Abstract
Isolated patellofemoral arthritis is a common debilitating condition in adults older than 40 years of age. Surgical options such as patellofemoral arthroplasty exist for those who failed to respond to nonoperative treatment. However, early patellofemoral arthroplasty techniques often resulted in poor outcomes due to mal-tracking and malalignment of components. Robotic-assisted surgery recently has been introduced as an alternative to classic patellofemoral arthroplasty, with the potential to improve the anatomical fit and reproducibility of implant positioning. We present the technique for minimally invasive robotic-assisted patellofemoral arthroplasty system.
Isolated patellofemoral arthritis occurs in nearly 10% of patients older than 40 years of age.1 Patellofemoral arthritis is defined as the loss of articular cartilage on the patella facets, the trochlear groove, or both. Although the etiology of patellofemoral arthritis is multifactorial, female sex, increased body mass, and previous trauma have been shown to increase rates of patellofemoral arthritis.2 Other risk factors include patients who have sustained a patella or quadricep tendon rupture or patellar instability. Congenital or developmental factors also play a role in the development of patellofemoral arthritis. These include such pathology as patella alta, trochlear dysplasia, increased Q angle, weak or hypoplastic vastus medialis, a contracted lateral retinaculum, or an absent/insufficient medial patellofemoral ligament.3,4 Women account for more than 75% of patients with isolated patellofemoral arthritis, hypothesized to be due to an increased Q angle and greater rates of trochlear dysplasia.5
Isolated patellofemoral arthritis can be debilitating and remains a treatment challenge.6,7 Conservative treatment, such as avoidance of activities that require deep knee flexion, bracing, use of an assistive device, and physical therapy, remains the first-line therapy.8 When conservative measures fail, a number of surgical options exist for suitable candidates. Although total knee arthroplasty (TKA) is considered the gold standard for degenerative knee arthritis, patellofemoral arthroplasty (PFA) is an alternative, especially in younger patients in whom there is concern for bone conservation and possible need for future surgery.9, 10, 11
PFA has existed since the 1950s. Historically, it has demonstrated suboptimal results, with high rates of failure. These historically poor results have been attributed to poor design features that were prone to complications such as mal-tracking and catching of the patella.12,13 Second-generation PFAs have existed since the 1990s, and early reports demonstrated improved survivorship over the initial designs.14, 15, 16 Current generations of patellofemoral prostheses have evolved to more accurately recreate pre-disease anatomy and joint function. These more anatomical implant designs have led to a renewed interest in PFA in recent years.17,18
Robotic-assisted minimally invasive surgery recently has been introduced as an accompaniment to PFA.19 These systems enable the user to perform preoperative implant planning using a patient-specific computed tomography (CT)-based bone model and virtual implant templates. The primary purpose of preoperative planning is to size, align, and position the implant to match the patients’ bony anatomy. A robotic arm is then used intraoperatively to guide bone cuts and help the surgeon place the implants in the preplanned position. This is hypothesized to potentially improve the anatomical fit and reproducibility of implant positioning.20,21 The following describes a technique for using the Stryker MAKOplasty system (Kalamazoo, MI) for robotic-assisted PFA.
Surgical Technique
Preoperative Investigation
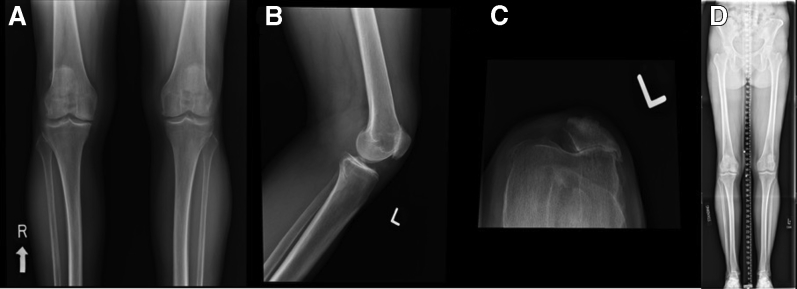
A routine series of radiographs is obtained of the knees, including standing anteroposterior, flexed posteroanterior, lateral, and sunrise views. Full-length standing radiographs should be obtained to assess overall mechanical axis. Lateral views may reveal patellofemoral osteophytes, joint space narrowing, and the presence of patella alta or baja. Sunrise views also may show a narrowed joint space as well as the presence of trochlear dysplasia, patellar tilt, or subluxation (Fig 1). In addition, each patient requires a preoperative CT scan to be used for robotic templating. Accurate and precise definition of bony landmarks from this CT allows for proper templating of the implant and reproducibility intraoperatively.
Fig 1.
A routine series of plain film radiographs of (A) standing anteroposterior bilateral knees; (B) lateral view left knee, which reveals patellofemoral osteophytes and joint-space narrowing; (C) sunrise left knee, which reveals a narrowed joint space as well as the presence of trochlear dysplasia and patellar tilt; and (d) long leg alignment preoperative radiographs. Full-length standing radiographs should be obtained to assess overall mechanical axis.
