Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) increases the risk of hepatocellular carcinoma, which is currently the leading cause of obesity-related cancer deaths in middle-aged men.
Methods
Probiotics with lipid-lowering function were screened from the fecal microbiota of healthy adults. Polysaccharide from different sources was screened for improving insulin resistance. The combination of probiotics and Salvia miltiorrhiza polysaccharide (LBM) was investigated for alleviating hepatic steatosis.
Results
First, Bifidobacterium bifidum V (BbV) and Lactobacillus plantarum X (LpX) were obtained from the fecal microbiota of healthy adults. Second, to improve insulin resistance, a Salvia miltiorrhiza Bunge polysaccharide showing good performance in reducing insulin resistance was obtained. The liver total cholesterol (TC) and total triglyceride (TG) levels and the serum levels of free fatty acid, alanine transaminase, aspartate transaminase, low density lipoprotein cholesterol, TG, and TC can be significantly reduced through supplementation with LpX-BbV (LB) in NAFLD mice. Interestingly, the function of the probiotic LB can be enhanced by S. miltiorrhiza Bunge polysaccharide. Furthermore, the gut microbiota was modulated by LpX-BbV+S. miltiorrhiza Bunge polysaccharide (LBM). The lipopolysaccharide concentration of the LBM group was decreased by 73.6% compared to the NAFLD group. Ultimately, the mRNA concentrations of the proinflammatory cytokines (tumor necrosis factor α, interleukin 1β [IL-1β], and IL-6) decreased with LB and LBM treatment.
Conclusion
The results of this this study indicate that the LBM combination can be used as a therapeutic for ameliorating NAFLD via modulating the gut microbiota and improving insulin resistance.
Keywords: Gastrointestinal microbiome, Insulin resistance, Non-alcoholic fatty liver disease, Probiotics
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is characterized by hepatic steatosis in the absence of a history of significant drinking (alcohol) or other known history liver diseases. NAFLD resembles alcohol-induced fatty liver damage, which can develop into nonalcoholic steatohepatitis (NASH) and, eventually, hepatocellular carcinoma [1]. NAFLD is the most common cause of chronic liver disease worldwide. Approximately 20% to 30% of adults in developed countries suffer from NAFLD [2], and the prevalence of NAFLD in obese adults and those suffering from type 2 diabetes mellitus has increased to 75% and 50% to 75%, respectively [3,4]. NAFLD is a multifactorial disease [5]. Inflammatory, environmental, metabolic, and various genetic factors and diets (the most important factor) have been considered in its pathogenesis. Up to 80% of obese people suffer from NAFLD disease [6,7]. NAFLD is a new challenge in the field of modern medicine. There are two routes to relieve NAFLD: one route is inhibiting triglyceride (TG) synthesis, and the other one is promoting the metabolism of TGs. Hepatic steatosis can be improved by inhibiting lipid synthesis and promoting fatty acid oxidation, especially β-oxidation [2].
NAFLD is an acquired metabolic stress-induced liver injury associated with insulin resistance and genetic susceptibility [8,9]. Endogenous ethanol [10], gut microbiota [5], and hepatic tumor necrosis factor α (TNF-α) [11] play important roles in the pathogenesis of NAFLD. Systemic inflammation can be induced by high-fat diets (HFDs); insulin resistance, weight gain, and nonalcoholic fatty liver can be caused by disturbed metabolic homeostasis and gut microbial derangements. In patients with fatty liver, intestinal bacterial overgrowth is common [12,13]. Intestinal permeability is associated with a fatty liver. The risk of spontaneous bacterial peritonitis can be increased by the increased intestinal permeability [14]. In addition, the bacterial endotoxin has been considered as a hepatotoxin [15], which is produced by gram-negative bacteria. Considerable evidence shows that the balance of gut microbiota and insulin resistance is considered a key role in the pathogenesis of NAFLD and NASH [5,16].
Fortunately, accumulating evidence has shown that probiotics are able to inhibit harmful bacteria and improve gastrointestinal barrier function [17]. In particular, various Lactobacilli isolates attenuated liver pathology via stabilization of the intestinal barrier and anti-inflammatory actions [18]. A study demonstrated that alanine transaminase (ALTase), aspartate transaminase (ASTase), TNF-α, and total cholesterol (TC) of NAFLD patients were reduced by Lactobacillus, Bifidobacterium, and Streptococcus [19]. Previous studies found that the serum lipid profiles can be influenced by Lactobacillus and Bifidobacterium [20].
Probiotics should impart the ability to tolerate gastric acidity and bile toxicity, survive under stressed conditions of the intestine and have antimicrobial effects against pathogens [21]. In this study, we attempt to relieve NAFLD by blocking TG synthesis (or enhancing TG metabolism) and reducing insulin resistance. The probiotics from the fecal microbiota of healthy adults were screened for cholesterol and TG clearance. Moreover, polysaccharides were also screened for insulin resistance improvement. We aim to quickly establish methods for probiotic screening, and the effect of a probiotic combination (Lactobacillus plantarum+Bifidobacterium bifidum+polysaccharide) on the mitigation of NAFLD was examined.
METHODS
Isolation of probiotics from fecal microbiota of healthy adults
Samples were collected from fecal microbiota in healthy adults. To ensure general health, the vital signs of volunteers were assessed and inclusion criteria were as follows: (1) 20 to 30 years of age; (2) free of known gastrointestinal and metabolic diseases, no history of gastrointestinal or metabolic disorders; (3) avoid taking medications; (4) limit alcohol consumption; (5) agree to avoid any changes in chronic medications; (6) willing to fill out all necessary research questionnaires and donate stool specimens; and (7) voluntary signing of written informed consent prior to participation in the study. Stool samples were harvested in sterile transparent-glass tubes (20 mL) and stored at 4℃. Stools were diluted by 10−4 to 10−6 with sterilized saline. Then, 50-µL dilutions were spread onto the MRS (de MAN, ROGOSA and SHARPE) broth, and MRSP (MRS broth was added with 1.8 mmol/L sodium thioglycolate, 2.8 mmol/L L-cysteine hydrochloride, and 0.9 mmol/L CaCl2) and M17 agar plates in anaerobic incubation at 37℃ for 48 hours.
