Visual Abstract
Key Words: adrenoreceptors, imaging, pulmonary hypertension, treatment
Abbreviations and Acronyms: β3AR, beta-3 adrenoreceptor; CCT, cardiac computed tomography; cGMP, cyclic guanosine monophosphate; CMR, cardiac magnetic resonance; CpcPH, combined pre- and post-capillary pulmonary hypertension; ECG, electrocardiography; HF, heart failure; IpcPH, isolated post-capillary pulmonary hypertension; ITT, intention to treat; LHD, left heart disease; LV, left ventricular; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PH, pulmonary hypertension; PP, Per protocol; PVR, pulmonary vascular resistance; RV, right ventricle
Highlights
-
•
CpcPH is a relatively common complication of chronic HF, is associated with poor survival, and has no specific pharmacological treatment.
-
•
ß3AR stimulation has shown improvement in pulmonary hemodynamics and RV performance in a translational large animal model mimicking this condition.
-
•
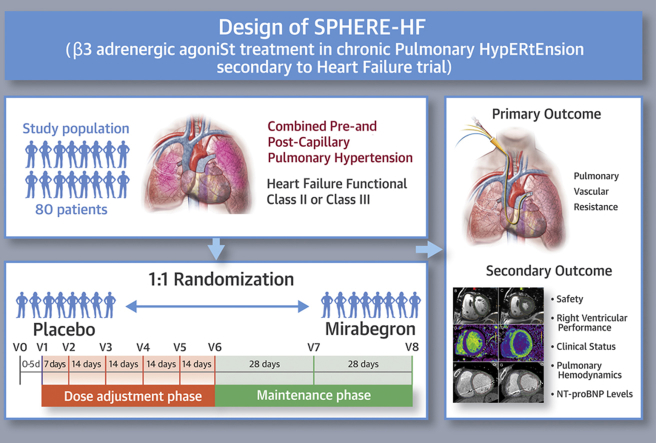
The SPHERE-HF trial is a Phase II randomized, double-blind clinical trial designed to evaluate the efficacy and safety of mirabegron (oral β3 AR agonist) in patients with CpcPH secondary to HF.
-
•
The SPHERE-HF trial will include 80 patients treated with mirabegron or placebo for 16 weeks.
-
•
The main outcome is the change in PVR. Secondary outcomes include changes in RV performance, clinical status, NT-proBNP levels, and additional pulmonary hemodynamic parameters.
Summary
Combined pre-and post-capillary hypertension (CpcPH) is a relatively common complication of heart failure (HF) associated with a poor prognosis. Currently, there is no specific therapy approved for this entity. Recently, treatment with beta-3 adrenergic receptor (β3AR) agonists was able to improve pulmonary hemodynamics and right ventricular (RV) performance in a translational, large animal model of chronic PH. The authors present the design of a phase II randomized clinical trial that tests the benefits of mirabegron (a clinically available β3AR agonist) in patients with CpcPH due to HF. The effect of β3AR treatment will be evaluated on pulmonary hemodynamics, as well as clinical, biochemical, and advanced cardiac imaging parameters. (Beta3 Agonist Treatment in Chronic Pulmonary Hypertension Secondary to Heart Failure [SPHERE-HF]; NCT02775539)
Pulmonary hypertension (PH) is a common complication of heart failure (HF) (1,2) that results in more severe symptoms, worse exercise tolerance, and increased risk of death (3, 4, 5). Initially, this isolated post-capillary PH (IpcPH) is purely passive but has the potential to progress to combined pre- and post-capillary PH (CpcPH), a progressive disease characterized by significant vasoconstriction and vascular remodeling with a worse prognosis than IpcPH (6,7). Although IpcPH can be treated by focusing only on the underlying condition (8), CpcPH requires treatment of both pulmonary vascular remodeling and the primary heart disease.
Currently, there are no specific pharmacological therapies approved for patients with CpcPH (8,9). Clinical studies performed with specific pulmonary vasodilators [i.e., prostanoids (10) and endothelin receptor blockers (11,12)] in cohorts with HF or PH secondary to HF have not shown positive results, primarily because of concomitant systemic hypotension and hepatic toxicity. Although preliminary data from small single-center studies (13,14) that tested phosphodiesterase type 5 inhibitors in PH secondary to HF were promising, more recent evidence (15, 16, 17) strongly discourages their use in this setting. In addition, neutral findings have been reported for cyclic guanosine monophosphate (cGMP) stimulation in PH secondary to HF, either with preserved or reduced left ventricular ejection fraction (LVEF) (18,19). Therefore, new treatments are needed for CpcPH.
The sympathetic nervous system is central to the neurohumoral regulation of cardiovascular function and is implicated in many cardiopulmonary diseases. Beta-3 adrenoreceptor (β3AR) expression has been demonstrated in the human myocardium and vessels, and it has been described to be upregulated in left heart disease (LHD) (20,21). Like other adrenoreceptors, β3ARs are coupled to G proteins, and the downstream activated pathway includes nitric oxide synthase, nitric oxide-activated guanylyl cyclase, and cGMP synthesis, as well as increased cyclic adenosine monophosphate synthesis (22). Loss of cGMP and cyclic adenosine monophosphate signaling represents a hallmark in PH. It is known that within the pulmonary circulation, cyclic nucleotides exert several favorable effects, including vasodilatation, inhibition of smooth muscle cell proliferation, and prevention of platelet aggregation (23).
In recent years, several publications have demonstrated the cardioprotective effect of β3AR stimulation in different experimental models of ischemia-reperfusion injury (24, 25, 26) and HF (27, 28, 29). Therefore, β3ARs have emerged as a potential therapeutic target in cardiovascular diseases. Recent experimental research has demonstrated that treatment with β3AR agonists produces a beneficial effect on hemodynamics, right ventricular (RV) remodeling, and pulmonary vascular proliferation in a translational porcine model of post-capillary chronic PH (30). In addition, several Phase II and III randomized clinical trials (31, 32, 33, 34, 35, 36, 37) have already confirmed the good safety profile of the oral β3AR agonist mirabegron in healthy subjects and in patients with overactive bladder syndrome. Mirabegron, the selective oral β3AR agonist tested in the present trial, is currently approved for the treatment of overactive bladder syndrome in Europe, Japan, and America. A recent study has also demonstrated a good safety profile of mirabegron in patients with HF and reduced LVEF (38).
