Abstract
Background
The detection of glutamic acid decarboxylase 65 (GAD65) autoantibodies is essential for the prediction and diagnosis of latent autoimmune diabetes in adults (LADA). The aim of the current study was to compare a newly developed electrochemiluminescence (ECL)-GAD65 antibody assay with the established radiobinding assay, and to explore whether the new assay could be used to define LADA more precisely.
Methods
Serum samples were harvested from 141 patients with LADA, 95 with type 1 diabetes mellitus, and 99 with type 2 diabetes mellitus, and tested for GAD65 autoantibodies using both the radiobinding assay and ECL assay. A glutamic acid decarboxylase antibodies (GADA) competition assay was also performed to assess antibody affinity. Furthermore, the clinical features of these patients were compared.
Results
Eighty-eight out of 141 serum samples (62.4%) from LADA patients were GAD65 antibody-positive by ECL assay. Compared with ECL-GAD65 antibody-negative patients, ECL-GAD65 antibody-positive patients were leaner (P<0.0001), had poorer β-cell function (P<0.05), and were more likely to have other diabetes-associated autoantibodies. The β-cell function of ECL-GAD65 antibody-positive patients was similar to that of type 1 diabetes mellitus patients, whereas ECL-GAD65 antibody-negative patients were more similar to type 2 diabetes mellitus patients.
Conclusion
Patients with ECL-GAD65 antibody-negative share a similar phenotype with type 2 diabetes mellitus patients, whereas patients with ECL-GAD65 antibody-positive resemble those with type 1 diabetes mellitus. Thus, the detection of GADA using ECL may help to identify the subtype of LADA.
Keywords: Autoantibodies, C-peptide, Glutamate decarboxylase, Latent autoimmune diabetes in adults
INTRODUCTION
Latent autoimmune diabetes in adults (LADA) is the most common term used to describe the disease in patients with a type 2 diabetes mellitus (T2DM) phenotype that is combined with the presence of islet autoantibodies and slowly progressing β-cell failure [1]. Most researchers believe that LADA is a subtype of type 1 diabetes mellitus (T1DM). However, some researchers believe that LADA is similar to T2DM, because both can initially be managed using diet and oral hypoglycemic agents, before the patient becomes insulin-dependent. In addition to “LADA,” this subtype of diabetes has been called “type 1.5 diabetes,” “latent type 1 diabetes,” “LADA-type 1 and LADA-type 2,” and “slowly progressive type 1 diabetes” [2].
The accurate diagnosis of LADA is difficult, but it must be distinguished from T1DM or T2DM for proper treatment [1]. The detection of islet autoantibodies, such as insulin autoantibody (IAA), glutamic acid decarboxylase antibodies (GADA), insulinoma-associated antigen-2 autoantibodies (IA-2A), and zinc transporter-8 autoantibodies (ZnT8A), is the most common method of diagnosing LADA. However, the majority of patients with LADA are only GADA-positive, probably because this is one of the most potent autoantigens involved in β-cell-specific autoimmunity [1,3]. Several cross-sectional studies have shown that GADA titer is associated with the phenotypic heterogeneity of LADA patients [4,5,6,7,8]. Furthermore, Krause et al. [9] found that GADA affinity varies widely (up to 10,000-fold) in GADA-positive LADA patients, and that it correlates inversely with β-cell function and is strongly associated with the subsequent need for insulin treatment. Additionally, they suggested that the epitope specificity of GADA is associated with the classification of adult-onset diabetes and can be used to predict the requirement for insulin therapy [10]. For example, antibodies against the central or C-terminal domains of glutamic acid decarboxylase 65 (GAD65) tend to be associated with a clinical phenotype of autoimmune T1DM and a need for insulin therapy, whereas antibodies against N-terminal epitopes tend to be associated with a similar clinical phenotype to T2DM and a lack of requirement for insulin.