Positioning and Preparation (With Video Illustration)
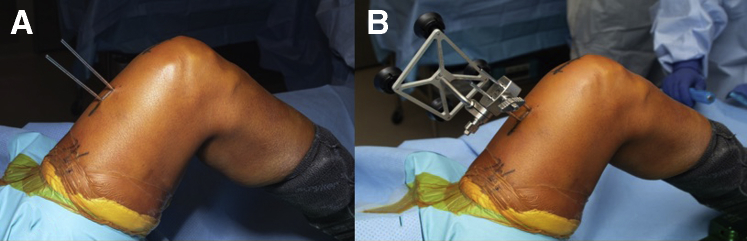
As shown in the technique video (Video 1), the patient should be placed supine on the table with a post of the surgeon’s preference placed on the operative side to assist in stabilizing the knee and allow for full knee range of motion or a leg positioner (Fig 2A). After induction of anesthesia, an examination of the knee should be carried out that includes range of motion, patellar tracking, patellar crepitation and alignment. A nonsterile tourniquet is placed, and the limb is prepped and draped in normal sterile fashion.
Fig 2.
(A) Patient draped and positioned with right leg prepped before docking robot. (B) Robot positioning adjacent to patient, on the operative side. (C) Operating room setup with view from receiver array, where industry representative would typically stand.
Preparation and Positioning of MAKO Robot and Staff
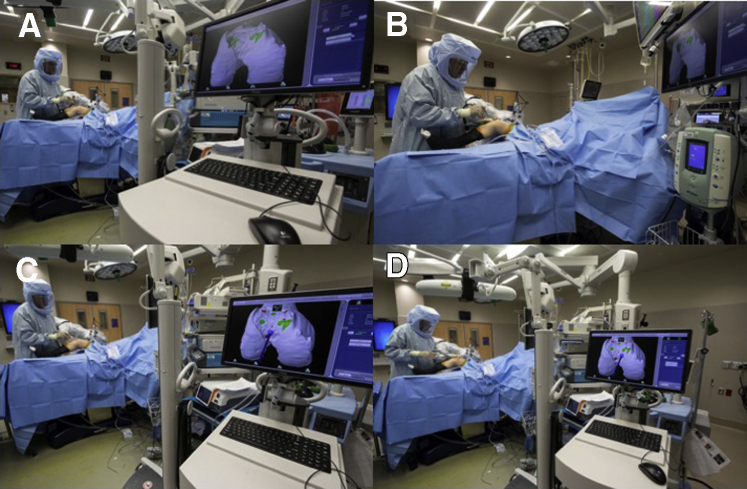
The arm of the robot is positioned on the operative side, while the computer referencing stand is located opposite the robotic arm. Since direct-line-of-sight is required for all tracking arrays, the nonoperative side must be free of camera obstruction during steps of the procedure that require real-time tracking. The camera is aimed toward the knee joint using the laser alignment guide on the camera. The surgeon’s monitor should be placed in a comfortable viewing location for the surgeon. The guidance module should be located where the representative professional can maintain direct observation of the surgeon (Fig 2B-C).
Operative Technique
Before the beginning the PFA, a diagnostic arthroscopy is performed. Arthroscopy using standard anterolateral and anteromedial portals is performed to examine all 3 compartments thoroughly. The medial and lateral compartments are the focus of increased scrutiny to determine whether preoperative imaging is reflective of true remaining cartilage. At this point, arthroscopic visualization of the articular surface allows for confirmation that the disease is isolated to the patellofemoral compartment and that the cruciate ligaments are intact.
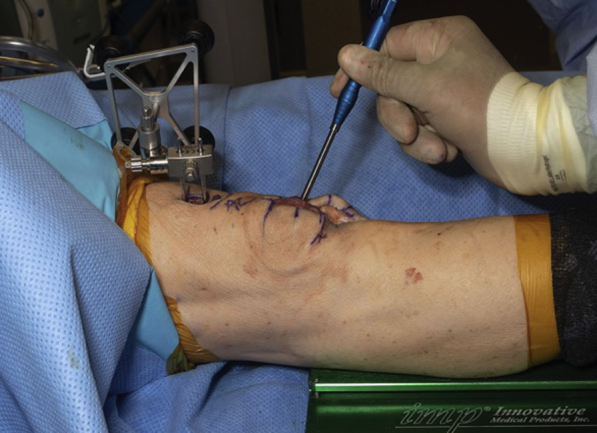
A standard medial-based parapatellar skin incision is made from approximately 3 cm above the superior pole of the patella to 1 cm proximal to the tibial tubercle distally. Next, 2 (3.0) threaded reference pins are placed in the central aspect of the femur and MAKO computer referencing guide is attached (Fig 3). MAKO registration of the femur is then performed by assessing the reference pins on the patient’s femur. Location of these points is directed by the navigation system and allows the system to match the preoperative CT image to the patient’s intraoperative bone position (Fig 4). An arthrotomy of the surgeon’s choice can now be performed. During arthrotomy, care should be taken to not cut the menisci, intermeniscal ligaments, or the articular cartilage. Next, some of the infrapatellar and supratrochlear fat pad should be excised to allow the patella to be subluxed and reflected laterally. Osteophytes, capsular adhesions, and osteochondral defects in the area are then removed using a rongeur. Soft tissue near the medial border of the patella should be preserved in case soft-tissue balancing is necessary.
