Abstract
While osteoarthritis is a common degenerative disease, ankle osteoarthritis is a subdivision that has received little attention. Two effective ways to treat osteoarthritis of the ankle are total ankle replacement (TAR) and ankle arthrodesis (AAD). Whether TAR or AAD is more beneficial for treatment is controversial. The purpose of this meta‐analysis was to compare the efficiency (clinical outcome and patient satisfaction) and safety (complications and survival) of these two procedures. The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement was performed as a guideline for this study. Three electronic databases, PubMed, Web of Science, and Cochrane Library, were searched up to May 2019, with no language restrictions. Prospective or retrospective comparative studies were identified. The outcomes included clinical outcome, patient satisfaction, complications, and survival. Review Manager (Revman) 5.3 software was used to conduct the data analysis. We only selected literature from the past 5 years (no earlier than 2015). Seven comparative studies were included. There were six cohort studies and one cross‐sectional study. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of cohort studies, and The Agency for Healthcare Research and Quality (AHRQ) checklist was chosen to assess the quality of cross‐sectional studies. No significant difference was observed for efficiency and safety. Clinical outcome was included in five studies with four different scoring systems. Two of them used the American Orthopaedic Foot & Ankle Society (AOFAS) questionnaire scores to assess the two procedures (mean difference, −4.26; 95% confidence interval [CI], −11.37–2.85; P = 0.24; I 2 = 1%). Patient satisfaction (risk ratio [RR], 0.96; 95% CI, 0.65–1.40; P = 0.82; I 2 = 54%), complications (RR, 1.15; 95% CI, 0.16–8.21; P = 0.89; I 2 = 84%), and survival (RR, 1.91; 95% CI, 0.33–11.08; P = 0.47; I 2 = 90%) showed no significant difference between the TAR group and the AAD group. This meta‐analysis showed no statistically significant difference between TAR and AAD in clinical outcome, patient satisfaction, complications, and survival. This revealed that TAR and AAD could appear to have similar results in these aspects. Therefore, the present results are not sufficient to conclude which of these two methods is better. Further studies are needed to obtain more clues.
Keywords: AAD, Ankle Arthrodesis, Meta‐analysis, Osteoarthritis, TAR, Total Ankle Replacement
Introduction
Osteoarthritis (OA) is a degenerative disease that affects joints and their cartilage, leading to the loss of structure and function1, 2. Osteoarthritis affects several joints, such as the knee, the hip, and the ankle. Because the incidence of ankle osteoarthritis is relatively low compared to other types of osteoarthritis, there is less discussion on this topic2. Two practical and well‐established ways to treat osteoarthritis of the ankle are total ankle replacement (TAR) and ankle arthrodesis (AAD).
AAD, also referred to as ankle fusion, is a widely used treatment for ankle osteoarthritis. The use of this type of treatment can result in good patient satisfaction and can provide pain relief and improvement in function, thus helping patients return to normal daily life3. It is regarded as a standard treatment for ankle osteoarthritis. TAR is also a useful surgical treatment strategy as an alternative treatment to AAD4. However, early ankle replacement was not acceptable nor popular due to the high probability of complications4. In recent years, this technique has been improved to achieve better results, including reducing pain and improving functional outcomes5. With the continuous progress and improvement in ankle replacement surgery, it is receiving more and more recognition.
Despite TAR and AAD being efficient surgical treatments for osteoarthritis, they have certain shortcomings. Ankle fusion is a traditional and popular operative treatment. However, it can lead to persistent alterations in gait, and may even lead to the development of osteoarthritis in other joints, such as the subtalar joint, the talonavicular, and the midfoot3, 5. For ankle replacement, the results for short‐term and mid‐term survivorship and functional outcomes are promising; however, the long‐term effects require further research, and the operation is complicated and challenging for doctors4, 5.
Because of the controversy over whether TAR or AAD is more advantageous and progressive, a comparative study of these two methods is vital to clinical research. There have been few previous studies and meta‐analyses comparing TAR and AAD; however, some new literature has been published. Therefore, it is necessary to conduct an updated systematic review and meta‐analysis. In our meta‐analysis, we included studies published in the past 5 years (from 2015 to 2019) and assessed clinical outcomes, patient satisfaction, complications, and survival to explore the efficiency and safety of these two procedures and then to determine which approach is more effective for osteoarthritis.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) statement was used to guide the study6.