Electrochemiluminescence (ECL) assay, an emerging method for islet autoantibody detection, can discriminate high-affinity, high-risk diabetes-specific antibodies from low-affinity, low-risk islet autoantibodies, and therefore be used to identify the initiation of islet autoimmunity at an earlier stage [11,12]. Miao et al. [12] found that ECL-GADA data are preferable for the prediction of the risk of progression to T1DM in relatives of diabetes patients or in the general population [13] than the current gold standard radiobinding assay (RBA). However, it is unclear whether ECL-GADA can be used to subtype patients with LADA, who constitute a heterogeneous group, or to predict the loss of β-cell function in LADA patients.
In this study, we used an ECL assay to detect GADA in patients with LADA, T1DM, and T2DM, and compared the clinical phenotypes of patients with LADA with those of patients with T1DM or T2DM.
METHODS
The quantity and characteristics of samples and patients
The assay was validated using serum samples from 141 LADA patients that were collected at The Second Xiangya Hospital of Central South University, The First Affiliated Hospital of Nanjing Medical University, and Sir Run Run Hospital, Nanjing Medical University. Samples were collected from a total of 95 T1DM and 99 T2DM patients attending Sir Run Run Hospital, Nanjing Medical University. LADA patients were included in the study if disease onset had been at >30 years of age; there was at least one circulating islet autoantibody to GAD, IA-2, or ZnT8, detected using RBA; and there was a lack of insulin dependency at diagnosis. T1DM patients were included if a clinical diagnosis of T1DM had been made using the 1999 World Health Organization (WHO) diagnostic criteria; there was at least one circulating islet autoantibody to GAD, IAA, IA-2, or ZnT8 detected using RBA; and they were insulin-dependent at diagnosis. T2DM patients were included if their diagnosis of diabetes had been on the basis of the WHO 1999 diagnostic criteria, no islet autoantibodies had been detected, and they were not dependent on insulin treatment at diagnosis. All LADA, T1DM, and T2DM patients were recruited in this study have disease duration within 1 year.
All participants in this study were given their written informed consent, and the study was carried out in accordance with the Declaration of Helsinki and approved by the Nanjing Medical University Ethics Committee (No. 2017-SR-003). All serum samples were tested for GAD, IA-2, and ZnT8 autoantibodies using RBA, and samples with at least one positive autoantibody were further detected GADA titer using ECL assay. The positive rates of GAD, IA-2, and ZnT8 autoantibodies by RBA assay were 73.0%, 27.0%, and 29.1% respectively in LADA patients, 54.7%, 38.9%, and 21.1% respectively in T1DM, and none in T2DM. Biochemical indices were measured and relevant clinical information was collected from all the participants. All the patients underwent a 75-g oral glucose tolerance test (OGTT), with two time points (0 and 120 minutes) for the measurement of C-peptide. In a subset of the participants, 49 with LADA, 39 with T2DM, and 13 with T1DM, this measurement was performed at five time points (0, 30, 60, 120, and 180 minutes).
Anthropometric measurements, including height and body mass, were performed by professional physicians. Body mass index (BMI) was calculated by dividing weight in kilograms by height in meters' squared. After a 12-hour fast, morning blood samples were obtained from an antecubital vein for glucose, C-peptide, and glycosylated hemoglobin (HbA1c) measurement, then the 75-g OGTT was performed, and venous blood samples were obtained 30, 60, 120, and 180 minutes after the glucose load for the measurement of plasma glucose and C-peptide.
RBA for islet autoantibodies
Islet autoantibodies (GAD, IA-2, and ZnT8) were detected using RBA [14] and the upper limit of normal was established as the 99th percentile in 200 healthy control subjects. Briefly, human 35S-labelled recombinant antigens were produced in an in vitro-coupled transcription and translation system using SP6 (GAD, IA2) or T7 (ZnT8) RNA polymerase and nuclease-treated rabbit reticulocyte lysate (Promega, Fitchburg, WI, USA). Five microliters of sera were incubated with 35S-antigens (≥20,000 of trichloroacetic acid-precipitable radioactivity). After an overnight incubation at 4℃, antibody-bound 35S-antigens were separated from unbound antigen by precipitation with protein A sepharose (GE Amersham Biosciences, Chicago, IL, USA) and the immunoprecipitated radioactivity was counted on a Wallac Microbeta Liquid Scintillation Counter (Perkin Elmer Life and Analytical Sciences Inc., Boston, MA, USA).