Based on the previously described concepts and evidence and the positive results of pre-clinical research, we designed a multicenter placebo-controlled Phase II randomized clinical trial to evaluate the efficacy and safety of mirabegron in patients with chronic CpcPH secondary to HF.
Methods
Study hypothesis
The main hypothesis of the SPHERE-HF (β3 Adrenergic Agonist Treatment in Chronic Pulmonary Hypertension Secondary to Heart Failure) trial is that maintenance treatment with a selective β3AR agonist (mirabegron) in patients with PH secondary to HF compared with placebo will result in a beneficial effect due to: 1) a reduction in pulmonary vascular resistance (PVR); 2) an increase in RV performance; 3) improvement in clinical status; and 4) no increase in adverse events.
Study endpoints
Efficacy measures
The primary outcome is the change in PVR on right heart catheterization, calculated in Wood units as: (mean pulmonary artery pressure (PAP) [mm Hg] − pulmonary capillary wedge pressure [mm Hg])/cardiac output [l/min]) from baseline to week 16. Secondary outcomes are change from baseline to week 16 in clinical status (measured by 6-min walk distance, New York Heart Association [NYHA] functional class, quality of life evaluated with the Spanish version of the Kansas City Cardiomyopathy Questionnaire, and the dyspnea Borg scale score), other hemodynamic variables assessed by right heart catheterization (mean PAP, transpulmonary gradient, diastolic pressure gradient, and cardiac output), RV performance (RV ejection fraction and cardiac output by cardiac magnetic resonance [CMR] or cardiac computed tomography [CCT]), as well as plasmatic levels of N-terminal pro-hormone of brain natriuretic peptide (NT-proBNP).
Safety measures include hospital admissions for HF or respiratory failure, death, urgent heart transplantation, initiation of intravenous therapy due to worsening HF (diuretics or inotropic drugs), adverse events, and adverse drug reactions, as well as monitorization of heart rate and the QTc interval on electrocardiography (ECG) (by Framingham method). External monitoring of all clinical events and ECG acquisitions will be performed.
Study population and inclusion/exclusion criteria
Patients have been identified and recruited from 4 different tertiary hospitals across Spain, all of which have a reference HF Unit. Inclusion and exclusion criteria are presented on Table 1. At the first protocol, an exclusion criterium of QTc interval on ECG >430 ms in men and >450 in women was included as a general safety standard. This criterion was later modified by an amendment to QTc >480 ms, based on the high percentage of patients with CpcPH who were excluded at baseline and the absence of data that suggested that mirabegron significantly increased QTc.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Written informed consent | Noncoronary cardiac surgery (e.g., valvular surgery) or noncoronary structural percutaneous procedure (e.g., Mitraclip) within the 12 months preceding recruitment or scheduled. |
| Age ≥18 years of age | Myocardial infarction or coronary revascularization within the 3 months preceding recruitment. |
| HF with reduced, intermediate or preserved LVEF, according to the definition of the European Society of Cardiology guidelines. | CRT implantation within the 6 months preceding recruitment |
| Combined pre- and post-capillary PH determined by RHC showing the following: | |
|
Sinus tachycardia or uncontrolled atrial fibrillation (HR >100 beats/min). |
| NYHA functional class II−IV | Uncontrolled systemic hypertension (systolic BP >180 mm Hg or diastolic BP >110 mm Hg) or symptomatic hypotension (systolic BP <90 mm Hg). |
| On optimized evidence-based pharmacological treatment | Diagnosis of infiltrative cardiomyopathy. |
| Stable clinical condition defined as no changes in therapeutic regimen for HF or hospitalization in the 30 days preceding recruitment and no current plan for changing therapy.∗ | Pre-menopausal women who have not undergone total hysterectomy |
| Expected survival <1 yr due to a disease other than HF. | |
| Severe renal failure (GFR <30 ml/min/1.73 m2). | |
| Severe hepatic impairment (transaminase elevation>3 times ULN). | |
| Prolonged QTc interval on the ECG (>430 ms in men or >450 ms in women)† | |
| Concomitant use with specific pulmonary vasodilators (sildenafil, bosentan, macicentan, riociguat, or other endothelin receptor blockers, phosphodiesterase 5 inhibitors or guanylate cyclase stimulators). | |
| Treatment with digoxin, flecainide, propafenone, dabigatran, tricyclic antidepressants or other CYP2D6 inhibitors (other than β-blockers). | |
| Severe COPD (FEV1/FVC ratio <0.7 together with FEV1 <50% predicted value). | |
| Severe restrictive lung disease (TLC <50%) | |
| Participation in another clinical trial | |
| Known allergy to mirabegron or any of the excipients |
BP = blood pressure; COPD = chronic obstructive pulmonary disease; CRT = cardiac resynchronization therapy; FEV1 = forced expiratory volume in the first second; FVC = forced vital capacity; GFR = glomerular filtration rate; HR = heart rate; LVEDP = left ventricular end-diastolic pressure; NYHA = New York Heart Association; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RHC = right heart catheterization; TLC = total lung capacity; ULN = upper limit of normal; WU = Wood units.
In the case of heart failure (HF) with preserved left ventricular ejection fraction (LVEF) it refers to an adequate control of comorbidity and optimized fluid status.
This criterion was changed by an amendment to QTc >480 ms.
Study design
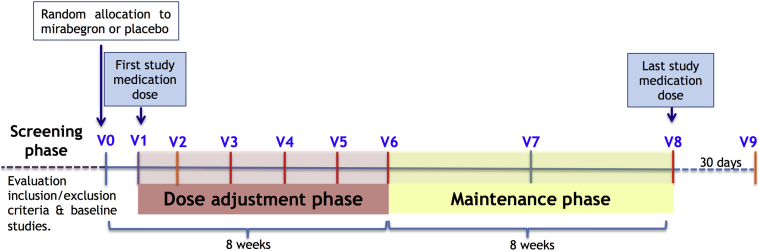
This trial is a Phase II double-blind multicenter, placebo-controlled randomized controlled trial. It consists of a screening phase and the randomized double-blind 16-week period (main study), which includes a dose titration phase and a maintenance phase (Figure 1).
