Central Illustration
Key Words: chronic kidney disease, dialysis, medial calcification, vascular calcification
Abbreviations and Acronyms: CAC, coronary artery calcification; CI, confidence interval; CKD, chronic kidney disease; CT, computed tomography; CV, cardiovascular; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; FGF, fibroblast growth factor; HR, hazard ratio; LDL-C, low-density lipoprotein cholesterol; MGP, matrix Gla protein; PTH, parathyroid hormone; VSMC, vascular smooth muscle cell
Highlights
-
•
Vascular calcification is a highly regulated, cell-mediated process that is strongly associated with CKD and confers increased risk for incident CV events.
-
•
Multiple pathways link vascular calcification with CKD; however, they remain incompletely understood, and the development of targeted therapies has been underwhelming.
-
•
Illumination of the causal steps and natural history that link vascular calcification to CV events will affirm its role as a CV risk factor and accelerate drug discovery and therapeutic translation.
Summary
Cardiovascular (CV) disease remains an important cause of morbidity and mortality for patients with chronic kidney disease (CKD). Although clustering of traditional risk factors with CKD is well recognized, kidney-specific mechanisms are believed to drive the disproportionate burden of CV disease. One perturbation that is frequently observed at high rates in patients with CKD is vascular calcification, which may be a central mediator for an array of CV sequelae. This review summarizes the pathophysiological bases of intimal and medial vascular calcification in CKD, current strategies for diagnosis and management, and posits vascular calcification as a risk marker and therapeutic target.
Chronic kidney disease (CKD) is a major global public health problem. Defined by the sustained presence of either kidney damage (albuminuria) or reduced kidney function (estimated glomerular filtration rate [eGFR] <60 ml/min/1.73 m2) (1), CKD is believed to affect 10% to 15% of the population and is estimated to contribute to 5 to 10 million deaths annually (2,3). Despite improvements in the care of patients with CKD, life expectancy remains significantly reduced across all stages of kidney disease (4), and the global burden of kidney disease continues to rise (5). In this context, cardiovascular disease (CVD) is a significant contributor to the high morbidity experienced by patients with CKD and CV death is the most common cause of death in this population (Figure 1).
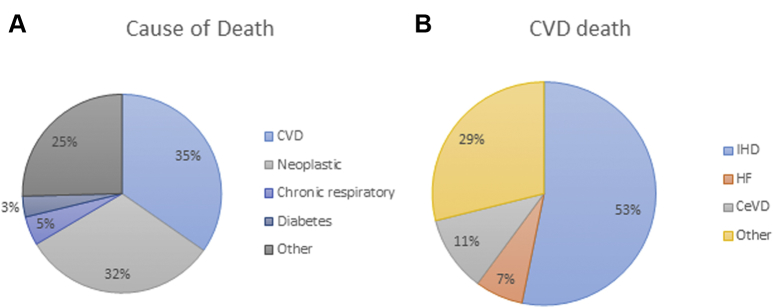
Figure 1.
Categorical and Cardiovascular Disease Cause-Specific Mortality
(A) Categorical and (B) cardiovascular disease (CVD) cause-specific mortality in an electronic health record-derived sample of >30,000 residents from Ohio with nondialysis chronic kidney disease (estimated glomerular filtration rate: 15 to 60 ml/min/1.73 m2) (140). CeVD = cerebrovascular disease; HF = heart failure; IHD = ischemic heart disease.
Large contemporary datasets have repeatedly shown linear relations between CV mortality and reduced eGFR and proteinuria or albuminuria (6). The effect is most pronounced for those with end-stage kidney disease (ESKD) in whom 85% of adults commencing dialysis at older than 45 years of age will have some form of CVD and experience a relative risk of CV death that exceeds 20 times that of the general population (7). In adults younger than 30 years of age, the relative risk is even more dramatic; incident ESKD confers a >150-fold risk of CV death compared with an age-matched population (8). Expressed another way, persons with mild to moderate (stages 1 to 3) CKD are at higher risk of CV events and CV mortality than they are for progression to ESKD (9,10).
CKD imparts increased risk for a wide array of CVDs (Figure 2). Although atherosclerotic CVD is the most frequent manifestation, leading some to consider CKD a coronary-artery risk equivalent (11), rates of heart failure (12), stroke (13), valvular heart disease (14), arrhythmia (15), and sudden cardiac death (16) are all significantly increased across the spectrum of CKD. Clustering of traditional risk factors such as type 2 diabetes mellitus and hypertension were originally believed to drive propensity for CVD in these patients; however, meta-analyses have consistently identified significant residual, CKD-specific risk (17,18). Once CVD is established, CKD confers a significantly worse prognosis with a more aggressive disease phenotype, higher risk of complications, and premature cause-specific death (18). Reduced efficacy of proven secondary preventative therapies (e.g., statins) likely also contribute to the divergent outcomes experienced by patients with CKD (19, 20, 21).
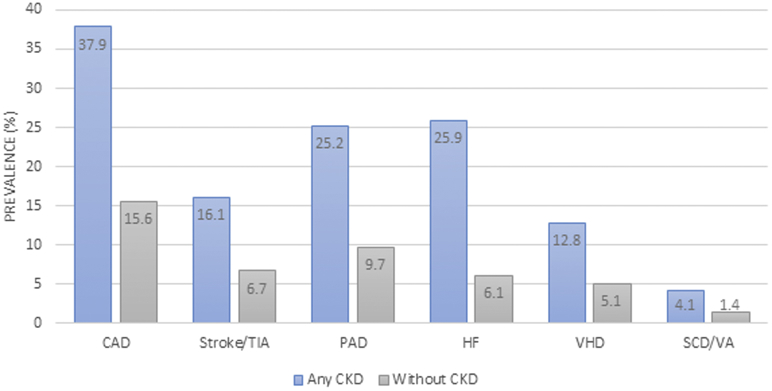
Figure 2.
Cardiovascular Disease Burden in a Sample of Medicare Beneficiaries
Cardiovascular disease burden in a sample of Medicare beneficiaries in the United States Renal Data System 2016 sample. CAD = coronary artery disease; PAD = peripheral arterial disease; SCD = sudden cardiac death; TIA = transient ischemic attack; VA = ventricular arrhythmia; VHD = valvular heart disease; other abbreviations as in Figure 1.
Multiple systemic perturbations observed in CKD can lead to cardiac and vascular damage, and likely underscore the increased risk of CV events observed in this population. Vascular calcification, the pathological deposition of calcium salts in the arterial wall, has been observed among patients with CKD at between 2- and 5-fold the rate of age-matched non-CKD patients. A large body of evidence has subsequently supported biologically plausible, temporal (22), and dose−response (23) relations between vascular calcification and CV risk in patients with CKD. However, whether the regression or halting of vascular calcification is possible and subsequently results in improved CV outcomes remains to be determined.
Types of Calcification
There are several distinct phenotypes of CV calcification—intimal vascular, medial vascular, and valvular. Although valvular calcification is increasingly recognized as an important contributor to morbidity and mortality in patients with CKD, particularly those on dialysis (24), for the purpose of this review we focused on the vascular entities of intimal and medial calcification. Intimal and medial vascular calcification are defined by their location within the arterial wall, which is a distinction that helps separate predisposing etiology, regional distribution, and clinical sequelae (Figure 3). Although this has intrinsic appeal, noninvasive imaging is unable to discern intimal from medial calcification; thus, our insights into the natural history and clinical relevance of each type of vascular calcification are limited.
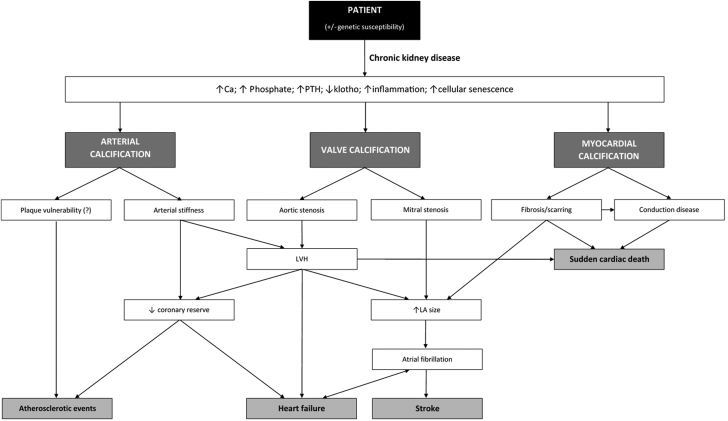
Figure 3.
Flowchart Linking Clinicopathological Calcification
Flowchart linking clinicopathological calcification. Ca = calcium; LA = left atrial; LVH = left ventricular hypertrophy; PTH = parathyroid hormone.
Intimal calcification
Intimal calcification occurs almost exclusively in the context of atherosclerosis, which has a predilection for medium to large arteries and areas of abnormal flow, such as arterial vessel bifurcations. Although generally increased in patients with CKD, intimal calcification is not specific to CKD and instead is associated with traditional atherogenic risk factors such as dyslipidemia, diabetes, hypertension, and cigarette smoking (25). Of note, adolescents on dialysis who do not have traditional risk factors develop medial calcification almost exclusively (26,27), which is similar to animal models of CKD that are not exposed to an additional metabolic stimulus (e.g., through low-density lipoprotein (LDL) receptor knockout or a high cholesterol diet) (28). CKD may promote, accelerate, or catalyze an already established, synchronous atherosclerotic calcification process rather than incite it (29). Regardless, the complications of intimal calcification are believed to be local and mediated through the development of luminal stenosis, obstruction, and distal ischemia or infarction (i.e., myocardial infarction, stroke, or limb events). In general, intimal calcification appears to be dynamic and may be considered a barometer for the atherosclerotic cycle; microscopic flecks are visible during the earliest stages of intimal thickening and diffuse, confluent regions are observed in the most advanced fibrocalcific lesions (30). Although spotty calcification (approximately 1 mm in size) observed on both computed tomography (CT) and intravascular imaging has been associated with plaque instability and propensity for atherothrombotic events, confluent calcific lesions appear to be associated with a more stable phenotype (30) and may instead represent a teleological response to injury (25). These confluent and often dense lesions on intravascular imaging are more common in patients with CKD (31). Whether the accelerated atherosclerotic or intimal calcification observed in CKD is causally linked to adverse CV outcomes or may be acting as a synchronous biomarker for medial calcification remains undetermined.