ECL-GADA assay
Twenty microliters of 5-fold diluted serum were mixed with 20 µL of antigen buffer containing both a sulfo-tag and biotin-labeled GAD65, and the mixture was shaken at room temperature on a low setting for 1 to 2 hours, followed by incubation at 4℃ overnight. On the same day, 150 µL of 3% Blocker A (Meso Scale Diagnostics [MSD], Gaithersburg, MD, USA) were added to each well to block the streptavidin-coated plate (MSD) overnight at 4℃. On the second day, the blocked plate was washed three times with phosphate buffer solution containing 0.05% Tween-20 (PBST) buffer, followed by the addition of 30 µL serum/antigen mixtures to each well. After shaking at room temperature for 1 hour on a low-speed setting, the plate was washed again with PBST three times to remove unbound GAD65. Finally, the read buffer was added and the plate was counted on an MSD Sector Imager 2400. Antibody values are shown as counts per second (CPS). The results are expressed as an index calculated using the following equation: Index value=[CPS (sample)−CPS (negative control)]/[CPS (positive control)−CPS (negative control)].
GADA competition assay
A standard curve was constructed for the GADA radioimmunoassay using serial dilutions of unlabeled antigen protein. Briefly, we selected nine samples for an ECL-GADA competition assay, five positive and four negative. Each serum sample was assessed in 14 separate wells, two without competition and 12 with competition, by adding unlabeled GAD65 (Diarect, Freiburg, Germany) at six different concentrations between 6.8×10−12 and 6.8×10−7 mol/L to the incubating serum. For the competition experiment, serum samples were mixed with radiolabeled and unlabeled GAD65 at the same time, and then incubated overnight at 4℃. The antibody-antigen complexes were precipitated using protein A/G sepharose (GE Healthcare, Little Chalfont, UK), and the radioactive signals were counted. The signal inhibitions of 50% by unlabeled GAD65 was calculated and compared for relative affinity of antibody.
Statistical analysis
All analyses were carried out using SPSS version 21.0 software (IBM Co., Armonk, NY, USA). Comparisons were performed by analysis of variance (ANOVA) or t-test. We used GraphPad Prism 5.0 software (GraphPad Software Inc., LaJolla, CA, USA) for all graphical representations. Normally distributed data are shown as mean±standard deviation, while variables with a skewed distribution are reported as median (interquartile range) and log transformed to approximate normality before analysis. Significance was accepted when P<0.05.
RESULTS
The results of ECL and RBA-GADA assays are comparable
With the specificity set at the 99th percentile for both assay, 62.4% (88 of 141) of LADA patients and 58.9% (56 of 95) of T1DM patients were positive by ECL-GADA assay, compared with 73.0% (103 of 141) of LADA patients and 54.7% (52 of 95) of T1DM patients by GADA radioassay. Fig. 1A shows a comparison of GADA positivity and concentration between the ECL and RBA assays in the LADA group. The sensitivity of the ECL-GADA assay was similar to that of the GADA radioassay, although a few discordant samples showed low levels of GADA in both assay.
Fig. 1. Comparison of the radiobinding assay (RBA) and electrochemiluminescence (ECL) assay and differences in the affinity and number of autoantibodies, the area under the curve (AUC) for C-peptide in the various patient groups. (A) Glutamic acid decarboxylase antibodies (GADA) concentrations obtained using RBA and ECL assay were compared in latent autoimmune diabetes in adult (LADA) patients. (B) Affinity data for GADA-positive samples, classified according to ECL-GADA status. Serum samples with positive GADA by RBA from nine subjects (five ECL-GADA+ and four ECL-GADA−) were incubated with a range of concentrations of unlabeled glutamic acid decarboxylase 65 (GAD65) protein and analyzed using standard RBA. Positive samples detected by ECL-GADA assay (solid line) required less unlabeled GAD65 to reach a 50% maximal inhibition than those found to be negative (dotted line), which means positive samples are consistent with higher affinity. Results are expressed as percentages of the signal not absorbed. (C) The number of positive diabetes-associated autoantibodies in 88 ECL-GADA+ and 53 ECL-GADA− LADA samples. GADA was detected by ECL assay, IA2, and ZnT8 antibodies were detected by radioassay. (D) The AUC of C-peptide in LADA patients (ECL-GADA+ and ECL-GADA−), type 1 diabetes mellitus (T1DM) patients, and type 2 diabetes mellitus (T2DM) patients. aP<0.01 compared to T1DM, bP<0.05 compared to ECL-GADA+, cP<0.05 compared to ECL-GADA−.
