Abstract
Alzheimer’s disease (AD) is a progressive age-related neurodegenerative disease characterized by memory loss and cognitive impairment. The major characteristics of AD are amyloid β plaques, apoptosis, autophagy dysfunction, neuroinflammation, oxidative stress, and mitochondrial dysfunction. These are mostly used as the significant indicators for selecting the effects of potential drugs. It is imperative to explain AD pathogenesis and realize productive treatments. Although the currently used chemical drugs for clinical applications of AD are effective in managing the symptoms, they are inadequate to achieve anticipated preventive or therapeutic outcomes. There are new strategies for treating AD. Traditional Chinese Medicine (TCM) has accumulated thousands of years of experience in treating dementia. Nowadays, numerous modern pharmacological studies have verified the efficacy of many bioactive ingredients isolated from TCM for AD treatment. In this review, representative TCM for the treatment of AD are discussed, and among these herbal medicines, the Lamiaceae family accounts for the highest proportion. It is concluded that monomers and extracts from TCM have potential therapeutic effect for AD treatment.
Keywords: Alzheimer’s disease, traditional Chinese medicine, β-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, mitochondrial dysfunction
Introduction
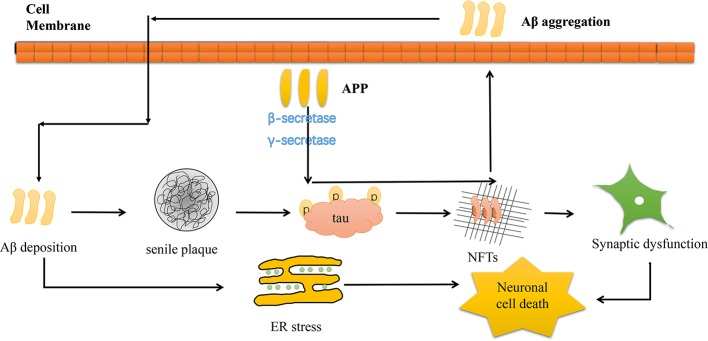
Alzheimer’s disease (AD) is the most common neurodegenerative disease with high mortality in adults (Van Cauwenberghe et al., 2016; El Kadmiri et al., 2018). The prevalence of AD has been increasing, and age is considered the major risk factor (Alzheimer’s Association, 2018). Current studies show that, globally, 44 million people live with dementia. Moreover, that number is expected to triple by 2050 as the population ages and the AD onset tends to be younger ages (Lane et al., 2018). The symptoms of AD include cognitive impairments, memory loss, and executive function loss, hence a hefty burden to the society (Alzheimer’s Association, 2018). The pathogenesis of AD is characterized by β-amyloid plaques deposition, tau protein hyperphosphorylation, neuroinflammation, mitochondrial dysfunction, autophagy dysfunction, and oxidative stress (Choi, 1995). The main cause of AD is mutation in either of these three genes―amyloid precursor protein (APP), presenilin 1 (PSEN1) or presenilin 2 (PSEN2) gene. The symptoms of fAD generally occur earlier than sporadic AD between the age of 30 and 50 years (Bateman et al., 2011). A key component of extracellular senile plaque is amyloid β peptide (Aβ). Amyloid plaques are Aβ with 40 or 42 amino acids (Aβ40 and Aβ42) that cluster abnormally extracellularly. The two metabolites are produced by APP after β-secretase and γ-secretase cleavage (Fleisher et al., 2008; Fan et al., 2017). Once APP levels are abnormal, Aβ accumulates causing tau phosphorylation and aggregation to form neurofibrillary tangles (NFTs). NFTs are insoluble twisted fibers made up of clustered hyperphosphorylated tau proteins in AD neurons. Normally, tau pathology begins at the medial temporal lobe allocortex and then spreads to the united neocortex (Lindwall and Cole, 1984; Serrano-Pozo et al., 2011). The Aβ and tau pathology build up causes endoplasmic reticulum stress (ER stress), leading to synaptic dysfunction and AD neurodegeneration (Richardson et al., 2015) (Figure 1). Besides, a recent study revealed that Aβ and reduced glutamate reuptake levels can trigger hyperexcitation in sensitive neurons. Inactive neurons are resistant to Aβ-mediated hyperactivation, whereas hyperactivity occurs in active neurons. This hyperactivation vicious cycle can be maintained by Aβ-induced AD brain extracts and Aβ-dimers (Zott et al., 2019).
Figure 1.
Schematic diagram depicting the pathogenesis of AD. Aβ generated from its precursor APP processing via cleavage by β-secretase and γ-secretase. Aβ deposition would cause senile plaque and further tau phosphorylation and aggregation to form NFTs, which lead to the loss of neurons and synaptic dysfunction. In addition, Aβ deposition induce mitochondrial injury and trigger ER stress, causing neuronal cell death.
The current approved drugs for the clinical treatment of AD are cholinesterase inhibitors (ChEIs) and N-methyl-d-aspartic acid (NMDA) receptor antagonists (Sun et al., 2012). Donepezil, rivastigmine, galantamine, and memantine are usually used to treat AD, but these drugs are all single-target drugs. However, they display modest and transitory symptoms improvement accompanied by side effects and hardly prevents or reverses the disease (Silva et al., 2014). Therefore, it is necessary to find better drugs for AD treatment.
Traditional Chinese medicine (TCM) has been established in China health care system over thousands of years (Tang et al., 2008; Xu and Yang, 2009). It has played a very important role in the treatment of chronic diseases such as lung cancer, coronary heart diseases, allergy, diabetes, and infections (Li and Brown, 2009; Li et al., 2009; Gu et al., 2010; Shi et al., 2012; Guo et al., 2012; Liu et al., 2014; Jiang et al., 2016; Zhu et al., 2017). TCM is usually viewed as more accessible, affordable, and acceptable form of treatment, and nearly a quarter of all modern drugs are derived from natural products. Thus, TCM is the basis of primary health care system and innovative medicines (Chan et al., 2014). TCM has been frequently applied in the treatment of dementia and has shown exceptional advantages due to its multi-target, multi-system, multi-link, and multi-pathway capacity. There are numerous prescriptions for treating dementia in the historical records (Luo et al., 2014), such as; Kaixin Powder, Naoling Decoction, Puzzle decoction, Huannaoyicong Decoction, and Compound Formula Rehmannia that have a significantly improve intelligence, anti-fatigue, enhance immunity, delay senility, improve memory, prevent, and treat dementia without noticeable side effects (Zang et al., 2016). Recently, some scholars conducted a general analysis on twenty eligible studies with 1,767 subjects in eight database searches. These studies investigated the combined use or compared application of TCM and clinical drugs, such as donepezil. They found out that TCM as adjuvant therapy exhibited an additive anti-AD advantage and was mainly safe and well tolerated in AD patients. These properties are not in the present approved drugs (Yang et al., 2017). According to reported evidences focus on treating AD is more on early detection of the pre-symptomatic phase and the prevalence of early dementia signs. However, TCM has wealthy clinical impact and experience in the prevention and management of chronic diseases including AD. Therefore, there is need to focus on the therapeutic potential of TCM for AD treatment (Hügel, 2015). Active compounds extracted from TCM have therapeutic effects on AD in vivo and in vitro, and some TCM drugs have been applied in clinical trials, providing an approach for AD drug development. Modern pharmacological researches confirmed that bioactive compounds extracted from TCM such as morroniside, curcumin, triptolide, and berberine (Ber) have anti-AD activity (Gu et al., 2004; Fan et al., 2017; Huang et al., 2017; Chen et al., 2018b). Thus, TCM is a potential source of AD drug. Therefore, it is important to analyze and summarize the research status of TCM as anti-AD drug.
In this review, we show TCM’s active components obvious effect on AD. These components are: monomers, such as safflower yellow (SY), crocin, β-asarone, matrine, linalool, icariin, and extracts like Dracoephalum moldavica L. flavonoid, Dendrobium nobile Lindl. alkaloids, Achyranthes bidentata Blume, and Coptis chinensis Franch. watery extract. It is shown that among these TCM that have therapeutic potential for AD, the Lamiaceae family accounts for the highest proportion, and for monomer components, the flavonoids, alkaloids, and polyphenols have significant activity in treating AD. We summarize these in prevention and treatment of AD by reducing Aβ production, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction.
AD Treatment According to Pathological Processes
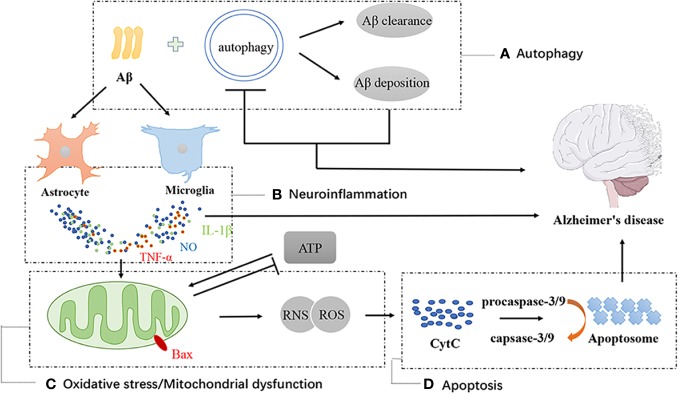
According to AD pathogenesis, the effective therapeutic effects of TCM’s monomers and extracts generally exert their effects in the following ways (Figure 2).
Figure 2.
Schematic diagram of autophagy, neuroinflammation, oxidative stress, mitochondrial dysfunction and apoptotic in AD. Autophagy is positive in alleviating AD through promoting Aβ degradation, but hyperactive autophagy is harmful to neuron survival (A). The depositions of Aβ activates the astrocytes and microglia which would secrete oxidative species, such as nitric oxide, and pro-inflammatory cytokines (B). Cytokines on the cell surface and activate pro-apoptotic signaling cascades. Mitochondrial dysfunction cause mitochondria to produce elevated levels of reactive oxygen and nitrogen species (ROS and RNS). Enhancement of ROS and RNS aggravates mitochondrial dysfunction (C, D), finally causing release of the pro-apoptotic signaling protein, CytC. CytC contributes to formation of the apoptosome (D). These factors all cause death of neuronal populations and lead to neurodegenerative disease.
β-Amyloid Production Reduction
Aβ plays a crucial role in AD pathogenesis, and neurotoxicity induced by Aβ is the chief cause of AD (Hung and Fu, 2017). Increase in Aβ production and a decrease in Aβ degrading enzymes possibly leads to Aβ aggregation. These leads to NFTs formation and neurodegeneration. APP accumulation causes Aβ deposition, leading to cognitive impairment (Selkoe and Hardy, 2016). Therefore, reducing Aβ deposition is one of the ways to treat AD.
Anti-Apoptosis Effect
Apoptosis is an active regulation process of cell death, and inducing apoptosis is an important part of Aβ-induced cell toxicity. Three major apoptotic pathways include mitochondrial pathway, endoplasmic reticulum pathway, and death receptors pathway. Mitochondrial pathway occurs mainly through reversing the mitochondrial membrane and the expression of cytochrome c (CytC). Endoplasmic reticulum pathway is mainly caused by Ca2+imbalance, which disrupts normal endoplasmic reticulum activities, causing an overload response in the endoplasmic reticulum, triggering the caspase receptor pathway cascade by activating caspase-3 and caspase-9 (Zhang et al., 2014). Generally, apoptosis occurs mainly through the cytochrome c/caspase-9 pathway and the caspase-8 pathway. When the cell receives signals to induce apoptosis, the expression of Bax in the outer membrane increases, accelerating CytC activity, leading to caspase-3 and caspase-9 activation (Nguyen et al., 2013). Apoptosis is a key link in Aβ-induced cytotoxicity, thus anti-apoptosis is recognized as an important way to treat AD.
Induce or Attenuate Autophagy Effect
Autophagy is a self-degradative process and a pervasive lysosomal degradation pathway to eliminate damaged organelles and proteins common in neurons. The process of autophagy can lead to recycling of cell material and homeostasis preservation (Rami, 2009; Hensley and Harris-White, 2015). The pathways involved in the regulation of autophagy are very complex, including phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mechanistic target of rapamycin (mTOR) pathway, AMP-activated protein kinase (AMPK) pathway, renin-angiotensyns-tem (RAS)/cyclic adenosine monophosphate (cAMP)/proteinkinase A (PKA) pathway, p53 pathway, Class I PI3K/PKB pathway, and PI3K III pathway. These pathways are associated with most neurodegenerative diseases including AD. Recent studies suggest that autophagy plays an important role in Aβ clearance. Aβ-containing autophagosomes bind to lysosomes and causes Aβ to degrade during autophagy (Xue et al., 2014). Not only is the APP protein processing related to autophagy, but the clearance of Aβ deposition and maintenance of neuron function is too closely related to autophagy (Pickford et al., 2008).
Anti-Neuroinflammation
Neuroinflammation has been identified as an important process in the pathogenesis of AD (Latta et al., 2015). Neuroinflammation is closely related to the activation of microglia and astrocytes. Activation of microglia and astrocytes produces a series of pro-inflammatory and cytotoxic factors, such as inducible nitric oxide synthase (iNOS), interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), cyclooxygenase-2 (COX-2), and interleukin-6 (IL-6) (Barger and Harmon, 1997; Wu et al., 2011; Lyman et al., 2014). These cytokines cause neuronal damage and eventually cell death. Overloaded Aβ leads to the activation of astrocytes and microglia and produces these factors. Neural inflammation in AD involves several important signaling pathways such as Toll-like receptor4 (TLR4) pathway and nuclear factor kappa B (NF-κB) pathway. TLR4-mediated signaling pathway comprises of two pathways: MyD88-dependent pathway and TRIF-dependent pathway. Currently, it is believed that proinflammatory cytokines activate microglial cells through TLR4 receptor. NF-κB is a common pathway for multiple signaling pathways that lead to inflammation and also a critical transcription factor involved in AD inflammatory processes (Granic et al., 2009). These suggest that inhibition of neuroinflammation and the NF-κB pathways is an effective way to treat AD.
Reduce Oxidative Stress
Oxidative stress is another important factor in the AD pathogenesis. TCM containing antioxidants reduces the risk of AD by inhibiting oxidative stress (Min and Min, 2014; Fan et al., 2017). Oxidative stress leads to excess reactive oxygen species (ROS) generation and DNA oxidation, and inhibits antioxidant substances, such as super oxide dismutase (SOD), glutathione peroxidase (GSH-Px). Enhancing ROS and nitrogen species (RNS) aggravates mitochondrial dysfunction and has a detrimental effect on the cellular DNA, proteins, and lipids. Deposited Aβ causes oxidative stress, subsequently increasing ROS levels (Miranda et al., 2000; Butterfield et al., 2006). Consequently, the high level of ROS and RNS lead to release of pro-apoptotic signaling protein, CytC. CytC accelerates the formation of apoptosome, causing neurodegenerative disease (Bhat et al., 2015).