Information Sources and Search Strategy
The relevant works of literature selected in this study were mainly from three electronic databases up to May 2019, with no language restrictions: PubMed, Web of Science, and Cochrane Library. The keywords identified in this search were Medical Subject Headings (MeSH) “Osteoarthritis,” and all synonyms (free terms), MeSH “Arthroplasty, Replacement, Ankle” and all synonyms (free terms), MeSH “Arthrodesis,” “Arthrodeses,” “Ankle Arthrodesis,” and “Ankle fusion.” The combination of these MeSH terms and free terms was then applied.
Eligibility Criteria
The following criteria for inclusion were applied: (i) population: adult patients with osteoarthritis; (ii) intervention: treatment with TAR; (iii) comparison: treatment with AAD; (iv) outcome: efficiency (clinical outcome and patient satisfaction) and safety (complications and survival); and (v) design: prospective or retrospective comparative studies (randomized controlled trials [RCT] and non‐randomized controlled studies [included observational studies]). The duplicated studies were excluded.
Data Collection
The first author extracted all information about patients and treatments. These data involved: (i) the first author, year of publication, study type, number of subjects, mean age of subjects, and follow up’ (ii) outcome measures; and (iii) intervention characteristics of the TAR groups and the AAD groups. The other author reviewed and checked the extracted data. Any disagreements between the two authors were resolved by discussion.
Quality Assessment
The Agency for Healthcare Research and Quality (AHRQ) checklist was chosen to assess the quality of cross‐sectional studies7. It included 11 quality items, and “yes,” “no,” and “unclear” could be applied to each item.
The Newcastle–Ottawa scale (NOS) was selected to assess the quality of other non‐randomized controlled studies8. There were three domains (selection, comparability, and outcome) and a total of eight detailed quality items in this scale. In “selection” and “outcome” domains, a maximum of one star could be awarded for each quality item. In the “comparability” domain, a maximum of two stars could be given. More stars obtained meant higher quality assessed.
For the included studies that were RCT, the Cochrane Collaboration's tool for assessing the risk of bias was appropriate9. The evaluation criteria were as follows: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. Because no RCT studies were included in this meta‐analysis, this quality assessment tool was not used.
Two authors conducted the quality assessment independently. Any disagreements between the two authors were resolved by discussion.
Data Analysis
The statistical software used in this study for data analysis was Review Manager (Revman) 5.3. For the dichotomous outcomes, the selected effect size was the risk ratio (RR) with a 95% confidence interval (CI), and the chosen method was the Mantel–Haenszel method. Weighted mean difference (WMD) with 95% CI was selected for the continuous outcomes. The I 2‐statistic test was used to detect the statistical heterogeneity between studies. If the value of I 2 was less than 50%, indicating that the heterogeneity was low, the fixed‐effects model was chosen. Otherwise, the random‐effects model was used because of the high heterogeneity. A P‐value less than 0.05 was considered statistically significant. Due to the small number of studies included (fewer than 10), the publication bias was not assessable, and the Begg funnel plots were not used to indicate potential publication bias.
Results
Study Selection
A total of 487 studies were identified and extracted from the initial three databases by using the inclusion criteria and the data collection strategy mentioned above. Among them, there were 242 works of literature from PubMed, 242 from Web of Science (only included trials), and 3 from Cochrane Library (only included trials). A total of 114 studies were excluded because of duplication and 333 studies were excluded after screening titles and abstracts because they did not study the two treatments (TAR and AAD) comprehensively or only studied one of them. The remaining 40 articles were reviewed by reading full texts to obtain more details. Of these, 20 were excluded because they were published earlier than 2015. There were 6 works of literature excluded because they did not clearly compare the efficiency and safety of the two therapies (TAR and AAD), and another 7 studies were excluded because they could not be studied in the quantitative synthesis. Finally, 7 studies10, 11, 12, 13, 14, 15, 16 were included in the meta‐analysis because they met the eligibility criteria. The process of the selection is shown in Fig. 1.
Figure 1.