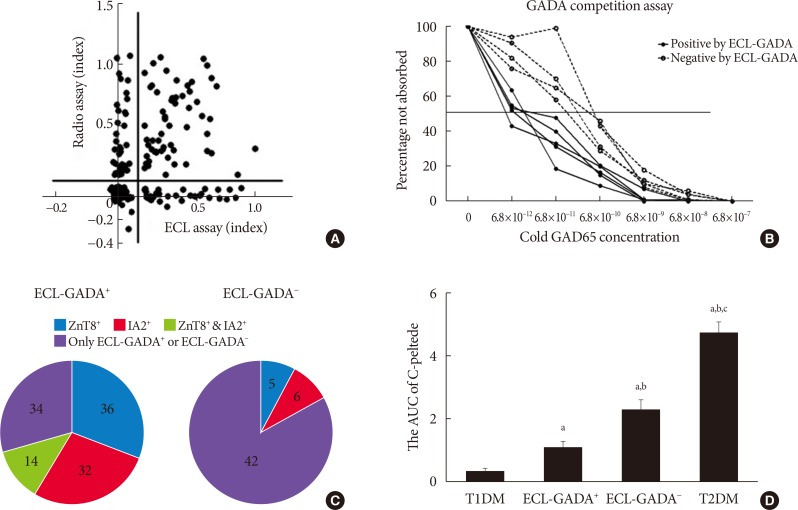
GADA positive samples demonstrate high-affinity binding in the ECL assay
Five ECL-GADA+ and four ECL-GADA− samples were used for the serial competition assay, all of which were RBA-GADA-positive. RBA GADA data were comparable between ECL-GADA+ and ECL-GADA− groups. The results of the competition assay are shown in Fig. 1B. ECL-GADA− samples required a concentration of unlabeled GAD65 protein (10−10 to 10−11 mol/L) that was greater than that for the ECL-GADA+ samples (10−12 mol/L), implying that ECL-GADA− samples require a 10 to 100-fold higher concentration of unlabeled GAD65 protein than ECL-GADA+ samples to achieve 50% inhibition of binding (P<0.05).
Comparison of the clinical features of LADA patients who were ECL-GADA+ or ECL-GADA−, and T1DM and T2DM patients
The LADA patients could be divided into two subgroups, depending on their ECL-GADA results. Patients who were ECL-GADA+ or ECL-GADA− had similar ages of onset and HbA1c concentrations. However, participants who were ECL-GADA+ had lower BMI, fasting C-peptide, 2-hour postprandial C-peptide concentration, and smaller area under the curve (AUC) of C-peptide (Fig. 1D), when compared to those who were ECL-GADA−, indicating that ECL-GADA+ patients were leaner (P<0.0001), and had poorer β-cell function than ECL-GADA− patients. In addition, patients who were ECL-GADA+ tended to be positive for more autoantibodies than ECL-GADA− patients (Fig. 1C). The β-cell function of ECL-GADA+ patients was similar to that of T1DM patients, whereas ECL-GADA− patients resembled to T2DM patients. Several T1DM and LADA patients suffer autoimmune thyroid disease or celiac disease, but no difference were found between these two groups (Table 1).
Table 1. Comparison of the clinical features of patients in various groups.