Reduce Mitochondrial Dysfunction
Mitochondrial dysfunction is another important factor in AD pathogenesis. Extracellular deposits of Aβ can access inside mitochondria and disrupt the normal state of mitochondria, leading to imbalanced mitochondrial membrane potential. The aggregation of Aβ in extracellular are associated with mitochondrial dysfunction and neuronal structural damage. Mitochondrial damage leads to the loss of adenosine triphosphate (ATP) and ROS increase, leading to the cell apoptosis (Reddy and Beal, 2008; Moreira et al., 2010; Onyango et al., 2016).
TCM for AD Treatment Via Reducing B-Amyloid Production
Ginseng Protein
Ginseng (Panax ginseng C.A. Meyer), which belongs to the Araliaceae family. In China, ginseng has a long history of use to prolong life and soothe the puzzle. It exhibits strong therapeutic effects on cognitive impairments and neurodegeneration (Huang et al., 2019a). Ginsenosides are considered the main bioactive constituent of ginseng, and some studies have reported their neuroprotective effect (Yang et al., 2012). Recent research indicates that ginseng protein (GP) has a very significant neuroprotective effect in the treatment of AD. It inhibits Aβ1-42 and tau pathology, increases the mRNA and protein expression of PI3K, p-Akt/Akt, and Bcl-2 (B-cell lymphoma 2)/Bax (Bcl-2 associated X) in the hippocampus. GP improves the memory capacity and cognitive function by activating PI3K/Akt signaling pathway (Li et al., 2016a).
The Total Flavonoid Extract from Dracoephalum moldavica L.
Dracoephalum moldavica L., a member of Lamiaceae family, possesses important medicinal value on refreshing body and mind as well as relieving sore throat and cough. In addition, D. moldavica has strong clinical effects on asthma, cardiovascular, and cerebral ischemia (Martínez-Vázquez et al., 2012). The flavonoids in D. moldavica have been a hotspot due to their extensive biological applications. The total flavonoid extract from D. moldavica (TFDM) mainly include apigenin, luteolin, acacetin, gardenin B, serophulein, salvigenin, isorhamnetin, tilianin, agastachoside, and kaempferol. These extracts are flavone, flavonol, and glycosides. The linked sugars are glucose, xylose, and their derivatives. TFDM are extracted from the aerial part of D. moldavica (Liu et al., 2019). A recent research revealed that TFDM treatment improved the memory capacity and inhibited neurodegeneration. TFDM decreased insoluble Aβ levels by reducing Aβ deposition and enhanced the antioxidant defense capacity. TFDM inhibited Aβ production pathway, which is related to the down-regulation of β-secretase and β-C-terminal fragments in the brain of APP/PS1 mice. TFDM treatment activated the nuclear translocation of phospho-extracellular signal-regulated kinase 1/2 (ERK1/2) that led to elevated brain-derived neurotrophic factor (BDNF) levels through enhanced cAMP response element-binding protein (CREB) activation. TFDM can protect injured cells, and is associated with reducing APP and Aβ1–42 levels, hence exerts beneficial effects (Liu et al., 2018).
Safflower Yellow
SY is the main active chalcone glycoside compound extracted from Carthamus tinctorius L. (safflower), and belongs to Compositae or Asteraceae family. It is widely used in TCM for management of dysmenorrhea, amenorrhea, and joint pain. Besides, safflower seeds have been reported to attenuate memory impairment (Kim et al., 2019). SY improved cognitive functions and ameliorated the memory loss of APP/PS1 mice. SY decreased the level of Aβ and overactivation of astrocytes in AD rats. Meanwhile, SY increased SOD and GSH-Px levels, and decreased malondialdehyde (MDA) and acetylcholinesterase (AChE) expression in brain tissues of AD mouse model. Moreover, SY was able to inhibit cyclin-dependent kinase 5 (CDK-5) and glycogen synthase kinase-3 (GSK-3) signaling pathways, which are upregulated in AD mouse model (Ma et al., 2015).
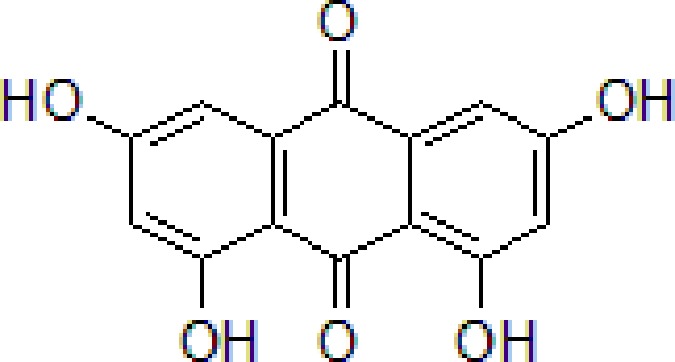
Emodin
Emodin is the main anthraquinone compound from Rheum officinale Baill. (Polygonaceae family) is extensively used in TCM and is the monarch drug in some TCM prescriptions with brain protection properties, such as Tiao-wei-cheng-qi Decoction, Da-cheng-qi Decoction (Gong et al., 2011). Emodin suppresses Aβ deposition and tau phosphorylation. Furthermore, emodin downregulates the activity of β-site APP-cleaving enzyme 1 (BACE1) and increases protein phosphatase 2A levels (Zeng et al., 2019).
Onjisaponin B
Onjisaponin B is the main active saponin constituent from Radix Polygalae. Radix Polygalae (the root of Polygala tenuifolia Willd.) belongs to Polygalaceae family and is a typical herbal medicine and has been extensively used for the treatment of dementia. Radix Polygalae plays an important role in most nootropics prescriptions (Zhang et al., 2008; Wang et al., 2015a; Wang et al., 2015b). Onjisaponin B reduces Aβ production without directly inhibiting of BACE1 and γ-secretase activities, promoting APP degradation (Li et al., 2016b) (Table 1).
Table 1.
TCM for treating AD by reducing β-Amyloid production.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Ginseng protein | — | 0.05–0.1 g/kg twice daily | Improve the memory ability and cognitive and reduce Aβ production | Inhibit Aβ1-42 and p-tau and increase the mRNA and PI3K, p-Akt/Akt, and Bcl-2/Bax | d-galactose/AlCl3 induced rat model | Li et al., 2016a |
| 2 |
Dracoephalum moldavica L. flavonoid |
— | 200 mg/kg | Reduce Aβ deposition and insoluble Aβ levels | Attenuate Aβ-induced toxicity through anti-amyloidogenesic and neurotrophic pathways | Heterozygous APPswe/PS1Δ9 transgenic founder mice | Liu et al., 2018 |
| 3 | Safflower yellow |

|
10–30 mg/kg | Improve cognitive function and ameliorate the learning and memory deficits | Decrease Aβ accumulation and overactivation of astrocytes | APP/PS1 transgenic mice | Ma et al., 2015 |
| 4 | Emodin |
|
80 mg/kg/day | Improve the memory ability and cognitive, reduce Aβ production | Reduce the levels of Aβ and tau phosphorylation | Hyperhomocysteinemia (HHcy) induced rats | Zeng et al., 2019 |
| 5 | Onjisaponin B |
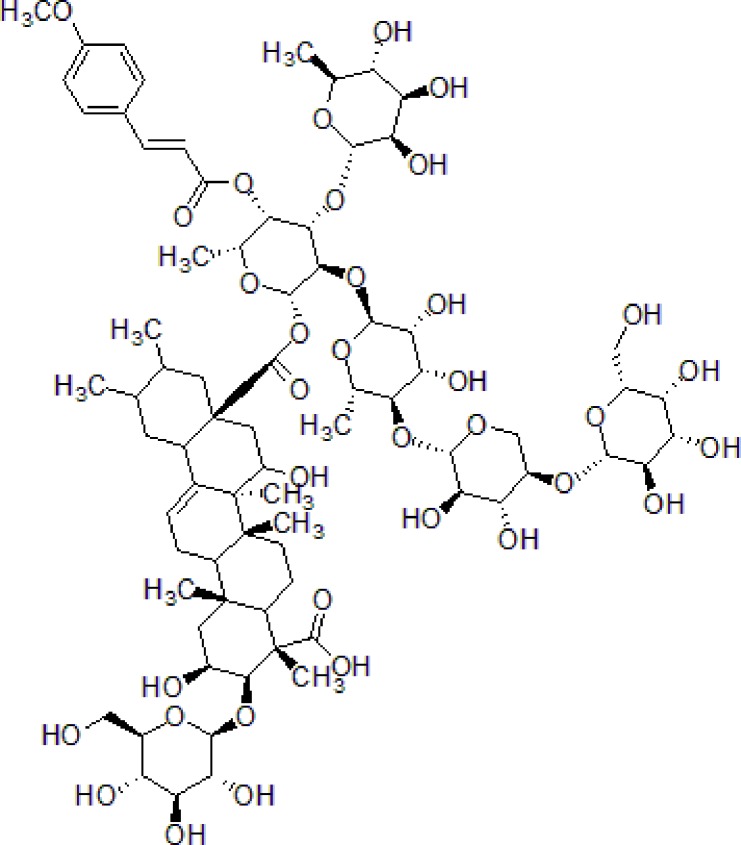
|
1mg/mL, 200μL | Suppress Aβ production, improve learning and memory capacity | Suppress Aβ production promoted the degradation of APP | The APPswe/PS1ΔE9 (APP/PS1) double-transgenic mice | Li et al., 2016a |
TCM for AD Treatment Via Anti-Apoptosis
Morroniside
Morroniside is a natural iridoid glycoside in Cornus officinalis Sieb. et Zucc (Cornaceae), which is widely used in TCM to treat dementia. The dry ripe sarcocarp of C. officinalis contains loganin, morroniside, and other bioactive compounds hence has great medicinal value (Wang et al., 2009). Morroniside prevents H2O2 or Aβ1-42-induced apoptosis through some complex apoptotic pathways, including Bcl-2, caspases, and mitochondria-dependent cell death pathways. Morroniside inhibits the expression of Bax, Cytc, cleaves caspase-3, and increases the level of Bcl-2. Morroniside exerts anti-apoptosis effect via attenuating c-Jun N-terminal kinase (JNK) and p38 MAPK phosphorylation in Aβ1-42-induced PC12 cells (Chen et al., 2018b).
Curcumin
Curcumin is an active polyphenol extraction isolated from Curcuma longa L. It is a member of ginger family and is important TCM with a variety of pharmacological activities, such as anti-inflammatory, anti-cancer, and dementia prevention. Several studies have shown that C. longa is a potential neuroprotective drug (Witkin and Li, 2013). Curcumin significantly decreased Aβ-induced cytotoxicity by inhibiting mitochondria-mediated apoptosis via regulation of Bcl-2 family. Curcumin inhibited Aβ-induced DNA damage by reducing of ROS generation through p38 MAPK and AKT pathways. Curcumin treatment also protected rat PC12 cells from Aβ25-35-induced reduction in cell viability, the level of lactate dehydrogenase (LDH) and MDA. This process was associated with high expression of N-methyl-d-aspartate receptor (NMDAR) and NMDAR subunit 2A (NR2A) (Fan et al., 2017). Furthermore, curcumin was found to protect neuronal cell effectively and attenuate apoptosis by regulating intracellular Ca2+ release, ROS, and mitochondrial membrane potential depolarization level in SH-SY5Y cells (Uğuz et al., 2016).
Triptolide
Triptolide is the main natural diterpene extracted from Tripterygium wilfordii Hook F. belonging to Celastraceae family, has been used in TCM for centuries. Recently, several reviews are published on T. wilfordii that exhibits therapeutic efficacies in the treatment of neurodegenerative diseases (Schwartz and Deczkowska, 2016). Triptolide remarkably inhibited the neuronal cells apoptosis and increased intracellular Ca2+ concentration induced by Aβ and Aβ25–35. Triptolide effectively ameliorates Aβ-induced cell injury by suppressing Aβ levels and chemokine receptor 2 (CXCR2) activity (Gu et al., 2004; Wang et al., 2015a).
Crocin
Crocin is a bioactive carotenoid extracted from Crocus sativus L. (Iridaceae family) and is an important TCM known for its medicinal properties in neuropsychiatric disorders, such as depression, seizure, anxiety, and neurodegenerative disease. (Modabbernia and Akhondzadeh, 2013). Crocin can improve memory impairment and learning ability by reducing neuron apoptosis and Bax levels as well as increasing the expression of Bcl-2 in hippocampus and prefrontal cortical neurons (PFC) (Lin et al., 2019a). Aβ administration in hippocampus significantly increases proteins and factors associated with autophagy and apoptosis, such as Beclin-1, LC3-phosphatidylethanolamine conjugate (LC3-II)/cytosolic LC3 (LC3-I) ratio, Bax/Bcl-2 ratio, and cleaved caspase-3 (Asadi et al., 2015). Essentially, crocin alleviates malathion-induced neurological alterations and cognitive impairment by exerting its anti-apoptotic effects (Mohammadzadeh et al., 2019). These show that crocin has potential therapeutic effects on AD (Table 2).
Table 2.