Probiotics and polysaccharide screening
The cholesterol-lowering capability of the candidate strains determined by the ferric ammonium sulfate [22,23]. Briefly, isolates were inoculated (1%, v/v) into MRSC broth (MRS broth supplemented with 1.0 g/L cholesterol) and incubated at 37℃ for 48 hours anaerobically. Next, 200-µL cultures were mixed with 4,800 µL ethanol after incubation. Then, the cells were removed by centrifugation (4,500 rpm, 20 minutes) at 4℃. Next, 4 mL of supernatant was slowly added to 2 mL of ferric ammonium sulfate reagent (Aladdin, Shanghai, China) and mixed well. The absorbance of the supernatant was measured by a spectrophotometer (Shimadzu, Kyoto, Japan) at 560 nm. The uninoculated samples were used as a negative control. Cholesterol clearance (CC) was calculated by the following formula: CC=[(Ao−A)/Ao]×100%, where Ao and A are the absorbance of uninoculated and inoculated samples, respectively.
The TG-reducing activity was tested according to the Saravanan and Ponmurugan [24] method. Isolates were inoculated (1%, v/v) into 25 mL of MRST broth (MRS broth supplemented with 1.0 g/L TG) and incubated at 37℃ for 48 hours. Samples cultured in MRS medium were used as a negative control. The supernatant of the cultures obtained by centrifugation (4,500 rpm, 20 minutes, 4℃) was saponified by adding 0.5 mL saponification reagent (50 g of potassium hydroxide, 400 mL isopropanol, 600 mL distilled water) followed by 0.5 mL of acetylacetone reagent (0.75 mL of acetylacetone, 40 mL of isopropanol, 60 mL distilled water). Evenly mixed mixtures were kept at 65℃ for 1 hour in an oil bath and cooled. The blank control and a series of standard concentrations of triolein were treated using the same method, and absorbance at 420 nm was read: C×T=cholesterol reduction rate (%)×TG reduction rate (%).
Polysaccharides from Dendrobium officinale, Talinum triangulare, and other sources were prepared according to the report [25]. Briefly, the D. officinale or other medicinal plant powder (20 g) was boiled three times with 600 mL distilled water for 3 hours. The polysaccharide was obtained by ethanol precipitation. After an 8-hour fast, mice were injected with glucose (3 g/kg), and after 15 minutes of glucose loading, an equal amount of polysaccharides was given by intragastric administration for 4 weeks. After the last administration, all the mice were fasted strictly but allowed free access to water as usual for 4 hours. Blood was collected for measurement of fasting glucose and insulin levels 12 hours after the last polysaccharide administration.
An insulin enzyme-linked immunosorbent assay (ELISA) kit (TSZ, San Francisco, CA, USA) was used to determine the fasting insulin (FINS). To select the polysaccharide with a good performance on reducing insulin resistance, the insulin sensitivity index (ISI) was calculated according to the following formulas: ISI=Ln [1/(FBG×FINS)] [26,27], where FBG was the level of fasting blood glucose (mg/dL) and FINS was the level of FINS (mIU/L). The index of insulin resistance was calculated by the following formulas [28]: homeostasis model assessment of insulin resistance (HOMA-IR)=FINS (mIU/L)×FBG (mmol/L)/22.5.
Screening and identification of the candidate strains
The effect of bile salts on the growth of these tested strains was carried out using a method described by Bao et al. [29]. After culturing in MRS broth at 37℃ for 12 hours, the bacterial concentration was adjusted to 108 colony-forming unit (CFU)/mL. Afterward, samples were inoculated into (1%, v/v) MRSB broth (MRS broth supplemented with 0.3% bile salts [oxgall]). Samples inoculated into MRS broth were used as controls. Strains were incubated anaerobically at 37℃ for 24 hours. To monitor bacterial growth, the optical density (OD620nm) was measured with a spectrophotometer. Survival percentage (%)=S/So×100%, where S was the OD620nm of the MRSB culture and So was the OD620nm of the MRS culture (control). Genomic DNA of the strain was extracted and amplified by polymerase chain reaction (PCR) according to the method of Huang et al. [30]. After cloning, the candidate strains were identified by DNA sequencing (Sangon Biotech, Shanghai, China) [31], and the sequence was compared to the National Center for Biotechnology Information (NCBI) database.
Oral glucose tolerance test and insulin tolerance test
Oral glucose tolerance tests (OGTTs) were measured at 0 to 6 weeks after treatments. Glucose load (2.0 g/kg of body weight) was conducted after 12 hours of fasting by oral gavage. After glucose administration, blood samples were collected at 0, 15, 30, 60, 90, and 120 minutes. Concentrations of glucose were measured, and the total glucose area under the curve (AUCglucose) were calculated. The insulin tolerance test (ITT) was completed after 6 hours of fasting. Next, 1.5 IU/kg insulin was infused into mice, and glucose was measured intraperitoneally. The constant rate for glucose disappearance during the ITT (KITT) was calculated using the formula 0·693/t1/2, where t1/2 was calculated from the slope of the least square analysis of the blood glucose levels during the linear decay phase of decline.
Mice and treatments
Forty male C57BL/6N mice (pathogen-free) were purchased from the Shanghai Laboratory Animal Center (Shanghai, China). The average initial body weight of the mice was approximately 18.5 g. The mice were housed in clean, sterile, polypropylene cages under controlled conditions with a room temperature of 20℃ to 24℃ and a cycle of 12/12 hours light-dark. Moreover, the experimental group and the control group were housed under the same conditions. During the first week, all mice were fed the same normal diet (ND). Afterward, the mice were randomly divided into four groups (n=10/group). The ND group received the normal diet, while the HFD group received the HFD diet; the HFD+(L. plantarum X [LpX]-B. bifidum V [BbV] [LB]) group received an HFD diet supplemented with LB via intragastric administration, and the HFD+(LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide [LBM]) group received an HFD supplemented with LBM via intragastric administration. LB was a mixture of 1×108 CFU/mL of LpX and 2×108 CFU/mL BbV; LBM was composed of LB (1×108 CFU/mL LpX+2×108 CFU/mL BbV) plus S. miltiorrhiza Bunge polysaccharide (50 mg/kg/day). After 30 minutes of LB administration, S. miltiorrhiza Bunge polysaccharide was given by intragastric administration. After the end of the experiment, all mice were sacrificed after fasting for 12 hours. The mice's livers were collected, weighed and stored at −80℃. For bacterial DNA and RNA extraction, the small intestinal and liver were frozen immediately in liquid nitrogen. All animal experiments were approved by the Ethics Committee of Southern Medical University Affiliated Nanfang Hospital (approval no. 20180415-NF325). All animal treatments were carried out in accordance with the Guidelines on Care and Use of Laboratory Animals issued by the Chinese Council on Animal Research and the Guidelines of Animal Care.