Medial calcification
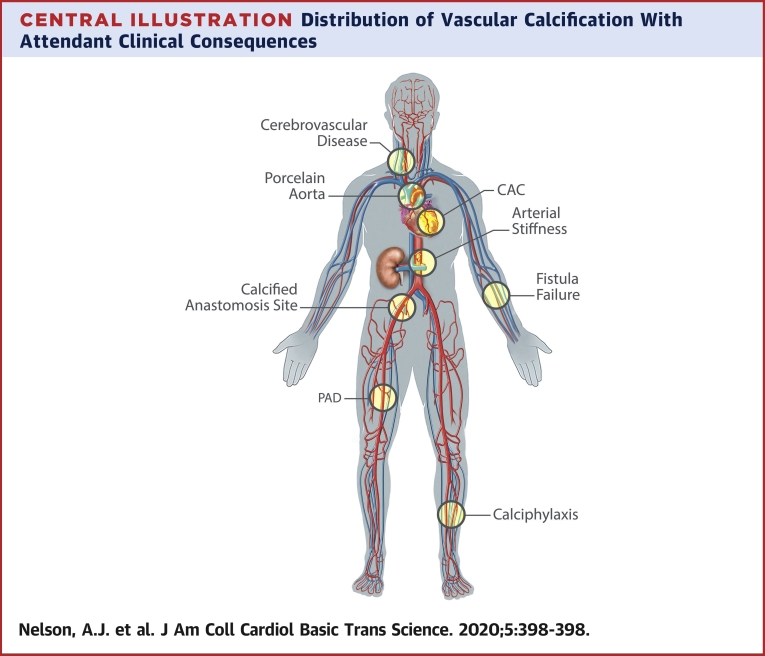
Unlike the often patchy distribution of intimal calcification, medial calcification tends to be more diffuse, forming sheets in topographic areas typically devoid of lipid or atherosclerotic change (26) (Central Illustration). Although medial calcification is seen in vessels of all calibers, it is conspicuous for location in territories usually spared from atherosclerosis, such as the internal mammary, radial, and digital arteries. Medial calcification is more specific to CKD because it appears to be associated with calcium-phosphate exposure, abnormal bone and mineral metabolism, severity of CKD, and dialysis vintage. In addition to CKD, medial calcification is also observed with diabetes mellitus and advanced age; these 3 entities potentially share linking pathophysiology of chronic inflammation and cellular senescence (32). Compared with intimal calcification, medial calcification is rarely associated with local luminal compromise, and instead is linked to the systemic manifestations of increased arterial stiffness. Following seminal observations linking the presence of (mostly medial) vascular calcification with increased arterial stiffness (33,34), the latter has been considered a functional surrogate for the former. A reduction in vascular compliance augments systolic blood pressure, which increases cardiac work and causes left ventricular hypertrophy (35) (Figure 3). This is a finding observed in up to 75% of patients with higher than or equal to stage 3 CKD (36), providing a mechanistic basis to the increased rates of heart failure (37) and atrial fibrillation observed in CKD (38).
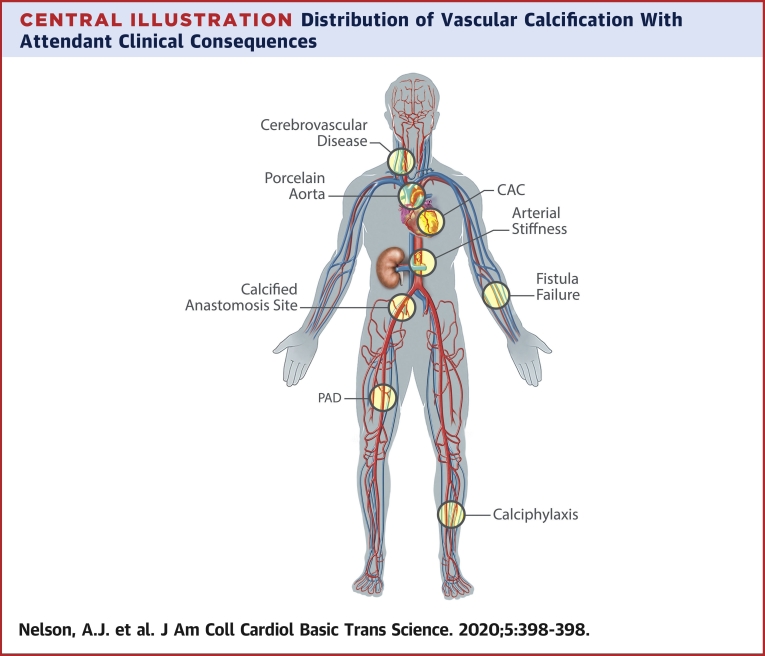
Central Illustration.
Distribution of Vascular Calcification With Attendant Clinical Consequences
Vascular calcification at the carotid vessels is associated with increased risk of stroke (141). Involvement of the proximal aorta can cause a porcelain aorta that can prohibit cardiothoracic surgery (142). Calcification of the coronary arteries has been linked to increased cardiovascular and all-cause mortality and locally may cause increased atherothrombosis. Calcification of the aorta and the distal vessels is associated with increased arterial stiffness. Calcification of the iliofemoral vessels at the site of anastomosis has been associated with graft failure and worse transplantation outcomes (143,144). Calcification of the radial artery and fistula site is more generally associated with early fistula failure (145,146). Calcification of the lower limb arteries is associated with the development of peripheral arterial disease (PAD) (claudication, limb ischemia) as well as arterial stiffness (147,148). Calciphylaxis is a severe and accelerated form of calcification, predominantly localized in the medial layer of skin arterioles and commonly affects the lower limbs but can occur anywhere. CAC = coronary artery calcification.
Detection and Prognosis
Most vascular calcification is detected incidentally through imaging obtained for other indications. In the research setting, several methods have been developed to quantitate vascular calcification and stratify risk. The most studied is the Agatston score (39) (often synonymous with “calcium score”) for coronary artery calcification on CT scans. However, the Kauppila index (40) for abdominal aortic calcification and the Adragao score (41) for both lower abdominal aorta and peripheral arteries have also been shown to have a prognostic value. As alluded to earlier, noninvasive imaging is unable to discern intimal from medial calcification; a limitation that is amplified in CKD in which there is a greater degree of coexistence of both processes, particularly in the coronary arteries, peripheral arteries, and the aorta. For this reason, there has been enthusiasm for evaluating arterial beds that are generally devoid of atherosclerosis, thereby providing more specific measures of medial calcification. Another approach has been to assess the functional consequences of calcification by evaluating indexes of arterial stiffness, reviewed elsewhere in detail (42).
CT coronary artery calcification score
Over 30 years of data in the general population has shown that CT coronary artery calcification (CAC) scoring with the Agatston score closely correlates with the atherosclerotic plaque burden, is linearly and independently associated with CV outcomes, and may outperform other established biomarkers for CV risk prognostication, including the ankle−brachial index, high-sensitivity C-reactive protein, and carotid intima media thickness (43). Seminal observations in the 1990s reported that patients with ESKD who received dialysis had an average CAC score that was an order of magnitude greater than what was generally considered a high-risk threshold (4,290 ± 1,509 Agatston units vs. >400 Agatston units) (44). Findings some years later affirmed the presence of a graded relationship between severity of CKD and CAC score (45,46). Until recently, data on the prognostic role of CAC in CKD was conflicting because of single-center studies, small sample sizes, or recruitment of narrow CKD severity grades (47). In the largest study of patients with CKD published in 2017, investigators evaluated the CAC scores of 1,541 participants with an eGFRs of 20 to 70 ml/min/1.73 m2 without established CVD as part of the CRIC Chronic Renal Insufficiency Cohort) trial (48). Over a median follow-up of 6 years, a total of 60 myocardial infarctions, 120 index heart failure events, 27 strokes, and 137 all-cause deaths occurred. There was a stepwise association between CAC severity (CAC score 0, 0 to 100, >100) and composite CV outcome. In multivariable modeling, the relative hazard ratios (HRs) associated with a 1 SD of log CAC were 1.40 (95% confidence interval [CI]: 1.16 to 1.69; p < 0.001) for the composite CVD outcome (myocardial infarction, heart failure, and stroke), 1.44 (95% CI: 1.02 to 2.02; p = 0.04) for myocardial infarction, and 1.39 (95% CI: 1.10 to 1.76; p = 0.006) for heart failure. CAC was not associated with all-cause mortality. This study from CRIC, as did another study (49), found CAC offered incremental prognostic value for CV events beyond traditional risk factors. The similar magnitude hazard ratios for atherosclerotic CVD and for HF suggest CAC scoring in the CKD population may be less specific for atherosclerotic burden and instead be an integrated marker of both medial and intimal calcification. This is consistent with autopsy findings of patients with established coronary artery disease in which high rates of coexistent medial calcification was observed only in those with CKD (50).