TCM for treating AD by anti-apoptosis.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Morroniside |
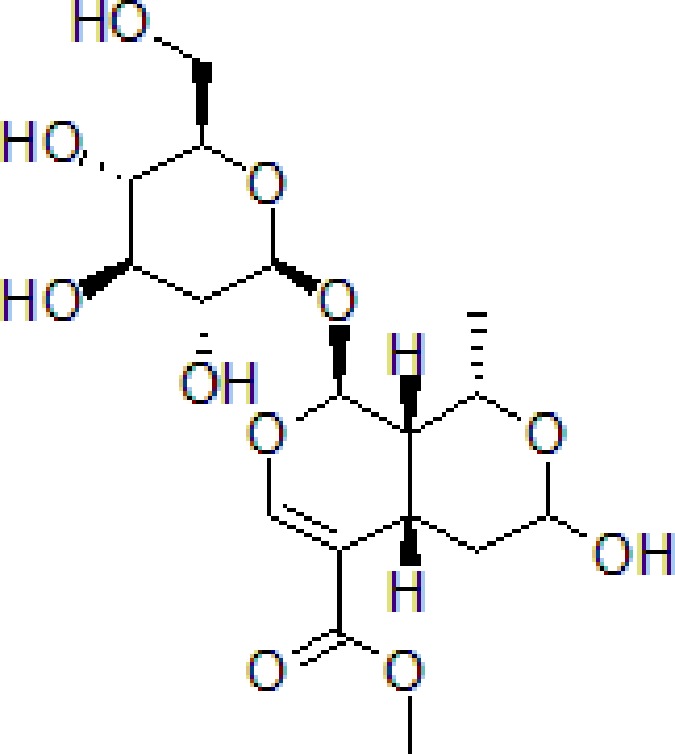
|
100 μM | Inhibit apoptosis | Inhibit apoptosis via attenuating JNK and p38 MAPK phosphorylation | PC12 cells | Chen et al., 2018b |
| 2 | Curcumin |
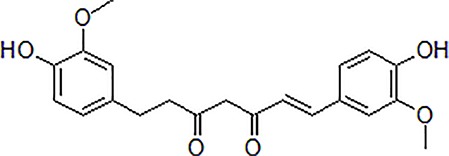
|
12.5–200 μM | Reversal neurotoxicity, inhibit apoptosis | Inhibit apoptosis though p38 MAPK and AKT pathways | PC12 cells | Uğuz et al., 2016; Fan et al., 2017 |
| 3 | Triptolide |
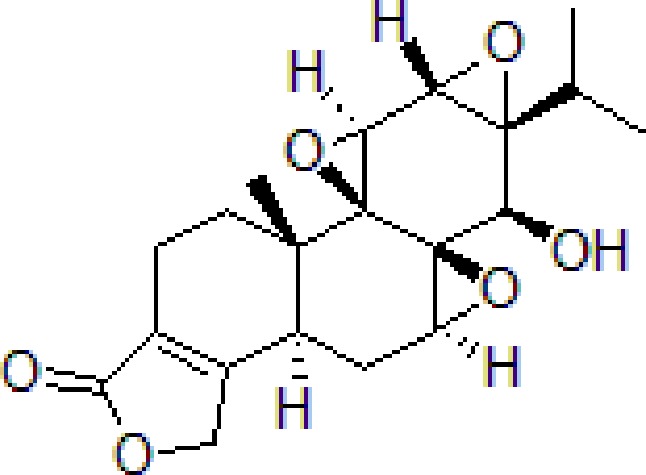
|
10−11 mol/L | Inhibit apoptosis | Inhibit intracellular Ca2+ and apoptosis | PC12 cells | Gu et al., 2004; Wang et al., 2015a; Xu et al., 2016 |
| 4 | Crocin |
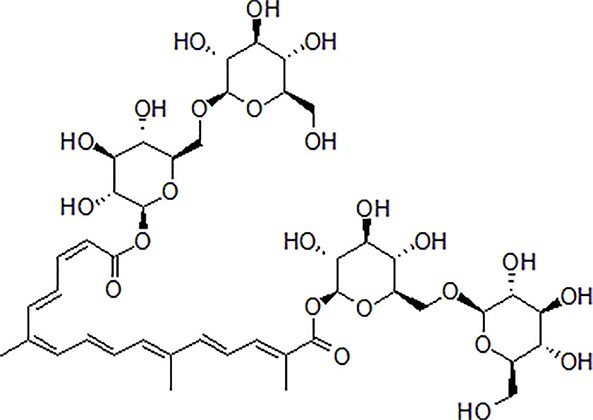
|
40 mg/kg | Regulate endoplasmic reticulum stress and apoptosis | Increase the autophagy and apoptosis biomarkers | Male Wistar rats | Asadi et al., 2015; Lin et al., 2019a; Lin et al., 2019b; Mohammadzadeh et al., 2019 |
TCM for AD Treatment via Autophagy Inducing or Attenuation
Berberine
Ber is an alkaloid extracted from Coptis chinensis Franch., a member of Ranunculaceae family extensively used in TCM. Coptis chinensis is a major ingredient of San-Huang-Xie-Xin-Tang, a traditional Chinese formula, used to manage cardiovascular and neurodegenerative diseases (Li et al., 2018). Ber improves memory retention and spatial learning capacity by promoting Aβ clearance. It exerts neuroprotective effect by facilitating autophagy through LC3-II, Beclin-1, hVps34, sequestosome 1 (p62), and Cathepsin-D activities as well as Bcl-2 brain levels reduction (Huang et al., 2017; Zhang et al., 2018).
Andrographolide
Andrographolide (Andro) is the main bioactive labdane diterpenoid component of Andrographis paniculata (Burm.f.) Nees, a typical TCM of Acanthaceae family. Recently, extensive researches have shown that A. paniculate can ameliorate cognitive impairment (Serrano et al., 2014; Thakur et al., 2016; Geng et al., 2018). Also, Andro can ameliorate cell viability and normalize the abnormal nuclear morphology by inhibiting Aβ1–42-induced abnormal changes in LDH, MDA, ROS, and mitochondrial membrane potential. Andro significantly improves Aβ1–42-induced cognitive impairment and learning function by activating autophagy-related genes and proteins, such as Beclin-1, LC3 nuclear factor, E2-related factor 2 (Nrf2), p62, and p21. In addition, Andro can exert autophagy effect via Nrf2-mediated p62 signaling pathway (Gu et al., 2018).
Geniposide
Geniposide is an iridoid glycoside component of Gardenia jasminoides Ellis. and is an essential component of traditional phytomedicines (Shan et al., 2017). It belonging to Rubiaceae family, and is widely used in TCM. Geniposide exhibits significant therapeutic effect in the treatment of brain disorders and neurodegenerative disease (Zhao et al., 2016). Geniposide improved cognitive function by decreasing Aβ1-40 deposition in the brain tissue of AD mouse model by activating glucagon-like peptide-1 (GLP-1) receptors, which regulates mTOR. Thus, down-regulating mTOR signaling leading to enhanced cellular autophagy and increased lysosomal clearance of Aβ fibrils. Geniposide increased p-Akt/Akt, p-mTOR/mTOR, expression of LC3-II and Beclin1. This indicates that geniposide has a therapeutic effect on the AD neuropathological damage (Zhang et al., 2019).
β-Asarone
β-asarone is the major component of Acorus tatarinowii Schott (Wu and Fang, 2004). Acorus tatarinowii, belongs to Acoraceae family, is commonly used as TCM. It is known for a variety of pharmacological activities, specifically in treatment of neurodegenerative diseases (Li et al., 2010). β-asarone increases cell viability by decreasing neuron specific enolase (NSE) levels and Beclin-1 in addition to Aβ1–42-induced autophagy attenuation through Akt-mTOR signaling pathway (Xue et al., 2014).
Dendrobium nobile Lindl Alkaloids
Dendrobium nobile Lindl. has been used for medicinal purpose for centuries and is a member of Orchidaceae family. It has been widely used for kidney injury treatment. Alkaloids are considered as the main characteristic potent ingredients of D. nobile (Ng et al., 2012). Dendrobium nobile Lindl alkaloids (DNLA) is an active alkaloid from D. nobile Lindl. and mainly includes dendrobine, dendroxine, nobiline, dendrine, 6-hydroxy-dendroxine, N-methyl-dendrobine, and N-isopentenyl-dendrobine (Inubushi et al., 1964; Granelli et al., 1970). DNLA are able to increase autophagic flux, inhibit axonal degeneration by increasing Beclin-1 expression. DNLA increases autophagosome formation and autophagosome-lysosome fusion in hippocampus region (Li et al., 2017).
Euxanthone
Euxanthone is a xanthone derivative extracted from Polygala caudata Rehder & E.H.Wilson mainly distributed in southwestern China that has been extensively used in TCM (Pan and Mao, 1984). uxanthone can attenuate memory impairment and learning dysfunction by reversing Aβ1-42-induced neuronal apoptosis and autophagy. Euxanthone protected PC12 cells against Aβ1-42-induced oxidative stress and apoptosis by inducing autophagy via LC3B-II enhancement and p62degradation (Yuan et al., 2018) (Table 3).
Table 3.
TCM for treating AD by inducing or attenuating autophagy effect.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Berberine |
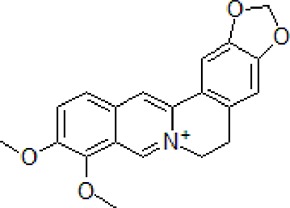
|
50–100 mg/kg/day | Promote Aβ clearance, improve learning and memory capacity | Induce autophagy by activating Bcl2/Beclin1 signaling | 3 × Tg-AD mice | Huang et al., 2017; Zhang et al., 2018 |
| 2 | Andrographolide |
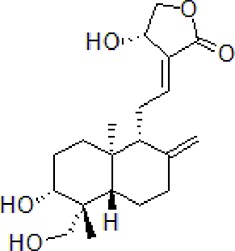
|
20 μM | Decrease cell death | Induce autophagy through activation of the Nrf2-mediated p62 signaling pathway | PC12 cells | Gu et al., 2018 |
| 3 | Geniposide |
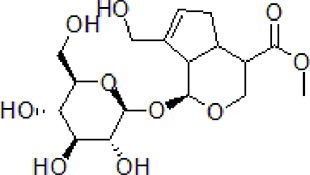
|
50 mg/kg/day | Reduce Aβ1–40 level, promote Aβ clearance, improve cognitive function | Induce autophagy by down regulation of mTOR | Age-matched C57BL/6 wild-type (WT) mice or APP/PS1 mice | Shan et al., 2017; Zhang et al., 2019 |
| 4 | β-asarone |
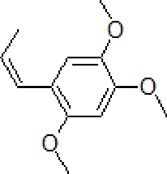
|
240 μg/mL | Increase cell viability, decrease NSE levels | Attenuate autophagy by Akt-mTOR signaling pathway | PC12 cells | Wu and Fang, 2004; Xue et al., 2014 |
| 5 | Dendrobium nobile Lindl. alkaloids | — | 3.5–350 ng/mL | Inhibit axonal degeneration | Induce autophagy by promoting Beclin1 | Aβ25–35-induced hippocampus primary neuron | Li et al., 2017 |
| 6 | Euxanthone | — | 30 mg/kg or 60 mg/kg | Attenuate memory and spatial learning dysfunction | Induce autophagy by increasing the expression level of LC3B-II and enhancing the degradation of p62 | Sprague-Dawley rats | Pan and Mao, 1984; Yuan et al., 2018 |
TCM for AD Treatment Via Anti-Neuroinflammation
Gypenoside
Gypenoside is the major active saponin ingredient of Gynostemma pentaphyllum (Thunb.) Makino, a member of Cucurbitaceae family known for strengthening effect on the heart and brain (Lin et al., 1993). Recent researches have shown that Gypenoside has a potential in the treatment of neurodegenerative diseases. The deposition of Aβ activated microglia and astrocyte, leading to the production of inflammatory factors, IL-1β, IL-6, and TNF-α which caused neuronal death (Kumar et al., 2018; Seo et al., 2018). Gypenoside attenuates Aβ-induced inflammation by downregulating the release of proinflammatory factors such as iNOS, TNF-α, IL-1, and IL-6, as well as increasing anti-inflammatory factors release, such as IL-10. Gypenoside can increase the levels of arginase-1 (Arg-1) protein, brain-derived neurotrophic factor (BDNF), and glial cell-derived neurotrophic factor (GDNF) secretions. The process is mediated by suppressing cell signaling protein 1 (SOCS1) (Cai et al., 2016).
Steroid-Enriched Fraction of Achyranthes bidentata Blume (ABS)
Achyranthes bidentata Blume (Amaranthaceae family) is commonly used in the treatment of dementia in TCM. The roots of A. bidentata are rich in pharmacological active ingredients (He et al., 2017). Recent reports have shown that ABS can attenuate cognitive dysfunction and neuroinflammation. ABS alone (50 mg/kg) reduced the levels of TNF-α in brain and decreased neuroinflammation by modulating ERK and NF-κB pathway (He et al., 2014; Xu X. X. et al., 2017; Lin et al., 2019b).
Matrine
Matrine is the major bioactive alkaloid of the Sophora flavescens Ait. (Fabaceae family), a famous traditional Chinese herbal medicine used to treat dementia. Matrine can improve cognitive deficits and learning ability. Matrine attenuated Aβ42-induced memory deficits, cytotoxicity, and the formation of senile plaques in AD transgenic mice. This was through reducing Aβ deposition and proinflammatory cytokines via glycation end products (RAGE) signaling pathway (Cui et al., 2017).
Loganin
Loganin is also a natural iridoid glycoside from Cornus officinalis Sieb. Loganin can attenuate Aβ- induced microglia activation, over-production of TLR4, Myeloid differentiation factor 88(MyD88), TNF receptor-associated factor 6 (TRAF6). The anti-inflammation effect of loganin is via inhibiting elevation of TNF-α, IL-6, macrophage chemotactic protein 1 (MCP-1), NO, prostaglandin E2 (PGE2), and up-regulating iNOS and COX-2 (Cui et al., 2018).
Scutellarein
Scutellarein is a natural ingredient extracted from Scutellaria baicalensis Georgi., and belongs to Lamiaceae family. It has been traditionally used to treat various diseases, including dementia. Recent reports revealed that flavonoids from S. baicalensis, such as baicalin, baicalein, and scutellarin, exert neuroprotective effects (Jin et al., 2019). Scutellarein, a hydrolysate of scutellarin, can also decrease hippocampal Aβ and MDA content while increasing superoxide dismutase (SOD) and acetylcholine (Ach) expression. Moreover, it has a protective effect on Aβ-exposed apoptosis, counteracts the Aβ-induced Bcl-2 expression decrease and inhibits the expression of Bax and cleaved caspase3. In PC12 cells, scutellarein attenuated Aβ-induced cell death, cognitive impairment, hippocampal alterations, hippocampal neuroinflammation, and NF-κB activation. In summary, scutellarein inhibited Aβ−induced PC12 cell apoptosis. This demonstrated that scutellarein has potential therapeutic application in AD (Huang et al., 2019b).
Oridonin
Oridonin (Ori) is the main active diterpenoid of Isodon rubescens (Hemsl.) H. Hara (syn. Rabdosia rubescens Hara) of Lamiaceae family and has been used as Chinese herbal medicine due to its biological activities (Zhang et al., 2004). Ori has been shown to attenuate memory impairment and has anti-inflammatory effects. Ori exerts anti-inflammatory effects by inhibiting the activation of glial, decreasing the release of inflammatory cytokines IL-1β, IL-6, and TNF-α. This process is potentially associated with the NF-κB pathway inhibition (Wang et al., 2014).
Hydroxy-Safflor Yellow A
Hydroxy-safflor yellow A (HSYA) is active chalcone glycoside extracted from Carthamus tinctorius L.(Sun et al., 2010). HSYA can ameliorate Aβ1–42-induced memory deficiency and microglia and astrocytes activation by downregulating the mRNA expression of pro-inflammatory cytokines. HSYA up-regulated the janus kinase-2 (JAK2) signal transducers and activators of transcription 3 (STAT3) pathway and inhibited the activation of NF-κB signaling pathways. HSYA protects Aβ1–42-induced AD model mice through JAK2/STAT3/NF-κB pathway (Zhang et al., 2014).