Measurement of biochemical parameters in the serum and liver
The mice were sacrificed by cervical dislocation. The mice's liver homogenate (1 g of liver tissue and 10 mL of sterilizing saline) was added into Folch solution (chloroform: formaldehyde=2:1 [v/v]), mixed well and centrifuged (4,500 ×g, 5 minutes). The supernatant of the liver was collected. The levels of high density lipoprotein cholesterol (HDL-C) and TC, TG and ASTase activity and ALTase activity were determined using test kits (Biosino Biotechnology and Science Inc., Beijing, China). Endotoxin levels (lipopolysaccharide [LPS]) were measured by using a limulus amebocyte lysate (LAL) assay endotoxin assay kit (Charles River Lab, Wilmington, MA, USA). The concentrations of acetate and butyrate were analyzed by Agilent 1260 Infinity high-performance liquid chromatography (HPLC; Agilent, Santa Clara, CA, USA) according to the report of Adler et al. [32].
Reverse-transcription quantitative polymerase chain reaction
Total RNA of the collected tissue was extracted by using a TranZol RNA Extraction Kit (Trans Gen Biotech, Beijing, China). Afterward, cDNA was obtained from the total RNA by reverse transcription using a cDNA synthesis kit (Takara Bio, Dalian, China). Interleukin 1β (IL-1β), TNF-α, and IL-6 were completed by using SYBR premix from Takara Bio. Primer sequences used for mRNA quantification are presented in Supplementary Table 1.
Sequences of the primers used in the lipid metabolism gene detection are presented in Supplementary Table 2. Fecal DNA was isolated using the QIAmp DNA stool mini kit (Qiagen, Hilden, Germany). Quantitative PCR analysis was performed using different genus- and phyla-specific primers for different bacterial groups according to the Kump et al. [33].
Statistical analysis
The software package SPSS version 13.0 (SPSS Inc., Chicago, IL, USA) was used for data analysis. All data are presented as the mean±standard deviation, and statistical significance was performed by one-way analysis of variance (ANOVA) followed by least significant difference for multiple comparisons. P values less than 0.05 were considered to be statistically significant.
RESULTS
Screening of probiotics from the fecal microbiota of healthy adults
High levels of cholesterol and TGs were associated with the formation of hyperlipidemia [34]. To obtain the strain with a highly effective lipid-lowering function, 47 candidates isolated from fecal samples were examined. The results are shown in Fig. 1A. Isolate V exhibited the highest activity on TG clearance (68% TG clearance, 25% CC). The IX isolate showed the highest activity on CC. Interestingly, isolate X exhibited outstanding performance, with cholesterol and TG clearance of 57% and 53%, respectively. The selected candidate isolates (X, IX, V) were cultured in the simulated gastric fluid. Fortunately, the survival rates of isolates V and X were up to 87.4% and 78.6%, respectively. However, the IX isolates had relatively low bile resistance (survival rates <20%). Meanwhile, Δlag phase of the tested isolates was investigated. The results showed that the growth of isolates X was slower than that of isolates V with a long Δlag phase of 2.1 hours (Fig. 1B). Based on the 16S rDNA sequence identification, strain V, X was B. bifidum and L. plantarum, respectively. To enhance the performances of the strains synergistically, the ratio of LB was optimized in vitro. The result showed that the cholesterol-TG clearance rate was up to the highest with a C×T value of 0.47, while the ratio of LB was 1:2 (Fig. 1C).
Fig. 1. (A) Strains screening. (B) The survival rate of three isolates in MRSB broth (MRS [de MAN, ROGOSA and SHARPE] broth containing 0.3% bile salts). (C) Effect of the ratio of Lactobacillus plantarum to Bifidobacterium bifidum on cholesterol-triglyceride reduction. (D) Effect of different polysaccharides on insulin resistance. (E) Effect of Salvia miltiorrhiza Bunge polysaccharide (M) dose on the insulin sensitivity index (ISI). The changes in (F) body weight and (G) food intake for 6 weeks. Food intake was estimated using the following formulas: Food intake=(initial weight of food provided–the final weight of food recovered [g]). MRSB was MRS broth supplemented with 0.3% bile salts after 3 hours at 37℃. The Δlag phase was calculated as the time needed to increase by 0.5 absorbance units at 620 nm in MRSB minus the time requirement in MRS broth according to Ding et al. [37]. C×T=cholesterol reduction rate (%)×triglyceride reduction rate (%). The ISI was calculated according to the following formulas: ISI=Ln [1/(FBG×FINS)], where FBG was the level of fasting blood glucose (mmol/L) and FINS was the level of fasting insulin (mU/L). All measurements were taken in triplicate, and experiments were repeated three times to evaluate the standard deviation. LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide; L. barbarum, Lycium barbarum; P. linteus, Phellinus linteus; M. charantia L., Momordicacharantia L.; C. militaris, Cordyceps militaris; A. membranaceus, Astragalus membranaceus (Fisch.) Bunge; S. miltiorrhiza, S. miltiorrhiza Bunge. aP<0.05, bP<0.01 indicates statistically significant differences compared with the high-fat diet (HFD) group (control), cP<0.01 indicates statistically significant differences compared with the normal diet (ND) group (control).