| Variable | Type 1 diabetes mellitus | LADA | Type 2 diabetes mellitus | |
|---|---|---|---|---|
| ECL-GADA+ | ECL-GADA− | |||
| Subjects no. of sera | 95 | 88 | 53 | 99 |
| Gender, male:female | 55:40 | 45:43 | 32:21 | 59:40 |
| Age at onset, yr | 28.90±15.81 | 45.57±14.17a | 46.69±13.71a | 43.64±12.97a |
| BMI, kg/m2 | 21.50±5.88 | 21.22±3.05 | 23.74±3.99a,b | 24.28±3.23a,b |
| Fasting C-peptide, nmol/L | 0.10±0.15 | 0.28±0.32a | 0.41±0.26a,b | 0.42±0.32a,b |
| Fasting blood glucose, mmol/L | 13.49±7.93 | 8.70±3.84a | 8.03±3.26a | 13.10±5.21b,c |
| 2-hr Postprandial C-peptide, nmol/L | 0.24±0.39 | 0.58±0.49a | 0.92±0.54a,b | 0.93±0.49a,b,c |
| 2-hr Postprandial blood glucose, mmol/L | 19.89±2.93 | 17.30±7.42 | 15.55±6.58a | 17.87±3.94 |
| HbA1c, % | 10.38±3.33 | 9.87±3.15 | 9.43±2.93 | 11.02±2.87b,c |
| AITD | 8 (8.42) | 4 (4.54) | 4 (7.54) | 0 |
| CD | 14 (14.73) | 3 (3.41) | 3 (5.66) | 0 |
Values are presented as mean±standard deviation or number (%).
LADA, latent autoimmune diabetes in adult; ECL-GADA, electrochemiluminescence-glutamic acid decarboxylase antibody; BMI, body mass index; HbA1c, glycosylated hemoglobin; AITD, autoimmune thyroid disease; CD, Celiac disease.
aP<0.05 compared to type 1 diabetes mellitus, bP<0.05 compared to ECL-GADA+, cP<0.05 compared to ECL-GADA−.
DISCUSSION
LADA patients show clear differences from classic T1DM and T2DM patients, because they have islet autoantibodies but often intact β-cell function [13]. However, they also exhibit significant clinical heterogeneity, variable β-cell function, and differing risk of progression to insulin dependence. The factors underlying this variation have yet to be determined. Thus, it is important to identify predictors of the progression of this disease and of the β-cell dysfunction. In this study, we used an ECL assay to detect islet antibodies and found that LADA patients could be divided into ECL-GADA+ and ECL-GADA− groups that had differing clinical phenotypes. Subjects who were ECL-GADA+ had poorer β-cell function, more islet autoantibodies, and a lower BMI than those who were ECL-GADA−. We have also shown differences in GADA detection between the RBA and ECL assays, and that the ECL-based GADA assay can be used to define and subtype LADA more precisely, because the data correspond more closely with the clinical features.
Some previous studies have shown that the results of ECL-GADA assays are preferable for the prediction of the risk of progression to classical T1DM in the relatives of diabetes patients or in the general population [12,13] than the current gold standard RBA. These showed that the ECL assay is superior for the detection of islet antibodies that are of higher disease relevance, because it discriminates between high-affinity, high-risk antibodies and low-risk, low-affinity antibodies, unlike the RBA [15]. Our results are consistent with these previous findings. However, we also assessed C-peptide concentrations and other clinical indices to evaluate β-cell function in LADA patients, and explored their relationships with ECL-GADA titers. β-Cell function may be associated with the epitope specificity of GADA, according to Achenbach et al. [10], who reported that the necessity for insulin therapy could be predicted by the detection of N-terminally-truncated GAD65 autoantibodies in LADA patients. However, the relationship between ECL-GADA titer and epitope specificity requires further investigation.
Some researchers [6,8,16] have also shown that the characteristics of patients with low GADA titers are similar to those of antibody-negative T2DM patients. Liu et al. [16] suggested that low-titer GADA detected by a radioligand assay might either represent a false positive, with signals resulting from immunization with a cross-reactive molecule, or a true positive resulting from immunization with the GAD autoantigen itself. Although competition assay is an indirect measurement to determine the antibody affinity, our findings showed that ECL-GADA− samples required a 10 to 100-fold higher concentration of unlabeled GAD65 protein than ECL-GADA+ samples to achieve 50% inhibition of binding which were similar to those given in previous reports [17,18,19,20]. We also compared the clinical features of the LADA patients who were ECL-GADA+ or ECL-GADA−, and patients with T1DM or T2DM, and found that the β-cell function, multiple positive islet autoantibodies, and low BMI of the ECL-GADA+ patients made them similar to the T1DM patients, whereas the ECL-GADA− patients resembled T2DM patients more closely. These findings further imply that the ECL-GADA assay could be used to subtype LADA more precisely.