Diammonium Glycyrrhizinate
Diammonium glycyrrhizinate (DG) is the most important active ingredient of Glycyrrhiza uralensis Fisch. ex DC. of the Fabaceae family. It has been widely used in TCM to treat variety of diseases. Recent pharmacological reports revealed that G. uralensis exerts neuroprotective effect in AD (Ahn et al., 2006). DG attenuated Aβ1–42-induced memory impairment and activation of microglia and inflammation in AD mice model by inhibiting the activation of MAPK and NF-κB signaling pathways (Zhao et al., 2013) (Table 4).
Table 4.
TCM for treating AD by anti-neuroinflammation.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Gypenoside |
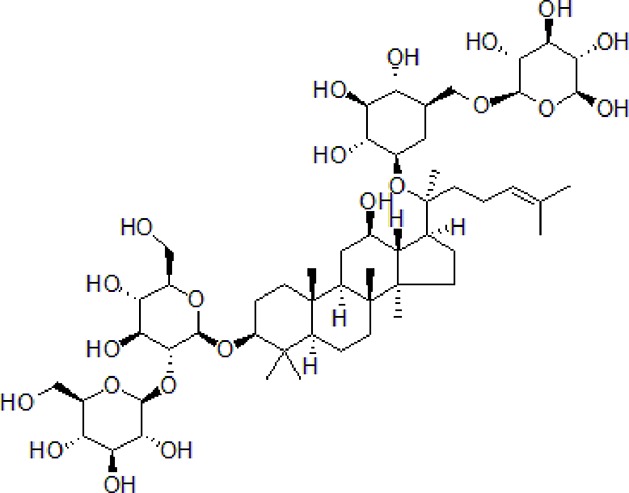
|
50 μg/mL | Attenuate inflammation | Attenuate Aβ induced inflammation via SOCS1 Signaling | N9 microglial cells | Cai et al., 2016; Kumar et al., 2018; Seo et al., 2018 |
| 2 | Achyranthes bidentata Blume | — | 50 mg/kg | Improve cognitive function, decrease neuroinflammation | Decrease oxidative stress and neuroinflammation through modulating ERK pathway, NF-κB phosphorylation, and translocation | Male Sprague-Dawley rats | Lin et al., 2019b |
| 3 | Matrine |
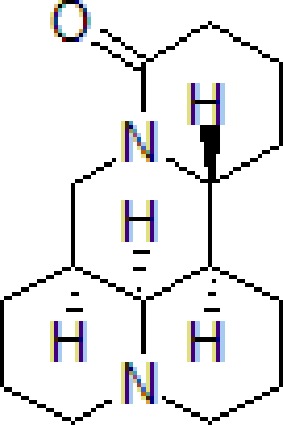
|
10–50 μM | Improve cognitive deficits and learning ability | Decrease neuroinflammation though Aβ/RAGE signaling pathway | SH-SY5Y cells | Cui et al., 2017 |
| 4 | Loganin |
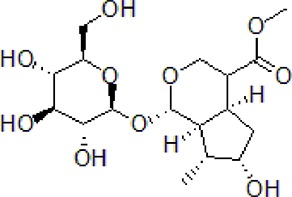
|
10–30 μM | Decrease neuroinflammation | Decrease neuroinflammation via regulating TLR4/TRAF6/NF-κB axis | BV-2 microglia cells | Cui et al., 2018 |
| 5 | Scutellarein |
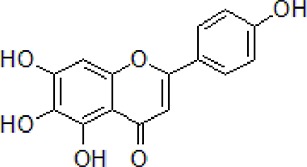
|
50 mg/kg, intraperitoneally | Suppress neuroinflammation | Increase Bcl-2 and suppress Beclin-1 expression via inhibition of the NF-κB pathway | PC12 cells, male Wistar rats | Huang et al., 2019b |
| 6 | Oridonin |
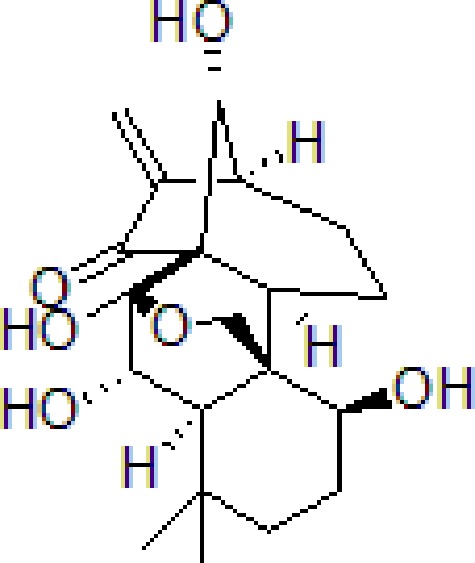
|
10 mg/kg/day, i.p. for 15 days | Inhibit glial activation, decrease the release of inflammatory cytokines and attenuate memory deficits | Attenuate Aβ1–42-induced neuroinflammation and inhibits NF-κB pathway | Male C57BL/6 (B6) mice | Wang et al., 2014 |
| 7 | Hydroxy-safflor yellow A |
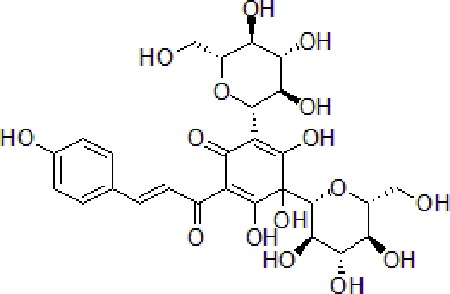
|
20 mg/kg per day, i.p. | Ameliorate the memory deficits and decrease the mRNA expression of pro-inflammatory mediators | Attenuate Aβ1-42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway | Male ICR mice | Sun et al., 2010; Zhang et al., 2014 |
| 8 | Diammonium Glycyrrhizinate |
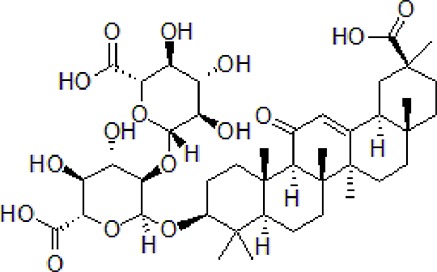
|
10 mg/kg per day, i.p. | Attenuate the memory deficits and suppress Aβ1–42-induced activation of microglia and inflammation | Attenuate Aβ1–42-induced neuroinflammation and regulate MAPK and NF-κB pathways | SH-SY5Y and HT-22 cells or male ICR mice | Zhao et al., 2013 |
TCM for AD Treatment Via Reducing Oxidative Stress
Schisanhenol
Schisanhenol is a lignan compound from Schisandra sphenanthera Rehder & E. H. Wilson of Schisandraceae family. It has been used extensively in TCM to treat age-related diseases (Hung et al., 2007). Schisanhenol can remove oxygen radicals and inhibit lipid peroxidation and apoptotic cell death induced by oxidative stress. In addition, schisanhenol can improve learning and memory as well as attenuate oxidative damage by reducing the activity of AChE via sirtuin 1 (SIRT1)- coactivator 1-α (PGC-1α)-Tau signaling pathway (Yu and Liu, 2008; Han et al., 2019).
Amentoflavone (AF)
AF is the major active flavonoid found in Selaginella tamariscina (P. Beauv.) Spring. This plant belongs to Schisandraceae family which has been found exert pleiotropic bioactivities. Recent pharmacological reports provide evidence that S. tamariscina could be an effective neuroprotective agent (Shin et al., 2006). It was found that AF decreased Aβ-induced learning and memory deficits and prevented neuronal cell apoptosis, oxidative stress in the hippocampus of rat model. AF increased Nrf2 expression and promoted AMPK signaling. The neuroprotective effect of AF against Aβ1–42-induced neurotoxicity is mediated by Nrf2 antioxidant pathways and AMPK signaling (Chen et al., 2018a).
Coptis chinensis Franch. Rhizome Watery Extract
Studies have reported that dried rhizome of Coptis chinensis Franch. possess pharmacologically active ingredients. Key among them include Ber, palmatine, coptisine, epiberberine, columbamine, and jatrorrhizine (Luo et al., 2011). Previously, extracts from Coptis chinensis Franch. rhizome watery extract (CRE) improved t-BOOH-induced cytotoxicity and cell injury by attenuating mitochondrial membrane depolarization, regulating Thioredoxin-interacting protein (TXNIP) and mitochondrially encoded NADH dehydrogenase. It also conferred neuroprotection by downregulating TXNIP levels (Friedemann et al., 2014).
Oxymatrine
Oxymatrine (OMT) is a bioactive quinolizidine alkaloid isolated from Sophora flavescens Aiton and Sophora alopecuroides L. (Qian et al., 2018). A study found that OMT significantly increased cell viability and the cognitive ability of rats. Moreover, treatment with OMT increased the ratio of Bcl-2/Bax and decreased the expression of caspase-3. The protective effect of OMT against Aβ1-42-induced neuronal toxicity was linked to the inhibition of MAPK and NF-κB signal pathways (Dong et al., 2019).
Shikonin
Shikonin is an active naphthoquinone ingredient found in Lithospermum erythrorhizon Siebold & Zucc., one of the most important Chinese herbal medicine belonging to the Boraginaceae family. This compound has long been used to treat allergic diseases. Recent reports have provided evidence that roots of L. erythrorhizon contain compounds with antioxidant activities (Papageorgiou et al., 2008; Yoshida et al., 2014). Shikonin markedly increased cell viability by upregulating the activity of SOD catalase and GSH-Px. It also decreased levels of MDA and ROS, and stabilized the mitochondrial membrane potential in Aβ1–42-treated PC12 cells. The neuroprotective effect of shikonin against Aβ-induced cell damage was due to inhibition of cleaved caspase-3 activity and reduction in ratio of Bcl-2/Bax all of which prevent antioxidants and apoptosis (Tong et al., 2018).
Linalool (LI)
LI is the main volatile monoterpene compound found in several aromatic plants, such as Lavandula angustifolia Mill., Rosmarinus officinalis L. and Coriandrum sativum L. (Kuroda et al., 2005; Batista et al., 2010; Gastón et al., 2016). In a previous study, LI significantly improved the cognitive performance and alleviated Aβ1-40-induced cell injury by increasing the levels of oxidative stress indicators such as SOD, GPX, and AChE. LI decreased the activity of cleaved caspase-3 and caspase-9 and increased the expression level of Nrf2 and heme oxygenase-1 (HO-1). Thus, LI confers neuroprotection by preventing apoptosis, oxidative stress, and activation of Nrf2/HO-1 signaling pathway (Xu P. et al., 2017).
Schisandrin C
Schisandrin C (SCH-C) is the main antioxidative lignan extracted from Schisandra chinensis (Trucz.) Baill. It attenuated impaired cognitive and learning ability by decreasing the activity of total cholinesterase (ChEtotal), significantly increasing the activities of SOD and GSH-Px glutathione. Thus, neuroprotective effect of SCH-C against Aβ1–42-induced cell injury is mediated by inhibition of ChEtotal and upregulation of the level of SOD and GSH-Px glutathione (Mao et al., 2015).
Acteoside
Acteoside, a phenylethanoid glycoside, is isolated from Cistanche deserticoLa Y. C. Ma. that belongs to Drobanchaceae family. It has been used to treat age-related disorders and improve kidney function (Jiang and Tu, 2009). Acteoside improved the cognitive function following treatment with d-galactose (d-gal) and AlCl3 by increasing the number of neurons and decreasing the level of nitric oxide (NO), nitric oxide synthase (NOS) and caspase-3 protein expression in hippocampal tissues. The mechanisms associated include suppression of oxidative stress and hence neuronal apoptosis (Peng et al., 2015).
Vanillic Acid
Vanillic acid (VA) is a natural benzoic acid derivative obtained from Angelica sinensis (Oliv.) Diels. which belongs to Apiaceae family. It has been used in TCM as a flavoring agent and to treat various diseases. Recent studies have provided evidence that Danggui Buxue Tang (derived from A. sinensis (Oliv.) Diels) has the potential to treat AD (Gong et al., 2019). VA significantly improved memory function by decreasing AChE, corticosterone, TNF-α, and inhibiting oxidative stress (Singh et al., 2015).
Protosappanin A
Protosappanin A (PTA) is the major biphenyl compound extracted from Caesalpinia sappan L. (Lignum Sappan), a member of Fabaceae family. This plant has traditionally been used to treat various diseases. Numerous lines of evidence also show that compounds found in Biancaea sappan (L.) Tod. (syn. Caesalpinia sappan L.) have antioxidant activities (Sasaki et al., 2007). Activation of microglia increases the level of ROS and NO, leading to neuronal and cell injury. PTA exerted immunosuppressive effects and anti-oxidative activities against lipopolysaccharide (LPS)-induced injury on BV-2 microglia by inhibiting the activation of microglia and suppressing ROS and NO levels. Moreover, PTA modulated the CD14/TLR4-dependent IκB-kinase (IKK)/nuclear factor κB (IκB)/NF-κB inflammation signaling pathway which decreased the expression of NADPH oxidase and iNOS (Zeng et al., 2012).
Salidroside
Salidroside is the main tyrosol-glucoside compound extracted from Rhodiola rosea L. that belongs to Crassulaceae family. Recently, it was reported that R. rosea contain various compounds such as salidroside with pharmacological properties, including anti-oxidative effects (Kanupriya et al., 2005). Salidroside confers neuroprotection by inducing thioredoxin (Trx), HO-1, and peroxiredoxin-I (PrxI). In addition, it suppresses Aβ25–35-induced neuronal injury be decreasing ROS production and restoring mitochondrial membrane potential (MMP) through JNK and p38 MAPK signaling pathways (Zhang et al., 2010) (Table 5).
Table 5.