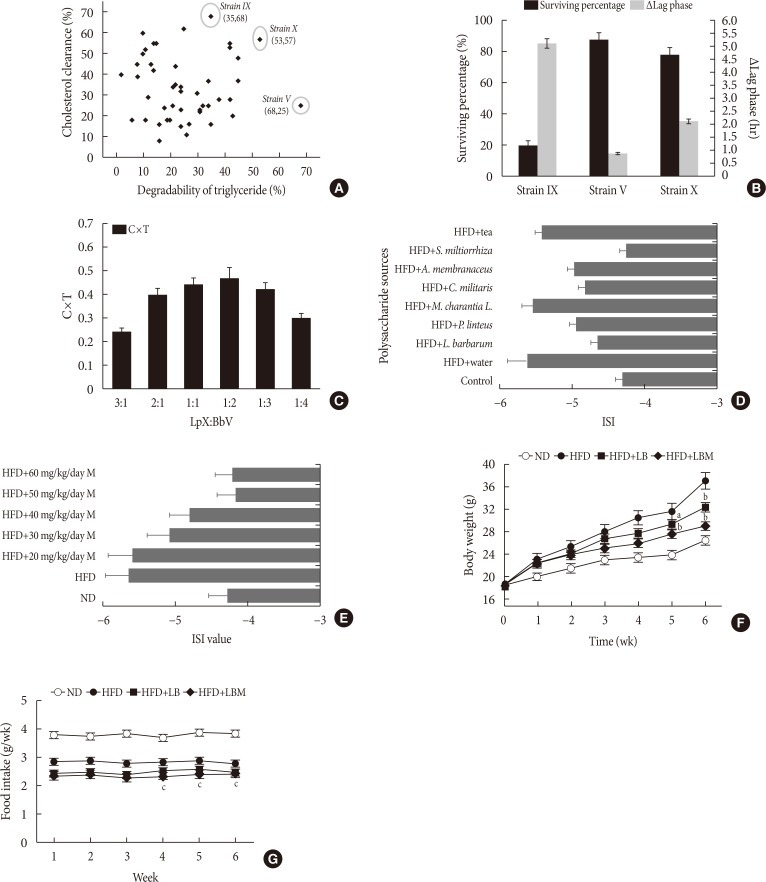
Insulin resistance is a cardinal feature of NAFLD [35]. Insulin resistance plays an important role during the development of NAFLD and progression to NASH [36]. To increase insulin sensitivity, polysaccharides derived from different sources were screened. The results showed that most of the tested polysaccharides can improve insulin resistance. In particular, compared to the other sources, the S. miltiorrhiza Bunge polysaccharide showed good performance with an ISI value of −4.26 was selected to reduce insulin resistance (Fig. 1D). Next, 50 mg/kg/day S. miltiorrhiza Bunge polysaccharide was observed to improve insulin resistance (Fig. 1E).
Effects of probiotic combination (LBM) on body index
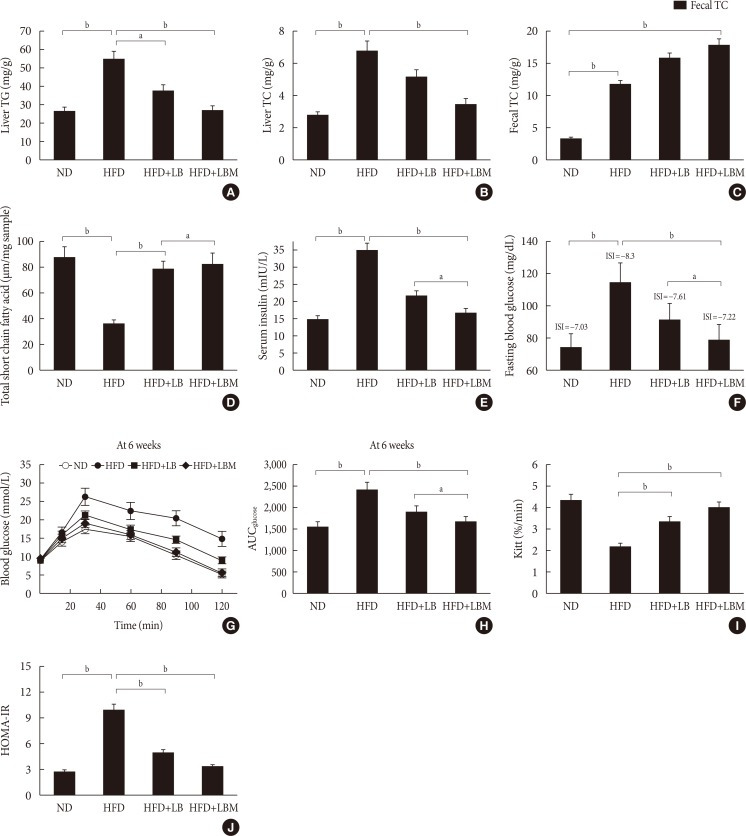
After HFD-induced treatment, the liver weight and body weight in the HFD group mice were significantly higher than those fed the ND. However, after LB or LBM treatment, a reduction in body weight was observed (Table 1, Fig. 1F). The total body weight of the LB group and LBM group was reduced by 12.5% and 21.5%, respectively, compared to the HFD mouse models. This weight loss was not related to reduced food intake (Fig. 1G) [37]. In addition, compared to the HFD group, a significant reduction of the liver was observed in the HFD+LB group. Moreover, S. miltiorrhiza Bunge polysaccharide contributed to reducing the liver index values of HFD groups; however, the promoting effect was not significant (Table 1). The liver TC levels and liver TG levels were increased in the HFD group compared to the control group. In contrast, the liver TC and TG levels can be decreased by LB (1:2) in mice fed a HFD (Fig. 2A and B). The lipids can be reduced by LB or LBM, where the increased total short-chain fatty acid concentration (the metabolism products of lipids) in cecal content has been observed (Fig. 2D). After 6 weeks of HFD consumption, the mice showed increased glucose blood levels and insulin resistance. After LB or LBM treatment, the blood glucose levels were reduced (close to the control group), and the insulin resistance was improved. Compared to the HFD group, HOMA-IR in the LBM group was reduced by 66.3%, LBM has the potential to reduce insulin resistance and improve insulin sensitivity (Fig. 2E, F, and J). In addition, NAFLD mice fed with LB (1:2) promoted excretion of cholesterol and total bile acid (Fig. 2C). Insulin sensitivity, as determined by the glucose disappearance rate (KITT) (Fig. 2I), ISI, or HOMA-IR (Fig. 2F and J), was decreased in HFD group mice and showed improvement after LB or LBM treatment. In the OGTT, the blood glucose increased to a maximum after 30 minutes of oral administration of glucose in all groups (Fig. 2G and H). This increased blood glucose in the HFD group was significantly decreased after administration of LBM (Fig. 2G). The inhibitory effect of LBM on blood glucose elevation by HFD treatment has been clearly reflected by the AUCglucose values (Fig. 2H).
Table 1. Body weight gain, liver weight, and liver index of mice in four groups.
| Group | BW gain, g | Liver weight, mg | Liver/BW, mg/g |
|---|---|---|---|
| ND | 7.92±0.42 | 1,030.00±20.1 | 38.66±0.35 |
| ND+LB | 7.88±0.35 | 1,029.00±18.5 | 38.76±0.30 |
| ND+LBM | 7.82±0.27 | 1,031.00±19.0 | 39.05±0.26 |
| HFD | 18.50±0.88 | 2,110.00±35 | 56.72±2.25 |
| HFD+LB | 14.00±0.49 | 1,324.00±8.5 | 40.68±0.58 |
| HFD+LBM | 10.55±0.43 | 1,128.00±6.5 | 38.64±0.31 |
Values are presented as mean±standard deviation. Liver index (liver/BW)=liver weight/final BW.