Reduction in radiation dose, application of semi-automated scoring mechanisms, ease of access, and validated age-standardization have resulted in the CT CAC score becoming the gold standard endpoint for trials that evaluated change in vascular calcification.
Plane radiography
The Kauppila score is a semiquantitative scoring method that attributes an ordinal value to calcification (0 to 3) at 8 sites along the abdominal aorta (total maximal score 24) as viewed on a lateral lumbar spine plane radiograph (40). Although the score has reasonable interobserver reproducibility, the progressive reduction in vertebral height with age (thus, length of quantified aorta) and superimposition of the vertebra on the aorta are known shortcomings. Studies in patients with ESKD have shown stepwise increases in abdominal aortic calcification (AAC) that correlate with CAC score (51) and that associate with worse CV outcomes (52). However, there are few data to support its accuracy at discerning temporal trends, particularly in non-ESKD cohorts in which calcification may be more modest, nor has there been formal validation of the Kauppila score against a quantitative method. Another x-ray−based scoring system that was described by Adragao involves semiquantitative scoring of linear calcification on pelvic and hand radiographs (41). The deliberate focus on pattern of calcification and inclusion of hand vessels increases the specificity for medial calcification, which is a theory supported by its correlation with arterial stiffness. In 1 study of 742 participants with ESKD, an elevation in either the Adragao (≥3) or Kauppila (≥6) score was associated with all-cause and CV mortality; however, only the Adragao score remained significant after adjustment for covariates (HR: 3.46; 95% CI: 1.27 to 9.45; p = 0.02) (53).
Breast imaging
Breast arterial calcification is an attractive method for measuring exclusively medial artery calcification because atherosclerosis has not been shown to occur in these vessels. Studies in patients with advanced CKD confirm mammography is not only capable of sensitive, temporal measurement of breast arterial calcification compared with CT (54), but also demonstrates that breast arterial calcification is strongly associated with both measures of peripheral arterial calcification (55) and CV outcomes (56). Although such a modality may inform mechanistic insights around the natural history of medial calcification, this is a modality limited to the assessment of women, and at present, has only been retrospectively analyzed.
Screening
Proponents of routine screening for vascular calcification among patients with CKD suggest there is significant incremental value in identifying a high-risk CV cohort. Conversely, others have argued that all patients with CKD should be considered at the highest CV risk and, in the absence of specific vascular calcification therapies, screening for vascular calcification does not influence management. Routine vascular calcification screening using either lateral pelvic x-ray or CT imaging received a weak (level 2C) recommendation in the 2017 Kidney Disease Improving Global Outcomes guidelines, reflecting the paucity of outcomes-driven data (57,58). The wording used in the 2017 updated guidelines was nearly identical to that used in the 2009 Guidelines, highlighting the lack of progress in the search for specific therapies proven to reverse, arrest, or attenuate vascular calcification (59).
Mechanisms
Our understanding of vascular calcification has evolved from a benign, passive deposition of minerals to what is now regarded as a pathological, tightly regulated, and cell-mediated process that resembles bone formation and turnover. Despite this growth in understanding, the temporal sequence of events and the exact cellular steps remain incompletely understood and the focus of ongoing research. Moreover, the observed events are unlikely to occur in a stepwise manner and instead are more likely to represent synchronous and interrelated processes (Table 1).
Table 1.
Key Mediators of Medial Vascular Calcification
| Phosphate, bone, and mineral metabolism |
| Failure of/reduction in calcification inhibitors |
| Matrix Gla protein |
| Fetuin-A |
| Klotho (± FGF-23) |
| Pyrophosphate |
| Osteopontin |
| Increase in calcification promoters |
| Osteocalcin |
| Alkaline phosphatase |
| Inflammatory cytokines |
| Runx2 |
| Phenotypic transdifferentiation of VSMCs (osteochondroblastic change) |
| Pro-calcific matrix vesicles |
| Uremic milieu |
| Cellular apoptosis |
| Pro-calcific miRNAs |
FGF = fibroblast growth factor; miRNA = micro RNA; VSMC = vascular smooth muscle cell.
Phosphate and mineral metabolism
Phosphate excretion is regulated at the proximal tubule of the kidney; however, the balance of bone formation and resorption is an important determinant of serum levels. Hormonal regulation of phosphate at the renal and gastrointestinal levels via parathyroid hormone (PTH), fibroblast growth factor (FGF)-23, klotho and 1,25-dihydroxyvitamin D (calcitriol) maintains this mineral in a narrow serum range (2.5 to 4.5 mg/dl). As impaired kidney function progresses to advanced stages, with reduction in functional nephron mass, phosphate excretion is impaired, bone remodeling slows, and together with persistent dietary intake, serum phosphate concentrations rise. Multiple studies have suggested that there is a link between hyperphosphatemia and the propensity for accelerated vascular calcification (46,60), particularly in patients on hemodialysis (61,62). Because phosphate is a key building block of hydroxyapatite crystals, it is conceivable that increased serum levels alone in the context of CKD could contribute directly to precipitation of hydroxyapatite in tissue (63). Although this may occur to some degree, not all patients with sustained hyperphosphatemia develop vascular calcification; seminal in vitro observations suggest a more active process wherein exposure to elevated phosphate induces a dose- and time-dependent phenotypic change in vascular smooth muscle cells (VSMCs) (64). The presence of a pro-calcific CKD milieu permits (and potentially promotes) the upregulation of phosphate channels (Pit-1 and Pit-2) which, in the context of hyperphosphatemia, mediates transdifferentiation of VSMCs to an osteochodrogenic cell phenotype (65); an event that can be, in part, inhibited by competitive antagonism of Pit-1 (64).
Beyond phosphate, other metabolic features of the mineral-bone axis have been implicated in vascular calcification, including calcium and PTH, although their effects may be more complex. Several lines of evidence support a direct role of PTH: elevated PTH is associated with higher rates of vascular calcification (66); and animal models of synthetic PTH infusion develop extensive calcification regardless of subtotal nephrectomy/sham and independent of presence or absence of hypercalcemia (67). In subtotally nephrectomized rats, submaximal suppression of PTH by treatment with a calcimimetic or parathyroidectomy slows rates of aortic calcification independent of serum calcium and phosphate concentrations (68). That stated, vascular calcification may also be potentiated in the setting of overzealous treatment of hyperparathyroidism, especially with excessive use of calcium-containing phosphate binders, which results in low bone turnover and reduced mineralization (69). Although complex and incompletely understood, states of low bone turnover and reduced mineralization are likely to attenuate the skeletal capacity for effective calcium and phosphate homeostasis. A reduction in skeletal buffering capacity may expose the vasculature to greater fluctuations in extracellular calcium and phosphate, thereby increasing the propensity for vascular calcification (70).
VSMCs
As alluded to, the transdifferentiation of VSMCs into an osteoblast-like secretory phenotype is central to the underlying pathophysiology of vascular calcification. This process is marked by downregulation of smooth muscle genes and subsequent expression of bone markers Runx2, osteopontin, osteocalcin, and alkaline phosphatase. The biology governing VSMC phenotypic switching remains to be fully appreciated; however, a number of triggers have been identified, including oxidative stress, imbalance of pro-calcific and/or anticalcific mediators (see the following), pro-calcific microRNAs, advanced glycation end products, cellular senescence [potentially through Prelamin A (71)], and hyperphosphatemia (as previously described). Once established, these osteoblast and/or chondrocyte-like cells behave in a manner consistent with other bone-forming cells by producing a collagen matrix through the secretion of calcium and phosphorous-laden vesicles (see the following).
Failure of inhibitory mechanisms
Mineralization occurs throughout the body at physiological calcium and phosphate levels; thus, a dynamic balance of promoters and inhibitors are required to ensure mineralization is supported at desired sites (bone) and is prevented from occurring elsewhere (ectopic, metastatic). Two prominent inhibitors are fetuin-A and matrix Gla protein (MGP). Fetuin-A is a glycoprotein synthesized in the liver that is recycled by VSMCs where it acts as a mineral chaperone, binding serum calcium in calciprotein complexes and preventing it from crystallization (72). Mice deficient in fetuin-A develop widespread calcification (73), whereas the addition of fetuin-A to bovine VSMC lines provides dose-dependent inhibition of calcification (74). Supporting data in patients with CKD suggest levels of fetuin-A are inversely associated with vascular calcification (75), and low levels have been linked to inflammation and all-cause mortality in the dialysis population (76).
MGP is a widely expressed protein and has been shown to accumulate in calcified tissue. Its precise role remains unconfirmed; however, the two prevailing, potentially synergistic, theories are that MGP may bind calcium ions and calcium crystals, thereby inhibiting crystal growth (77), or that MGP may interfere with bone morphogenic protein(s) signaling and prevent unwanted cell-induced mineralization (78). Animal MGP-knockout models developed rapid and extensive aortic medial calcification, which is associated with rupture and early hemorrhagic death (79). In vitro studies showed that MGP was downregulated in states of vitamin K deficiency and had less affinity for calcium in the setting of hyperphosphatemia, which are 2 common abnormalities observed in CKD (80,81). MGP requires vitamin K−dependent carboxylation to assume its inhibitory qualities, thus a reduction in MGP activity may not necessarily relate to absolute levels but to functional decreases through persistence of a decarboxylated state. Supportive translational data demonstrated the administration of vitamin K antagonists (e.g., warfarin) was associated with increased rates of vascular calcification in cell lines, animal models, and patients (82,83). These findings subsequently spawned interest in the potential therapeutic capacity of vitamin K supplementation (see the following).