Fig 3.
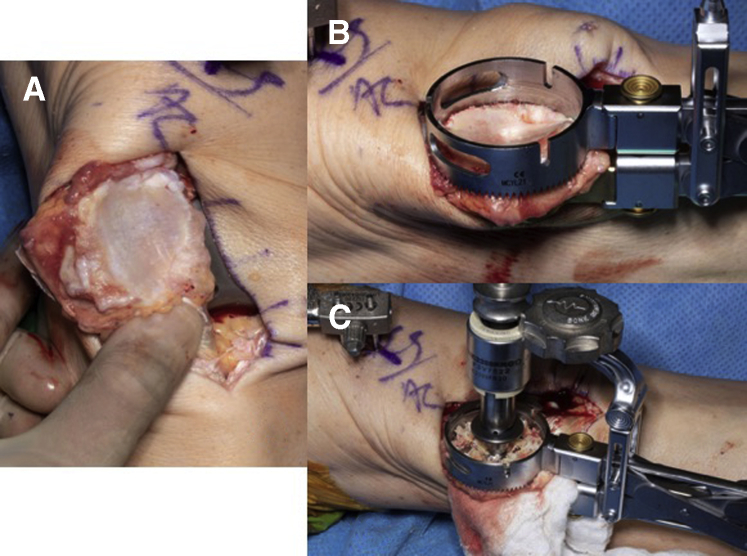
(A) Mako guide pin attached through right femur without receiver. (B) Mako guide pins attached through right femur with receiver in place. Receiver must face the receiver array without any obstruction, as this can introduce interference in the navigation system.
Fig 4.
Mako Registration to sync to preoperative computed tomography as seen from the navigation console.
After arthritis is confirmed to be isolated to the patellofemoral joint, then setting the reference points for the trochlea may begin. While holding the Knee End Effector Array near the knee joint, place the blunt probe tip into the Knee End Effector Array divot. When both the Knee End Effector Array and probe tip are visible to the camera, the system will automatically check probe tip accuracy. Repeat for the sharp probe (Fig 5).
Fig 5.
Mako probe registering on articular surface of the right knee through medial parapatellar approach.
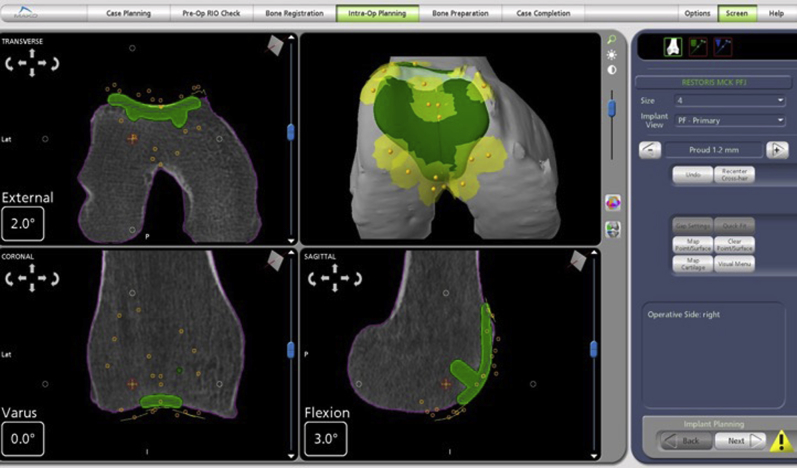
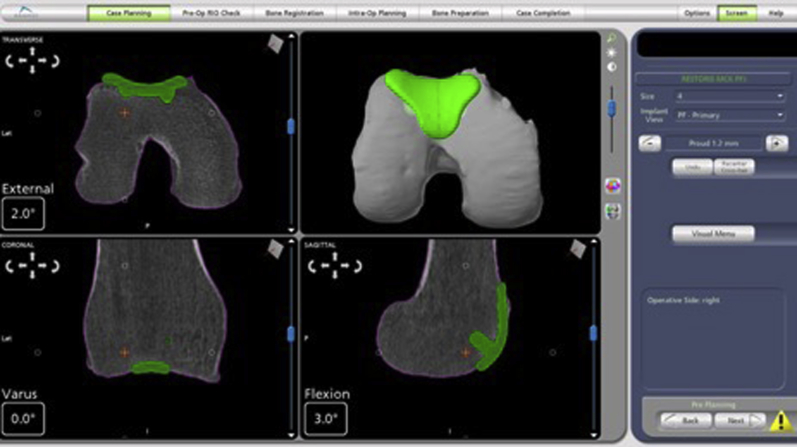
Now, using the sharp probe, the trochlea is mapped by first marking the 2 points at the medial and lateral superior edge. Next, 5 points are marked along the deepest points of the trochlear groove, from most anterior to most distal. Lastly, 3 points are marked along each side of the medial and lateral transition edges. These reference points are matched with the preoperative CT (Fig 6). Of note, it is important to use the sharp probe to push through cartilage and down to subchondral bone to accurately align with preoperative CT, as cartilage is not seen on CT.