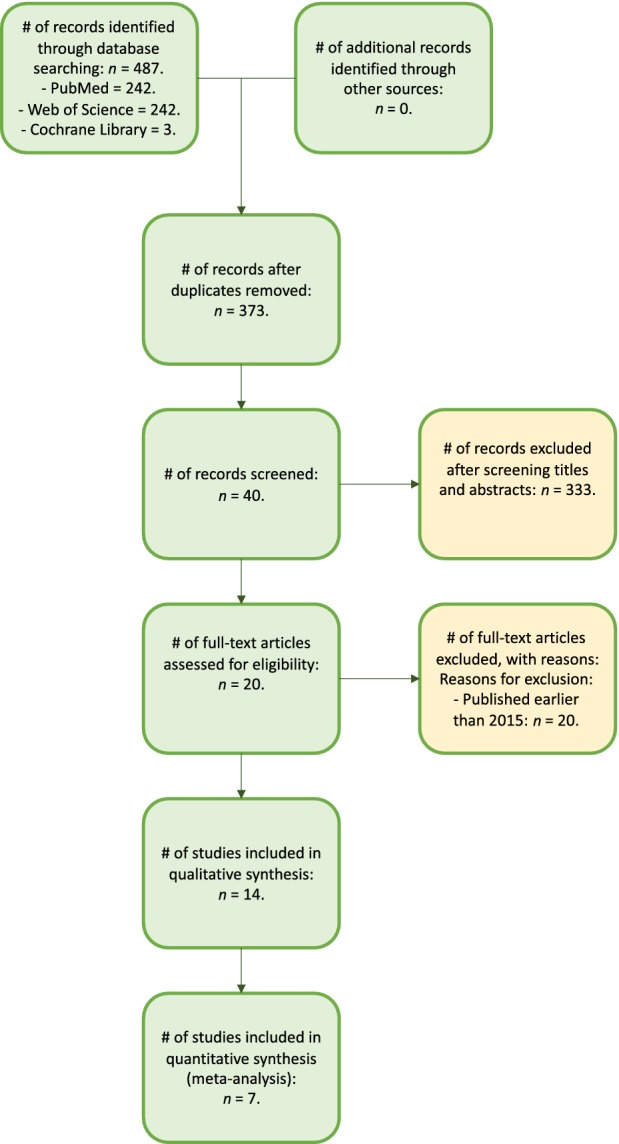
Study selection flow diagram (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses [PRISMA]).
Study Characteristics
Only studies from 2015 to 2019 (the past 5 years) were included. A total of 1280 patients were included in the 7 studies selected, of which 927 were treated with TAR and 353 with AAD. The follow‐up cycles were provided in all 7 studies, with the shortest one being 12.0 months and the longest being 77.0 months, while 5 studies12, 13, 14, 15, 16 showed the average age of patients. Five studies10, 12, 13, 15, 16 involved clinical outcome, 2 studies12, 13 presented patient satisfaction, two studies13, 14 compared complications, and four studies11, 12, 13, 14 reported survival. Because of the lack of directly given data, the standard deviations were estimated for the clinical outcome part of the studies by Mehdi and Pedowitz13, 15. Table 1 depicts the specific study characteristics and more details.
Table 1.
