathways. J. Biol. Chem. 286 26987–26995. 10.1074/jbc.M111.223610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy T. A., Quintana J. L. J., Reens A. L., Crooks A. L., Detweiler C. S. (2019). Autophagy induction by a small molecule inhibits Salmonella survival in macrophages and mice. Antimicrob. Agents Chemother. 63:e01536-19. 10.1128/AAC.01536-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasone N., Higa N., Toma C., Ogura Y., Suzuki T., Yamashiro T., et al. (2017). Epigallocatechin gallate inhibits the type III secretion system of Gram-negative enteropathogenic bacteria under model conditions. FEMS Microbiol. Lett. 364:fnx111. 10.1093/femsle/fnx111 [DOI] [PubMed] [Google Scholar]
- Owen K. A., Meyer C. B., Bouton A. H., Casanova J. E. (2014). Activation of focal adhesion kinase by Salmonella suppresses autophagy via an Akt/mTOR signaling pathway and promotes bacterial survival in macrophages. PLoS Pathog. 10:e1004159. 10.1371/journal.ppat.1004159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen K. A., Pixley F. J., Thomas K. S., Vicente-Manzanares M., Ray B. J., Horwitz A. F., et al. (2007). Regulation of lamellipodial persistence, adhesion turnover, and motility in macrophages by focal adhesion kinase. J. Cell Biol. 179 1275–1287. 10.1083/jcb.200708093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin A. J., Jiang X. J., Birmingham C. L., So N. S. Y., Brumell J. H. (2004). Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 14 806–811. 10.1016/j.cub.2004.04.033 [DOI] [PubMed] [Google Scholar]
- Qiu Y. H., Lu C. B., Chen P., Sun F. F., Wang D. D., Wang Z. J., et al. (2019). Synergistic clearance of intracellular pathogens by hyaluronan-streptomycin micelles encapsulated with rapamycin. Carbohydr. Polym. 210 364–371. 10.1016/j.carbpol.2019.01.068 [DOI] [PubMed] [Google Scholar]
- Rathinavelan T., Lara-Tejero M., Lefebre M., Chatterjee S., McShan A. C., Guo D. C., et al. (2014). NMR model of PrgI-SipD interaction and its implications in the needle-tip assembly of the Salmonella type III secretion system. J. Mol. Biol. 426 2958–2969. 10.1016/j.jmb.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravenhill B. J., Boyle K. B., von Muhlinen N., Ellison C. J., Masson G. R., Otten E. G., et al. (2019). The Cargo receptor NDP52 initiates selective autophagy by recruiting the ULK complex to cytosol-invading bacteria. Mol. Cell 74 320–329. 10.1016/j.molcel.2019.01.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter B., Sliter D. A., Herhaus L., Stolz A., Wang C. X., Beli P., et al. (2016). Phosphorylation of OPTN by TBK1 enhances its binding to Ub chains and promotes selective autophagy of damaged mitochondria. Proc. Natl. Acad. Sci. U.S.A. 113 4039–4044. 10.1073/pnas.1523926113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogov V. V., Suzuki H., Fiskin E., Wild P., Kniss A., Rozenknop A., et al. (2013). Structural basis for phosphorylation-triggered autophagic clearance of Salmonella. Biochem. J. 454 459–466. 10.1042/BJ20121907 [DOI] [PubMed] [Google Scholar]
- Shahzada K., Shigemoto F., Tetsuro M., Akira N., Katsuhiko O., Tomoaki I., et al. (2018). Reactive persulfides from Salmonella Typhimurium downregulate autophagy-mediated innate immunity in macrophages by inhibiting electrophilic signaling. Cell Chem. Biol. 25 1403–1413.e4. 10.1016/j.chembiol.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Shi S. R., Wu S., Shen Y. R., Zhang S., Xiao Y. Q., He X., et al. (2018). Iron oxide nanozyme suppresses intracellular Salmonella enteritidis growth and alleviates infection in vivo. Theranostics 8 6149–6162. 10.7150/thno.29303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X. C., Sun Q. S., An Y. N., Zhang Q. L., Guo N., Yu L. (2016). Enhancement of intracellular bacteria killing ability in macrophages by triclosan-induced autophagy via a mTOR-independent pathway. J. Jilin Agric. Univ. 38. [Google Scholar]