Fig 6.
Positioning of implant (green template) on the trochlea suing computed tomography guidance.
After reference points are obtained, intraoperative templating is adjusted until surgeon satisfaction. Once plan is confirmed, the MAKO assisted burring arm is brought into position. The robotic base is centered at the patient’s hip, perpendicular to the surgical table, and about 1 to 2 meters from the surgical table. The cutting handle is then placed directly above knee center with approximately 10 cm of space between the bottom of the cutting handle and the knee joint (Fig 7A).
Fig 7.
(A) Robotic arm in position for trochlear resection view from professional representative’s monitor; (B) robotic arm with burr in use on right knee with view from cross-table; (C) robotic arm with burr in use view from representative’s monitor; and (D) view of professional representative’s monitor and receiver array. These views demonstrate a clear line of site between the receiver located on the patient’s knee and the receiver array from the representative’s monitor.
Once in correct position, the haptically controlled burr is used to remove bone precisely from the location where the trochlear implant will fit (Fig 7B-C). The arm does not allow the burr to remove bone outside the predetermined space based on the template. The implant lug holes also are created with the burr (Fig 8). Of note, to create the proper press fit for the implant, the burr should only be plunged at the plug site once, otherwise a larger hole than is needed will be created. After adequate bone removal, the trochlear trial implant is inserted, and smooth tracking of the patella is confirmed.
Fig 8.
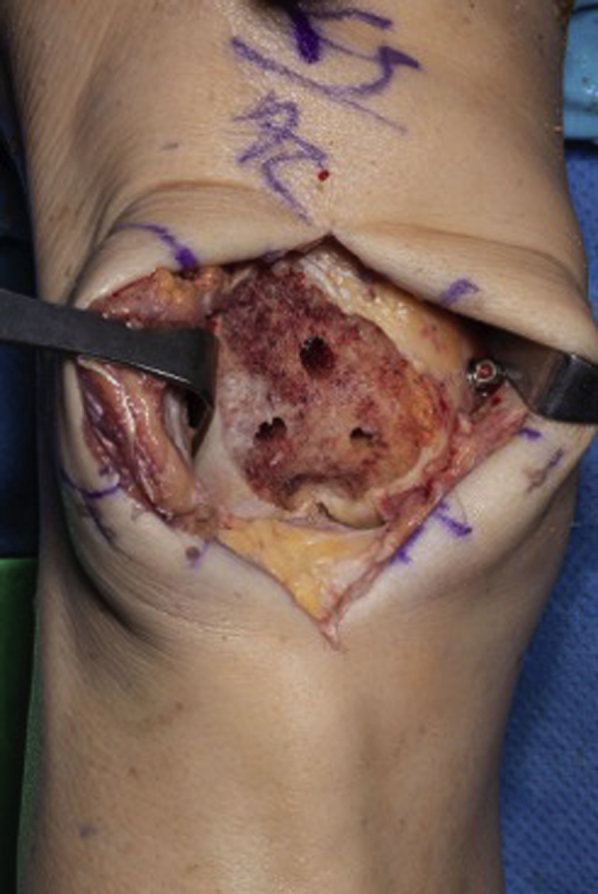
Trochlea status postresection with retractors in soft tissue to allow for visualization. Three peg hole burr cuts visible.
Attention is then turned to the patella. The patella should be everted and measured for maximum patella thickness using a caliper in both the lateral and medial aspect. The patella implant size is estimated by measuring the superior-inferior height of the patella articular surface, and the optimal patella size is the largest size that does not overhang the bone superior-inferiorly. Once the patella is sized, it may be prepared either with reaming or with the use of a saw. A patellar reamer guide assembly is then firmly clamped onto the patella so that the spikes fully engage onto the patella and the drill guide sits flat on the bone surface. For proper patella tracking, the drill guide should be aligned with the former patella ridge (medialized on the patellar bone). This will lateralize the remaining patella bone and avoid tightening of the lateral quadriceps and risk of lateral subluxation. Once in proper position, the 3 peg holes are drilled, and the patella trial is placed (Fig 9 A-C). Thickness of the patella with the trial is then remeasured to confirm that appropriate thickness was achieved. A manual range of motion check is now performed to ensure proper patella tracking and patella transitioning.