Other known inhibitors of vascular calcification include pyrophosphate and osteopontin. Pyrophosphate is produced by arterial smooth muscle and directly inhibits hydroxyapatite formation (84). Osteopontin is a phosphoprotein that regulates mineralization through multiple functions, including mediating angiogenesis and responses to inflammation and mechanical stress. The relationship between osteopontin and vascular calcification appears complex. Although there is evidence of upregulation in calcified vessels (85), serum levels do not readily correlate with calcific burden (86).
Matrix vesicles and apoptosis
Matrix vesicles, a form of extracellular vesicle observed in the vascular wall, have been more broadly implicated in both transdifferentiation of VSMCs and the calcification environment (87). Extracellular vesicles are phospholipid membrane-bound particles, have molecular cargo (protein, RNA, or lipid), and are released by many cells and cell types in response to cell activation or apoptosis. Under pro-calcifying conditions the vesicles released by VSMCs are altered and resemble those released by osteoblasts (88). These vesicles act as nucleating points that have the capacity for calcium binding and extracellular matrix production. There is emerging evidence that these vesicles may permit cross-talk among VSMCs but potentially also between endothelial cells and other vascular cells (89), lending biological support for bidirectional positive feedback between intimal and medial calcification (90). In vesicles that contain genotypic and/or phenotypic information such as microRNA, the content of the specific sequences released by VMSCs may either induce or inhibit nearby pro-calcific phenotypic change (91). An additional paracrine-type effect may be mediated through osteoblastic- and/or osteochrondoblastic-like cell activity on surrounding tissue. Subsequent changes in relative collagen composition and the degradation of elastin (92) have been shown to promote hydroxyapatite formation and provide scaffolding for further mineralization (93).
FGF-23 and klotho
Most FGF-23 is produced by bone (94) and although the mediators of its release remain incompletely understood (95), associations with PTH (96), iron deficiency (97), calcium (98), and vitamin D (99) have been consistently observed. The primary function of FGF-23 is to orchestrate phosphate and calcium homeostasis by stimulating urinary phosphate excretion and suppressing circulating concentrations of calcitriol (100). The activity of FGF-23 to regulate phosphate homeostasis in the kidney requires the presence of klotho, a co-receptor that facilitates binding of FGF-23 to the FGF-receptor (101). FGF-23 excess is associated with poor outcomes in CKD (102), although its links to vascular calcification are less clear, and multiple studies have conveyed conflicting results. Human and animal VSMCs exposed to FGF-23 in the presence or absence of klotho, and in the presence of normal or high phosphate, showed increased, decreased, or no effect at all on vascular calcification (103, 104, 105, 106, 107). These conflicting results posit a more complex role of FGF-23 in vascular calcification; however, it is also possible that FGF-23 is not causal factor at all. Unlike FGF-23, in vitro and in vivo evidence support a protective role of klotho in vascular calcification. Addition of klotho to a rat VSMC line directly suppressed Pit-1 and Pit-2 activity and subsequently prevented phosphate-induced osteogenic transdifferentiation. In addition, klotho knockout models demonstrated increased expression of Pit-1 and Pit-2 receptors, which suggested that the progressive klotho deficiency observed in advancing CKD might result in upregulation of these receptors, which would subsequently promote phosphate uptake and drive VSMC transdifferentiation (108).
Therapeutic Approaches
A large investment into targeting intermediates of bone and mineral metabolism over several years has yielded little more than possible slowing of vascular calcification. Critically, neither evidence of halting nor evidence of regression has been observed. Further data questioning the validity of a passive (or restorative) therapeutic approach comes from elegant animal work in which calcified aortas from uremic mice were orthotopically transplanted into non-CKD mice and observed for 34 weeks (109). Although there was some superficial loss of calcium, there was no significant active resorption or regression of established calcified salts.
Despite the failures to date, novel therapeutic approaches are emerging that actively target calcified material and may offer fresh insights into drug discovery in this area of unmet need.
Calcimimetics
The calcium-sensing receptor (CaR) is expressed in multiple tissues but particularly in the parathyroid glands, which makes it an effective target for treating secondary hyperparathyroidism and disordered mineral metabolism in patients with ESKD. Calcimimetics bind to the CaR and allosterically increase the parathyroid cell sensitivity to extracellular calcium, thereby suppressing the release of PTH and resulting in a reduction of serum calcium. In patients with ESKD, calcimimetics also decrease serum phosphate, presumably through a reduction in PTH-mediated bone resorption, which otherwise releases bone phosphate into the circulation. Beyond modulating PTH release, the CaR is also found on VSMCs (110) and its stimulation is associated with a reduction in phosphate- and calcium-driven vascular mineralization both in vitro and in animal models (111,112).
These findings prompted the design and execution of 2 key clinical studies that evaluated cinacalcet: ADVANCE (A Randomised Study to Evaluate the Effects of Cinacalcet Plus Low Dose Vitamin D on Vascular Calcification in Subjects with Chronic Kidney Disease Receiving Hemodialysis) and EVOLVE (Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events). ADVANCE enrolled 360 patients who received hemodialysis across 3 continents with secondary hyperparathyroidism and CAC (113). Participants were randomized to open-label cinacalcet and low-dose vitamin D analogs or flexible vitamin D therapy and were followed for 12 months. At follow-up, there was a smaller numerical increase (i.e., attenuation of progression) in the CAC score of the cinacalcet-treated patients compared with those treated with flexible vitamin D therapy; however, this did not reach statistical significance (progression of CAC: 24%; 95% CI: −22% to 119% vs. 31% (95% CI: −9% to 179%; p = 0.073). A secondary analysis by CAC volume score in the coronaries and the aortic valve suggested a potential beneficial effect of cinacalcet, although this was only of nominal significance. It remained unclear if excess vitamin D use might have mitigated the benefits of cinacalcet, or whether a 12-month follow-up might have been too short to observe a significant change. In comparison, the EVOLVE trial randomized 3,883 patients with secondary hyperparathyroidism on maintenance hemodialysis to cinacalcet or placebo in the setting of conventional therapy (e.g., phosphate binders and calcitriol or active vitamin D analogs) (114). After a median follow-up of 17 (placebo) to 21 (cinacalcet) months, there was an observed trend for relative reduction in the HR for the primary composite CV outcome (i.e., time until death, myocardial infarction, hospitalization for unstable angina, heart failure, or a peripheral vascular event) (HR: 0.93; 95% CI: 0.85 to 1.02; p = 0.11). Post hoc adjustment for age yielded lower relative HRs with nominally significant results in favor of cinacalcet, as did the analyses that accounted for differential withdrawal of study drug and commercial use of cinacalcet in the placebo arm.
Etelcalcetide, an intravenous calcimimetic that acts at a different site on the CaR, was highly efficacious at lowering PTH and FGF-23 in placebo-controlled trials (115,116), and in a head-to-head study, was more effective than cinacalcet on biochemical endpoints (117). The effects of etelcalcetide on vascular calcification or clinical events have not been evaluated.
Vitamin K
As previously described, vitamin K is required to decarboxylate MGP to obtain its calcification inhibitory capacity. Consistent with this physiology, both vitamin K antagonism and vitamin K deficiency have been linked to vascular calcification (118,119). In a biochemical proof-of-concept study conducted in 53 patients who underwent hemodialysis, daily supplementation of vitamin K was safe and resulted in a significant increase in MGP activation (120). Whether increased MGP activation translates into slowing of calcification is being tested in the VitaVasK study. This trial enrolled 348 patients who underwent hemodialysis with a CAC score of at least 100 and randomized them to either oral vitamin K1 orally thrice weekly supplementation or placebo (121). The primary endpoint is progression of thoracic aortic calcification and CAC at 18 months. Major adverse CV events will be assessed at 3 to 5 years after treatment initiation; results are expected towards the end of 2020. The other way of testing this hypothesis is to evaluate for differential effects on vascular calcification in patients who receive direct acting oral anticoagulants compared with vitamin K antagonists (e.g., warfarin). The largest trial evaluating this hypothesis is the IRIVASC-Trial (Rivaroxaban Compared to Vitamin K Antagonist Treatment Upon Development of Cardiovascular Calcification; NCT02066662), which has enrolled participants with an eGFR >15 ml/min/1.73 m2 and who have either atrial fibrillation or pulmonary embolism that requires systemic anticoagulation.
Phosphate binders
Patients with advanced CKD who have developed hyperphosphatemia frequently require treatment with phosphate binders when dietary restriction is inadequate. Because phosphate and the attendant increase in FGF-23 and PTH have been linked to vascular calcification (46,64) (as previously described), lowering or even maintaining stable phosphate levels near normal could be associated with favorable overall vascular outcomes. Serum phosphate can be reduced with either calcium-containing binders (acetate, carbonate) or calcium-free binders (sevelamer, lanthanum, iron compounds, magnesium). In the dialysis population, the totality of data suggest exposure to exogenous calcium through calcium-containing binders is associated with higher rates of vascular calcification (62,122,123). Although subsequent meta-analyses have shown a mortality benefit for calcium-free binders (124), it remains unclear whether this is due to increased risk in the comparator arms due to excess calcium loading or to an absolute benefit in the calcium free arms. Moreover, because of the degree of phosphate lowering has been similar across most patients in the trials, it is unclear whether any benefits at all are due to the management of phosphatemia (or potentially its effect on intermediates, such as FGF-23) or are instead due to agent-specific effects, such as improvements in lipid profile and C-reactive protein observed with sevalemer. Importantly, no clinical trial has demonstrated a long-term benefit of phosphate binders on clinical outcomes in ESKD nor has any trial established the optimal serum phosphate target for ESKD.