- Slowicka K., van Loo G. (2018). Optineurin functions for optimal immunity. Front. Immunol. 9:769. 10.3389/fimmu.2018.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowicka K., Vereecke L., Mc Guire C., Sze M., Maelfait J., Kolpe A., et al. (2016). Optineurin deficiency in mice is associated with increased sensitivity to Salmonella but does not affect proinflammatory NF-κB signaling. Eur. J. Immunol. 46 971–980. 10.1002/eji.201545863 [DOI] [PubMed] [Google Scholar]
- Sorbara M. T., Girardin S. E. (2015). Emerging themes in bacterial autophagy. Curr. Opin. Microbiol. 23 163–170. 10.1016/j.mib.2014.11.020 [DOI] [PubMed] [Google Scholar]
- Steele-Mortimer O. (2008). The Salmonella-containing vacuole: moving with the times. Curr. Opin. Microbiol. 11 38–45. 10.1016/j.mib.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattoli I., Sorbara M. T., Philpott D. J., Girardin S. E. (2012a). Bacterial autophagy: the trigger, the target and the timing. Autophagy 8 1848–1850. 10.4161/auto.21863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattoli I., Sorbara M. T., Vuckovic D., Ling A., Soares F., Carneiro L. A. M., et al. (2012b). Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe 11 563–575. 10.1016/j.chom.2012.04.012 [DOI] [PubMed] [Google Scholar]
- Thurston T. L., Ryzhakov G., Bloor S., von Muhlinen N., Randow F. (2009). The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin-coated bacteria. Nat. Immunol. 10 1215–1221. 10.1038/ni.1800 [DOI] [PubMed] [Google Scholar]
- Thurston T. L., Wandel M. P., von Muhlinen N., Foeglein Á., Randow F. (2012). Galectin 8 targets damaged vesicles for autophagy to defend cells against bacterial invasion. Nature 482 414–418. 10.1038/nature10744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres A., Luke J. D., Kullas A. L., Kapilashrami K., Botbol Y., Koller A., et al. (2016). Asparagine deprivation mediated by Salmonella asparaginase causes suppression of activation-induced T cell metabolic reprogramming. J. Leukoc. Biol. 99 387–398. 10.1189/jlb.4A0615-252R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbarello D. A., Manna P. T., Allen M., Bycroft M., Arden S. D., Kendrick-Jones J., et al. (2015). The autophagy receptor TAX1BP1 and the molecular Motor Myosin VI are required for clearance of Salmonella Typhimurium by autophagy. PLoS Pathog. 11:e1005174. 10.1371/journal.ppat.1005174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac P., Grégoire I. P., Azocar O., Petkova D. S., Baguet J., Viret C., et al. (2015a). Autophagy receptor NDP52 regulates pathogen-containing autophagosome maturation. Cell Host Microbe 17 515–525. 10.1016/j.chom.2015.02.008 [DOI] [PubMed] [Google Scholar]
- Verlhac P., Viret C., Faure M. (2015b). Dual function of CALCOCO2/NDP52 during xenophagy. Autophagy 11 965–966. 10.1080/15548627.2015.1046672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlhac P., Viret C., Faure M. (2015c). Handcuffs for bacteria-NDP52 orchestrates xenophagy of intracellular Salmonella. Microb. Cell 2 214–215. 10.15698/mic2015.06.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Muhlinen N., Akutsu M., Ravenhill B. J., Foeglein Á., Bloor S., Rutherford T. J., et al. (2012). LC3C, bound selectively by a noncanonical LIR motif in NDP52, is required for antibacterial autophagy. Mol. Cell 48 329–342. 10.1016/j.molcel.2012.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Muhlinen N., Akutsu M., Ravenhill B. J., Foeglein Á., Bloor S., Rutherford T. J., et al. (2013). An essential role for the ATG8 ortholog LC3C in antibacterial autophagy. Autophagy 9 784–786. 10.4161/auto.23698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Muhlinen N., Thurston T., Ryzhakov G., Bloor S., Randow F. (2010). NDP52, a novel autophagy receptor for ubiquitin-decorated cytosolic bacteria. Autophagy 6 288–289. 10.4161/auto.6.2.11118 [DOI] [PubMed] [Google Scholar]