Fig 9.
(A) Native patella before osteophyte resection; (B) patella with drilling clamp applied; and (C) patella with 3 peg holes being drilled.
If satisfactory, both the trochlear and patellar trials are removed, and bone surfaces are irrigated. Once the surfaces are free of debris, cement is mixed and applied to both the trochlear implant, as well as the resected cavity. The trochlear implant is then placed and impacted. Excess cement is removed, and the implant is held stationary until the cement has cured. Cement is then applied to the resected patella surface, drilled peg holes, and patella implant. The patella component is then placed onto the resurfaced patella and a cement clamp assembly may be used to apply adequate force to the patella implant. Excess cement is removed and after the cement has sufficiently cured, the cement clamp assembly is removed (Fig 10).
Fig 10.
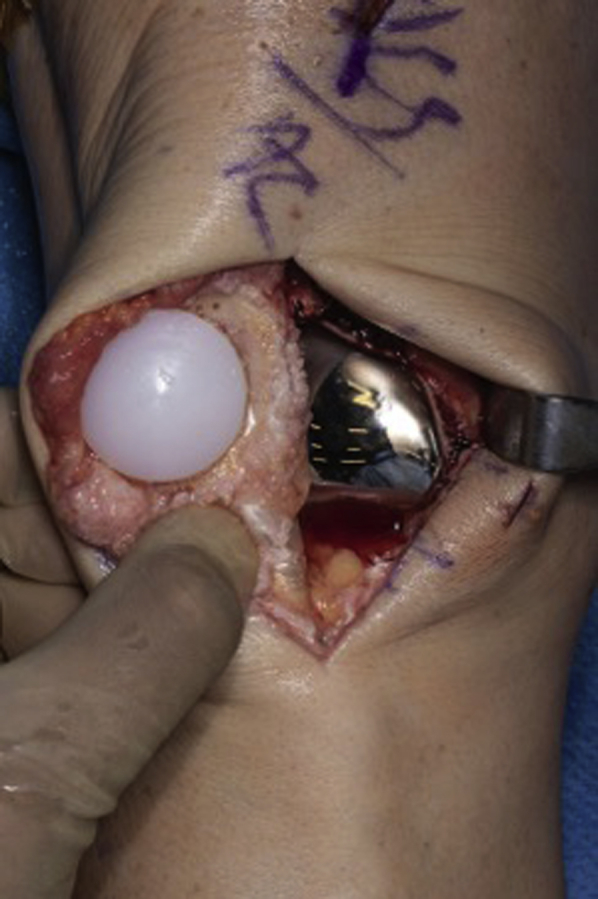
Final components in position. Trochlear implant is in correct position after impaction and removal of excess cement. Patellar button is visualized, with all excess cement removed.
The joint is copiously irrigated once again, and a final range of motion check is performed, and verification of implant fixation is made prior to wound closure. There should be no tilt or subluxation as the knee is put through a full range of motion. The medial parapatellar incision is then closed with an interrupted horizontal mattress pattern to tighten the medial soft tissues. Arthrotomy closure is made using a #2 ETHIBOND figure-of-eight suture followed by running locking #0 VICRYL suture. Once completed, the balance is again checked with range of motion, and possible patellar mal-tracking, medial and patella glide, or instability is assessed. Layered closure is then performed with #0 VICRYL sutures followed by 2-0 undyed VICRYL sutures, followed by skin staples. Sterile dressing is placed per surgeon’s preference.
Rehabilitation
Postoperatively, patients are made weight-bearing as tolerated with emphasis on range of motion exercises immediately. We begin physical therapy postoperative week 1, again with emphasis on range of motion and quadriceps strengthening. Patients are liberalized to stationary bike activity at 4 weeks postoperative and gradually return to full unrestricted activity 4 to 6 weeks postoperatively.