Figure 1.
Summary of the Study Conduct
During the screening phase, eligible patients, usually identified after a right heart catheterization, are invited to participate in the study. After providing written informed consent, patients undergo the following baseline procedures and assessments (all must be completed within 4 weeks before random allocation): demographic and medical history data collection; physical examination (including blood pressure, heart rate, and pulse oximetry); NYHA functional class; blood sample analysis including NT-proBNP; ECG; echocardiography; right heart catheterization; 6-min walking test, and CMR. Those patients with formal contraindications for CMR can undergo a dedicated CCT examination to measure RV volumes and function (Table 2, Figure 2).
Table 2.
Overview of Study Visits and Examinations
| V0−V1 | V2 | V3 | V4 | V5 | V6 | V7 | V8 | V9 | |
|---|---|---|---|---|---|---|---|---|---|
| Informed consent | X | ||||||||
| Inclusion/exclusion criteria | X | ||||||||
| Demographic variables, prior medical history and medication | X | ||||||||
| Anamnesis | X | X | X | X | X | X | X | X | X |
| Physical examination | X | X | X | X | X | X | X | X | X |
| Laboratory | X | X | X | X | X | X | |||
| ECG | X | X | X | X | X | X | X | X | X |
| Kansas City Cardiomyopathy Questionnaire | X | X | |||||||
| Echocardiogram | X | X | |||||||
| Right heart catheterization | X | X | |||||||
| NT-proBNP | X | X | |||||||
| 6-min walking test | X | X | |||||||
| CMR | X | X |
CMR = cardiac magnetic resonance; ECG = electrocardiogram; NT-proBNP = N-terminal pro-hormone of brain natriuretic peptide; V = visit.
Figure 2.
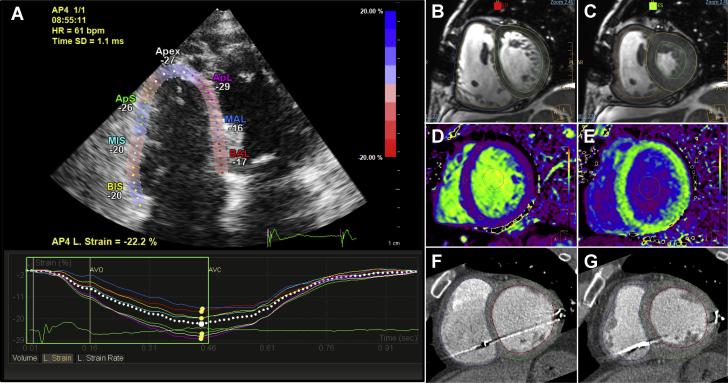
Multimodality Imaging Evaluation of RV Performance in SPHERE-HF Trial
(A) Right ventricular (RV) speckle tracking−derived strain from an apical 4-chamber view on echocardiography. (B and C) End-diastolic and end-systolic frames from the cine sequence at the mid-ventricular level to calculate biventricular volumes and ejection fraction with cardiac magnetic resonance (CMR). (D and E) T1 maps before (D) and 15 min after contrast administration (E) for estimation of extracellular volume using CMR. (F and G) Cardiac computed tomographic images from end-diastolic and end-systolic frames to calculate biventricular volumes and ejection fraction in a patient who could not undergo CMR due to an implantable cardiac resynchronization therapy device. SPHERE-HF = β3 Adrenergic Agonist Treatment in Chronic Pulmonary Hypertension Secondary to Heart Failure.
Patients who fulfill all inclusion criteria and none of the exclusion criteria are randomized to receive either mirabegron (50 mg) or placebo once daily in a blinded fashion (visit 0). Visit 1 represents the day of the first study medication dose and must take place in the 5 days after visit 0. A safety visit is performed 1 week after initiation of therapy (visit 2) because, according to the medication technical sheet, steady-state concentrations are achieved at 7 days of once-daily dosing with mirabegron. Thereafter, medication dose is titrated every 2 weeks for 8 weeks (visits 3, 4, and 5) based on patients’ monitoring of blood pressure, heart rate, QTc interval, blood analysis, and clinical status assessed at that visit (Table 3 shows the recommended titration algorithm). At the end of the dose titration phase (visit 6), all patients are expected to have reached their optimal dose. To assure blinding of the treatment arms, patients allocated to the placebo group undergo titration from visit 3 onwards, following the same algorithm.
Table 3.
Recommended Medication Dose Titration Algorithm
| Clinical Assessment | Recommended Action |
|---|---|
| Normal BP (systolic BP ≥95 and ≤135 mm Hg) AND HR ≤90 beats/min AND QTc interval∗ <430 ms in men or <450 ms in women AND blood analysis within normality AND patient asymptomatic | Up titrate study medication in 50 mg/day |
| If the patient develops any of the following: significant hypotension (systolic BP <80 mm Hg) or hypertension (systolic BP >145 mm Hg) OR tachycardia (HR >100 beat/min) OR prolonged QTc interval∗ (>430 ms in men or >450 ms in women) OR worsening of renal function/transaminase elevation on blood analysis OR symptoms associated with medication | Reduce or stop study medication dose |
| If the patient presents with mild hypotension (systolic BP ≥80 and <90 mm Hg) OR mild hypertension (systolic BP >135 and ≤145 mm Hg) AND/OR HR 90−100 beats/min (with a QTc interval∗ <430 ms in men and <450 ms in women) AND blood analysis is within normality | Maintain study medication dose |
During the maintenance phase (8 weeks), patients continue receiving the same dose assigned at visit 6, unless a decrease in dose is required for safety purposes. An intermediate clinical visit is performed halfway through the maintenance phase (visit 7). At visit 8 (end of the study), patients undergo all study examinations performed at baseline and stop the study medication. The last visit (visit 9) takes place 30 days after the last study medication dose for security monitoring, according to clinical trials regulation.
Table 2 shows a detailed calendar of the procedures that are performed at each study visit.