One limitation of this study is that we did not follow up these patients and observe the progression of their disease. Future studies should prospectively follow patients and record any changes in phenotype in similar patient groups.
In conclusion, detection of GADA using ECL may help to identify the subtype of LADA. Patients who are ECL-GADA− show similar phenotypic features to T2DM patients and may develop mild β-cell dysfunction, whereas patients who are ECL-GADA+ are more similar to T1DM patients, showing a rapid loss of β-cells, and being more likely to require insulin treatment sooner. Thus, precise subtyping of LADA patients will contribute to the optimization of treatment strategy and more accurate prognostication.
ACKNOWLEDGMENTS
This study was supported by grants from the National Key Research and Development Program of China (2016YFC1305000, 2016YFC1305005), Nanjing Science and Technology Committee (201605084), and The National Natural Science Foundation of China (81770778, 81570704).
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
- Conception or design: T.Y., G.H., Z.Z., Y.L.
- Acquisition, analysis, or interpretation of data: Y.Z., L.Q., Q.L., J.Z., Y.Z., Y.L.
- Drafting the work or revising: L.Q., Y.Z., Y.L.
- Final approval of the manuscript: Y.L.
References
- 1.Fourlanos S, Dotta F, Greenbaum CJ, Palmer JP, Rolandsson O, Colman PG, Harrison LC. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48:2206–2212. doi: 10.1007/s00125-005-1960-7. [DOI] [PubMed] [Google Scholar]
- 2.Palmer JP, Hirsch IB. What's in a name: latent autoimmune diabetes of adults, type 1.5, adult-onset, and type 1 diabetes. Diabetes Care. 2003;26:536–538. doi: 10.2337/diacare.26.2.536. [DOI] [PubMed] [Google Scholar]
- 3.Cernea S, Buzzetti R, Pozzilli P. Beta-cell protection and therapy for latent autoimmune diabetes in adults. Diabetes Care. 2009;32:S246–S252. doi: 10.2337/dc09-S317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin P, Huang G, Lin J, Yang L, Xiang B, Zhou W, Zhou Z. High titre of antiglutamic acid decarboxylase autoantibody is a strong predictor of the development of thyroid autoimmunity in patients with type 1 diabetes and latent autoimmune diabetes in adults. Clin Endocrinol (Oxf) 2011;74:587–592. doi: 10.1111/j.1365-2265.2011.03976.x. [DOI] [PubMed] [Google Scholar]
- 5.Buzzetti R, Di Pietro S, Giaccari A, Petrone A, Locatelli M, Suraci C, Capizzi M, Arpi ML, Bazzigaluppi E, Dotta F, Bosi E Non Insulin Requiring Autoimmune Diabetes Study Group. High titer of autoantibodies to GAD identifies a specific phenotype of adult-onset autoimmune diabetes. Diabetes Care. 2007;30:932–938. doi: 10.2337/dc06-1696. [DOI] [PubMed] [Google Scholar]
- 6.Hawa MI, Kolb H, Schloot N, Beyan H, Paschou SA, Buzzetti R, Mauricio D, De Leiva A, Yderstraede K, Beck-Neilsen H, Tuomilehto J, Sarti C, Thivolet C, Hadden D, Hunter S, Schernthaner G, Scherbaum WA, Williams R, Brophy S, Pozzilli P, Leslie RD Action LADA consortium. Adult-onset autoimmune diabetes in Europe is prevalent with a broad clinical phenotype: Action LADA 7. Diabetes Care. 2013;36:908–913. doi: 10.2337/dc12-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lohmann T, Kellner K, Verlohren HJ, Krug J, Steindorf J, Scherbaum WA, Seissler J. Titre and combination of ICA and autoantibodies to glutamic acid decarboxylase discriminate two clinically distinct types of latent autoimmune diabetes in adults (LADA) Diabetologia. 2001;44:1005–1010. doi: 10.1007/s001250100602. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z, Xiang Y, Ji L, Jia W, Ning G, Huang G, Yang L, Lin J, Liu Z, Hagopian WA, Leslie RD LADA China Study Group. Frequency, immunogenetics, and clinical characteristics of latent autoimmune diabetes in China (LADA China study): a nationwide, multicenter, clinic-based cross-sectional study. Diabetes. 