Discussion
The incidence of isolated patellofemoral arthritis in patients older than 55 has been reported to be anywhere from 2% to 11% in men and 8% to 24% in women.22 For patients who have isolated patellofemoral arthritis and have not responded to nonoperative treatments, surgical interventions may be considered. Multiple different surgical techniques have been described but have shown varying results.23, 24, 25
The main surgical options include TKA or PFA. The first generation of PFA began in 1955; however, many of the first-generation devices failed for reasons such as mal-tracking, polyethylene wear, component mal-positioning, and poor implant design.26 Tauro et al.27 retrospectively reviewed 76 cases of first-generation PFA and found a 65% survivorship at 5-year follow-up. Seventy-one percent of failures (15/21) were due to patellofemoral mal-tracking. Second-generation implants were introduced in the 1990s and showed improved outcomes.3 The goals of second generation implant designs were to more closely resemble the patient’s normal anatomy. This theoretically allowed smooth patellar tracking and minimized the chance of subluxation or dislocation.28 Ackroyd et al.29 reported a 96% 5-year survivorship in 306 patients with second-generation PFA. Other studies have reported similar results at 5-year follow-up.30,31
With long-term outcome data now available, the development of femorotibial osteoarthritis has been determined to be the most common reason for failure of second generation PFA, with conversion to TKA.32 Conversion to TKA was seen in 13% of PFA in the study by van Jonbergen et al.33 Conversion rates of 1 in 5 also have been reported after an average of 7 to 16 years.34
The decision to perform a PFA or a TKA remains controversial; however, PFA may be a suitable option in select younger patients, as it is bone conserving and is associated with shorter postoperative rehabilitation. In addition, PFA has been associated with better knee kinematics in the sagittal plane because of the preservation of the tibiofemoral articulation, menisci, and ligaments.35 One retrospective study compared outcomes in 45 patients undergoing PFA or TKA at mean follow-up of 2.5 years found similar Knee Society and pain scores, but the PFA group had significantly greater activity scores.9 A recent meta-analysis of 28 studies compared complications with PFA and TKA performed for isolated patellofemoral arthritis and found no significant differences in reoperation, revision, pain, or mechanical complications. With subgroup analysis, first generation inlay-style prostheses had greater than 4-fold higher rates of significant complications than second-generation prostheses. The significant failure rates and patellar tracking complications that occurred in early inlay PFA designs appear to have been minimized with the modern generation of onlay-style prostheses. These factors suggest that PFA is an improving and viable alternative to TKA.36
The recent introduction of robot-assisted PFA may deliver some unique advantages (Table 1). The ability to preoperatively plan on a 3-dimensional image allows for more precise implant sizing. Intraoperatively, cartilage mapping and then precise removal of bone allows for consistent alignment, position, and a smooth transition zone distally. This eliminates a source of error that can cause ongoing symptoms in patients who undergo PFA.19,20 In a study by Turktas et al.37 examining 30 knees following robotically-assisted PFA with a mean follow-up of 15.9 months showed no sign of malalignment or mal-tracking. On average, the postoperative Oxford Knee Score was 33.5 compared with a preoperative score of 21.7. Ackroyd et al.16 reported similar postoperative Oxford Knee Score results following nonrobotic PFA, which were 30.5 and 37 respectively. These scores show that the outcomes of robotically assisted PFA are similar to other successful techniques. To help guide adopters of this technique, pearls and pitfalls to this technique have been included in Table 2.
Table 1.
Advantages and Disadvantages of a Minimally Invasive Robotic-Assisted Patellofemoral Arthroplasty
| Advantages |
| More anatomical implant design and fixation than previous techniques |
| Reduces inconsistencies such as malalignment or mal-tracking |
| Encouraging 5-year follow-up |
| Disadvantages |
| Longer-term follow-up not yet available |
| Significant capital investment required to acquire robot |
| Requires preoperative computed tomography scan |
Table 2.
Pearls and Pitfalls of a Minimally Invasive Robotic-Assisted Patellofemoral Arthroplasty
| Pearls | Pitfalls |
|---|---|
| Patient selection is key | Avoid oblique placement or placement of reference pins >3.0, as these can create stress risers/fractures |
| Pain in the patellofemoral joint | |
| Age | |
| Less than grade 3 changes in medial and lateral compartment | |
| Arthroscopy before confirm candidacy for procedure | |
| Intraoperative positioning crucial to allow full-knee ROM/robot positioning | Soft-tissue balancing needs to be performed in patellofemoral joint to avoid early wear |
| Use 3.0 reference pins in distal femur | Check soft-tissue balancing before resurfacing patella |
| Pie-crust medial patella soft tissue after implant placement for soft-tissue balancing | Take care with patella resurfacing as often the lateral facet is deficient secondary to patellar DJD and therefore less resection may be required |
| When mapping cartilage, ensure probe is down to bone completely through cartilage layer; patella resurfacing is similar to TKA |
DJD, degenerative joint disease; ROM, range of motion; TKA, total knee arthroplasty.
As with any newer technology, there are a few limitations that should be discussed (Table 1). Newer-style only implants will still benefit from increased long-term follow-up. The technique presented requires significant capital investment to purchase the robot needed for this technique. This could limit its usefulness across a wide range of hospitals. Finally, our technique recommends knee arthroscopy before proceeding with PFA to assess the other compartments for degree of arthritis. This could potentially add to the cost of the overall care.
The use of robotic-assisted PFA for isolated patella-femoral arthritis has the potential to provide more anatomical implant design and fixation than previous surgical techniques. Further clinical studies are warranted to validate whether increased anatomic alignment and reproducibility from robotic PFA results in improved clinical outcomes when compared with nonrobotic techniques.
Footnotes
The authors report the following potential conflict of interest or source of funding: A.C. receives consultant fees from Arthrex, Trice Medical, and Zimmer Biomet. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
This is a visual demonstration of technique to perform a robotic patellofemoral arthroplasty using the Stryker MAKOplasty system. This was performed for a patient who had isolated patellofemoral arthritis. Diagnostic arthroscopy is performed on the patient before the beginning of the procedure. Attention is then turned to the setup and manipulation of the Stryker MAKOplasty system.