Sample size
A sample size sample size of 31 subjects per group achieves 80% power to reject the null hypothesis of zero treatment difference when the 2-sided significance level (alpha) is 0.05 and 2.1 Wood units, which are the minimal clinically important difference between groups. We assumed a SD of 3.2 Wood units in PVR (39) and a correlation between baseline and 16 weeks of 0.6. Assuming a 20% dropout rate, the estimated number of patients needed is 40 per group (80 total). Calculations were made using the power.t.test function available in R software (R Foundation, Vienna, Austria).
Manufacturing of study medication
Manufacturing of mirabegron and placebo is carried out in the pharmaceutical area of the Hospital Clínic Pharmacy department. The excipient for placebo capsules (mixture of microcrystalline cellulose and colloidal silica) is received with the corresponding certificate of analysis from the manufacturer (Fagron Iberica S.A.U, Tarrasa, Spain). The material the lot number and expiration date are verified for each entry, and the corresponding certificate of analysis is archived. Similarly, at the reception of mirabegron (Betmiga; bought from Astelas Pharma [Chuo, Tokyo, Japan] for 28.9€/box containing 30 pills of 50 mg of Betmiga), the batch and expiration date are checked and recorded. Betmiga pills are taken out of the blisters and automatically encapsulated (a single pill into a capsule). Similarly, the excipient is included in the capsules. Immediately, capsules including mirabegron or excipient are introduced in polyethylene bottles (30 capsules each) that are labeled as study medication, including the batch number and expiration. The bottles are kept in the clinical trials area in conservation conditions (atmosphere temperature <25 ºC).
Polyethylene bottles containing 30 capsules of mirabegron or placebo are labeled in a blinded manner, so both the physicians and patients are blinded to the study medication. Thereby, titration is performed in the same way for both active drug and placebo by increasing the number of capsules a day, and there is no option that unblinding is lost in the up titration phase.
Statistical methods
Randomization procedure
Participants will be assigned, on an individual basis, to mirabegron or placebo using randomly selected block sizes stratified by center using the Blockrand package (R Foundation). The randomization list will be provided exclusively to the pharmacy department responsible for the medication preparation, which will prepare the medication kits by identifying them with a unique sequential number for the entire study and will provide the kits to the centers. Researchers will assign received medication kits in a sequential order and record the kit number provided to each patient in the data collection system.
Statistical analysis
The following analysis populations are pre-defined for this study: intention to treat (ITT) population―all randomized patients; per protocol (PP) population―patients from the ITT group who have the final (16-week) measurement of the primary outcome and who have taken at least 80% of all the medication doses; and safety population―all patients who have taken ≥1 doses of the assigned treatment.
Baseline descriptive statistics will be calculated according to standard methods on the ITT and PP populations. Qualitative variables will be described as absolute number (n) and frequency (%), whereas quantitative variables will be described by mean ± SD or median (interquartile range), depending on normality (assessed using the Shapiro-Wilk test). Efficacy measures will be calculated on the ITT and PP analyses. The treatment effect on the primary outcome measure will be analyzed using a linear regression model that includes the baseline value, treatment, baseline × treatment interaction, center, and center × treatment interaction. Interaction terms will be removed if the Wald test is not statistically significant (p > 0.10). The efficacy analyses done on the PP set will be considered confirmatory (main analyses). For the ITT population, in those cases with missing final values of the primary outcome measure, these values will be replaced by the baseline data (last observation carried forward).
The following exploratory analyses of the primary outcome measure will be contemplated: LVEF (LVEF <40% vs. ≥40%); and the maximum tolerated dose (mg/d) subgroups and their interaction with treatment. Secondary outcome measures will be analyzed similarly using linear models for quantitative normal variables or generalized models for quantitative non-normal variables and logistic regression for dichotomous values. The analysis of the secondary variables will be carried out using the available information, without missing imputation techniques.
A descriptive analysis will be performed to evaluate safety of the treatment using the safety population. No interim analyses are planned. A 2-tailed p value < 0.05 will be considered statistically significant, unless otherwise specified.
Description of the study procedures and examinations
Right heart catheterization
The procedure will be performed using a Swan-Ganz catheter introduced via the internal jugular vein using a standard methodology. Hemodynamic measurements will include right atrial pressure, systolic, diastolic, and mean PAP, as well as pulmonary capillary wedge pressure at end-expiration. Zero level will be set at the level of the anterior axillar line while lying flat; 2 different mean PAP values will be registered over a 5- to 10-min period, and the average calculated. Cardiac output will be quantified by the thermodilution method (5 measurements, average of 3, excluding the highest and lowest values).
6-min walking test
Tests will be carried out following the recommendations of the American and European Respiratory Societies (40). Patients will be instructed to walk along a 30-m long corridor and to walk as far as possible for 6 min. Tests will be supervised by a nurse, and heart rate and oxygen saturation will be recorded.
Quality of life questionnaire
The Kansas City Cardiomyopathy Questionnaire, which has been validated in the Spanish population, will be used to assess quality of life.
Physical examination
Blood pressure will be measured at every visit using an automated sphygmomanometer in a quiet room. Three measurements will be performed over a 10-min period, and the average between the second and third measurement will be registered. Heart rate and oxygen saturation (using a pulse oxymeter) will be also entered into the electronic case report form. Patients will be weighed on every study visit, and functional class (following the NYHA classification), as well as HF signs and symptoms will be recorded.
Blood samples
A venous sample will be obtained at visits 0, 3, 4, 5, 6, and 8 and tested for complete blood count, serum glucose, as well as renal and liver function. NT-proBNP measurement will also be performed at visits 0 and 8.
ECG
Heart rhythm, heart rate, QT, and QTc interval will be measured.