TCM for treating AD by reducing oxidative stress.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Schisanhenol |
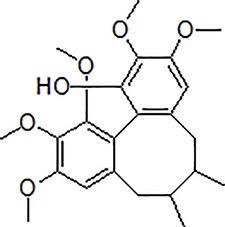
|
10–100 mg/kg | Improve learning memory and attenuate oxidative damage | Reduce acetylcholinesterase activity and attenuate oxidative damage through SIRT1-PGC-1α-Tau signaling pathway | Scopolamine-treated Kunming mice | Han et al., 2019 |
| 2 | Amentoflavone |
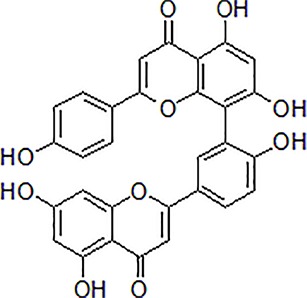
|
80 mg/kg | Ameliorate memory deficits and oxidative stress | Ameliorate memory deficits and oxidative stress by inducing Nrf2 antioxidant pathways via AMPK signaling activation | PC12 cells and rat | Chen et al., 2018a |
| 3 | Coptis chinensis Franch. watery extract | — | 100 mg/mL | Neuroprotective and against oxidative stress | Have neuroprotective and against oxidative stress and down regulation of TXNIP | SHSY5Y cells | Thomas Friedemann et al., 2014 |
| 4 | Oxymatrine |
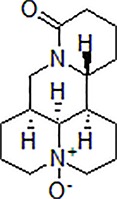
|
30–120 mg/kg | Increase cell viability and SOD activity | Improve the cognitive ability of rats and has a protective effect on Aβ1-42-induced primary neuronal cell by inhibiting MAPK and NF-κB signal pathways | Sprague-Dawley rats | Qian et al., 2018; Dong et al., 2019 |
| 5 | Shikonin |
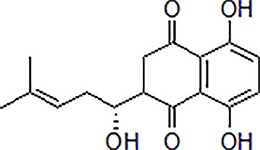
|
3.47, 10.42, 34.72 µM | Improve cell viability, decrease the MDA and ROS content, and stabilize the mitochondrial membrane potential | Reduce the activity of caspase-3 and moderate the ratio of Bcl-2/Bax through antioxidant and antiapoptotic activities | PC12 cells | Tong et al., 2018 |
| 6 | Linalool |

|
100 mg/kg per day, i.p. | Improve the cognitive performance and reverse the Aβ1-40 induced hippocampal cell injury, apoptosis and changes of oxidative stress indicators | Alleviate apoptosis, oxidative stress via activation of Nrf2/HO-1 signaling | C57BL/6J mice | Kuroda et al., 2005; Batista et al., 2010; Gastón et al., 2016; Xu P. et al., 2017 |
| 7 | Schisandrin C |
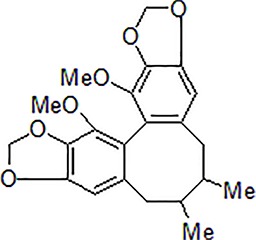
|
150 μg/kg, 10 mL/kg, injection | Improve the cognitive function and working memory | Inhibit ChEtotal, increased SOD and GSH-px, GSH | Male Kunming mice | Mao et al., 2015 |
| 8 | Acteoside |
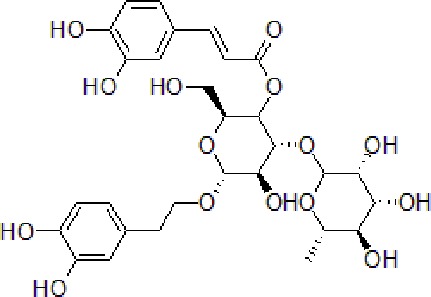
|
30, 60, and 120 mg/kg/day, for 30 days | Attenuate cognitive impairment and increase the numbers of neurons and Nissl bodies | Decrease the content of NO, the activity of NOS and the expression of caspase-3 protein due to oxidative stress | Kunming mice | Peng et al., 2015 |
| 9 | Vanillic acid |
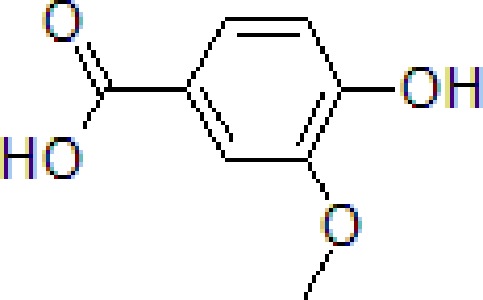
|
50 and 100 mg/kg | Improve spatial learning and memory | Improve the habituation memory, decrease the AChE, corticosterone, TNF-α by preventing oxidative stress | Swiss albino male mice | Singh et al., 2015 |
| 10 | Protosappanin A |
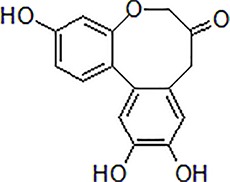
|
10 mg/kg, 5–50 μM | Inhibit ROS and NO production by suppression of NADPH oxidase and iNOS activity | Modulate IKK/IκB/NF-κB inflammation signal pathway to inhibit the activity and expressions of NADPH oxidase and iNOS | BV-2 cells or ICR mice | ZeNg et al., 2012 |
| 11 | Salidroside |

|
10, 50, 100 mM | Protect neurons from oxidative stress via the induction of antioxidant enzymes, Trx, HO-1, and PrxI | Inhibit Aβ25–35-induced phosphorylation of JNK and p38 MAP kinase and oxidative stress. | SH-SY5Y cells | Zhang et al., 2010 |
TCM Improves AD by Correcting Mitochondrial Dysfunction
Icariin
Icariin is the principal flavonoid in Epimedium brevicornu Maxim. This plant is a member of the Berberidaceae family commonly used to formulate Chinese herbal drugs for various diseases. Accumulating evidence demonstrates that E. brevicornu confer beneficial effects on the nervous systems (Ma et al., 2011). Icariin administration effectively decreased Aβ deposition in mitochondria and reversed the decline in levels of pyruvate dehydrogenase component α (PDHE1α) and cytochrome c oxidase IV (COX IV) in 3×Tg-AD mice. In summary, Icariin improves cognitive functions and neuronal cell activity, enhancing electron transport chain, N-acetylaspartate level, and brain ATP metabolism (Lustbader et al., 2004; Reddy and Beal, 2008; Chen et al., 2016).
Salvianolic Acid B
Salvianolic acid B (SalB) is the main active polyphenol found in Salvia miltiorrhiza Bge. a member of Lamiaceae family. Epidemiological evidence demonstrates that the roots of S. miltiorrhiza contain pharmacologically active ingredients with curative potential for various diseases, including AD (Wang et al., 2016). It was previously found that SalB alleviated Aβ-induced increase in glutathione (GSH) activity, lipid oxidation, axonal mitochondrial fragmentation, stabilized mitochondrial membrane potential, improved ATP production, and activity of CytC oxidase, all of which prevented cytotoxicity. Administration of SalB restores the synaptic density of Aβ-treated neurons (He et al., 2018).
Ligustilide
Ligustilide (LIG) is the main bioactive lipophilic component of Radix angelicae sinensis, Angelica sinensis (Oliv.) Diels. LIG reduces Aβ expression and that of dynamin-related protein 1 (Drp1) and increases levels of mitofusin 1 (Mfn1), mitofusin 2 (Mfn2), and Optic atrophy 1(Opa1) in APP/PS1 transgenic mice. LIG exerts anti-oxidant effects by decreasing MDA and ROS levels while increasing the level of Mn-SOD. It also improves memory deficits and mitochondrial function in APP/PS1 mice by reducing Aβ levels, enhancing mitochondrial motility, and restoring synaptic structure (Xu et al., 2018).
Tetrahydroxy Stilbene Glycoside
Tetrahydroxy stilbene glycoside (TSG) is one of the active polyhydroxystibene component isolated from Reynoutria multiflora (Thunb.) Moldenke (syn. Polygonum multiflorum), a member of the Polygonaceae family. It has been used to treat liver and kidney injury and as an antiaging agent for centuries. Recent reports show that TSG improves cognitive deficits in AD (Um et al., 2006). It also alleviates cell oxidative stress injury by attenuating LDH release, ROS production, and MDA leakage. TSG canceled Aβ-induced loss of MMP, alleviated the release of CytC from mitochondria to cytosol. It also increases the activity of caspase-3 and Bax expression, whereas it decreases Bcl-2 expression. TSG prevents neuronal cell injury by stabilizing mitochondrial function via Nrf2-HO-1 pathway (Jiao et al., 2017).
Hopeahainol A
Hopeahainol A (Hop A) is a potential AChE inhibitor and anti-oxidative polyphenol isolated from Hopea hainanensis Merrill & Chun. A previous study revealed that Hop A not only suppressed Aβ levels, but also inhibited the interaction between Aβ1-42 and Aβ-bound alcohol dehydrogenase (ABAD), which partially improved mitochondrial function and oxidative damage. These results reveal that Hop A balances synaptic function and improves memory deficits in APP/PS1 mice. (Zhu et al., 2013) (Table 6).
Table 6.
TCM for treating AD by reducing mitochondrial dysfunction.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Icariin |
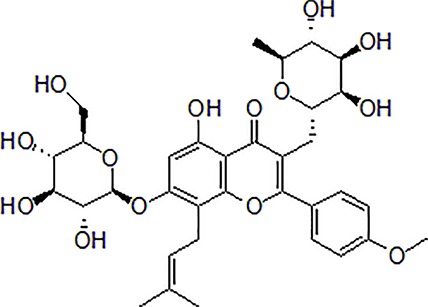
|
65 mg/kg, 20 μmol/kg/day | Improve spatial learning and memory retention | Ameliorate Aβ elevation in mitochondria and regulate the activity and expression of key mitochondrial enzymes | 3 × Tg-AD mice | Lustbader et al., 2004; Reddy and Beal, 2008; Chen et al., 2016 |
| 2 | Salvianolic acid B |
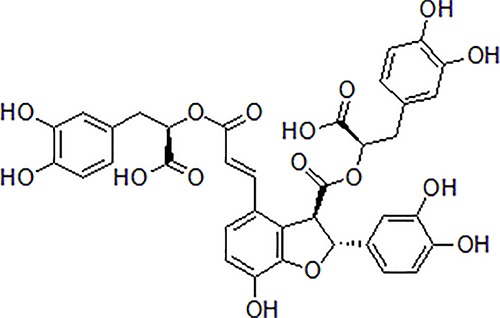
|
50 μM | Inhibit axonal mitochondrial fragmentation | Attenuate axonal mitochondrial fragmentation and increase kinesin-like protein 1 phosphorylation and restore the synaptic density | Primary cultured mouse neurons cell | He et al., 2018 |
| 3 | Ligustilide |
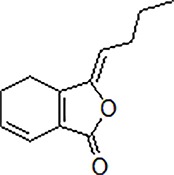
|
10 or 40 mg/kg | Reduce the level of Drp1 and increase levels of Mfn1, Mfn2, and Opa1 | Exerts an antioxidation effect via reducing the levels of MDA and ROS and increasing the activity of Mn-SOD | The APPswe/PS1dE9 (APP/PS1) transgenic mouse | Xu et al., 2018 |
| 4 | Tetrahydroxy stilbene glycoside |
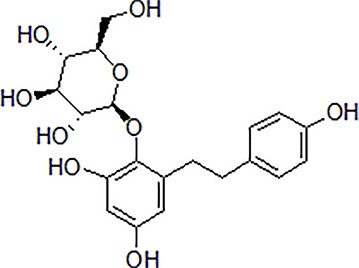
|
100 μg/mL, 5, 10, 30, 60, 90 μmol/L | Alleviate cell oxidative stress injury and mitochondrial membrane potential | Restore Aβ-induced hippocampal neuronal cell damage by restoring mitochondrial function via Nrf2-HO-1 pathway | HT-22 cell | Jiao et al., 2017 |
| 5 | Hopeahainol A |
|
4 mg/kg/day | Attenuate memory deficits | Reduce mitochondrial dysfunction and oxidative stress | APP/PS1 transgenic mice | Zhu et al., 2013 |
TCM for AD Treatment Through Various Mechanisms
Osthole
Osthole, a derivative of coumarin extracted from Cnidium monnieri (L.) Cusson, a member of Apiaceae family. Osthole up-regulates miR-107 and inhibits cleaving enzyme 1. It also increases cell survival, reduces LDH leakage. Osthole decreased Aβ levels by up-regulating miR-107 (Jiao et al., 2016).
Dendrobium officinale Polysaccharides
Dendrobium officinale Kimura & Migo is one of the most important TCM used to treat age-related disorders. Dendrobium officinale polysaccharides (DOP) remarkably attenuated cognitive decline and activated hippocampal microglial by downregulating IL-1β, TNF-α, and IL-6 while upregulating interleukin-10 (IL-10), neprilysin (NEP), and insulin-degrading enzyme (IDE) (Feng et al., 2019) (Table 7).
Table 7.
TCM for treating AD by other ways.
| Numbers | Compounds | Chemistry structure | Dosages | Activities | Molecular mechanism | Models | References |
|---|---|---|---|---|---|---|---|
| 1 | Osthole |
|
10–100 μM | Decrease Aβ levels | Decrease Aβ levels though up-regulation of miR-107 | APP/PS1 transgenic mice and SH-SY5Y cells, HEK293 cell | Jiao et al., 2016 |
| 2 | Dendrobium officinale polysaccharides | — | 5–10 μg/mL | Attenuate cognitive impairment | Attenuate cognitive impairment via modulation of microglial activation | BV2 cells, SAMP8 mice | Feng et al., 2019 |
Conclusions
AD is a progressive neurodegenerative disease characterized by progressive loss of memory and cognitive function. Several studies have investigated the pathogenesis of AD in order to develop strategies to treat AD. So far, it has been reported that β-Amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction participate in the pathogenesis of AD. The current drugs used to control symptoms of AD are often accompanied by side effects and are not sufficiently effective. This is because the of pathogenesis is complicated and is not fully understood. Recent research has provided evidence that natural active ingredients in TCM drugs have multi-target therapeutic effects. Thus, important active monomers and bioactive compounds extracted from TCM herbs have the potential to be new drugs for treating AD. We show that a variety of bioactive components from TCM such as Polygala tenuifolia, Tripterygium wilfordii, Andrographis paniculata, Gynostemma pentaphyllum, Schisandra sphenanthera, and Reynoutria multiflora can improve AD. The monomers and extracts of TCM mentioned in this paper regulate AD by decreasing β-Amyloid production, autophagy, apoptosis, neuroinflammation, oxidative stress as well as mitochondrial dysfunction (Figure 3).
Figure 3.
Mechanism in treating AD based on monomer and extract of TCM.
In this study, we found that compounds extracted from Lamiaceae family are potential treatments for AD. For example, Dracoephalum moldavica L., suppresses AD progression by reducing Aβ production, whereas Scutellaria baicalensis Georgi. and Isodon rubescens (Hemsl.) H.Hara improve AD by preventing neuroinflammation. Lavandula angustifolia Mill. and Rosmarinus officinalis L. confer therapeutic benefits on AD by reducing oxidative stress whereas Salvia miltiorrhiza Bge. suppresses the progression of AD by resolving mitochondrial dysfunction. This may be associated with this plant contains aromatic oils and other active ingredients. In addition, extracts from Fabaceae family and Polygonaceae family have beneficial effects on AD. It should be noted that TCM exhibit multi-target, multi-pathway, and multi-system characteristics, and their monomers have same mechanism of action but different structures. Among TCM compounds, flavonoids, alkaloids, and polyphenols have the most significant effects on AD. Further studies are advocated to explore further mechanisms of these drugs on AD.