References
- 1.Grelsamer R.P., Stein D.A. Patellofemoral arthritis. J Bone Joint Surg. 2006;88:1849–1860. doi: 10.2106/JBJS.E.01394. [DOI] [PubMed] [Google Scholar]
- 2.Cooper C., McAlindon T., Snow S. Mechanical and constitutional risk factors for symptomatic knee osteoarthritis: Differences between medial tibiofemoral and patellofemoral disease. J Rheumatol. 1994;21:307–313. [PubMed] [Google Scholar]
- 3.van Jonbergen H.-P.W., Poolman R.W., van Kampen A. Isolated patellofemoral osteoarthritis. Acta Orthop. 2010;81:199–205. doi: 10.3109/17453671003628756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minkowitz R.B., Bosco J.A. Patellofemoral arthritis. Bull NYU Hosp Jt Dis. 2009;67:30–38. [PubMed] [Google Scholar]
- 5.Lonner J.H. Patellofemoral arthroplasty. J Am Acad Orthop Surg. 2007;15:495–506. doi: 10.5435/00124635-200708000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Fulkerson J.P. Alternatives to patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;436:76–80. doi: 10.1097/01.blo.0000172305.20156.ba. [DOI] [PubMed] [Google Scholar]
- 7.Tarassoli P., Punwar S., Khan W., Johnstone D. Patellofemoral arthroplasty: A systematic review of the literature. Open Orthop J. 2012;6:340–347. doi: 10.2174/1874325001206010340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller M., Thompson S. Ed 3. Saunders; Philadelphia: 2019. DeLee & Drez’s orthopaedic sports medicine. [Google Scholar]
- 9.Dahm D.L., Al-Rayashi W., Dajani K., Shah J.P., Levy B.A., Stuart M.J. Patellofemoral arthroplasty versus total knee arthroplasty in patients with isolated patellofemoral osteoarthritis. Am J Orthop. 2010;39:487–491. [PubMed] [Google Scholar]
- 10.Wetzels T., Bellemans J. Patellofemoral osteoarthritis treated by partial lateral facetectomy: Results at long-term follow up. Knee. 2012;19:411–415. doi: 10.1016/j.knee.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Choo K.J., Lonner J.H. Treating patellofemoral arthritis with patellofemoral arthroplasty. In: Gerlinger T., editor. Unicompartmental knee arthroplasty. Springer International Publishing; New York: 2020. pp. 107–120. [Google Scholar]
- 12.Lonner J.H. Patellofemoral arthroplasty: The impact of design on outcomes. Orthop Clin North Am. 2008;39:347–354. doi: 10.1016/j.ocl.2008.02.002. vi. [DOI] [PubMed] [Google Scholar]
- 13.Krajca-Radcliffe J.B., Coker T.P. Patellofemoral arthroplasty. A 2- to 18-year followup study. Clin Orthop Relat Res. 1996;(330):143–151. [PubMed] [Google Scholar]
- 14.Argenson J.-N.A., Flecher X., Parratte S., Aubaniac J.-M. Patellofemoral arthroplasty: An update. Clin Orthop Relat Res. 2005;440:50–53. doi: 10.1097/01.blo.0000187061.27573.70. [DOI] [PubMed] [Google Scholar]
- 15.Halai M., Ker A., Anthony I., Holt G., Jones B., Blyth M. Femoro patella vialla patellofemoral arthroplasty: An independent assessment of outcomes at minimum 2-year follow-up. World J Orthop. 2016;7:487–493. doi: 10.5312/wjo.v7.i8.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackroyd C.E., Newman J.H., Evans R., Eldridge J.D.J., Joslin C.C. The Avon patellofemoral arthroplasty: Five-year survivorship and functional results. J Bone Joint Surg Br. 2007;89:310–315. doi: 10.1302/0301-620X.89B3.18062. [DOI] [PubMed] [Google Scholar]
- 17.Lonner J.H. Patellofemoral arthroplasty: Pros, cons, and design considerations. Clin Orthop Relat Res. 2004;428:158–165. [PubMed] [Google Scholar]
- 18.Rodriguez-Merchan E.C. Surgical treatment of isolated patellofemoral osteoarthritis. HSS J. 2014;10:79–82. doi: 10.1007/s11420-013-9375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustig S. Patellofemoral arthroplasty. Orthop Traumatol Surg Res. 2014;100(suppl 1):S35–S43. doi: 10.1016/j.otsr.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 20.Cobb J., Henckel J., Gomes P. Hands-on robotic unicompartmental knee replacement: A prospective, randomised controlled study of the acrobot system. J Bone Joint Surg Br. 2006;88:188–197. doi: 10.1302/0301-620X.88B2.17220. [DOI] [PubMed] [Google Scholar]