Echocardiography
Studies will be performed according to standard American Society of Echocardiography and European Association of Cardiovascular Imaging guidelines, with special dedication to the evaluation of RV performance (41,42). The acquisition protocol is standardized across all centers, and studies are digitally stored for dedicated offline analysis at the Imaging Core Laboratory located at Centro Nacional de Investigaciones Cardiovasculares. Left ventricular (LV) volumes and ejection fractions will be measured using the modified Simpson rule (biplane method). Left and right atrial areas will be obtained at ventricular end-systole, excluding the pulmonary vein confluence and the left atrial appendage. RV dimensions, end-systolic and end-diastolic RV areas, and the tricuspid annular plane systolic excursion will be measured from a RV-focused apical 4-chamber view. Fractional area change will be calculated as the difference in RV end-diastolic area and RV end-systolic area divided by the RV end-systolic area. RV index of myocardial performance (Tei index) and the tricuspid lateral annular systolic velocity wave (S') will be measured using Doppler tissue imaging velocity of the lateral tricuspid annulus. Valvular disease and diastolic function will be graded according to the American Society of Echocardiography and European Association of Cardiovascular Imaging standards (43, 44, 45). A 3-beat 2-dimensional digital clip of a RV-focused, 4-chamber view will be acquired for quantification of RV strain by speckle tracking echocardiography (Figure 2A). All analyses will be performed using the EchoPAC v112 (GE Healthcare, Chicago, Illinois) or Xcelera R4.1 and QLAB 10.2 (Philips, Amsterdam, the Netherlands) software.
CMR
CMR studies will be performed with either 1.5- or 3.0-T magnets, using dedicated surface coils for cardiac studies and retrospective electrocardiographic gating. The acquisition protocol is standardized across all centers, and all studies are digitally stored for dedicated offline analysis at the Imaging Core Laboratory located at Centro Nacional de Investigaciones Cardiovasculares (CNIC) (Madrid, Spain).
The CMR examination includes the following sequences. A steady-state free precession cine sequence is used to acquire 15 contiguous short-axis slices covering both ventricles from base to apex and reconstructed into 25 cardiac phases each for the evaluation of biventricular volumes and function. Two-dimensional flow imaging (phase contrast) is performed perpendicular to the main pulmonary artery with a velocity-encoded gradient echo sequence and an upper velocity limit of 100 cm/s (with further increases for any signal aliasing). For this purpose, 2 double-oblique orthogonal views oriented along the main axis of the pulmonary artery trunk are acquired with a standard steady-state free precession cine sequence and used as the reference to prescribe a plane truly perpendicular to the main pulmonary artery for the acquisition of phase contrast images. Care is taken to ensure that the imaging plane remains between the pulmonary valve and pulmonary artery bifurcation throughout the cardiac cycle. In addition, aortic velocity and LV cardiac output are systematically measured (46). For delayed gadolinium enhancement imaging, patients receive an intravenous bolus of 0.125 mmol/kg of gadolinium followed by 20 ml of saline. After 5 to 10 min, contiguous short-axis views matching the cine images are acquired using a phase-sensitive inversion−recovery fast gradient echo sequence for the evaluation of delayed gadolinium enhancement. Typical breath-hold times range from 8 to 14 s. In patients with an inability to perform such breath-holds, delayed gadolinium enhancement images are repeated with a single-shot inversion−recovery steady-state free precession sequence during free breathing. T1 mapping sequences will be acquired just before contrast administration and 15 min after contrast administration in a short-axis view at the level of the papillary muscles for estimation of the extracellular volume fraction.
CMR analysis
Cine, 2-dimensional flow imaging and delayed enhancement will be analyzed using specialized software (IntelliSpacePortal v9.0, Philips) by blinded investigators in the Imaging Core Laboratory at CNIC. On cine images, end-diastolic and end-systolic frames will be selected based on visual assessment of largest and smallest LV volumes, respectively, and on the opening or closing of atrioventricular and semilunar valves. Biventricular endocardial contours will be manually traced in end-diastole and end-systole, and Simpson’s method will be used to automatically calculate end-diastolic volumes, end-systolic volumes, and ejection fractions. RV trabeculations will be adjudicated to the blood pool (Figures 2B and 2C). Similarly, the inner contours of the main pulmonary artery cross section will be outlined in each cardiac phase. Through integration of pulmonary artery areas and flow, the following parameters will be quantified: peak velocity; average velocity during the complete cardiac cycle; minimum and maximum areas; and pulmonary artery net forward volume. RV-arterial coupling (the ratio of pulmonary artery effective elastance to RV maximal end-systolic elastance) will be estimated as (end-systolic volume/stroke volume) (47). Regions of interest will be drawn on T1 maps in the myocardial anterior and inferior RV insertion points, interventricular septum, LV lateral wall and LV cavity blood pool before and after contrast administration for estimation of the extracellular volume, as previously described (Figures 2D and 2E) (48).
CCT
CCT studies are performed using at least a 64-slice scanner with retrospective electrocardiographic gating mode acquisition (ideally with modulation dose). CCT images are acquired from the level of the aortic arch to the dome of the diaphragm in a breath-hold mode. Nonionic iodinated contrast agent (approximately 80 ml) is injected at a rate of 5 ml/s with a power injector, followed by a saline 40 ml flush. A bolus tracking technique is used to trigger image acquisition once attenuation in a region of interest placed in the descending aorta has reached a pre-set threshold of 100 HU.
Axial images are reconstructed with an image matrix of 512 × 512 pixels and a slice thickness of 1.25 mm from 0% to 90% of the cardiac cycle to calculate RV function. Post-processing of CCT images will be performed on a dedicated workstation with specific RV function quantification package (IntelliSpacePortal v9.0, Philips) (Figures 2F and 2G).
Duration of the study
The SPHERE-HF clinical trial started recruitment in June 2017, and it is expected that the inclusion phase will end by June 2020. As of December 18, 2019, a total of 61 patients have been included in the study.
Discussion
PH associated with HF has repeatedly been shown to decrease survival, particularly in patients with CpcPH (6,39). Although IpcPH is entirely reversible when pulmonary arterial wedge pressure is normalized by medical or surgical interventions, this is not the case for CpcPH, due to associated pulmonary arterial remodeling. The latter correlates with pulmonary vascular gradients and increased PVR (49). Approximately 12% to 14% of patients with PH and HF present with CpcPH, a prevalence similarly distributed in reduced or preserved HF (6). As underlined by Vanderpool and Naeije (50), knowledge of the specific phenotype of CpcPH is of great importance to the design of future trials of targeted therapies for PH and HF.