Author Contributions
S-YC and YG prepared the draft manuscript. J-YS and DY searched the database and extracted literature. L-HF summarized all the tables. LX, PW, and X-LM revised the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (NO. 81903902 and 81773974), “Xing Lin scholar” discipline talents, Research ability Enhancement Program of Chengdu University of Traditional Chinese Medicine (BSH2019001) and Project funded by China Postdoctoral Science Foundation (00808412).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahn J., Um M., Choi W., Kim S., Ha T. (2006). Protective effects of Glycyrrhiza uralensis Fisch. on the cognitive deficits caused by beta-amyloid peptide 25-35 in young mice. Biogerontology 7, 239–247. 10.1007/s10522-006-9023-0 [DOI] [PubMed] [Google Scholar]
- Alzheimer’s Association Report (2018). 2018 Alzheimer’s disease facts and figures. Alzheimer‘s Dement. 14, 367–429. 10.1016/j.jalz.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Asadi F., Jamshidi A. H., Khodagholi F., Yans A., Azimi L., Faizi M., et al. (2015). Reversal effects of crocin on amyloid β-induced memory deficit: Modification of autophagy or apoptosis markers. Pharmacol. Biochem. Behav. 139, 47–58. 10.1016/j.pbb.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Barger S. W., Harmon A. D. (1997). Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature 388, 878–881. 10.1038/42257 [DOI] [PubMed] [Google Scholar]
- Bateman R. J., Aisen P. S., De Strooper B., Fox N. C., Lemere C. A., Ringman J. M., et al. (2011). Autosomal-dominant Alzheimer’s disease: a review and proposal for the prevention of Alzheimer’s disease. Alzheimers Res. Ther. 3, 1. 10.1186/alzrt59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista P. A., Werner M. F., Oliveira E. C., Burgos L., Pereira P., Brum L. F., et al. (2010). The antinociceptive effect of (-)-linalool in models of chronic inflammatory and neuropathic hypersensitivity in mice. J. Pain 11, 1222–1229. 10.1016/j.jpain.2010.02.022 [DOI] [PubMed] [Google Scholar]
- Bhat A. H., Dar K. B., Anees S., Zargar M. A., Masood A., Sof M. A., et al. (2015). Oxidative stress, mitochondrial dysfunction and neurode-generative diseases; a mechanistic insight. Biomed. Pharmacother. 74, 101–110. 10.1016/j.biopha.2015.07.025 [DOI] [PubMed] [Google Scholar]
- Butterfield D. A., Perluigi M., Sultana R. (2006). Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur. J. Pharmacol. 545, 39–50. 10.1016/j.ejphar.2006.06.026 [DOI] [PubMed] [Google Scholar]
- Cai H., Liang Q., Ge G. (2016). Gypenoside attenuates β amyloid-induced inflammation in N9 microglial cells via SOCS1 signaling. Neural. Plast. 2016, 6362707. 10.1155/2016/6362707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M., Alan L., Fan T. P., Josephine B., Liu L., Lu A. P., et al. (2014). The Art and Science of Traditional Medicine Part 1-TCM Today - A Case for Integration. Science 346 (6216), 1569. 10.1126/science.346.6216.1569-d. [DOI] [Google Scholar]
- Chen Y. J., Zheng H. Y., Huang X. X., Han S. X., Zhang D. S., Ni J. Z., et al. (2016). Neuroprotective effects of icariin on brain metabolism, mitochondrial functions, and cognition in triple-transgenic Alzheimer’s disease mice. CNS Neurosci. Ther. 22, 63–73. 10.1111/cns.12473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Li B., Cheng G., Yang X., Zhao N., Shi R. (2018. a). Amentoflavone ameliorates Aβ1–42-induced memory deficits and oxidative stress in cellular and rat model. Neurochem. Res. 43, 857–868. 10.1007/s11064-018-2489-8 [DOI] [PubMed] [Google Scholar]
- Chen K., Lu Y., Liu C., Zhang L., Fang Z., Yu G. (2018. b). Morroniside prevents H2O2 or Aβ1–42-induced apoptosis via attenuating JNK and p38 MAPK phosphorylation. Eur. J. Pharmacol. 834, 295–304. 10.1016/j.ejphar.2018.07.047 [DOI] [PubMed] [Google Scholar]
- Choi B. H. (1995). Oxidative stress and Alzheimer’s disease. Neurobiol. Aging 16, 675–678. 10.1016/0197-4580(95)00065-m [DOI] [PubMed] [Google Scholar]
- Cui L., Cai Y., Cheng W., Liu G., Zhao J., Cao H., et al. (2017). A novel, multi-target natural drug candidate, matrine, improves cognitive deficits in Alzheimer’s Disease transgenic mice by inhibiting Aβ aggregation and blocking the RAGE/Aβ axis. Mol. Neurobiol. 54, 1939–1952. 10.1007/s12035-016-9783-8 [DOI] [PubMed] [Google Scholar]
- Cui Y., Wang Y., Zhao D., Feng X., Zhang L., Liu C. (2018). Loganin prevents BV-2 microglia cells from Aβ1-42-induced inflammation via regulating TLR4/TRAF6/NF-κB axis. Cell. Biol. Int. 42, 1632–1642. 10.1002/cbin.11060 [DOI] [PubMed] [Google Scholar]
- Dong P., Ji X., Han W., Han H. (2019). Oxymatrine attenuates Aβ1-42-induced neurotoxicity in primary neuronal cells and memory impairment in rats. Can. J. Physiol. Pharmacol. 97, 99–106. 10.1139/cjpp-2018-0299 [DOI] [PubMed] [Google Scholar]
- El Kadmiri N., Said N., Slassi I., El, Moutawakil B., Nadifi S. (2018). Biomarkers for Alzheimer Disease: Classical and novel candidates’ review. Neuroscience 370, 181–190. 10.1016/j.neuroscience [DOI] [PubMed] [Google Scholar]
- Fan C. D., Li Y., Fu X. T., Wu Q. J., Hou Y. J., Yang M. F., et al. (2017). Reversal of beta-Amyloid-induced neurotoxicity in PC12 cells by curcumin, the important role of ROS-mediated signaling and ERK pathway. Cell. Mol. Neurobiol. 37, 211–222. 10.1007/s10571-016-0362-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng C. Z., Cao L., Luo D., Ju L. S., Yang J. J., Xu X. Y., et al. (2019). Dendrobium polysaccharides attenuate cognitive impairment in senescence-accelerated mouse prone 8 mice via modulation of microglial activation. Brain Res. 1704, 1–10. 10.1016/j.brainres [DOI] [PubMed] [Google Scholar]
- Fleisher A. S., Raman R., Siemers E. R., Becerra L., Clark C. M., Dean R. A., et al. (2008). Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease. Arch. Neurol. 65, 1031–1038. 10.1001/archneur.65.8.1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedemann T., Otto B., Klätschke K., Schumacher U., Tao Y., Leung A. K., et al. (2014). Coptis chinensis Franch. exhibits neuroprotective properties against oxidative stress in human neuroblastoma cells. J. Ethnopharmacol. 155, 607–615. 10.1016/j.jep.2014.06.004 [DOI] [PubMed] [Google Scholar]
- Gastón M. S., Cid M. P., Vázquez A. M., Decarlini M. F., Demmel G. I., Rossi L. I., et al. (2016). Sedative effect of central administration of Coriandrum sativum essential oil and its major component linalool in neonatal chicks. Pharm. Biol. 54, 1954–1961. 10.3109/13880209.2015.1137602 [DOI] [PubMed] [Google Scholar]
- Geng J., Liu W., Xiong Y., Ding H., Jiang C., Yang X., et al. (2018). Andrographolide sulfonate improves Alzheimer-associated phenotypes and mitochondrial dysfunction in APP/PS1 transgenic mice. BioMed. Pharmacother. 97, 1032–1039. 10.1016/j.biopha.2017.11.039 [DOI] [PubMed] [Google Scholar]
- Gong H., Tang W., Wang H., Xia Q., Huang X. (2011). Effects of food and gender on the pharmacokinetics of rhein and emodin in rats after oral dosing with Da-Cheng-Qi decoction. Phytother. Res. 25, 74–80. 10.1002/ptr.3223 [DOI] [PubMed] [Google Scholar]
- Gong G., Qi B., Liang Y. T., Dong T. T. X., Wang H. Y., Tsim K. W. K., et al. (2019). Danggui Buxue Tang, an ancient Chinese herbal decoction, protects β-amyloid-induced cell death in cultured cortical neurons. BMC Complement. Altern. 19, 9. 10.1186/s12906-018-2411-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granelli I., Leander K., Lüning B. (1970). Studies on orchidaceae alkaloids. XVI. A new alkaloid, 2-hydroxydendrobine, from Dendrobium findlayanum par. et Rchb. f. Acta Chem. Scand. 24, 1209–1212. 10.3891/acta.chem.scand.24-1209 [DOI] [PubMed] [Google Scholar]
- Granic I., Dolga A. M., Nijholt I. M., van Dijk G., Eisel U. L. (2009). Inflammation and NF-κB in Alzheimer’s disease and diabetes. J. Alzheimers Dis. 16, 809–821. 10.3233/JAD-2009-0976 [DOI] [PubMed] [Google Scholar]
- Gu M., Zhou H. F., Xue B., Niu D. B., He Q. H., Wang X. M. (2004). Effect of Chinese herb Tripterygium wilfordii Hook F monomer triptolide on apoptosis of PC12 cells induced by Abeta1-42. Sheng Li Xue Bao 56, 73–78. [PubMed] [Google Scholar]
- Gu Y., Zhang Y., Shi X., Li X., Hong J., Chen J., et al. (2010). Effect of traditional Chinese medicine berberine on type 2 diabetes based on comprehensive metabonomics. Talanta 81, 766–772. 10.1016/j.talanta.2010.01.015 [DOI] [PubMed] [Google Scholar]
- Gu L., Yu Q., Li Q., Zhang L., Lu H., Zhang X. (2018). Andrographolide protects PC12 cells against β-Amyloid-induced autophagy-associated cell death through activation of the Nrf2-mediated p62 signaling pathway. Int. J. Mol. Sci. 19, pii: E2844. 10.3390/ijms19092844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Yu S., Guan Y., Li Y. Y., Lu Y. Y., Zhang H., et al. (2012). Molecular mechanisms of same TCM syndrome for different diseases and different TCM syndrome for same disease in chronic hepatitis B and liver cirrhosis. Evid. Based Complement. Alternat. Med. 2012, 120350–120359. 10.1155/2012/120350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y., Yang H., Li L., Du X., Sun C. (2019). Schisanhenol improves learning and memory in scopolamine-treated mice by reducing acetylcholinesterase activity and attenuating oxidative damage through SIRT1-PGC-1α-Tau signaling pathway. Int. J. Neurosci. 129, 110–118. 10.1080/00207454.2018 [DOI] [PubMed] [Google Scholar]
- Hügel H. M. (2015). Brain Food for Alzheimer-Free Ageing: Focus on Herbal Medicines. Adv. Exp. Med. Biol. 863, 95–116. 10.1007/978-3-319-18365-7_5 [DOI] [PubMed] [Google Scholar]
- He G., Guo W., Lou Z., Zhang H. (2014). Achyranthes bidentata saponins promote osteogenic differentiation of bone marrow stromal cells through the ERK MAPK signaling pathway. Cell. Biochem. Biophys. 70, 467–473. 10.1007/s12013-014-9942-3 [DOI] [PubMed] [Google Scholar]
- He X., Wang X., Fang J., Chang Y., Ning N., Guo H., et al. (2017). The genus Achyranthes: A review on traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol. 203, 260–278. 10.1016/j.jep.2017.03.035 [DOI] [PubMed] [Google Scholar]
- He Y., Jia K., Li L., Wang Q., Zhang S., Du J., et al. (2018). Salvianolic acid B attenuates mitochondrial stress against Aβ toxicity in primary cultured mouse neurons. Biochem. Biophys. Res. Commun. 498, 1066–1072. 10.1016/j.bbrc.2018.03.119 [DOI] [PubMed] [Google Scholar]
- Hensley K., Harris-White M. E. (2015). Harris-White. Redox regulation of autophagy in healthy brain and neurodegeneration. Neurobiol. Dis. 84, 50–59. 10.1016/j.nbd.2015.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Jiang X., Liang Y., Liu Q., Chen S., Guo Y. (2017). Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of beta-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 91, 25–33. 10.1016/j.exger.2017.02.004 [DOI] [PubMed] [Google Scholar]
- Huang X., Li N., Pu Y., Zhang T., Wang B. (2019. a). Neuroprotective Effects of Ginseng Phytochemicals: Recent Perspectives. Molecules 24, 2939–2959. 10.3390/molecules24162939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X. W., Xu Y., Sui X., Lin H., Xu J. M., Han D., et al. (2019. b). Scutellarein suppresses Aβ induced memory impairment via inhibition of the NF-κB pathway in vivo and in vitro. Oncol. Lett. 17, 5581–5589. 10.3892/ol.2019.10274 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Hung S. Y., Fu W. M. (2017). Drug candidates in clinical trials for Alzheimer’s disease. J. Biomed. Sci. 24, 47–59. 10.1186/s12929-017-0355-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung T. M., Na M., Min B. S., Ngoc T. M., Lee I., Zhang X., et al. (2007). Acetylcholinesterase inhibitory effect of lignans isolated from Schizandra chinensis. Arch. Pharm. Res. 30, 685–690. 10.1007/bf02977628 [DOI] [PubMed] [Google Scholar]
- Inubushi Y., Ishii H., Yasui B., Konita T., Harayama T. (1964). Isolation and characterization of alkaloids of the Chinese drug Chin-Shih-Hu. Chem. Pharm. Bull. 12, 1175–1180. 10.1248/cpb.12.1175 [DOI] [PubMed] [Google Scholar]
- Jiang Y., Tu P. F. (2009). Analysis of chemical constituents in Cistanche species. J. Chromatogr. A. 1216, 1970–1979. 10.1016/j.chroma.2008.07.031 [DOI] [PubMed] [Google Scholar]
- Jiang T. T., Wei L. L., Shi L. Y., Chen Z. L., Wang C., Liu C. M., et al. (2016). Microarray expression profile analysis of mRNAs and long noncoding RNAs in pulmonary tuberculosis with different traditional Chinese medicine syndromes. BMC Complement. Altern. Med. 16, 472–484. 10.1186/s12906-016-1436-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y., Kong L., Yao Y., Li S., Tao Z., Yan Y., et al. (2016). Osthole decreases beta amyloid levels through up-regulation of miR-107 in Alzheimer’s disease. Neuropharmacology 108, 332–344. 10.1016/j.neuropharm.2016.04.046 [DOI] [PubMed] [Google Scholar]