BW, body weight; ND, normal diet; LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide; HFD, high-fat diet.
Fig. 2. Liver (A) total cholesterol (TC) and (B) triglyceride (TG) levels in mice with different treatments. (C) Fecal TC of the four groups at the end of feeding period, mice orally fed high-fat diet (HFD) supplemented with a mixture of L. plantarum-B. bifidum (1:2) and Salvia miltiorrhiza Bunge polysaccharide. (D) Total short-chain fatty acid concentration (the metabolized products of lipids) in cecal content. (E) Serum insulin; (F) fasting blood glucose (FBG). (G) Oral glucose tolerance test (OGTT) at 6 weeks after treatment and (H) the area under the curve (AUC) of OGTT at 6 weeks. (I) The glucose disappearance rate during the insulin tolerance test (KITT). (J) Homeostasis model assessment of insulin resistance (HOMA-IR). Insulin sensitivity index (ISI)=−ln (FPG×fasting insulin [FINS]). HOMA-IR=FINS (mIU/L)×FBG (mmol/L)/22.5. Data are expressed as the mean±standard deviation. ND, normal diet; LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide. aP<0.05, bP<0.01.
Effects of probiotic combination (LBM) on lipid profiles
The results showed that the serum TC, low density lipoprotein cholesterol (LDL-C), and TG levels of the HFD group decreased significantly by treatment with probiotic LB (1:2) (Table 2). Compared to the ND group, the HDL level decreased significantly in the HFD group. In contrast, the HDL level of HFD-treated mice increased when supplemented with LB (1:2). Furthermore, serum free fatty acid (FFA), ALTase, ASTase, LDL-C, TG and TC levels in the HFD group were reduced by supplementation with LBM significantly.
Table 2. Serum lipid levels in the four groups.
| Group | TG, mg/dL | TC, mg/dL | LDL-C, mg/dL | HDL-C, mg/dL | ALTase, U/L | ASTase, U/L |
|---|---|---|---|---|---|---|
| ND | 92.9±1.0 | 84.7±0.58 | 25.9±0.15 | 57.5±0.6 | 26.3±0.5 | 55.1±1.2 |
| HFD | 115.1±2.1 | 168.5±3.2 | 65.5±0.82 | 25.9±2.1 | 83.5±2.5 | 128.5±12.2 |
| HFD+LB | 104.7±1.5 | 114.3±2.5 | 39.8±0.40 | 55.8±1.7 | 34.9±1.2 | 68.2±2.9 |
| HFD+LBM | 101.2±1.5 | 100.1±1.2 | 38.2±0.25 | 62.7±2.4 | 23.8±1.1 | 62.5±1.2 |
Values are presented as mean±standard deviation.
TG, triglyceride; TC, total cholesterol; LDL-C, low density lipoprotein cholesterol; HDL-C, high density lipoprotein cholesterol; ALTase, alanine transaminase; ASTase, aspartate transaminase; ND, normal diet; HFD, high-fat diet; LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide.
Effect of combination (LBM) on gut microbiota
The relative abundance of gut microbiota at the phylum and genus levels was investigated. For the ND group, Firmicutes and Bacteroidetes (with 80% to 90% abundance) were the main members of the gut bacteria community presenting in the feces at the phylum level. Moreover, compared to the ND group, the abundance of Cyanobacteria was increased by 68.75% in the HFD group (Fig. 3A). After administering the probiotic combination (LBM), the abundance of Cyanobacteria in HFD-fed mice was reduced.
Fig. 3. Effect of probiotic combination (LBM) on gut microbiota in the intestines of tested mice. (A) The relative abundance of four groups at the phyla level and (B) the relative abundance of four groups at the genus level. ND, normal diet; HFD, high-fat diet; LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide.
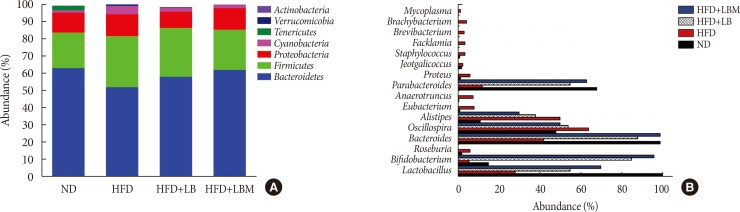
Moreover, the ratio of Bacteroidetes/Firmicutes in the HFD group decreased by 42.6% compared to the ND group. However, the ratio of Bacteroidetes/Firmicutes was increased by 14.3% and 34.04% relative to the HFD group via administration probiotics LB and LBM, respectively. At the genus level, the expression profiles of each group (16 highly abundant species >1.0% abundance), including the abundance of Bacteroides, Lactobacillus, Parabacteroides, Oscillospira, and Alistipes, were analyzed (Fig. 3B). Of those species, the abundance of Bacteroides, Lactobacillus, and Parabacteroides was increased by treatment with LB probiotics, while the Oscillospira and Alistipes were decreased by LBM.
Effects of probiotic combination (LBM) on endotoxin and liver inflammation
The concentrations of acetate, butyrate, and LPS were investigated. The total concentrations of acetate and butyrate were significantly reduced by 81.7% and 80% in the HFD groups compared to the ND group. The acetate and butyrate concentrations of NAFLD mice were increased by supplementation with LBM (Fig. 4A and B). Moreover, the HFD group with a high LPS concentration was significantly reduced by probiotic (LB). Moreover, the ability of probiotic LB was enhanced by S. miltiorrhiza Bunge polysaccharide, the LPS concentration of LB group (0.23 endotoxin unit [EU]/mL) decreased by 73.6% compared to the HFD group (0.87 EU/mL); however, the difference between LB and LBM is not significant (P>0.05) (Fig. 4C).