Summary of characteristics of the studies included in the meta‐analysis
| The First author | Year | Study type | Number of subjects | Mean age of subjects | Follow‐up (months) | Clinical outcome | Patient satisfaction | Complications | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Chorpa10 | 2017 | Retrospective |
TAR:12 AAD:12 |
TAR:NR AAD:NR |
TAR:56.4 AAD:56.4 |
FAAM | NR | NR | NR |
| Croft11 | 2017 | Retrospective |
TAR:362 AAD:169 |
TAR:NR AAD:NR |
TAR:40.8 AAD:40.8 |
NR | NR | NR |
TAR:62 AAD:8 |
| Henricson12 | 2016 | NR |
TAR:7 AAD:7 |
TAR:61.1 AAD:61.1 |
TAR:77.0 AAD:66.4 |
SEFAS |
TAR:7 AAD:6 |
NR |
TAR:2 AAD:1 |
| Mehdi13 | 2019 | Retrospective |
TAR:25 AAD:25 |
TAR:60.0 AAD:62.0 |
TAR:65.0 AAD:68.0 |
AOFAS; Pain VAS |
TAR:16 AAD:20 |
TAR:7 AAD:2 |
TAR:9 AAD:1 |
| Norvell14 | 2018 | Prospective |
TAR:395 AAD:98 |
TAR:63.3 AAD:54.2 |
TAR:12.0 AAD:12.0 |
NR | NR |
TAR:33 AAD:17 |
TAR:16 AAD:13 |
| Pedowitz15 | 2016 | Retrospective |
TAR:41 AAD:27 |
TAR:65.0 AAD:55.0 |
TAR:33.7 AAD:40.3 |
SF‐12MCS; SF‐12PCS; VAS; FAAM‐ADL; FAAM‐Sports | NR | NR | NR |
| Pinsker16 | 2015 | Prospective |
TAR:85 AAD:15 |
Total:61.2 |
TAR:30.0 AAD:30.0 |
SMFA; WOMAC; AOFAS; LEFS; FFI; AOS | NR | NR | NR |
Survival = revision, re‐operation, or operation failure. ADL, activities of daily living; AOFAS, American Orthopaedic Foot & Ankle Society questionnaire; AOS, ankle osteoarthritis scale; FAAM, foot and ankle ability measure score; FFI, foot function index; LEFS, lower extremity functional scale; MCS, mental component summary; NR, not reported; PCS, physical component summary; SEFAS, self‐reported foot and ankle score; SF‐12, Short Form‐12; SMFA, short musculoskeletal function assessment; VAS, visual analogue scale; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Quality Assessment
The quality of the 7 studies was evaluated, with 6 cohort studies10, 11, 12, 13, 14, 15 and 1 cross‐sectional study16 included. Cohort studies were assessed using the NOS, and the cross‐sectional study was assessed using AHRQ checklist7, 8. The evaluation results and summary are shown in Tables 2 and 3.
Table 2.
The Newcastle–Ottawa Scale (NOS) for assessing the quality of cohort studies
Table 3.
Agency for Healthcare Research and Quality (AHRQ) checklist for assessing the quality of cross‐sectional studies
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pinsker (2015)16 | + | + | + | U | U | U | − | + | − | + | U |
Yes = +; no = −; unclear = U.
Data Analysis
Clinical Outcomes
Of 7 studies, 5 studies involved clinical outcomes, but they used various scoring systems. Two of them used the American Orthopaedic Foot & Ankle Society (AOFAS) questionnaire scores to assess the two groups: the TAR group and the AAD group (mean difference, −4.26; 95% CI, −11.37–2.85; P = 0.24; I 2 = 1%) (Fig. 2).
Figure 2.
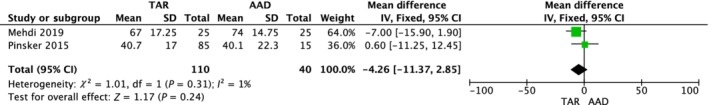
Forest plot for clinical outcome (American Orthopaedic Foot & Ankle Society [AOFAS] questionnaire scores) comparison between total ankle replacement (TAR) and ankle arthrodesis (AAD) groups.
Patient Satisfaction
Two studies reported on patient satisfaction, involving 32 patients of the TAR group and 32 patients of the AAD group. Of these, 23 TAR patients were satisfied, and 26 AAD patients were satisfied.
The result showed that there was no statistically significant difference between the TAR group and the AAD group (RR, 0.96; 95% CI, 0.65–1.40; P = 0.82; I 2 = 54%) (Fig. 3).
Figure 3.
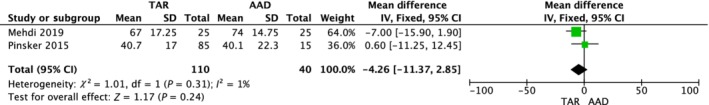
Forest plot for patient satisfaction comparison between total ankle replacement (TAR) and ankle arthrodesis (AAD) groups. [Correction added on 20 April 2020, after first online publication: figure 3 image has been corrected.]
Complications
Two studies involved complications, including 420 TAR subjects and 123 AAD subjects. The pooled data revealed that there was no statistical significance (RR, 1.15; 95% CI, 0.16–8.21; P = 0.89; I 2 = 84%) (Fig. 4).
Figure 4.