Several randomized controlled trials tested the effect of pulmonary vasodilators in HF but few of them focused in patients with confirmed PH and only a minority of studies focused on CpcPH. Studies that evaluated the effect of prostanoids (epoprostenol) (10) and endothelin-1 receptor antagonists (bosentan) (11,51) in patients with advanced HF and severe LV systolic dysfunction were terminated early due to an increased rate of adverse events in the investigational drug group compared with the control group. In the recent MELODY (Macitentan in Pulmonary Hypertension due to Left Ventricular Dysfunction) trial (12), which randomized 63 patients with CpcPH to macitentan or placebo, there were no differences in the main endpoint (composite of fluid retention or worsening in NYHA functional class) between groups. Phosphodiesterase-type 5 inhibitors also failed to show a consistent effect in PH secondary to HF (13, 14, 15, 16, 17). Moreover, in the recent Spanish multicenter SIOVAC (Sildenafil for Improving Outcomes After Valvular Correction) trial that included 200 patients with residual PH after surgically treated valvular heart disease, sildenafil was associated with worse outcomes than placebo (17). Regarding soluble guanylate cyclase stimulators (riociguat), neutral findings were reported in PH secondary to HF with preserved and reduced LVEFs (18,19). Several limitations of the previous trials should be noted: 1) in most of them, the target population was not selected based on the pulmonary hemodynamic status (confirmed diagnosis of PH) but included the full spectrum of patients with LHD, and only a minority were targeted at patients with CpcPH; 2) most studies were single center, which limited the extrapolation of results; 3) few required optimization of HF therapy before initiating the study medication; and 4) most lacked a comprehensive evaluation of hemodynamics, exercise capacity, and imaging of the RV. Based on this evidence, targeted treatment using pulmonary arterial hypertension-approved therapies on PH secondary to LHD is discouraged by the current PH guidelines (8).
The SPHERE-HF trial addresses a new approach to treat PH secondary to HF and overcomes most of the previously mentioned limitations. SPHERE-HF is based on solid large animal experimentation data (30). SPHERE-HF is a multicenter randomized controlled trial that targets patients with LHD and confirmed PH with an established pre-capillary component (PVR >3 Wood units and/or diastolic gradient ≥7 mm Hg or transpulmonary gradient ≥12 mmHg, criteria based on PH guidelines valid at the time of the trial design). In this way, SPHERE-HF targets those patients who are most likely to develop pulmonary vascular remodeling and impaired RV function, and therefore, may potentially benefit most from β3AR stimulation. As opposed to other trials, the primary outcome measure in SPHERE-HF is the change in PVR rather than mean PAP. We consider PVR a more robust endpoint because it recapitulates pulmonary pressures and the degree of RV dysfunction and has demonstrated prognostic relevance in PH (52), and particularly in PH secondary to LHD (3,8,9,53, 54, 55). In addition, cardiac imaging is a critical part of the SPHERE-HF clinical trial. The effect of mirabegron on RV performance will be evaluated using state-of-the art imaging techniques (CMR, CCT, and echocardiography) and several markers of subclinical myocardial disease (e.g., speckle tracking−derived strain and extracellular volume quantified by T1-mapping CMR) will be assessed to quantify subtle changes in RV function under treatment. Finally, despite the limitations derived from the sample size, important patient-centric outcomes (e.g., functional class or quality of life) have been pre-specified as secondary outcomes of the current SPHERE-HF.
Study limitations
First, the trial focuses in patients with CpcPH by including patients with reduced or preserved LVEF based on a common hemodynamic and biological phenotype (56). Because we recognize that it may include some heterogeneity, a pre-specified subanalysis regarding LVEF (<40% vs. ≥40%) will be considered. Second, this is a proof-of-concept trial with a hemodynamic endpoint; if positive, a Phase III trial with clinical patient-center endpoints will be performed.
The dose selection for this study was based on previously reported safety data, and particularly, cardiovascular safety for mirabegron up to 200 mg/day has been established (31, 32, 33,38).
Conclusions
SPHERE-HF will test the hypothesis that in patients with CpcPH secondary to HF, therapy with a β3AR agonist improves pulmonary hemodynamics, RV performance, and functional status with a good safety profile.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: CpcPH is a relatively common complication of chronic HF that is associated with poor survival and no specific pharmacological treatment. Recently, it was shown that β3AR stimulation improves pulmonary hemodynamics and RV performance in a translational large animal model mimicking this condition. The SPHERE-HF trial is a phase-2 randomized clinical trial designed to evaluate the efficacy and security of mirabegron (oral β3AR agonist) in patients with CpcPH secondary to HF.
TRANSLATIONAL OUTLOOK: SPHERE-HF is a pilot clinical trial that has been developed after experimental evidence of the beneficial effect of beta3AR agonist treatment in chronic PH, following our translational program at CNIC. If the effect of this new pharmacological approach is confirmed in SPHERE-HF, a larger clinical trial with a patient-centric outcome, ideally including hospital admissions and mortality, will be undertaken.
Footnotes
The SPHERE-HF trial is an investigator-initiated noncommercial trial independent of the pharmaceutical industry. This work was funded by a grant from Fundació La Marató de TV3 (20151730-31-32). The CNIC is supported by the Ministerio de Ciencia, Innovación y Universidades and the Pro CNIC Foundation and is a Severo Ochoa Center of Excellence (SEV-2015-0505). IDIBAPS belongs to the CERCA Programme and receives partial funding from the Generalitat de Catalunya. Drs. Ibanez, García-Álvarez, and Fuster are co-inventors of a patent for the use of beta-3 agonists for the treatment of pulmonary hypertension. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Contributor Information
Borja Ibanez, Email: bibanez@cnic.es.
Ana García-Álvarez, Email: agarciaa@cnic.es, anagarci@clinic.cat.