Fig. 4. Effect of probiotic combination LBM on the concentration of short-chain organic acids and endotoxin (lipopolysaccharide [LPS]) in cecal content. (A) Levels of butyrate in cecal content, (B) levels of acetate in cecal content, (C) LPS, (D) mRNA expression of inflammatory genes in the liver of mice. (E, F) Effect of LBM on the expression of the beginning synthesis genes and lipid oxidation genes. Relative mRNA levels are expressed as a ratio relative to β-actin. Values are expressed as the mean±standard deviation. ND, normal diet; HFD, high-fat diet; LB, Lactobacillus plantarum X (LpX)-Bifidobacterium bifidum V (BbV); LBM, LpX-BbV+Salvia miltiorrhiza Bunge polysaccharide; TNF-α, tumor necrosis factor α; IL-1β, interleukin 1β; IL-6, interleukin 6; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase; SREBP-1c, sterol regulatory element binding protein 1c; SCD-1, stearoyl-CoA desaturase-1; PPARα, peroxisome proliferator-activated receptor-α; CPT-1a, carnitine palmitoyltransferase-1a. aP<0.05, bP<0.01.
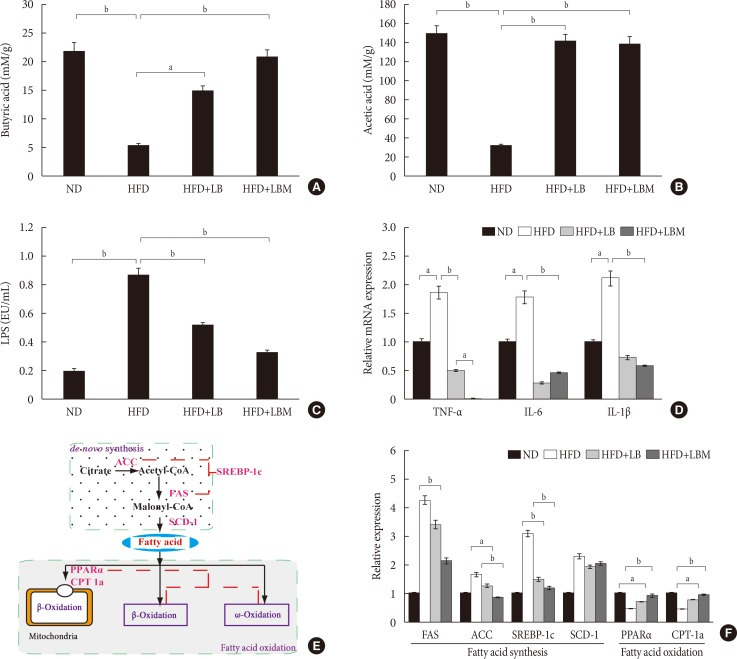
Based on the changes in short-chain organic acid concentrations and gut microbial abundance in HFD-treated mice, we were trying to reveal the global influence of LBM on liver inflammation. Inflammatory markers (TNF-α, IL-1β, and IL-6) of the liver were investigated. The results showed that inflammatory markers were modulated by the probiotic combination (LBM). Compared to the HFD group, the mRNA concentrations of cytokines (TNF-α, IL-1β, IL-6) were reduced by LB and LBM (Fig. 4D). Interestingly, the TNF-α concentration in the LBM group was significantly lower than that in the ND group.
The effect of LBM on lipid metabolism was investigated (Fig. 4E), and the results are shown in Fig. 4F. In the way of de novo synthesis, increased hepatic expression of the four genes (acetyl-CoA carboxylase [ACC], fatty acid synthase [FAS], stearoyl-CoA desaturase-1 [SCD-1], and sterol regulatory element binding protein 1c [SREBP-1c]) in the HFD group was observed compared with the ND group. The hepatic expression of ACC, FAS, and SREBP-1c was clearly reduced after LBM treatment. The change in SCD-1 gene expression between the HFD group and the HFD supplemented with LB or LBM group was not significant (P>0.05). Compared to the HFD group, a significant decrease in the hepatic expression of SREBP-1c was induced by LBM (P<0.01) (Fig. 4E and F). Regarding oxidation metabolism, genes associated with fatty acid oxidation (peroxisome proliferator-activated receptor-α [PPARα] and carnitine palmitoyltransferase-1a [CPT-1a]) showed a significant improvement with the help of LBM (P<0.05) (Fig. 4F).
DISCUSSION
Blood cholesterol can be reduced by various strains of Lactobacilli and Bifidobacteria, which is safe for consumption, such as Lactobacilli rhamnosus, L. paracasei, L. casei, and L. plantarum [38,39]. Fecal microbiota transplantation has been used for the treatment of Clostridium difficile-associated diarrhea and pseudomembranous colitis [40], which highlighted a disease improvement or resolution rate of 83% to 92% [41]. Therefore, the fecal microbiota of healthy adults was selected for the screening of probiotics (Lactobacillus), and most of the isolates performed a high CC capability in this study.
In addition, insulin resistance has been regarded as a key factor in the pathogenesis of metabolic syndrome [11]. Hepatocyte injury and inflammation could be promoted by hepatic insulin resistance [16,42]. It has been reported that different sources of polysaccharides (such as Ganoderma lucidum) could improve insulin resistance by regulating gut microbiota composition and inflammatory cytokines [43]. In the present study, different polysaccharides were screened, and the insulin sensitivity was improved by polysaccharides from S. miltiorrhiza Bunge, which was the outstanding performer among the tested polysaccharides. Finally, the FFA, ALTase, ASTase, LDL-C, TG and TC levels of HFD-treated mice in serum were significantly reduced by LB (1:2). The function of the probiotic LB was enhanced by S. miltiorrhiza Bunge polysaccharide. In previous research, polysaccharides have been used to reduce IL-6, IL-1β, and TNF-α concentrations in plasma [43]. TNF-α expression and hepatic inflammation can be reduced by L. rhamnosus and other probiotics [44]. Our research showed that the expression levels of TNF-α, IL-1β, and IL-6 can be reduced by LBM; we speculate that it was the result of the synergy between L. plantarum-B. bifidum and S. miltiorrhiza Bunge. Furthermore, it was shown that the composition of the intestinal microbiota can be regrouped by LBM in this study. The abundance of Cyanobacteria in HFD-fed mice was reduced by administering LBM. Previous studies indicated that microbiota composition is related to insulin resistance. Increased Cyanobacteria (gram-negative bacteria) have been demonstrated as contributors to increased LPS levels, and all types of Cyanobacteria can produce various toxins, which are involved in various human diseases via increasing the hepatic damage triggered by hepatotoxins [45]. Moreover, we found that Bacteroidetes/Firmicutes can be increased by LB and LBM. Numerous studies have suggested that Firmicutes/Bacteroidetes ratios are a compositional biomarker for obesity [46] and type 1 diabetes mellitus [47]. Moreover, fecal extracts of ulcerative colitis patients were characterized by reduced levels of butyrate, acetate, methylamine, and trimethylamine in comparison with a control population [48,49]. The acetate and butyrate concentrations of NAFLD mice were increased by supplementation LBM in this study. This result was consistent with the report of Turnbaugh et al. [49] and de Wit et al. [50]. Therefore, LBM combination has the potential to be used as a therapeutic for ameliorating NAFLD via modulation of the gut microbiota and improvement of insulin resistance.