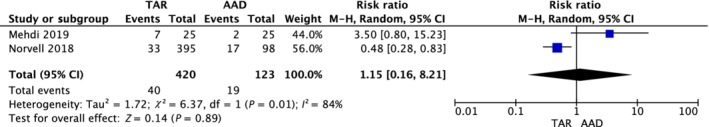
Forest plot for complication comparison between total ankle replacement (TAR) and ankle arthrodesis (AAD) groups.
Survival
Four studies presented survival details; that is, revision, re‐operation, or operation failure. Although there was no statistically significant difference found between the two groups, the RR showed that the risk of survival in the TAR group was relatively higher than that of the AAD group (RR, 1.91; 95% CI, 0.33–11.08; P = 0.47; I 2 = 90%) (Fig. 5).
Figure 5.
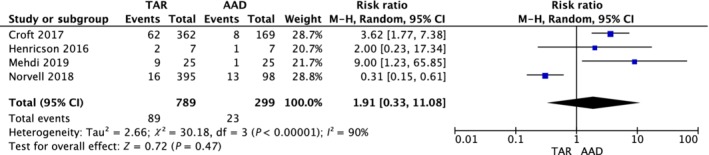
Forest plot for survival comparison between total ankle replacement (TAR) and ankle arthrodesis (AAD) groups.
Discussion
This is an updated systematic review and meta‐analysis reporting on the comparison of the efficiency and safety of TAR and AAD in the treatment of osteoarthritis. This meta‐analysis was based on 7 studies published in the past 5 years (2015–2019).
Kim et al. conducted a similar meta‐analysis comparing TAR and AAD for end‐stage ankle arthritis in 201617. The main finding of our study was relatively consistent with this previous meta‐analysis: TAR and AAD shared similar efficiency and safety in some aspects. Kim's work, like ours, concluded that TAR and AAD had no significant difference in clinical outcomes and patient satisfaction, but their conclusions showed that TAR patients had more re‐operations and complications than AAD17. In contrast, our results revealed no statistical difference between them. This discrepancy might occur because the works of literature included in our meta‐analysis were published more recently, and the surgical techniques might be improving. This might also be because the focuses on the details were different; for example, they were concerned about end‐stage ankle arthritis, while we were concerned about osteoarthritis.
Over time, TAR has made progress and has improved significantly in many areas over its predecessors18, 19, 20. Surgeons’ experience has also been increasing19. The same is true for AAD, where fusion rates have increased with the advent of new surgical techniques21. These factors can all lead to different results. This meta‐analysis only included studies published in the past 5 years because of the continuous progress of the procedures. Some early operations might be immature and showed poor results. If improvements in technology are not taken into account, the results may be biased. Therefore, we think it is necessary to select only recent studies for analysis. Besides, we did the screening of time periods after reading titles and abstracts, which could help not miss any high‐quality literature.
There are some potential limitations of this study. First, this meta‐analysis had a limited sample size. A small number of studies was included, and each study reported different outcome measures so that each outcome had a relatively small sample size. In addition, some of the included studies had few subjects. This might lead to potential bias or heterogeneity. For instance, various scoring systems were used to assess clinical outcomes in the included studies, and only two of the studies shared the same scoring system, AOFAS scores. For some rare complications, such a limited sample size might not be adequate. Besides, due to the different focuses of each included study, some assessments could not be performed because of the insufficient data. Therefore, further research is needed to resolve this issue.
Second, there was no RCT among the 7 studies included in this meta‐analysis, which were all observational studies. RCT is a relatively challenging type of research, so there are fewer RCT studies than other types of studies. Due to the characteristics of this study, ethical issues also need to be considered. RCT research focuses on randomness, and the existence of ethical problems makes such research difficult. Therefore, we found few relevant RCT studies, which led to higher heterogeneity.
Then, the search was not comprehensive enough. This was due to the imperfect retrieval strategy: only limited network databases (Cochrane Library, PubMed, Web of Science) were used in this meta‐analysis without searching published physical books.
In conclusion, although each therapy tends to have relatively better performance in some of the above aspects, it is difficult to judge which of the two is superior: there was no statistically significant difference between the two treatments. The current limitations of this meta‐analysis, mentioned above, indicate that it is necessary to increase the sample size and improve the retrieval strategy to reduce heterogeneity and bias. Further studies are necessary to assess and compare the efficiency and safety of TAR and AAD.