- Walthers D., Carroll R. K., Navarre W. W., Libby S. J., Fang F. C., Kenney L. J. (2007). The response regulator SsrB activates expression of diverse Salmonella pathogenicity island 2 promoters and counters silencing by the nucleoid-associated protein H-NS. Mol. Microbiol. 65 477–493. 10.1111/j.1365-2958.2007.05800.x [DOI] [PubMed] [Google Scholar]
- Wang L. D., Li Y. L., Liu Y. H., Zuo L. L., Li Y. Y., Wu S. Y., et al. (2019). Salmonella spv locus affects type I interferon response and the chemotaxis of neutrophils via suppressing autophagy. Fish Shellfish Immunol. 87 721–729. 10.1016/j.fsi.2019.02.009 [DOI] [PubMed] [Google Scholar]
- Wang S. G., Yao J. Y., Zhou B., Yang J. X., Chaudry M. T., Wang M., et al. (2018). Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J. Food Prot. 81 68–78. 10.4315/0362-028X.JFP-17-214 [DOI] [PubMed] [Google Scholar]
- Weidberg H., Elazar Z. (2011). TBK1 mediates crosstalk between the innate immune response and autophagy. Sci. Signal. 4:e39 10.1126/scisignal.2002355 [DOI] [PubMed] [Google Scholar]
- Wild P., Farhan H., McEwan D. G., Wagner S., Rogov V. V., Brady N. R., et al. (2011). Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science 333 228–233. 10.1126/science.1205405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K., Gokulan K., Shelman D., Akiyama D., Khan A., Khare S. (2015). Cytotoxic mechanism of cytolethal distending toxin in nontyphoidal Salmonella serovar (Salmonella Javiana) during macrophage infection. DNA Cell Biol. 34 113–124. 10.1089/dna.2014.2602 [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Chu Y. Y., Yang Y. R., Li Y. Y., He P. Y., Zheng Y. J., et al. (2014). Inhibition of macrophage autophagy induced by Salmonella enterica serovar Typhi plasmid. Front. Biosci. 19 490–503. 10.2741/4220 [DOI] [PubMed] [Google Scholar]
- Wu S. Y., Wang L. D., Li J. L., Xu G. M., He M. L., Li Y. Y., et al. (2016). Salmonella spv locus suppresses host innate immune responses to bacterial infection. Fish Shellfish Immunol. 58 387–396. 10.1016/j.fsi.2016.09.042 [DOI] [PubMed] [Google Scholar]
- Xia Y., Liu N., Xie X. X., Bi G. Y., Ba H. P., Li L., et al. (2019). The macrophage-specific V-ATPase subunit ATP6V0D2 restricts inflammasome activation and bacterial infection by facilitating autophagosome-lysosome fusion. Autophagy 15 960–975. 10.1080/15548627.2019.1569916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X. Q., Li F. X., Wang Y. Y., Wang Y. L., Lin Z. J., Cheng X. F., et al. (2015). Molecular basis of ubiquitin recognition by the autophagy receptor CALCOCO2. Autophagy 11 1775–1789. 10.1080/15548627.2015.1082025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z. P., Klionsky D. J. (2007). Autophagosome formation: core machinery and adaptation. Nat. Cell Biol. 9 1102–1109. 10.1038/ncb1007-1102 [DOI] [PubMed] [Google Scholar]
- Yang Z. F., Klionsky D. J. (2010). Eaten alive: a history of macroautophagy. Nat. Cell Biol. 12 814–822. 10.1038/ncb0910-814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H. B., Croxen M. A., Marchiando A. M., Ferreira R. B. R., Cadwell K., Foster L. J., et al. (2014). Autophagy facilitates Salmonella replication in HeLa cells. mBio 5:e00865-14. 10.1128/mBio.00865-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. C., Tang X. D., Guo N., An Y. N., Chen X. R., Shi C., et al. (2018). Biochanin a enhances the defense against Salmonella enterica infection through AMPK/ULK1/mTOR-mediated autophagy and extracellular traps and reversing SPI-1-dependent macrophage (MΦ) M2 polarization. Front. Cell. Infect. Microbiol. 8:318. 10.3389/fcimb.2018.00318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y. Y. T., Shahnazari S., Brech A., Lamark T., Johansen T., Brumell J. H., et al. (2009). The adaptor protein p62/SQSTM1 targets invading bacteria to the autophagy pathway. J. Immunol. 183 5909–5916. 10.4049/jimmunol.0900441 [DOI] [PubMed] [Google Scholar]