ACKNOWLEDGMENTS
Funding for this study was provided by the Chinese Foundation for Hepatitis Prevention and Control–TianQing Liver Disease Research Fund Subject TQGB20190130 (http://www.cfhpc.org). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Conception or design: W.W., F.C.Z.
- Acquisition, analysis, or interpretation of data: Y. L., S.F.X.
- Drafting the work or revising: W.W., A.L.X., Z.C.L.
- Final approval of the manuscript: H.C.S., F.C.Z.
SUPPLEMENTARY MATERIALS
Supplementary materials related to this article can be found online at https://doi.org/10.4093/dmj.2019.0042.
Primers used for mRNA detection
Sequences of the primers used in the lipid metabolism gene detection
References
- 1.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. Obes Facts. 2016;9:65–90. doi: 10.1159/000443344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nomura K, Yamanouchi T. The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J Nutr Biochem. 2012;23:203–208. doi: 10.1016/j.jnutbio.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Athyros VG, Tziomalos K, Gossios TD, Griva T, Anagnostis P, Kargiotis K, Pagourelias ED, Theocharidou E, Karagiannis A, Mikhailidis DP GREACE Study Collaborative Group. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: a post-hoc analysis. Lancet. 2010;376:1916–1922. doi: 10.1016/S0140-6736(10)61272-X. [DOI] [PubMed] [Google Scholar]
- 4.Michelotti GA, Machado MV, Diehl AM. NAFLD, NASH and liver cancer. Nat Rev Gastroenterol Hepatol. 2013;10:656–665. doi: 10.1038/nrgastro.2013.183. [DOI] [PubMed] [Google Scholar]
- 5.Abu-Shanab A, Quigley EM. The role of the gut microbiota in nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:691–701. doi: 10.1038/nrgastro.2010.172. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed M. Non-alcoholic fatty liver disease in 2015. World J Hepatol. 2015;7:1450–1459. doi: 10.4254/wjh.v7.i11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaggini M, Morelli M, Buzzigoli E, DeFronzo RA, Bugianesi E, Gastaldelli A. Non-alcoholic fatty liver disease (NAFLD) and its connection with insulin resistance, dyslipidemia, atherosclerosis and coronary heart disease. Nutrients. 2013;5:1544–1560. doi: 10.3390/nu5051544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–690. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 10.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–1347. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 11.Kakino S, Ohki T, Nakayama H, Yuan X, Otabe S, Hashinaga T, Wada N, Kurita Y, Tanaka K, Hara K, Soejima E, Tajiri Y, Yamada K. Pivotal role of TNF-α in the development and progression of nonalcoholic fatty liver disease in a murine model. Horm Metab Res. 2018;50:80–87. doi: 10.1055/s-0043-118666. [DOI] [PubMed] [Google Scholar]
- 12.Bauer TM, Schwacha H, Steinbruckner B, Brinkmann FE, Ditzen AK, Aponte JJ, Pelz K, Berger D, Kist M, Blum HE. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol. 2002;97:2364–2370. doi: 10.1111/j.1572-0241.2002.05791.x. [DOI] [PubMed] [Google Scholar]
- 13.Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long-term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. Am J Gastroenterol. 2001;96:1251–1255. doi: 10.1111/j.1572-0241.2001.03636.x. [DOI] [PubMed] [Google Scholar]
- 14.Thalheimer U, De Iorio F, Capra F, del Mar Lleo M, Zuliani V, Ghidini V, Tafi MC, Caburlotto G, Gennari M, Burroughs AK, Vantini I. Altered intestinal function precedes the appearance of bacterial DNA in serum and ascites in patients with cirrhosis: a pilot study. Eur J Gastroenterol Hepatol. 2010;22:1228–1234. doi: 10.1097/MEG.0b013e32833b4b03. [DOI] [PubMed] [Google Scholar]
- 15.Kirsch R, Clarkson V, Verdonk RC, Marais AD, Shephard EG, Ryffel B, de la M Hall P. Rodent nutritional model of steatohepatitis: effects of endotoxin (lipopolysaccharide) and tumor necrosis factor alpha deficiency. J Gastroenterol Hepatol. 2006;21:174–182. doi: 10.1111/j.1440-1746.2005.04220.x. [DOI] [PubMed] [Google Scholar]
- 16.Pagano G, Pacini G, Musso G, Gambino R, Mecca F, Depetris N, Cassader M, David E, Cavallo-Perin P, Rizzetto M. Nonalcoholic steatohepatitis, insulin resistance, and metabolic syndrome: further evidence for an etiologic association. Hepatology. 2002;35:367–372. doi: 10.1053/jhep.2002.30690. [DOI] [PubMed] [Google Scholar]
- 17.Karczewski J, Troost FJ, Konings I, Dekker J, Kleerebezem M, Brummer RJ, Wells JM. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am J Physiol Gastrointest Liver Physiol. 2010;298:G851–G859. doi: 10.1152/ajpgi.00327.2009. [DOI] [PubMed] [Google Scholar]
- 18.Xu RY, Wan YP, Fang QY, Lu W, Cai W. Supplementation with probiotics modifies gut flora and attenuates liver fat accumulation in rat nonalcoholic fatty liver disease model. J Clin Biochem Nutr. 2012;50:72–77. doi: 10.3164/jcbn.11-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911–6918. doi: 10.3748/wjg.v19.i40.6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malaguarnera M, Vacante M, Antic T, Giordano M, Chisari G, Acquaviva R, Mastrojeni S, Malaguarnera G, Mistretta A, Li Volti G, Galvano F. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci. 2012;57:545–553. doi: 10.1007/s10620-011-1887-4. [DOI] [PubMed] [Google Scholar]
- 21.Cani PD, Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–1558. doi: 10.2174/138161209788168164. [DOI] [PubMed] [Google Scholar]