In the nondialysis CKD population, data are even less convincing. A recent trial that compared phosphate control with either calcium-containing or calcium-free binders against placebo in patients with stage 3 to 4 CKD showed only a modest reduction in phosphate and a paradoxical increase in CAC (albeit largest in those receiving calcium-containing binders) (125). A further trial is currently underway, IMPROVE-CKD (Impact of Phosphate Reduction on Vascular Endpoints in Chronic Kidney Disease), which is comparing lanthanum against placebo in patients with stage 3 to 4 CKD. This trial will evaluate arterial stiffness as the primary endpoint in 488 participants at 96 weeks with secondary endpoints that include aortic calcification, left ventricular mass using magnetic resonance imaging, and bone and/or phosphate markers among others (126). Whether IMPROVE-CKD will fill the evidence gap between control (or reduction) of serum phosphate with slowing or halting of vascular calcification, or potentially further highlight the limitations of serum phosphate as an overall measure of the total phosphate pool (and thus, propensity to calcification) remains to be seen. Adding complexity is the recently completed COMBINE (CKD Optimal Management With Binders and Nicotinamide study) trial that evaluated a similar cohort of patients with stage 3 to 4 CKD; not only was lanthanum poorly tolerated (despite the addition of nicotinamide), there was minimal effect on phosphate or FGF-23 over 12 months (127).
Magnesium
Recent studies have highlighted a potential role for magnesium to prevent vascular calcification. In vitro (128, 129, 130) and animal studies (131) showed that magnesium modulates the development of phosphate-induced calcification in a dose-dependent manner. Small human clinical studies have shown that oral administration of magnesium to patients with moderate CKD through to ESKD, either as a phosphate binder or as a supplement, directly slowed CAC progression or indirectly reduced the propensity for calcification (132, 133, 134). Based on this favorable signal, an open-label randomized controlled trial evaluated the effect of magnesium oxide on CAC progression in patients with CKD stages 3 to 4 (135). The trial was stopped early when the control arm demonstrated a median CAC change of 39.5% (interquartile range: 19.0% to 81.3%), whereas the magnesium arm showed a progression of only 11.3% (interquartile range: 0% to 30.8%; p < 0.001). Because this was an open label trial that was halted early and included <100 participants, larger studies are required to confirm and progress these findings.
SNF472: myo-inositol hexaphosphate
A compound with promising results in early phase studies is SNF472, which is a hexasodium salt of the active ingredient, myo-inositol hexaphosphate (IP6), or phytate. SNF472 inhibits the development and progression of calcification by binding to growth sites of hydroxyapatite crystal; this mechanism appears to be agnostic to the underlying cause of calcification and may represent an opportunity to inhibit the final common pathway of vascular calcification.
Pre-clinical studies demonstrated that the intravenous administration of SNF472 to vitamin D−supplemented rodent models reduced the development of CV calcification (136) and prevented progression of established calcification (137). Recently, the results of the CaLIPSO (Cal for calcium and ipso meaning the item itself) trial were reported; in this Phase IIb study, 274 patients with ESKD who underwent dialysis were randomized to SNF472 administered at 2 different doses (300 mg or 600 mg) or placebo (1:1:1) for 52 weeks (138). For the primary endpoint at 12 months, using data from the combined dose group, administration of SNF472 resulted in significant slowing of progression of CAC (11%: 95% CI: 7% to 15% vs. 20%; 95% CI 14% to 26%; p = 0.016). Secondary endpoints also showed slowing of progression of aortic valve calcification (14%; 95% CI: 5% to 24% vs. 98%; 95% CI: 77% to 123%; p < 0.001) and a directionally consistent but nonsignificant difference in progression of thoracic aorta calcification (23%; 95% CI: 16% to 30% vs. 28%; 95% CI: 19% to 38%; p = 0.40). Notwithstanding these promising data, larger studies are required to delineate the clinical efficacy and safety of this compound.
Conclusions
Biologically plausible, temporal, and dose-dependent relationships exist between vascular calcification and CV outcomes in patients with CKD. Despite a large investment in potential therapeutic strategies, little more than slowing of calcification has been documented in only a handful of previous studies.
The reasons for a lack of progress are multiple and varied. At a pre-clinical level, there are a lack of animal models that faithfully recapitulate a chronic, progressive CKD calcification process. The extension of pre-clinical findings derived from the current acute injury models may be misleading and translate to early clinical phase failure. The modification of an adenine murine model that does not require transgenic manipulation or surgical intervention appears promising (139). From an imaging perspective, a metric that reliably and quantitatively measures medial calcification with fidelity over time would be a welcome addition to trial endpoints of novel therapies. That said, currently enrolling studies have included imaging of multiple vascular beds or included functional assessments of arterial stiffness. A combination of these predominantly intimal versus predominantly medial modalities will provide unique insights into natural history, relative modifiability and potential differential drug efficacy.
Furthermore, conventional thinking about vascular calcification has been relatively uni-dimensional, aiming to restore or reverse components of the uremic milieu in individuals in whom calcification is established, and inhibitory mechanisms exhausted. The underwhelming results may be due to late intervention at a point of inexorable vascular damage, in which case trials such as IMPROVE-CKD may demonstrate the potential benefits of earlier, more preventative intervention. Alternatively, because of the multiple and synergistic processes involved in the development of vascular calcification in CKD, addressing individual upstream factors in isolation, even if dominant, may not be sufficient. Instead, actively targeting the final common pathway of calcification, such as with an anticalcifying agent agnostic to the upstream processes, may provide critical insight on this type of therapeutic approach.
After decades of translational research fueling heated debate and polarized opinion, the next wave of evidence may finally provide the answer to whether arterial calcification, particularly in the media, sits on the causal pathway of CVD in CKD. Perhaps the more critical question in managing the growing number of patients with CKD is whether targeting vascular calcification will lead to meaningful improvements in CV outcomes.
Footnotes
Dr. Raggi has been a member of the Advisory Board for Sanifit. Dr. Chertow has been an advisor for Sanifit. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
References
- 1.Levey A.S., Eckardt K.U., Tsukamoto Y. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 2.Luyckx V.A., Tonelli M., Stanifer J.W. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96:414−22D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills K.T., Xu Y., Zhang W. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. doi: 10.1038/ki.2015.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turin T.C., Tonelli M., Manns B.J., Ravani P., Ahmed S.B., Hemmelgarn B.R. Chronic kidney disease and life expectancy. Nephrol Dial Transplant. 2012;27:3182–3186. doi: 10.1093/ndt/gfs052. [DOI] [PubMed] [Google Scholar]
- 5.Xie Y., Bowe B., Mokdad A.H. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Matsushita K., Sang Y., Ballew S.H. Subclinical atherosclerosis measures for cardiovascular prediction in CKD. J Am Soc Nephrol. 2015;26:439–447. doi: 10.1681/ASN.2014020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cozzolino M., Mangano M., Stucchi A., Ciceri P., Conte F., Galassi A. Cardiovascular disease in dialysis patients. Nephrol Dial Transplant. 2018;33:iii28−34. doi: 10.1093/ndt/gfy174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modi Z.J., Lu Y., Ji N. Risk of cardiovascular disease and mortality in young adults with end-stage renal disease: an analysis of the US Renal Data System. JAMA Cardiol. 2019;4:353–362. doi: 10.1001/jamacardio.2019.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargiulo R., Suhail F., Lerma E.V. Cardiovascular disease and chronic kidney disease. Dis Mon. 2015;61:403–413. doi: 10.1016/j.disamonth.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Keith D.S., Nichols G.A., Gullion C.M., Brown J.B., Smith D.H. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med. 2004;164:659–663. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 11.Briasoulis A., Bakris G.L. Chronic kidney disease as a coronary artery disease risk equivalent. Curr Cardiol Rep. 2013;15:340. doi: 10.1007/s11886-012-0340-4. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw S.M., Cruz D.N., Aspromonte N. Epidemiology of cardio-renal syndromes: workgroup statements from the 7th ADQI Consensus Conference. Nephrol Dial Transplant. 2010;25:1406–1416. doi: 10.1093/ndt/gfq066. [DOI] [PubMed] [Google Scholar]