- 21.Lonner J.H. Modular bicompartmental knee arthroplasty with robotic arm assistance. Am J Orthop. 2009;38(suppl 2):28–31. [PubMed] [Google Scholar]
- 22.McAlindon T.E., Snow S., Cooper C., Dieppe P.A. Radiographic patterns of osteoarthritis of the knee joint in the community: The importance of the patellofemoral joint. Ann Rheum Dis. 1992;51:844–849. doi: 10.1136/ard.51.7.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hamlin B. Patellofemoral arthroplasty: NAVIO. In: Lonner J.H., editor. Robotics in knee and hip arthroplasty. Springer; 2019. pp. 109–114. [Google Scholar]
- 24.Law J., Hofmann A., Stevens B., Myers A. Patellofemoral arthroplasty technique: Mako. In: Lonner J.H., editor. Robotics in knee and hip arthroplasty. Springer; New York: 2019. pp. 115–122. [Google Scholar]
- 25.Iranpour F., Aframian A., Cobb J.P. Patellofemoral osteoarthritis: Patellofemoral arthroplasty. In: Rodriguez-Merchan E.C., Liddle A.D., editors. Disorders of the patellofemoral joint. Springer; New York: 2019. pp. 129–134. [Google Scholar]
- 26.Argenson J.N., Guillaume J.M., Aubaniac J.M. Is there a place for patellofemoral arthroplasty? Clin Orthop Rel Res. 1995;321:162–167. [PubMed] [Google Scholar]
- 27.Tauro B., Ackroyd C.E., Newman J.H., Shah N.A. The Lubinus patellofemoral arthroplasty. A five- to ten-year prospective study. J Bone Joint Surg Br. 2001;83:696–701. doi: 10.1302/0301-620x.83b5.11577. [DOI] [PubMed] [Google Scholar]
- 28.Lonner J.H., Bloomfield M.R. The clinical outcome of patellofemoral arthroplasty. Orthop Clin North Am. 2013;44:271–280. doi: 10.1016/j.ocl.2013.03.002. vii. [DOI] [PubMed] [Google Scholar]
- 29.Ackroyd C.E., Chir B. Development and early results of a new patellofemoral arthroplasty. Clin Orthop Relat Res. 2005;436:7–13. doi: 10.1097/01.blo.0000171914.94503.d1. [DOI] [PubMed] [Google Scholar]
- 30.Merchant A.C. Early results with a total patellofemoral joint replacement arthroplasty prosthesis. J Arthroplasty. 2004;19:829–836. doi: 10.1016/j.arth.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Leadbetter W.B., Kolisek F.R., Levitt R.L. Patellofemoral arthroplasty: A multi-centre study with minimum 2-year follow-up. Int Orthop. 2009;33:1597–1601. doi: 10.1007/s00264-008-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker T., Perkinson B., Mihalko W.M. Patellofemoral arthroplasty: The other unicompartmental knee replacement. J Bone Joint Surg Am. 2012;94:1712–1720. doi: 10.2106/JBJS.L.00539. [DOI] [PubMed] [Google Scholar]
- 33.van Jonbergen H.P., Werkman D.M., Barnaart L.F., van Kampen A. Long-term outcomes of patellofemoral arthroplasty. J Arthroplasty. 2010;25:1066–1071. doi: 10.1016/j.arth.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 34.Kooijman H.J., Driessen A.P.P.M., van Horn J.R. Long-term results of patellofemoral arthroplasty. A report of 56 arthroplasties with 17 years of follow-up. J Bone Joint Surg Br. 2003;85:836–840. [PubMed] [Google Scholar]
- 35.Hollinghurst D., Stoney J., Ward T., Pandit H., Beard D., Murray D.W. In vivo sagittal plane kinematics of the Avon patellofemoral arthroplasty. J Arthroplasty. 2007;22:117–123. doi: 10.1016/j.arth.2006.02.160. [DOI] [PubMed] [Google Scholar]
- 36.Dy C.J., Franco N., Ma Y., Mazumdar M., McCarthy M.M., Gonzalez Della Valle A. Complications after patello-femoral versus total knee replacement in the treatment of isolated patello-femoral osteoarthritis. A meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:2174–2190. doi: 10.1007/s00167-011-1677-8. [DOI] [PubMed] [Google Scholar]
- 37.Turktas U., Piskin A., Poehling G.G. Short-term outcomes of robotically assisted patello-femoral arthroplasty. Int Orthop. 2016;40:919–924. doi: 10.1007/s00264-015-2786-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This is a visual demonstration of technique to perform a robotic patellofemoral arthroplasty using the Stryker MAKOplasty system. This was performed for a patient who had isolated patellofemoral arthritis. Diagnostic arthroscopy is performed on the patient before the beginning of the procedure. Attention is then turned to the setup and manipulation of the Stryker MAKOplasty system.