- Jiao C., Gao F., Ou L., Yu J., Li M., Wei P., et al. (2017). Tetrahydroxy stilbene glycoside (TSG) antagonizes Aβ-induced hippocampal neuron injury by suppressing mitochondrial dysfunction via Nrf2-dependent HO-1 pathway. Biomed. Pharmacother. 96, 222–228. 10.1016/j.biopha.2017.09.134 [DOI] [PubMed] [Google Scholar]
- Jin X., Liu M. Y., Zhang D. F., Zhong X., Du K., Qian P., et al. (2019). Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci. Ther. 25, 575–590. 10.1111/cns.13086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanupriya, Prasad ,. D., Sai, Ram M., Kumar R., Sawhney R. C., Sharma S. K., et al. (2005). Cytoprotective and antioxidant activity of Rhodiola imbricata against tert-butyl hydroperoxide induced oxidative injury in U-937 human macrophages. Mol. Cell. Biochem. 275, 1–6. 10.1007/s11010-005-7637-1 [DOI] [PubMed] [Google Scholar]
- Kim J. H., He M. T., Kim M. J., Yang C. Y., Shin Y. S., Yokozawa T., et al. (2019). Safflower (Carthamus tinctorius L.) seed attenuates memory impairment induced by scopolamine in mice via regulation of cholinergic dysfunction and oxidative stress. Food Funct. 10, 3650–3659. 10.1039/c9fo00615j [DOI] [PubMed] [Google Scholar]
- Kumar K., Kumar A., Keegan R. M., Deshmukh R. (2018). Recent advances in the neurobiology and neuropharmacology of Alzheimer’s disease. Biomed. Pharmacother. 98, 297–307. 10.1016/j.biopha.2017.12.053 [DOI] [PubMed] [Google Scholar]
- Kuroda K., Inoue N., Ito Y., Kubota K., Sugimoto A., Kakuda T., et al. (2005). Sedative effects of the jasmine tea odor and (R)- (-)-linalool, one of its major odor components, on autonomic nerve activity and mood states. Eur. J. Appl. Physiol. 95, 107–114. 10.1007/s00421-005-1402-8 [DOI] [PubMed] [Google Scholar]
- Lane C. A., Hardy J., Schott J. M. (2018). Alzheimer’s disease. Eur. J. Neurol. 25, 59–70. 10.1111/ene.13439 [DOI] [PubMed] [Google Scholar]
- Latta C. H., Brothers H. M., Wilcock D. M. (2015). Neuroinflammation in Alzheimer’s disease; a source of heterogeneity and target for personalized therapy. Neuroscience 302, 103–111. 10.1016/j.neuroscience.2014.09.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. M., Brown L. (2009). Efficacy and mechanisms of action of traditional Chinese medicines for treating asthma and allergy. J. Allergy Clin. Immunol. 123, 297–306. 10.1016/j.jaci.2008.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. X., Sze S. C., Tong Y., Ng T. B. (2009). Production of Th1- and Th2-dependent cytokines induced by the Chinese medicine herb. Rhodiola algida, on human peripheral blood monocytes. J. Ethnopharmacol. 123, 257–266. 10.1016/j.jep.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Li C., Xing G., Dong M., Zhou L., Li J., Wang G., et al. (2010). Beta-asarone against beta-amyloid-induced neurotoxicity in PC12 cells via JNK signaling and modulation of Bcl-2 family proteins. Eur. J. Pharmacol. 635, 96–102. 10.1016/j.ejphar.2010.03.013 [DOI] [PubMed] [Google Scholar]
- Li H., Kang T., Qi B., Kong L., Jiao Y., Cao Y., et al. (2016. a). Neuroprotective effects of ginseng protein on PI3K/Akt signaling pathway in the hippocampus of d-galactose / AlCl3 inducing rats model of Alzheimer’s disease. J. Ethnopharmacol. 179, 162–169. 10.1016/j.jep.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Li X., Cui J., Yu Y., Li W., Hou Y., Wang X., et al. (2016. b). Traditional Chinese nootropic medicine radix Polygalae and its active constituent Onjisaponin B reduce β-Amyloid production and improve cognitive impairments. PloS One 11, e0151147. 10.1371/journal.pone.0151147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L. S., Lu Y. L., Nie J., Xu Y. Y., Zhang W., Yang W. J., et al. (2017). Dendrobium nobile Lindl. alkaloid, a novel autophagy inducer, protects against axonal degeneration induced by Abeta25-35 in hippocampus neurons in vitro. CNS Neurosci. Ther. 23, 329–340. 10.1111/cns.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Guan S., Liu C., Chen X., Zhu Y., Xie Y., et al. (2018). Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer’s disease (AD). Int. J. Biol. Macromol. 113, 991–995. 10.1016/j.ijbiomac.2018.03.035 [DOI] [PubMed] [Google Scholar]
- Lin J. M., Lin C. C., Chiu H. F., Yang J. J., Lee S. G. (1993). Evaluation of the anti-inflammatory and liver-protective effects of anoectochilus formosanus, ganoderma lucidum and gynostemma pentaphyllum in rats. Am. J. Chin. Med. 21, 59–69. 10.1142/S0192415X9300008X [DOI] [PubMed] [Google Scholar]
- Lin L., Liu G., Yang L. (2019. a). Crocin improves cognitive behavior in rats with Alzheimer’s disease by regulating endoplasmic reticulum stress and apoptosis. Biomed. Res. Int. 26, 9454913. 10.1155/2019/9454913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L. W., Tsai F. H., Lan W. C., Cheng Y. D., Lee S. C., Wu C. R. (2019. b). Steroid-Enriched Fraction of Achyranthes bidentata protects Amyloid β peptide 1–40-induced cognitive dysfunction and neuroinflammation in rats. Mol. Neurobiol. 56, 5671–5688. 10.1007/s12035-018-1436-7 [DOI] [PubMed] [Google Scholar]
- Lindwall G., Cole R. D. (1984). Phosphorylation affects the ability of tau protein to promote microtubule assembly. J. Biol. Chem. 259, 5301–5305. [PubMed] [Google Scholar]
- Liu J., Li Y., Wei L., Yang X., Xie Z., Jiang T., et al. (2014). Screening and identification of potential biomarkers and establishment of the diagnostic serum proteomic model for the traditional Chinese medicine syndromes of tuberculosis. J. Ethnopharmacol. 155, 1322–1331. 10.1016/j.jep.2014.07.025 [DOI] [PubMed] [Google Scholar]
- Liu Q. S., Jiang H. L., Wang Y., Wang L. L., Zhang J. X., He C. H., et al. (2018). Total flavonoid extract from Dracoephalum moldavica L. attenuates β-amyloid-induced toxicity through anti amyloidogenesic and neurotrophic pathways. Life Sci. 193, 214–225. 10.1016/j.lfs.2017.10.041 [DOI] [PubMed] [Google Scholar]
- Liu Y., Jin M., Wang Z. L. (2019). Advances in the Study of Flavonoids Content and Pharmacological Action of Dracocephalum Moldavica. Chin. J. Ethnomed. Ethnopharm. 28, 68–71. [Google Scholar]
- Luo T., Zhang H., Zhang W. W., Huang J. T., Song E. L., Chen S. G., et al. (2011). Neuroprotective effect of Jatrorrhizine on hydrogen peroxide-induced cell injury and its potential mechanisms in PC12 cells. Neurosci. Lett. 498, 227–231. 10.1016/j.neulet.2011.05.017 [DOI] [PubMed] [Google Scholar]
- Luo J., Shang Q., Han M., Chen K., Xu H. (2014). Traditional Chinese medicine injection for angina pectoris: An overview of systematic reviews. Am. J. Chin. Med. 42, 37–59. 10.1142/S0192415X14500037 [DOI] [PubMed] [Google Scholar]
- Lustbader J. W., Cirilli M., Lin C., Xu H. W., Takuma K., Wang N., et al. (2004). ABAD directly links Abeta to mitochondrial toxicity in Alzheimer’s disease. Science 304, 448–452. 10.1126/science.1091230 [DOI] [PubMed] [Google Scholar]
- Lyman M., Lloyd D. G., Ji X., Vizcaychipi M. P., Ma D. (2014). Neuroinflammation: The role and consequences. Neurosci. Res. 79, 1–12. 10.1016/j.neures.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Ma H., He X., Yang Y., Li M., Hao D., Jia Z. (2011). The genus Epimedium: an ethnopharmacological and phytochemical review. J. Ethnopharmacol. 134, 519–541. 10.1016/j.jep.2011.01.001 [DOI] [PubMed] [Google Scholar]
- Ma Q., Ruan Y. Y., Xu H., Shi X. M., Wang Z. X., Hu Y. L. (2015). Safflower yellow reduces lipid peroxidation, neuropathology, tau phosphorylation and ameliorates amyloid β-induced impairment of learning and memory in rats. Biomed. Pharmacother. 76, 153–164. 10.1016/j.biopha.2015.10.004 [DOI] [PubMed] [Google Scholar]
- Mao X., Liao Z., Guo L., Xu X., Wu B., Xu M., et al. (2015). Schisandrin C ameliorates learning and memory deficits by Aβ1–42-induced oxidative stress and neurotoxicity in mice. Phytother. Res. 29, 1373–1380. 10.1002/ptr.5390 [DOI] [PubMed] [Google Scholar]
- Martínez-Vázquez M., Estrada-Reyes R., Martínez-Laurrabaquio A., López-Rubalcava C., Heinze G. (2012). Neuropharmacological study of Dracocephalum moldavica L. (Lamiaceae) in mice: sedative effect and chemical analysis of an aqueous extract. J. Ethnopharmacol. 141, 908–917. 10.1016/j.jep.2012.03.028 [DOI] [PubMed] [Google Scholar]
- Min J. Y., Min K. B. (2014). Serum lycopene, lutein and zeaxanthin, and the risk of Alzheimer’s disease mortality in older adults. Dement. Geriatr. Cogn. Disord. 37, 246–256. 10.1159/000356486 [DOI] [PubMed] [Google Scholar]
- Miranda S., Opazo C., Larrondo L. F., Munoz F. J., Ruiz F., Leighton F., et al. (2000). The role of oxidative stress in the toxicity induced by amyloid beta-peptide in Alzheimer’s disease. Prog. Neurobiol. 62, 633–648. 10.1016/s0301-0082(00)00015-0 [DOI] [PubMed] [Google Scholar]
- Modabbernia A., Akhondzadeh S. (2013). Saffron, passionflower, valerian and sage for mental health. Psychiatr. Clin. North. Am. 36, 85–91. 10.1016/j.psc.2012.12.007 [DOI] [PubMed] [Google Scholar]
- Mohammadzadeh L., Abnous K., Razavi B. M., Hosseinzadeh H. (2019). Crocin-protected malathion-induced spatial memory deficits by inhibiting TAU protein hyperphosphorylation and antiapoptotic effects. Nutr. Neurosci. 21, 1–16. 10.1080/1028415X.2018.1492772 [DOI] [PubMed] [Google Scholar]
- Moreira P. I., Carvalho C., Zhu X., Smith M. A., Perry G. (2010). Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta 1802, 2–10. 10.1016/j.bbadis.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Ng T. B., Liu J., Wong J. H., Ye X., Wing, Sze S. C., Tong Y., et al. (2012). Review of research on Dendrobium, a prized folk medicine. Appl. Microbiol. Biotechnol. 93, 1795–1803. 10.1007/s00253-011-3829-7 [DOI] [PubMed] [Google Scholar]
- Nguyen K. C., Willmore W. G., Tayabali A. F. (2013). Cadmium telluride quantum dots cause oxidative stress leading to extrinsic and intrinsic apoptosis in hepatocellular carcinoma HepG2 cells. Toxicology 306, 114–123. 10.1016/j.tox.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Onyango I. G., Dennis J., Khan S. M. (2016). Mitochondrial dysfunction in Alzheimer’s disease and the rationale for bioenergetics based therapies. Aging Dis. 7, 201–214. 10.14336/AD.2015.1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan M. D., Mao Q. (1984). Isolation and identification of wubangziside A and B from Polygala caudata Rehd et Wils. Yao Xue Xue Bao 19, 899–903. [PubMed] [Google Scholar]
- Papageorgiou V. P., Assimopoulou A. N., Ballis A. C. (2008). Alkannins and shikonins: a new class of wound healing agents. Curr. Med. Chem. 15, 3248–3267. 10.2174/092986708786848532 [DOI] [PubMed] [Google Scholar]
- Peng X. M., Gao L., Huo S. X., Liu X. M., Yan M. (2015). The mechanism of memory enhancement of Acteoside (Verbascoside) in the senescent mouse model induced by a combination of D-gal and AlCl3. Phytother. Res. 29, 1137–1144. 10.1002/ptr.5358 [DOI] [PubMed] [Google Scholar]
- Pickford F., Masliah E., Britschgi M., Lucin K., Narasimhan R., Jaeger P. A., et al. (2008). The autophagy related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid beta accumulation in mice. J. Clin. Invest. 118, 2190–2199. 10.1172/JCI33585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L., Li X., Ye P., Wang G., Dai W., Liu Y., et al. (2018). Oxymatrine induces apoptosis and inhibits invasion in Gallbladder carcinoma via PTEN/PI3K/AKT pathway. Cytotechnology 70, 83–94. 10.1007/s10616-017-0153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rami A. (2009). Review: autophagy in neurodegeneration: firefighter and/or incendiarist? Neuropathol. Appl. Neurobiol. 35, 449–461. 10.1111/j.1365-2990.2009.01034.x [DOI] [PubMed] [Google Scholar]
- Reddy P. H., Beal M. F. (2008). Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 14, 45–53. 10.1016/j.molmed.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A., Galvan V., Lin A. L., Oddo S. (2015). How longevity research can lead to therapies for Alzheimer’s disease: the rapamycin story. Exp. Gerontol. 68, 51–58. 10.1016/j.exger.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Hosokawa T., Nagai M., Nagumo S. (2007). In vitro study for inhibition of NO production about constituents of Sappan Lignum. Biol. Pharm. Bull. 30, 193–196. 10.1248/bpb.30.193 [DOI] [PubMed] [Google Scholar]
- Schwartz M., Deczkowska A. (2016). Neurological Disease as a Failure of Brain-Immune Crosstalk: The Multiple Faces of Neuroinflammation. Trends Immunol. 37, 668–679. 10.1016/j.it.2016.08.001 [DOI] [PubMed] [Google Scholar]
- Selkoe D. J., Hardy J. (2016). The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 8, 595–608. 10.15252/emmm.201606210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E. J., Fischer N., Efferth T. (2018). Phytochemicals as inhibitors of NF-κB for treatment of Alzheimer’s disease. Pharmacol. Res. 129, 262–273. 10.1016/j.phrs.2017.11.030 [DOI] [PubMed] [Google Scholar]