- 13.Dad T., Weiner D.E. Stroke and chronic kidney disease: epidemiology, pathogenesis, and management across kidney disease stages. Semin Nephrol. 2015;35:311–322. doi: 10.1016/j.semnephrol.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herzog C.A., Asinger R.W., Berger A.K. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 15.Das M., Aronow W.S., McClung J.A., Belkin R.N. Increased prevalence of coronary artery disease, silent myocardial ischemia, complex ventricular arrhythmias, atrial fibrillation, left ventricular hypertrophy, mitral annular calcium, and aortic valve calcium in patients with chronic renal insufficiency. Cardiol Rev. 2006;14:14–17. doi: 10.1097/01.crd.0000148162.88296.9f. [DOI] [PubMed] [Google Scholar]
- 16.Pun P.H., Smarz T.R., Honeycutt E.F., Shaw L.K., Al-Khatib S.M., Middleton J.P. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76:652–658. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox C.S., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380:1662–1673. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmoodi B.K., Matsushita K., Woodward M. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without hypertension: a meta-analysis. Lancet. 2012;380:1649–1661. doi: 10.1016/S0140-6736(12)61272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fellstrom B.C., Jardine A.G., Schmieder R.E. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 21.Wanner C., Krane V., Marz W. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 22.Sigrist M., Bungay P., Taal M.W., McIntyre C.W. Vascular calcification and cardiovascular function in chronic kidney disease. Nephrol Dial Transplant. 2006;21:707–714. doi: 10.1093/ndt/gfi236. [DOI] [PubMed] [Google Scholar]
- 23.Garland J.S., Holden R.M., Groome P.A. Prevalence and associations of coronary artery calcification in patients with stages 3 to 5 CKD without cardiovascular disease. Am J Kidney Dis. 2008;52:849–858. doi: 10.1053/j.ajkd.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 24.Raggi P., Bellasi A., Gamboa C. All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol. 2011;6:1990–1995. doi: 10.2215/CJN.01140211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sage A.P., Tintut Y., Demer L.L. Regulatory mechanisms in vascular calcification. Nat Rev Cardiol. 2010;7:528–536. doi: 10.1038/nrcardio.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Amann K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin J Am Soc Nephrol. 2008;3:1599–1605. doi: 10.2215/CJN.02120508. [DOI] [PubMed] [Google Scholar]
- 27.Shroff R., Long D.A., Shanahan C. Mechanistic insights into vascular calcification in CKD. J Am Soc Nephrol. 2013;24:179–189. doi: 10.1681/ASN.2011121191. [DOI] [PubMed] [Google Scholar]
- 28.Tvedegaard E. Arterial disease in chronic renal failure--an experimental study in the rabbit. Acta Pathol Microbiol Immunol Scand A. 1987;290:1–28. [PubMed] [Google Scholar]
- 29.Bernelot Moens S.J., Verweij S.L., van der Valk F.M. Arterial and cellular inflammation in patients with CKD. J Am Soc Nephrol. 2017;28:1278–1285. doi: 10.1681/ASN.2016030317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori H., Torii S., Kutyna M., Sakamoto A., Finn A.V., Virmani R. Coronary artery calcification and its progression: what does it really mean? J Am Coll Cardiol Img. 2018;11:127–142. doi: 10.1016/j.jcmg.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Baber U., Stone G.W., Weisz G. Coronary plaque composition, morphology, and outcomes in patients with and without chronic kidney disease presenting with acute coronary syndromes. J Am Coll Cardiol Img. 2012;5:S53−61. doi: 10.1016/j.jcmg.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 32.Shanahan C.M. Mechanisms of vascular calcification in CKD-evidence for premature ageing? Nat Rev Nephrol. 2013;9:661–670. doi: 10.1038/nrneph.2013.176. [DOI] [PubMed] [Google Scholar]
- 33.Blacher J., Guerin A.P., Pannier B., Marchais S.J., London G.M. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38:938–942. doi: 10.1161/hy1001.096358. [DOI] [PubMed] [Google Scholar]
- 34.Guerin A.P., London G.M., Marchais S.J., Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15:1014–1021. doi: 10.1093/ndt/15.7.1014. [DOI] [PubMed] [Google Scholar]
- 35.Nelson A.J., Worthley S.G., Cameron J.D. Cardiovascular magnetic resonance-derived aortic distensibility: validation and observed regional differences in the elderly. J Hypertens. 2009;27:535–542. doi: 10.1097/hjh.0b013e32831e4599. [DOI] [PubMed] [Google Scholar]
- 36.Paoletti E., Bellino D., Cassottana P., Rolla D., Cannella G. Left ventricular hypertrophy in nondiabetic predialysis CKD. Am J Kidney Dis. 2005;46:320–327. doi: 10.1053/j.ajkd.2005.04.031. [DOI] [PubMed] [Google Scholar]
- 37.Ritz E. Left ventricular hypertrophy in renal disease: beyond preload and afterload. Kidney Int. 2009;75:771–773. doi: 10.1038/ki.2009.35. [DOI] [PubMed] [Google Scholar]
- 38.Baber U., Howard V.J., Halperin J.L. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 40.Kauppila L.I., Polak J.F., Cupples L.A., Hannan M.T., Kiel D.P., Wilson P.W. New indices to classify location, severity and progression of calcific lesions in the abdominal aorta: a 25-year follow-up study. Atherosclerosis. 1997;132:245–250. doi: 10.1016/s0021-9150(97)00106-8. [DOI] [PubMed] [Google Scholar]
- 41.Adragao T., Pires A., Birne R. A plain X-ray vascular calcification score is associated with arterial stiffness and mortality in dialysis patients. Nephrol Dial Transplant. 2009;24:997–1002. doi: 10.1093/ndt/gfn584. [DOI] [PubMed] [Google Scholar]
- 42.Townsend R.R. Arterial stiffness in CKD: a review. Am J Kidney Dis. 2019;73:240–247. doi: 10.1053/j.ajkd.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeboah J., McClelland R.L., Polonsky T.S. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA. 2012;308:788–795. doi: 10.1001/jama.2012.9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun J., Oldendorf M., Moshage W., Heidler R., Zeitler E., Luft F.C. Electron beam computed tomography in the evaluation of cardiac calcification in chronic dialysis patients. Am J Kidney Dis. 1996;27:394–401. doi: 10.1016/s0272-6386(96)90363-7. [DOI] [PubMed] [Google Scholar]
- 45.Budoff M.J., Rader D.J., Reilly M.P. Relationship of estimated GFR and coronary artery calcification in the CRIC (Chronic Renal Insufficiency Cohort) Study. Am J Kidney Dis. 2011;58:519–526. doi: 10.1053/j.ajkd.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raggi P., Boulay A., Chasan-Taber S. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J Am Coll Cardiol. 2002;39:695–701. doi: 10.1016/s0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 47.Bashir A., Moody W.E., Edwards N.C., Ferro C.J., Townend J.N., Steeds R.P. Coronary artery calcium assessment in CKD: utility in cardiovascular disease risk assessment and treatment? Am J Kidney Dis. 2015;65:937–948. doi: 10.1053/j.ajkd.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 48.Chen J., Budoff M.J., Reilly M.P. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol. 2017;2:635–643. doi: 10.1001/jamacardio.2017.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liabeuf S., Desjardins L., Diouf M. The addition of vascular calcification scores to traditional risk factors improves cardiovascular risk assessment in patients with chronic kidney disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura S., Ishibashi-Ueda H., Niizuma S., Yoshihara F., Horio T., Kawano Y. Coronary calcification in patients with chronic kidney disease and coronary artery disease. Clin J Am Soc Nephrol. 2009;4:1892–1900. doi: 10.2215/CJN.04320709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bellasi A., Ferramosca E., Muntner P. Correlation of simple imaging tests and coronary artery calcium measured by computed tomography in hemodialysis patients. Kidney Int. 2006;70:1623–1628. doi: 10.1038/sj.ki.5001820. [DOI] [PubMed] [Google Scholar]
- 52.Niu Q., Hong Y., Lee C.H., Men C., Zhao H., Zuo L. Abdominal aortic calcification can predict all-cause mortality and CV events in dialysis patients: a systematic review and meta-analysis. PLoS One. 2018;13 doi: 10.1371/journal.pone.0204526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorriz J.L., Molina P., Cerveron M.J. Vascular calcification in patients with nondialysis CKD over 3 years. Clin J Am Soc Nephrol. 2015;10:654–666. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manzoor S., Ahmed S., Ali A. Progression of medial arterial calcification in CKD. Kidney Int Rep. 2018;3:1328–1335. doi: 10.1016/j.ekir.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duhn V., D'Orsi E.T., Johnson S., D'Orsi C.J., Adams A.L., O'Neill W.C. Breast arterial calcification: a marker of medial vascular calcification in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:377–382. doi: 10.2215/CJN.07190810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abou-Hassan N., Tantisattamo E., D'Orsi E.T., O'Neill W.C. The clinical significance of medial arterial calcification in end-stage renal disease in women. Kidney Int. 2015;87:195–199. doi: 10.1038/ki.2014.187. [DOI] [PubMed] [Google Scholar]
- 57.Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2017;7:1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Uhlig K., Berns J.S., Kestenbaum B. KDOQI US commentary on the 2009 KDIGO Clinical practice guideline for the diagnosis, evaluation, and treatment of ckd-mineral and bone disorder (CKD-MBD) Am J Kidney Dis. 2010;55:773–799. doi: 10.1053/j.ajkd.2010.02.340. [DOI] [PubMed] [Google Scholar]
- 59.Kidney Disease: Improving Global Outcomes CKD-MBD Update Work Group KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD) Kidney Int Suppl. 2009:S1−130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 60.Adeney K.L., Siscovick D.S., Ix J.H. Association of serum phosphate with vascular and valvular calcification in moderate CKD. J Am Soc Nephrol. 2009;20:381–387. doi: 10.1681/ASN.2008040349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Block G.A., Klassen P.S., Lazarus J.M., Ofsthun N., Lowrie E.G., Chertow G.M. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 62.Chertow G.M., Raggi P., Chasan-Taber S., Bommer J., Holzer H., Burke S.K. Determinants of progressive vascular calcification in haemodialysis patients. Nephrol Dial Transplant. 2004;19:1489–1496. doi: 10.1093/ndt/gfh125. [DOI] [PubMed] [Google Scholar]