2013;62:543–550. doi: 10.2337/db12-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krause S, Landherr U, Agardh CD, Hausmann S, Link K, Hansen JM, Lynch KF, Powell M, Furmaniak J, Rees-Smith B, Bonifacio E, Ziegler AG, Lernmark A, Achenbach P. GAD autoantibody affinity in adult patients with latent autoimmune diabetes, the study participants of a GAD65 vaccination trial. Diabetes Care. 2014;37:1675–1680. doi: 10.2337/dc13-1719. [DOI] [PubMed] [Google Scholar]
- 10.Achenbach P, Hawa MI, Krause S, Lampasona V, Jerram ST, Williams AJK, Bonifacio E, Ziegler AG, Leslie RD Action LADA consortium. Autoantibodies to N-terminally truncated GAD improve clinical phenotyping of individuals with adult-onset diabetes: Action LADA 12. Diabetologia. 2018;61:1644–1649. doi: 10.1007/s00125-018-4605-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu L. Islet autoantibody detection by electrochemiluminescence (ECL) assay. Methods Mol Biol. 2016;1433:85–91. doi: 10.1007/7651_2015_296. [DOI] [PubMed] [Google Scholar]
- 12.Miao D, Steck AK, Zhang L, Guyer KM, Jiang L, Armstrong T, Muller SM, Krischer J, Rewers M, Yu L Type 1 Diabetes TrialNet Study Group. Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technol Ther. 2015;17:119–127. doi: 10.1089/dia.2014.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Groop LC, Bottazzo GF, Doniach D. Islet cell antibodies identify latent type I diabetes in patients aged 35-75 years at diagnosis. Diabetes. 1986;35:237–241. doi: 10.2337/diab.35.2.237. [DOI] [PubMed] [Google Scholar]
- 14.Bonifacio E, Yu L, Williams AK, Eisenbarth GS, Bingley PJ, Marcovina SM, Adler K, Ziegler AG, Mueller PW, Schatz DA, Krischer JP, Steffes MW, Akolkar B. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab. 2010;95:3360–3367. doi: 10.1210/jc.2010-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu Y, Zhao Z, Miao D, High H, Yang T, Yu L. Electrochemiluminescence assays for human islet autoantibodies. J Vis Exp. 2018;23:e57227. doi: 10.3791/57227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Li X, Xiang Y, Huang G, Lin J, Yang L, Zhao Y, Yang Z, Hou C, Li Y, Liu J, Zhu D, Leslie RD, Wang X, Zhou Z. Latent autoimmune diabetes in adults with low-titer GAD antibodies: similar disease progression with type 2 diabetes: a nationwide, multicenter prospective study (LADA China Study 3) Diabetes Care. 2015;38:16–21.:LADA China Study Group. doi: 10.2337/dc14-1770. [DOI] [PubMed] [Google Scholar]
- 17.Mayr A, Schlosser M, Grober N, Kenk H, Ziegler AG, Bonifacio E, Achenbach P. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes. 2007;56:1527–1533. doi: 10.2337/db06-1715. [DOI] [PubMed] [Google Scholar]
- 18.Achenbach P, Koczwara K, Knopff A, Naserke H, Ziegler AG, Bonifacio E. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. J Clin Invest. 2004;114:589–597. doi: 10.1172/JCI21307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curnock RM, Reed CR, Rokni S, Broadhurst JW, Bingley PJ, Williams AJ. Insulin autoantibody affinity measurement using a single concentration of unlabelled insulin competitor discriminates risk in relatives of patients with type 1 diabetes. Clin Exp Immunol. 2012;167:67–72. doi: 10.1111/j.1365-2249.2011.04495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siljander H, Harkonen T, Hermann R, Simell S, Hekkala A, Salonsaari RT, Simell T, Simell O, Ilonen J, Veijola R, Knip M. Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes Metab Res Rev. 2009;25:615–622. doi: 10.1002/dmrr.998. [DOI] [PubMed] [Google Scholar]