- Serrano F. G., Tapia-Rojas C., Carvajal F. J., Hancke J., Cerpa W., Inestrosa N. C., et al. (2014). Andrographolide reduces cognitive impairment in young and mature AβPPswe/PS-1 mice. Mol. Neurodegener. 9, 61. 10.1186/1750-1326-9-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A., Frosch M. P., Masliah E., Hyman B. T. (2011). Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189. 10.1101/cshperspect.a006189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan M., Yu S., Yan H., Guo S., Xiao W., Wang Z., et al. (2017). A Review on the phytochemistry, pharmacology, pharmacokinetics and toxicology of geniposide, a natural product. Molecules 22, pii: E1689. 10.3390/molecules22101689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Zhao H., Chen J., Ma X., Yang Y., Zheng C., et al. (2012). Study on TCM syndrome identification modes of coronary heart disease based on data mining. Evid. Based Complement. Alternat. Med. 2012, 697028. 10.1155/2012/697028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D. H., Bae Y. C., Kim-Han J. S., Lee J. H., Choi I. Y., Son K. H., et al. (2006). Polyphenol amentoflavone affords neuroprotection against neonatal hypoxic-ischemic brain damage via multiple mechanisms. J. Neurochem. 96, 561–572. 10.1111/j.1471-4159.2005.03582.x [DOI] [PubMed] [Google Scholar]
- Silva T., Reis J., Teixeira J., , Borges F. (2014). Alzheimer’s disease, enzyme targets and drug discovery struggles: From natural products to drug prototypes. Ageing Res. Rev. 15, 116–145. 10.1016/j.arr.2014.03.008 [DOI] [PubMed] [Google Scholar]
- Singh J. C., Kakalij R. M., Kshirsagar R. P., Kumar B. H., Komakula S. S., Diwan P. V. (2015). Cognitive effects of vanillic acid against streptozotocin-induced neurodegeneration in mice. Pharm. Biol. 53, 630–636. 10.3109/13880209.2014.935866 [DOI] [PubMed] [Google Scholar]
- Sun X., Wei X., Qu S., Zhao Y., Zhang X. (2010). Hydroxysafflor Yellow A suppresses thrombin generation and inflammatory responses following focal cerebral ischemia-reperfusion in rats. Bioorg. Med. Chem. Lett. 20, 4120–4124. 10.1016/j.bmcl.2010.05.076 [DOI] [PubMed] [Google Scholar]
- Sun X., Jin L., Ling P. (2012). Review of drugs for Alzheimer’s disease. Drug Discovery Ther. 6, 285–290. 10.5582/ddt.2012.v6.6.285 [DOI] [PubMed] [Google Scholar]
- Tang J. L., Liu B. Y., Ma K. W. (2008). Traditional Chinese medicine. Lancet 372, 1938–1940. 10.1016/S0140-6736(08)61354-9 [DOI] [PubMed] [Google Scholar]
- Thakur A. K., Rai G., Chatterjee S. S., Kumar V. (2016). Beneficial effects of an Andrographis paniculata extract and andrographolide on cognitive functions in streptozotocin-induced diabetic rats. Pharm. Biol. 54, 1528–1538. 10.3109/13880209.2015.1107107 [DOI] [PubMed] [Google Scholar]
- Tong Y., Bai L., Gong R., Chuan J., Duan X., Zhu Y. (2018). Shikonin protects PC12 Cells against β-amyloid peptide-induced cell injury through antioxidant and antiapoptotic activities. Sci. Rep. 8, 26–36. 10.1038/s41598-017-18058-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uğuz A. C., Öz A., Nazıroğlu M. (2016). Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J. Recept. Signal Transduction Res. 36, 395–401. 10.3109/10799893.2015.1108337 [DOI] [PubMed] [Google Scholar]
- Um M. Y., Choi W. H., Aan J. Y., Kim S. R., Ha T. Y. (2006). Protective effect of Polygonum multiflorum Thunb on amyloid beta-peptide25-35 induced cognitive deficits in mice. J. Ethnopharmacol. 104, 144–148. 10.1016/j.jep.2005.08.054 [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. (2016). The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet. Med. 18, 421–430. 10.1038/gim.2015.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Sun F., An Y., Ai H., Zhang L., Huang W., et al. (2009). Morroniside protects human neuroblastoma SH-SY5Y cells against hydrogen peroxide-induced cytotoxicity. Eur. J. Pharmacol. 613, 19–23. 10.1016/j.ejphar.2009.04.013 [DOI] [PubMed] [Google Scholar]
- Wang S., Yang H., Yu L., Jin J., Qian L., Zhao H., et al. (2014). Oridonin attenuates Aβ1–42-induced neuroinflammation and inhibits NF-κB pathway. PloS One 9, e104745. 10.1371/journal.pone.0104745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Cao L., Xu L. M., Cao F. F., Peng B., Zhang X. (2015. a). Celastrol ameliorates EAE induction by suppressing pathogenic T cell responses in the peripheral and central nervous systems. J. Neuroimmune Pharm. 10, 506–516. 10.1007/s11481-015-9598-9 [DOI] [PubMed] [Google Scholar]
- Wang Y., Wang Y., Sui Y., Yu H. S., Shen X. H., Chen S. D., et al. (2015. b). The combination of aricept with a traditional Chinese medicine formula, smart soup, may be a novel way to treat Alzheimer’s disease. J. Alzheimers Dis. 45, 1185–1195. 10.3233/Jad-143183 [DOI] [PubMed] [Google Scholar]
- Wang Z. Y., Liu J. G., Li H., Yang H. M. (2016). Pharmacological Effects of Active Components of Chinese Herbal Medicine in the Treatment of Alzheimer’s Disease: A Review. Am. J. Chin. Med. 44, 1525–1541. 10.1142/S0192415X16500853 [DOI] [PubMed] [Google Scholar]
- Witkin J. M., Li X. (2013). Curcumin, an active constituent of the ancient medicinal herb Curcuma longa L.: some uses and the establishment and biological basis of medical efficacy. CNS Neurol. Disord. Drug Targets 12, 487–497. 10.2174/1871527311312040007 [DOI] [PubMed] [Google Scholar]
- Wu H. B., Fang Y. Q. (2004). Pharmacokinetics of β-asarone in rats. Yao Xue Xue Bao 39, 836–838. [PubMed] [Google Scholar]
- Wu J., Wang A., Min Z., Xiong Y., Yan Q., Zhang J., et al. (2011). Lipoxin A4 inhibits the production of proinflammatory cytokines induced by beta-amyloid in vitro and in vivo. Biochem. Biophys. Res. Commun. 408, 382–387. 10.1016/j.bbrc.2011.04.013 [DOI] [PubMed] [Google Scholar]
- Xu J., Yang Y. (2009). Traditional Chinese medicine in the Chinese health care system. Health Policy 90, 133–139. 10.1016/j.healthpol.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Wang H., Li Z., Yang Z. (2016). Triptolide attenuated injury via inhibiting oxidative stress in Amyloid-Beta25–35-treated differentiated PC12 cells. Life Sci. 145, 19–26. 10.1016/j.lfs.2015.12.018 [DOI] [PubMed] [Google Scholar]
- Xu P., Wang K., Lu C., Dong L., Gao L., Yan M., et al. (2017). Protective effects of linalool against amyloid beta-induced cognitive deficits and damages in mice. Life Sci. 1, 21–27. 10.1016/j.lfs.2017.02.010 [DOI] [PubMed] [Google Scholar]
- Xu X. X., Zhang X. H., Diao Y., Huang Y. X. (2017. b). Achyranthes bidentate saponins protect rat articular chondrocytes against IL-1β-induced inflammation and apoptosis in vitro. Kaohsiung J. Med. Sci. 33, 62–68. 10.1016/j.kjms.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. J., Mei Y., Qu Z. L., Zhang S. J., Zhao W., Fang J. S., et al. (2018). Ligustilide Ameliorates Memory Deficiency in APP/PS1 Transgenic Mice via Restoring Mitochondrial Dysfunction. Biomed. Res. Int. 2018, 4606752. 10.1155/2018/4606752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Z., Guo Y., Zhang S., Huang L., He Y., Fang R., et al. (2014). Beta-asarone attenuates amyloid beta-induced autophagy via Akt/mTOR pathway in PC12 cells. Eur. J. Pharmacol. 741, 195–204. 10.1016/j.ejphar.2014.08.006 [DOI] [PubMed] [Google Scholar]
- Yang X. L., Guo T. K., Wang Y. H., Huang Y. H., Liu X., Wang X. X., et al. (2012). Ginsenoside Rd attenuates the inflammatory response via modulating p38 and JNK signaling pathways in rats with TNBS-induced relapsing colitis. Int. Immunopharmacol. 12, 408–414. 10.1016/j.intimp.2011.12.014 [DOI] [PubMed] [Google Scholar]
- Yang W. T., Zheng X. W., Chen S., Shan C. S., Xu Q. Q., Zhu J. Z., et al. (2017). Chinese herbal medicine for Alzheimer’s disease: Clinical evidence and possible mechanism of neurogenesis. Biochem. Pharmacol. 141, 143–155. 10.1016/j.bcp.2017.07.002 [DOI] [PubMed] [Google Scholar]
- Yoshida L. S., Kohri S., Tsunawaki S., Kakegawa T., Taniguchi T., Takano-Ohmuro H., et al. (2014). Evaluation of superoxide anion radical scavenging activity of shikonin by electron spin resonance. J. Clin. Biochem. Nutr. 55, 90–96. 10.3164/jcbn.13-107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L. H., Liu G. T. (2008). Schisanhenol attenuated ox-LDL-induced apoptosis and reactive oxygen species generation in bovine aorta endothelial cells in vitro. J. Asian Nat. Prod. Res. 10, 799–806. 10.1080/10286020802031072 [DOI] [PubMed] [Google Scholar]
- Yuan H., Jiang C., Zhao J., Zhao Y., Zhang Y., Xu Y., et al. (2018). Euxanthone Attenuates Aβ1-42-Induced Oxidative Stress and Apoptosis by Triggering Autophagy. J. Mol. Neurosci. 66, 512–523. 10.1007/s12031-018-1175-2 [DOI] [PubMed] [Google Scholar]
- Zang C. X., Bao X. Q., Sun H., Zhang D. (2016). Research progress of traditional Chinese medicine compound for Alzheimer’s disease. Pharmacol. Clin. Chin. Master Clin. Med. 32, 157–161. 10.13412/j.cnki.zyyl.2016.04.047 [DOI] [Google Scholar]
- Zeng K. W., Zhao M. B., Ma Z. Z., Jiang Y., Tu P. F. (2012). Protosappanin A inhibits oxidative and nitrative stress via interfering the interaction of transmembrane protein CD14 with Toll-like receptor-4 in lipopolysaccharide-induced BV-2 microglia. Int. Immunopharmacol. 14, 558–569. 10.1016/j.intimp.2012.09.004 [DOI] [PubMed] [Google Scholar]
- Zeng P., Shi Y., Wang X. M., Lin L., Du Y. J., Tang N., et al. (2019). Emodin rescued hyperhomocysteinemia-induced dementia and Alzheimer’s disease-like features in rats. Int. J. Neuropsychopharmacol. 22, 57–70. 10.1093/ijnp/pyy090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. L., Wu L. J., Tashiro S., Onodera S., Ikejima T. (2004). Oridonin induced A375-S2 cell apoptosis via BAX-regulated caspase pathway activation, dependent on the cytochrome c/caspase-9 apoptosome. J. Asian Nat. Prod. Res. 6, 127–138. 10.1080/1028602031000147375 [DOI] [PubMed] [Google Scholar]
- Zhang H., Han T., Zhang L., Yu C. H., Wan D. G., Rahman K., et al. (2008). Effects of tenuifolin extracted from radix polygalae on learning and memory: a behavioral and biochemical study on aged and amnesic mice. Phytomedicine 15, 587–594. 10.1016/j.phymed.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Zhang L., Yu H., Zhao X., Lin X., Tan C., Cao G., et al. (2010). Neuroprotective effects of salidroside against beta-amyloid-induced oxidative stress in SH-SY5Y human neuroblastoma cells. Neurochem. Int. 57, 547–555. 10.1016/j.neuint.2010.06.021 [DOI] [PubMed] [Google Scholar]
- Zhang Z. H., Yu L. J., Hui X. C., Wu Z. Z., Yin K. L., Yang H., et al. (2014). Hydroxy-safflor yellow A attenuates Aβ1-42-induced inflammation by modulating the JAK2/STAT3/NF-κB pathway. Brain Res. 1563, 72–80. 10.1016/j.brainres.2014.03.036 [DOI] [PubMed] [Google Scholar]
- Zhang H. N., Sun Y. J., He H. Q., Li H. Y., Xue Q. L., Liu Z. M., et al. (2018). Berberine promotes nerve regeneration through IGFR mediated JNK AKT signal pathway. Mol. Med. Rep. 18, 5030–5036. 10.3892/mmr.2018.9508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Wang X., Zhang D., Liu Y., Li L. (2019). Geniposide-mediated protection against amyloid deposition and behavioral impairment correlates with downregulation of mTOR signaling and enhanced autophagy in a mouse model of Alzheimer’s disease. Aging (Albany NY) 11, 536–548. 10.18632/aging.101759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Wang S. L., Qian L., Jin J. L., Li H., Xu Y., et al. (2013). Diammonium glycyrrhizinate attenuates Aβ (1-42) -induced neuroinflammation and regulates MAPK and NF-κB pathways in vitro and in vivo. CNS Neurosci. Ther. 19, 117–124. 10.1111/cns.12043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Lv C., Li H., Du S., Liu X., Li Z., et al. (2016). Geniposide protects primary cortical neurons against oligomeric Aβ1-42-induced neurotoxicity through a mitochondrial pathway. PloS One 11, e0152551. 10.1371/journal.pone.0152551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X., Ye L., Ge H., Chen L., Jiang N., Qian L., et al. (2013). Hopeahainol A attenuates memory deficits by targeting β-amyloid in APP/PS1 transgenic mice. Aging Cell. 12, 85–92. 10.1111/acel.12022 [DOI] [PubMed] [Google Scholar]
- Zhu ,. Y. J., Zhang H. B., Liu L. R., Liu Y. H., Zhang F. L., Bai J. P., et al. (2017). Yin-cold or Yang-heat syndrome type of traditional Chinese medicine was associated with the epidermal growth factor receptor gene status in non-small cell lung cancer patients: confirmation of a TCM concept. Evid. Based Complement Alternat. Med. 2017, 7063859. 10.1155/2017/7063859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zott B., Simon M. M., Hong W., Unger F., Chen-Engerer H. J., Frosch M. P., et al. (2019). A vicious cycle of β amyloid-dependent neuronal hyperactivation. Science 365, 559–565. 10.1126/science.aay0198 [DOI] [PMC free article] [PubMed] [Google Scholar]