- 63.Shanahan C.M., Crouthamel M.H., Kapustin A., Giachelli C.M. Arterial calcification in chronic kidney disease: key roles for calcium and phosphate. Circ Res. 2011;109:697–711. doi: 10.1161/CIRCRESAHA.110.234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jono S., McKee M.D., Murry C.E. Phosphate regulation of vascular smooth muscle cell calcification. Circ Res. 2000;87:E10−7. doi: 10.1161/01.res.87.7.e10. [DOI] [PubMed] [Google Scholar]
- 65.Villa-Bellosta R., Bogaert Y.E., Levi M., Sorribas V. Characterization of phosphate transport in rat vascular smooth muscle cells: implications for vascular calcification. Arterioscler Thromb Vasc Biol. 2007;27:1030–1036. doi: 10.1161/ATVBAHA.106.132266. [DOI] [PubMed] [Google Scholar]
- 66.Jean G., Bresson E., Lorriaux C. Increased levels of serum parathyroid hormone and fibroblast growth factor-23 are the main factors associated with the progression of vascular calcification in long-hour hemodialysis patients. Nephron Clin Pract. 2012;120:c132–c138. doi: 10.1159/000334424. [DOI] [PubMed] [Google Scholar]
- 67.Neves K.R., Graciolli F.G., dos Reis L.M. Vascular calcification: contribution of parathyroid hormone in renal failure. Kidney Int. 2007;71:1262–1270. doi: 10.1038/sj.ki.5002241. [DOI] [PubMed] [Google Scholar]
- 68.Jung S., Querfeld U., Muller D., Rudolph B., Peters H., Kramer S. Submaximal suppression of parathyroid hormone ameliorates calcitriol-induced aortic calcification and remodeling and myocardial fibrosis in uremic rats. J Hypertens. 2012;30:2182–2191. doi: 10.1097/HJH.0b013e328357c049. [DOI] [PubMed] [Google Scholar]
- 69.Raggi P., James G., Burke S.K. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 70.Persy V., D'Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med. 2009;15:405–416. doi: 10.1016/j.molmed.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 71.Lin Y.J., Lo M.T., Lin C. Prevalence, characteristics, mapping, and catheter ablation of potential rotors in nonparoxysmal atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6:851–858. doi: 10.1161/CIRCEP.113.000318. [DOI] [PubMed] [Google Scholar]
- 72.Heiss A., Pipich V., Jahnen-Dechent W., Schwahn D. Fetuin-A is a mineral carrier protein: small angle neutron scattering provides new insight on fetuin-A controlled calcification inhibition. Biophys J. 2010;99:3986–3995. doi: 10.1016/j.bpj.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schafer C., Heiss A., Schwarz A. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. J Clin Invest. 2003;112:357–366. doi: 10.1172/JCI17202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moe S.M., Reslerova M., Ketteler M. Role of calcification inhibitors in the pathogenesis of vascular calcification in chronic kidney disease (CKD) Kidney Int. 2005;67:2295–2304. doi: 10.1111/j.1523-1755.2005.00333.x. [DOI] [PubMed] [Google Scholar]
- 75.Shroff R.C., McNair R., Figg N. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation. 2008;118:1748–1757. doi: 10.1161/CIRCULATIONAHA.108.783738. [DOI] [PubMed] [Google Scholar]
- 76.Ketteler M., Bongartz P., Westenfeld R. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003;361:827–833. doi: 10.1016/S0140-6736(03)12710-9. [DOI] [PubMed] [Google Scholar]
- 77.Price P.A., Urist M.R., Otawara Y. Matrix Gla protein, a new gamma-carboxyglutamic acid-containing protein which is associated with the organic matrix of bone. Biochem Biophys Res Commun. 1983;117:765–771. doi: 10.1016/0006-291x(83)91663-7. [DOI] [PubMed] [Google Scholar]
- 78.Zebboudj A.F., Imura M., Bostrom K. Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem. 2002;277:4388–4394. doi: 10.1074/jbc.M109683200. [DOI] [PubMed] [Google Scholar]
- 79.Luo G., Ducy P., McKee M.D. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 80.Paloian N.J., Giachelli C.M. A current understanding of vascular calcification in CKD. Am J Physiol Renal Physiol. 2014;307:F891−900. doi: 10.1152/ajprenal.00163.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Roy M.E., Nishimoto S.K. Matrix Gla protein binding to hydroxyapatite is dependent on the ionic environment: calcium enhances binding affinity but phosphate and magnesium decrease affinity. Bone. 2002;31:296–302. doi: 10.1016/s8756-3282(02)00821-9. [DOI] [PubMed] [Google Scholar]
- 82.Kruger T., Oelenberg S., Kaesler N. Warfarin induces cardiovascular damage in mice. Arterioscler Thromb Vasc Biol. 2013;33:2618–2624. doi: 10.1161/ATVBAHA.113.302244. [DOI] [PubMed] [Google Scholar]
- 83.Rennenberg R.J., van Varik B.J., Schurgers L.J. Chronic coumarin treatment is associated with increased extracoronary arterial calcification in humans. Blood. 2010;115:5121–5123. doi: 10.1182/blood-2010-01-264598. [DOI] [PubMed] [Google Scholar]
- 84.Meyer J.L. Can biological calcification occur in the presence of pyrophosphate? Arch Biochem Biophys. 1984;231:1–8. doi: 10.1016/0003-9861(84)90356-4. [DOI] [PubMed] [Google Scholar]
- 85.Giachelli C.M., Speer M.Y., Li X., Rajachar R.M., Yang H. Regulation of vascular calcification: roles of phosphate and osteopontin. Circ Res. 2005;96:717–722. doi: 10.1161/01.RES.0000161997.24797.c0. [DOI] [PubMed] [Google Scholar]
- 86.Barreto D.V., Lenglet A., Liabeuf S. Prognostic implication of plasma osteopontin levels in patients with chronic kidney disease. Nephron Clin Pract. 2011;117:c363−72. doi: 10.1159/000321520. [DOI] [PubMed] [Google Scholar]
- 87.Schurgers L.J., Akbulut A.C., Kaczor D.M., Halder M., Koenen R.R., Kramann R. Initiation and propagation of vascular calcification is regulated by a concert of platelet- and smooth muscle cell-derived extracellular vesicles. Front Cardiovasc Med. 2018;5:36. doi: 10.3389/fcvm.2018.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kapustin A.N., Chatrou M.L., Drozdov I. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res. 2015;116:1312–1323. doi: 10.1161/CIRCRESAHA.116.305012. [DOI] [PubMed] [Google Scholar]
- 89.Lin X., He Y., Hou X., Zhang Z., Wang R., Wu Q. Endothelial cells can regulate smooth muscle cells in contractile phenotype through the miR-206/ARF6&NCX1/exosome axis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bardeesi A.S.A., Gao J., Zhang K. A novel role of cellular interactions in vascular calcification. J Transl Med. 2017;15:95. doi: 10.1186/s12967-017-1190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goettsch C., Hutcheson J.D., Aikawa E. MicroRNA in cardiovascular calcification: focus on targets and extracellular vesicle delivery mechanisms. Circ Res. 2013;112:1073–1084. doi: 10.1161/CIRCRESAHA.113.300937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hodroge A., Trecherel E., Cornu M. Oligogalacturonic acid inhibits vascular calcification by two mechanisms: inhibition of vascular smooth muscle cell osteogenic conversion and interaction with collagen. Arterioscler Thromb Vasc Biol. 2017;37:1391–1401. doi: 10.1161/ATVBAHA.117.309513. [DOI] [PubMed] [Google Scholar]
- 93.Pai A.S., Giachelli C.M. Matrix remodeling in vascular calcification associated with chronic kidney disease. J Am Soc Nephrol. 2010;21:1637–1640. doi: 10.1681/ASN.2010040349. [DOI] [PubMed] [Google Scholar]
- 94.Yoshiko Y., Wang H., Minamizaki T. Mineralized tissue cells are a principal source of FGF23. Bone. 2007;40:1565–1573. doi: 10.1016/j.bone.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 95.Martin A., David V., Quarles L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kawata T., Imanishi Y., Kobayashi K. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 97.Block G.A., Pergola P.E., Fishbane S. Effect of ferric citrate on serum phosphate and fibroblast growth factor 23 among patients with nondialysis-dependent chronic kidney disease: path analyses. Nephrol Dial Transplant. 2019;34:1115–1124. doi: 10.1093/ndt/gfy318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Edmonston D., Wolf M. FGF23 at the crossroads of phosphate, iron economy and erythropoiesis. Nat Rev Nephrol. 2020;16:7–19. doi: 10.1038/s41581-019-0189-5. [DOI] [PubMed] [Google Scholar]
- 99.Liu S., Tang W., Zhou J. Fibroblast growth factor 23 is a counter-regulatory phosphaturic hormone for vitamin D. J Am Soc Nephrol. 2006;17:1305–1315. doi: 10.1681/ASN.2005111185. [DOI] [PubMed] [Google Scholar]
- 100.Wolf M. Update on fibroblast growth factor 23 in chronic kidney disease. Kidney Int. 2012;82:737–747. doi: 10.1038/ki.2012.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen G., Liu Y., Goetz R. alpha-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signalling. Nature. 2018;553:461–466. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Olauson H., Larsson T.E. FGF23 and Klotho in chronic kidney disease. Curr Opin Nephrol Hypertens. 2013;22:397–404. doi: 10.1097/MNH.0b013e32836213ee. [DOI] [PubMed] [Google Scholar]
- 103.Jimbo R., Kawakami-Mori F., Mu S. Fibroblast growth factor 23 accelerates phosphate-induced vascular calcification in the absence of klotho deficiency. Kidney Int. 2014;85:1103–1111. doi: 10.1038/ki.2013.332. [DOI] [PubMed] [Google Scholar]