References
- 1.Ghio S., Gavazzi A., Campana C. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol. 2001;37:183–188. doi: 10.1016/s0735-1097(00)01102-5. [DOI] [PubMed] [Google Scholar]
- 2.Lam C.S., Roger V.L., Rodeheffer R.J., Borlaug B.A., Enders F.T., Redfield M.M. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53:1119–1126. doi: 10.1016/j.jacc.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guazzi M., Borlaug B.A. Pulmonary hypertension due to left heart disease. Circulation. 2012;126:975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 4.Bursi F., McNallan S.M., Redfield M.M. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59:222–231. doi: 10.1016/j.jacc.2011.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grigioni F., Potena L., Galie N. Prognostic implications of serial assessments of pulmonary hypertension in severe chronic heart failure. J Heart Lung Transplant. 2006;25:1241–1246. doi: 10.1016/j.healun.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 6.Gerges M., Gerges C., Pistritto A.M. Pulmonary hypertension in heart failure. Epidemiology, right ventricular function, and survival. Am J Respir Crit Care Med. 2015;192:1234–1246. doi: 10.1164/rccm.201503-0529OC. [DOI] [PubMed] [Google Scholar]
- 7.Tatebe S., Fukumoto Y., Sugimura K. Clinical significance of reactive post-capillary pulmonary hypertension in patients with left heart disease. Circ J. 2012;76:1235–1244. doi: 10.1253/circj.cj-11-1288. [DOI] [PubMed] [Google Scholar]
- 8.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 9.Vachiery J.L., Adir Y., Barbera J.A. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62:D100−8. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 10.Califf R.M., Adams K.F., McKenna W.J. A randomized controlled trial of epoprostenol therapy for severe congestive heart failure: the Flolan International Randomized Survival Trial (FIRST) Am Heart J. 1997;134:44–54. doi: 10.1016/s0002-8703(97)70105-4. [DOI] [PubMed] [Google Scholar]
- 11.Kalra P.R., Moon J.C., Coats A.J. Do results of the ENABLE (Endothelin Antagonist Bosentan for Lowering Cardiac Events in Heart Failure) study spell the end for non-selective endothelin antagonism in heart failure? Int J Cardiol. 2002;85:195–197. doi: 10.1016/s0167-5273(02)00182-1. [DOI] [PubMed] [Google Scholar]
- 12.Vachiery J.L., Delcroix M., Al-Hiti H. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur Respir J. 2018;51 doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 13.Lewis G.D., Shah R., Shahzad K. Sildenafil improves exercise capacity and quality of life in patients with systolic heart failure and secondary pulmonary hypertension. Circulation. 2007;116:1555–1562. doi: 10.1161/CIRCULATIONAHA.107.716373. [DOI] [PubMed] [Google Scholar]
- 14.Guazzi M., Vicenzi M., Arena R., Guazzi M.D. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124:164–174. doi: 10.1161/CIRCULATIONAHA.110.983866. [DOI] [PubMed] [Google Scholar]
- 15.Redfield M.M., Chen H.H., Borlaug B.A. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309:1268–1277. doi: 10.1001/jama.2013.2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoendermis E.S., Liu L.C., Hummel Y.M. Effects of sildenafil on invasive haemodynamics and exercise capacity in heart failure patients with preserved ejection fraction and pulmonary hypertension: a randomized controlled trial. Eur Heart J. 2015;36:2565–2573. doi: 10.1093/eurheartj/ehv336. [DOI] [PubMed] [Google Scholar]
- 17.Bermejo J., Yotti R., Garcia-Orta R. Sildenafil for improving outcomes in patients with corrected valvular heart disease and persistent pulmonary hypertension: a multicenter, double-blind, randomized clinical trial. Eur Heart J. 2018;39:1255–1264. doi: 10.1093/eurheartj/ehx700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonderman D., Pretsch I., Steringer-Mascherbauer R. Acute hemodynamic effects of riociguat in patients with pulmonary hypertension associated with diastolic heart failure (DILATE-1): a randomized, double-blind, placebo-controlled, single-dose study. Chest. 2014;146:1274–1285. doi: 10.1378/chest.14-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonderman D., Ghio S., Felix S.B. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128:502–511. doi: 10.1161/CIRCULATIONAHA.113.001458. [DOI] [PubMed] [Google Scholar]
- 20.Moniotte S., Kobzik L., Feron O., Trochu J.N., Gauthier C., Balligand J.L. Upregulation of beta(3)-adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation. 2001;103:1649–1655. doi: 10.1161/01.cir.103.12.1649. [DOI] [PubMed] [Google Scholar]
- 21.Dessy C., Moniotte S., Ghisdal P., Havaux X., Noirhomme P., Balligand J.L. Endothelial beta3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation. 2004;110:948–954. doi: 10.1161/01.CIR.0000139331.85766.AF. [DOI] [PubMed] [Google Scholar]
- 22.Rozec B., Gauthier C. Beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–673. doi: 10.1016/j.pharmthera.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Baliga R.S., MacAllister R.J., Hobbs A.J. New perspectives for the treatment of pulmonary hypertension. Br J Pharmacol. 2011;163:125–140. doi: 10.1111/j.1476-5381.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aragon J.P., Condit M.E., Bhushan S. Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 2011;58:2683–2691. doi: 10.1016/j.jacc.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garcia-Prieto J., Garcia-Ruiz J.M., Sanz-Rosa D. β3 adrenergic receptor selective stimulation during ischemia/reperfusion improves cardiac function in translational models through inhibition of mPTP opening in cardiomyocytes. Basic Res Cardiol. 2014;109:422. doi: 10.1007/s00395-014-0422-0. [DOI] [PubMed] [Google Scholar]
- 26.Niu X., Zhao L., Li X. β3-adrenoreceptor stimulation protects against myocardial infarction injury via eNOS and nNOS activation. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bundgaard H., Liu C.C., Garcia A. β(3) adrenergic stimulation of the cardiac Na+-K+ pump by reversal of an inhibitory oxidative modification. Circulation. 2010;122:2699–2708. doi: 10.1161/CIRCULATIONAHA.110.964619. [DOI] [PubMed] [Google Scholar]