- 22.Azat R, Liu Y, Li W, Kayir A, Lin DB, Zhou WW, Zheng XD. Probiotic properties of lactic acid bacteria isolated from traditionally fermented Xinjiang cheese. J Zhejiang Univ Sci B. 2016;17:597–609. doi: 10.1631/jzus.B1500250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mei L, Tang Y, Li M, Yang P, Liu Z, Yuan J, Zheng P. Co-Administration of cholesterol-lowering probiotics and anthraquinone from Cassia obtusifolia L. Ameliorate non-alcoholic fatty liver. PLoS One. 2015;10:e0138078. doi: 10.1371/journal.pone.0138078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saravanan G, Ponmurugan P. Ameliorative potential of S-allylcysteine: effect on lipid profile and changes in tissue fatty acid composition in experimental diabetes. Exp Toxicol Pathol. 2012;64:639–644. doi: 10.1016/j.etp.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Zheng L, Zhang Z, Hai CX. Protective effect of a water-soluble polysaccharide from Salvia miltiorrhiza Bunge on insulin resistance in rats. Carbohydr Polym. 2012;89:890–898. doi: 10.1016/j.carbpol.2012.04.027. [DOI] [PubMed] [Google Scholar]
- 26.Xing XY, Li YF, Fu ZD, Chen YY, Wang YF, Liu XL, Liu WY, Li GW. Antihypertensive effect of metformin in essential hypertensive patients with hyperinsulinemia. Zhonghua Nei Ke Za Zhi. 2010;49:14–18. [PubMed] [Google Scholar]
- 27.Zhang Y, Hu T, Zhou H, Zhang Y, Jin G, Yang Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int J Biol Macromol. 2016;83:126–132. doi: 10.1016/j.ijbiomac.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 28.Ren D, Hu Y, Luo Y, Yang X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015;6:3342–3350. doi: 10.1039/c5fo00557d. [DOI] [PubMed] [Google Scholar]
- 29.Bao Y, Zhang Y, Zhang Y, Liu Y, Wang S, Dong X, Wang Y, Zhang H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control. 2010;21:695–701. [Google Scholar]
- 30.Huang Y, Wang X, Wang J, Wu F, Sui Y, Yang L, Wang Z. Lactobacillus plantarum strains as potential probiotic cultures with cholesterol-lowering activity. J Dairy Sci. 2013;96:2746–2753. doi: 10.3168/jds.2012-6123. [DOI] [PubMed] [Google Scholar]
- 31.Janda JM, Abbott SL. 16S rRNA gene sequencing for bacterial identification in the diagnostic laboratory: pluses, perils, and pitfalls. J Clin Microbiol. 2007;45:2761–2764. doi: 10.1128/JCM.01228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adler P, Frey LJ, Berger A, Bolten CJ, Hansen CE, Wittmann C. The key to acetate: metabolic fluxes of acetic acid bacteria under cocoa pulp fermentation-simulating conditions. Appl Environ Microbiol. 2014;80:4702–4716. doi: 10.1128/AEM.01048-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kump PK, Grochenig HP, Lackner S, Trajanoski S, Reicht G, Hoffmann KM, Deutschmann A, Wenzl HH, Petritsch W, Krejs GJ, Gorkiewicz G, Hogenauer C. Alteration of intestinal dysbiosis by fecal microbiota transplantation does not induce remission in patients with chronic active ulcerative colitis. Inflamm Bowel Dis. 2013;19:2155–2165. doi: 10.1097/MIB.0b013e31829ea325. [DOI] [PubMed] [Google Scholar]
- 34.Watts GF, Burke V. Lipid-lowering trials in the primary and secondary prevention of coronary heart disease: new evidence, implications and outstanding issues. Curr Opin Lipidol. 1996;7:341–355. doi: 10.1097/00041433-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91:4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 36.Peverill W, Powell LW, Skoien R. Evolving concepts in the pathogenesis of NASH: beyond steatosis and inflammation. Int J Mol Sci. 2014;15:8591–8638. doi: 10.3390/ijms15058591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding W, Shi C, Chen M, Zhou J, Long R, Guo X. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J Funct Foods. 2017;32:324–332. [Google Scholar]
- 38.Ooi LG, Liong MT. Cholesterol-lowering effects of probiotics and prebiotics: a review of in vivo and in vitro findings. Int J Mol Sci. 2010;11:2499–2522. doi: 10.3390/ijms11062499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu W, Xi X, Sudu Q, Kwok L, Guo Z, Hou Q, Menhe B, Sun T, Zhang H. High-throughput sequencing reveals microbial community diversity of Tibetan naturally fermented yak milk. Ann Microbiol. 2015;65:1741–1751. [Google Scholar]
- 40.Palmer R. Fecal matters. Nat Med. 2011;17:150–152. doi: 10.1038/nm0211-150. [DOI] [PubMed] [Google Scholar]
- 41.Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011;53:994–1002. doi: 10.1093/cid/cir632. [DOI] [PubMed] [Google Scholar]
- 42.Farrell GC. Signalling links in the liver: knitting SOCS with fat and inflammation. J Hepatol. 2005;43:193–196. doi: 10.1016/j.jhep.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Xu S, Dou Y, Ye B, Wu Q, Wang Y, Hu M, Ma F, Rong X, Guo J. Ganoderma lucidum polysaccharides improve insulin sensitivity by regulating inflammatory cytokines and gut microbiota composition in mice. J Funct Foods. 2017;38(Pt A):545–552. [Google Scholar]
- 44.Bluemel S, Williams B, Knight R, Schnabl B. Precision medicine in alcoholic and nonalcoholic fatty liver disease via modulating the gut microbiota. Am J Physiol Gastrointest Liver Physiol. 2016;311:G1018–G1036. doi: 10.1152/ajpgi.00245.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Durai P, Batool M, Choi S. Structure and effects of cyanobacterial lipopolysaccharides. Mar Drugs. 2015;13:4217–4230. doi: 10.3390/md13074217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 47.Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, Hu C, Wong FS, Szot GL, Bluestone JA, Gordon JI, Chervonsky AV. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455:1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marchesi JR, Holmes E, Khan F, Kochhar S, Scanlan P, Shanahan F, Wilson ID, Wang Y. Rapid and noninvasive metabonomic characterization of inflammatory bowel disease. J Proteome Res. 2007;6:546–551. doi: 10.1021/pr060470d. [DOI] [PubMed] [Google Scholar]
- 49.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 50.de Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, de Vogel-van den Bosch J, Kleerebezem M, Muller M, van der Meer R. Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol. 2012;303:G589–G599. doi: 10.1152/ajpgi.00488.2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers used for mRNA detection
Sequences of the primers used in the lipid metabolism gene detection