- 104.Lim K., Lu T.S., Molostvov G. Vascular klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 105.Lindberg K., Olauson H., Amin R. Arterial klotho expression and FGF23 effects on vascular calcification and function. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Scialla J.J., Lau W.L., Reilly M.P. Fibroblast growth factor 23 is not associated with and does not induce arterial calcification. Kidney Int. 2013;83:1159–1168. doi: 10.1038/ki.2013.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu D., Mackenzie N.C., Millan J.L., Farquharson C., MacRae V.E. A protective role for FGF-23 in local defence against disrupted arterial wall integrity? Mol Cell Endocrinol. 2013;372:1–11. doi: 10.1016/j.mce.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu M.C., Shi M., Zhang J. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lomashvili K.A., Manning K.E., Weitzmann M.N., Nelea V., McKee M.D., O'Neill W.C. Persistence of vascular calcification after reversal of uremia. Am J Pathol. 2017;187:332–338. doi: 10.1016/j.ajpath.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lindberg J.S., Culleton B., Wong G. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16:800–807. doi: 10.1681/ASN.2004060512. [DOI] [PubMed] [Google Scholar]
- 111.Alam M.U., Kirton J.P., Wilkinson F.L. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovasc Res. 2009;81:260–268. doi: 10.1093/cvr/cvn279. [DOI] [PubMed] [Google Scholar]
- 112.Ivanovski O., Nikolov I.G., Joki N. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE-/-) mice. Atherosclerosis. 2009;205:55–62. doi: 10.1016/j.atherosclerosis.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 113.Raggi P., Chertow G.M., Torres P.U. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 114.Investigators E.T., Chertow G.M., Block G.A. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367:2482–2494. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 115.Block G.A., Bushinsky D.A., Cunningham J. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317:146–155. doi: 10.1001/jama.2016.19456. [DOI] [PubMed] [Google Scholar]
- 116.Wolf M., Block G.A., Chertow G.M. Effects of etelcalcetide on fibroblast growth factor 23 in patients with secondary hyperparathyroidism receiving hemodialysis. Clin Kidney J. 2020;13:75–84. doi: 10.1093/ckj/sfz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Block G.A., Bushinsky D.A., Cheng S. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317:156–164. doi: 10.1001/jama.2016.19468. [DOI] [PubMed] [Google Scholar]
- 118.Schurgers L.J., Barreto D.V., Barreto F.C. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shea M.K., Holden R.M. Vitamin K status and vascular calcification: evidence from observational and clinical studies. Adv Nutr. 2012;3:158–165. doi: 10.3945/an.111.001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Westenfeld R., Krueger T., Schlieper G. Effect of vitamin K2 supplementation on functional vitamin K deficiency in hemodialysis patients: a randomized trial. Am J Kidney Dis. 2012;59:186–195. doi: 10.1053/j.ajkd.2011.10.041. [DOI] [PubMed] [Google Scholar]
- 121.Krueger T., Schlieper G., Schurgers L. Vitamin K1 to slow vascular calcification in haemodialysis patients (VitaVasK trial): a rationale and study protocol. Nephrol Dial Transplant. 2014;29:1633–1638. doi: 10.1093/ndt/gft459. [DOI] [PubMed] [Google Scholar]
- 122.Chertow G.M., Burke S.K., Raggi P., Treat to Goal Working Group Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62:245–252. doi: 10.1046/j.1523-1755.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 123.Di Iorio B., Bellasi A., Russo D. Mortality in kidney disease patients treated with phosphate binders: a randomized study. Clin J Am Soc Nephrol. 2012;7:487–493. doi: 10.2215/CJN.03820411. [DOI] [PubMed] [Google Scholar]
- 124.Jamal S.A., Vandermeer B., Raggi P. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382:1268–1277. doi: 10.1016/S0140-6736(13)60897-1. [DOI] [PubMed] [Google Scholar]
- 125.Block G.A., Wheeler D.C., Persky M.S. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23:1407–1415. doi: 10.1681/ASN.2012030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lioufas N., Toussaint N.D., Pedagogos E. Can we IMPROVE cardiovascular outcomes through phosphate lowering in CKD? Rationale and protocol for the IMpact of Phosphate Reduction On Vascular End-points in Chronic Kidney Disease (IMPROVE-CKD) study. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2018-024382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Isakova T., Ix J.H., Sprague S.M. Rationale and approaches to phosphate and fibroblast growth factor 23 reduction in CKD. J Am Soc Nephrol. 2015;26:2328–2339. doi: 10.1681/ASN.2015020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kircelli F., Peter M.E., Sevinc Ok E. Magnesium reduces calcification in bovine vascular smooth muscle cells in a dose-dependent manner. Nephrol Dial Transplant. 2012;27:514–521. doi: 10.1093/ndt/gfr321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Louvet L., Buchel J., Steppan S., Passlick-Deetjen J., Massy Z.A. Magnesium prevents phosphate-induced calcification in human aortic vascular smooth muscle cells. Nephrol Dial Transplant. 2013;28:869–878. doi: 10.1093/ndt/gfs520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu J., Bai Y., Jin J. Magnesium modulates the expression levels of calcification-associated factors to inhibit calcification in a time-dependent manner. Exp Ther Med. 2015;9:1028–1034. doi: 10.3892/etm.2015.2215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 131.Diaz-Tocados J.M., Peralta-Ramirez A., Rodriguez-Ortiz M.E. Dietary magnesium supplementation prevents and reverses vascular and soft tissue calcifications in uremic rats. Kidney Int. 2017;92:1084–1099. doi: 10.1016/j.kint.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 132.Bressendorff I., Hansen D., Schou M. Oral magnesium supplementation in chronic kidney disease stages 3 and 4: efficacy, safety, and effect on serum calcification propensity-a prospective randomized double-blinded placebo-controlled clinical trial. Kidney Int Rep. 2017;2:380–389. doi: 10.1016/j.ekir.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Spiegel D.M., Farmer B. Long-term effects of magnesium carbonate on coronary artery calcification and bone mineral density in hemodialysis patients: a pilot study. Hemodial Int. 2009;13:453–459. doi: 10.1111/j.1542-4758.2009.00364.x. [DOI] [PubMed] [Google Scholar]
- 134.Tzanakis I.P., Stamataki E.E., Papadaki A.N. Magnesium retards the progress of the arterial calcifications in hemodialysis patients: a pilot study. Int Urol Nephrol. 2014;46:2199–2205. doi: 10.1007/s11255-014-0751-9. [DOI] [PubMed] [Google Scholar]
- 135.Sakaguchi Y., Hamano T., Obi Y. A Randomized trial of magnesium oxide and oral carbon adsorbent for coronary artery calcification in predialysis CKD. J Am Soc Nephrol. 2019;30:1073–1085. doi: 10.1681/ASN.2018111150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Grases F., Sanchis P., Perello J. Effect of crystallization inhibitors on vascular calcifications induced by vitamin D: a pilot study in Sprague-Dawley rats. Circ J. 2007;71:1152–1156. doi: 10.1253/circj.71.1152. [DOI] [PubMed] [Google Scholar]
- 137.Ferrer M.D., Ketteler M., Tur F. Characterization of SNF472 pharmacokinetics and efficacy in uremic and non-uremic rats models of cardiovascular calcification. PLoS One. 2018;13 doi: 10.1371/journal.pone.0197061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raggi P., Bellasi A., Bushinsky D. Slowing progression of cardiovascular calcification with SNF472 in patients on hemodialysis: results of a randomized, phase 2b study. Circulation. 2020;141:728–739. doi: 10.1161/CIRCULATIONAHA.119.044195. [DOI] [PubMed] [Google Scholar]
- 139.Tani T., Orimo H., Shimizu A., Tsuruoka S. Development of a novel chronic kidney disease mouse model to evaluate the progression of hyperphosphatemia and associated mineral bone disease. Sci Rep. 2017;7:2233. doi: 10.1038/s41598-017-02351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Navaneethan S.D., Schold J.D., Arrigain S., Jolly S.E., Nally J.V., Jr. Cause-specific deaths in non-dialysis-dependent CKD. J Am Soc Nephrol. 2015;26:2512–2520. doi: 10.1681/ASN.2014101034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cardellini M., Rovella V., Scimeca M. Chronic kidney disease is linked to carotid nodular calcification, an unstable plaque not correlated to inflammation. Aging Dis. 2019;10:71–81. doi: 10.14336/AD.2018.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abramowitz Y., Jilaihawi H., Chakravarty T., Mack M.J., Makkar R.R. Porcelain aorta: a comprehensive review. Circulation. 2015;131:827–836. doi: 10.1161/CIRCULATIONAHA.114.011867. [DOI] [PubMed] [Google Scholar]
- 143.Aitken E., Ramjug S., Buist L., Kingsmore D. The prognostic significance of iliac vessel calcification in renal transplantation. Transplant Proc. 2012;44:2925–2931. doi: 10.1016/j.transproceed.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 144.Droupy S., Eschwege P., Hammoudi Y., Durrbach A., Charpentier B., Benoit G. Consequences of iliac arterial atheroma on renal transplantation. J Urol. 2006;175:1036–1039. doi: 10.1016/S0022-5347(05)00325-3. [DOI] [PubMed] [Google Scholar]
- 145.Choi S.J., Yoon H.E., Kim Y.S. Pre-existing arterial micro-calcification predicts primary unassisted arteriovenous fistula failure in incident hemodialysis patients. Semin Dial. 2015;28:665–669. doi: 10.1111/sdi.12365. [DOI] [PubMed] [Google Scholar]
- 146.Georgiadis G.S., Georgakarakos E.I., Antoniou G.A. Correlation of pre-existing radial artery macrocalcifications with late patency of primary radiocephalic fistulas in diabetic hemodialysis patients. J Vasc Surg. 2014;60:462–470. doi: 10.1016/j.jvs.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 147.Bos D., Leening M.J., Kavousi M. Comparison of atherosclerotic calcification in major vessel beds on the risk of all-cause and cause-specific mortality: the Rotterdam Study. Circ Cardiovasc Imaging. 2015;8 doi: 10.1161/CIRCIMAGING.115.003843. [DOI] [PubMed] [Google Scholar]
- 148.O'Neill W.C., Han K.H., Schneider T.M., Hennigar R.A. Prevalence of nonatheromatous lesions in peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2015;35:439–447. doi: 10.1161/ATVBAHA.114.304764. [DOI] [PubMed] [Google Scholar]