- 28.Niu X., Watts V.L., Cingolani O.H. Cardioprotective effect of beta-3 adrenergic receptor agonism: role of neuronal nitric oxide synthase. J Am Coll Cardiol. 2012;59:1979–1987. doi: 10.1016/j.jacc.2011.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belge C., Hammond J., Dubois-Deruy E. Enhanced expression of beta3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation. 2014;129:451–462. doi: 10.1161/CIRCULATIONAHA.113.004940. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Alvarez A., Pereda D., Garcia-Lunar I. Beta-3 adrenergic agonists reduce pulmonary vascular resistance and improve right ventricular performance in a porcine model of chronic pulmonary hypertension. Basic Res Cardiol. 2016;111:49. doi: 10.1007/s00395-016-0567-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malik M., van Gelderen E.M., Lee J.H. Proarrhythmic safety of repeat doses of mirabegron in healthy subjects: a randomized, double-blind, placebo-, and active-controlled thorough QT study. Clin Pharmacol Ther. 2012;92:696–706. doi: 10.1038/clpt.2012.181. [DOI] [PubMed] [Google Scholar]
- 32.Chapple C.R., Amarenco G., Lopez Aramburu M.A. A proof-of-concept study: mirabegron, a new therapy for overactive bladder. Neurourol Urodyn. 2013;32:1116–1122. doi: 10.1002/nau.22373. [DOI] [PubMed] [Google Scholar]
- 33.Chapple C.R., Dvorak V., Radziszewski P. A phase II dose-ranging study of mirabegron in patients with overactive bladder. Int Urogynecol J. 2013;24:1447–1458. doi: 10.1007/s00192-013-2042-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chapple C.R., Kaplan S.A., Mitcheson D. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63:296–305. doi: 10.1016/j.eururo.2012.10.048. [DOI] [PubMed] [Google Scholar]
- 35.Herschorn S., Barkin J., Castro-Diaz D. A phase III, randomized, double-blind, parallel-group, placebo-controlled, multicentre study to assess the efficacy and safety of the beta(3) adrenoceptor agonist, mirabegron, in patients with symptoms of overactive bladder. Urology. 2013;82:313–320. doi: 10.1016/j.urology.2013.02.077. [DOI] [PubMed] [Google Scholar]
- 36.Khullar V., Amarenco G., Angulo J.C. Efficacy and tolerability of mirabegron, a beta(3)-adrenoceptor agonist, in patients with overactive bladder: results from a randomised European-Australian phase 3 trial. Eur Urol. 2013;63:283–295. doi: 10.1016/j.eururo.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Nitti V.W., Auerbach S., Martin N., Calhoun A., Lee M., Herschorn S. Results of a randomized phase III trial of mirabegron in patients with overactive bladder. J Urol. 2013;189:1388–1395. doi: 10.1016/j.juro.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Bundgaard H., Axelsson A., Hartvig Thomsen J. The first-in-man randomized trial of a beta3 adrenoceptor agonist in chronic heart failure: the BEAT-HF trial. Eur J Heart Fail. 2017;19:566–575. doi: 10.1002/ejhf.714. [DOI] [PubMed] [Google Scholar]
- 39.Gerges C., Gerges M., Lang M.B. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143:758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 40.Holland A.E., Spruit M.A., Troosters T. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44:1428–1446. doi: 10.1183/09031936.00150314. [DOI] [PubMed] [Google Scholar]
- 41.Lang R.M., Badano L.P., Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 42.Rudski L.G., Lai W.W., Afilalo J. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. quiz 86−8. [DOI] [PubMed] [Google Scholar]
- 43.Zoghbi W.A., Adams D., Bonow R.O. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017;30:303–371. doi: 10.1016/j.echo.2017.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Nagueh S.F., Smiseth O.A., Appleton C.P. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17:1321–1360. doi: 10.1093/ehjci/jew082. [DOI] [PubMed] [Google Scholar]
- 45.Baumgartner H., Hung J., Bermejo J. Recommendations on the echocardiographic assessment of aortic valve stenosis: a focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2017;30:372–392. doi: 10.1016/j.echo.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Alvarez A., Fernandez-Friera L., Garcia-Ruiz J.M. Noninvasive monitoring of serial changes in pulmonary vascular resistance and acute vasodilator testing using cardiac magnetic resonance. J Am Coll Cardiol. 2013;62:1621–1631. doi: 10.1016/j.jacc.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 47.Sanz J., Garcia-Alvarez A., Fernandez-Friera L. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart. 2012;98:238–243. doi: 10.1136/heartjnl-2011-300462. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Alvarez A., Garcia-Lunar I., Pereda D. Association of myocardial T1-mapping CMR with hemodynamics and RV performance in pulmonary hypertension. J Am Coll Cardiol Img. 2015;8:76–82. doi: 10.1016/j.jcmg.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 49.Heath D., Edwards J.E. Histological changes in the lung in diseases associated with pulmonary venous hypertension. Br J Dis Chest. 1959;53:8–18. doi: 10.1016/s0007-0971(59)80105-4. [DOI] [PubMed] [Google Scholar]
- 50.Vanderpool R.R., Naeije R. Progress in pulmonary hypertension with left heart failure. Beyond new definitions and acronyms. Am J Respir Crit Care Med. 2015;192:1152–1154. doi: 10.1164/rccm.201507-1509ED. [DOI] [PubMed] [Google Scholar]
- 51.Packer M., McMurray J., Massie B.M. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 52.Swiston J.R., Johnson S.R., Granton J.T. Factors that prognosticate mortality in idiopathic pulmonary arterial hypertension: a systematic review of the literature. Respir Med. 2010;104:1588–1607. doi: 10.1016/j.rmed.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Chang P.P., Longenecker J.C., Wang N.Y. Mild vs severe pulmonary hypertension before heart transplantation: different effects on posttransplantation pulmonary hypertension and mortality. J Heart Lung Transplant. 2005;24:998–1007. doi: 10.1016/j.healun.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 54.Rosenkranz S., Gibbs J.S., Wachter R., De Marco T., Vonk-Noordegraaf A., Vachiery J.L. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;37:942–954. doi: 10.1093/eurheartj/ehv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vonk Noordegraaf A., Westerhof B.E., Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol. 2017;69:236–243. doi: 10.1016/j.jacc.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 56.Assad T.R., Hemnes A.R., Larkin E.K. Clinical and biological insights into combined post- and pre-capillary pulmonary hypertension. J Am Coll Cardiol. 2016;68:2525–2536. doi: 10.1016/j.jacc.2016.09.942. [DOI] [PMC free article] [PubMed] [Google Scholar]