References
- 1. Hochberg MC, Altman RD, Brandt KD, et al Guidelines for the medical management of osteoarthritis. Arthritis Rheum, 1995, 38: 1535–1540. [DOI] [PubMed] [Google Scholar]
- 2. Horisberger M, Valderrabano V, Hintermann B. Posttraumatic ankle osteoarthritis after ankle‐related fractures. J Orthop Trauma, 2009, 23: 60–67. [DOI] [PubMed] [Google Scholar]
- 3. Fuentes‐Sanz A, Moya‐Angeler J, López‐Oliva F, Forriol F. Clinical outcome and gait analysis of ankle arthrodesis. Foot Ankle Int, 2012, 33: 819–827. [DOI] [PubMed] [Google Scholar]
- 4. Bestic JM, Peterson JJ, DeOrio JK, Bancroft LW, Berquist TH, Kransdorf MJ. Postoperative evaluation of the total ankle arthroplasty. Am J Roentgenol, 2008, 190: 1112–1123. [DOI] [PubMed] [Google Scholar]
- 5. Demetracopoulos CA, Halloran JP, Maloof P, Adams SB, Parekh SG. Total ankle arthroplasty in end‐stage ankle arthritis. Curr Rev Musculoskelet Med, 2013, 6: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Ann Intern Med, 2009, 151: 264–269. [DOI] [PubMed] [Google Scholar]
- 7. Rostom A, Dubé C, Cranney A, et al Quality assessment forms In: Celiac Disease. Rockville, MD: Agency for Healthcare Research and Quality, 2004. [Google Scholar]
- 8. Wells GA. The Newcastle–Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta‐analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2001.
- 9. Higgins JP, Altman DG, Gøtzsche PC, et al The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ, 2011, 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chopra S, Favre J, Crevoisier X. Qualitative analysis of foot intersegment coordination in the sagittal plane following surgery for end‐stage ankle osteoarthrosis. J Orthop Res, 2017, 35: 1304–1310. [DOI] [PubMed] [Google Scholar]
- 11. Croft S, Wing KJ, Daniels TR, Glazebrook M, et al Association of ankle arthritis score with need for revision surgery. Foot Ankle Int, 2017, 38: 939–943. [DOI] [PubMed] [Google Scholar]
- 12. Henricson A, Fredriksson M, Carlsson Å. Total ankle replacement and contralateral ankle arthrodesis in 16 patients from the Swedish Ankle Registry: self‐reported function and satisfaction. Foot Ankle Surg, 2016, 22: 32–34. [DOI] [PubMed] [Google Scholar]
- 13. Mehdi N, Bernasconi A, Laborde J, Lintz F. Comparison of 25 ankle arthrodeses and 25 replacements at 67 months’ follow‐up. Orthop Traumatol Surg Res, 2019, 105: 139–144. [DOI] [PubMed] [Google Scholar]
- 14. Norvell DC, Shofer JB, Hansen ST, et al Frequency and impact of adverse events in patients undergoing surgery for end‐stage ankle arthritis. Foot Ankle Int, 2018, 39: 1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pedowitz DI, Kane JM, Smith GM, Saffel HL, Comer C, Raikin SM. Total ankle arthroplasty versus ankle arthrodesis: a comparative analysis of arc of movement and functional outcomes. Bone Joint J, 2016, 98: 634–640. [DOI] [PubMed] [Google Scholar]
- 16. Pinsker E, Inrig T, Daniels TR, Warmington K, Beaton DE. Reliability and validity of 6 measures of pain, function, and disability for ankle arthroplasty and arthrodesis. Foot Ankle Int, 2015, 36: 617–625. [DOI] [PubMed] [Google Scholar]
- 17. Kim HJ, Suh DH, Yang JH, et al Total ankle arthroplasty versus ankle arthrodesis for the treatment of end‐stage ankle arthritis: a meta‐analysis of comparative studies. Int Orthop, 2017, 41: 101–109. [DOI] [PubMed] [Google Scholar]
- 18. Henne TD, Anderson JG. Total ankle arthroplasty: a historical perspective. Foot Ankle Clin, 2002, 7: 695–702. [DOI] [PubMed] [Google Scholar]
- 19. Gougoulias NE, Khanna A, Maffulli N. History and evolution in total ankle arthroplasty. Br Med Bull, 2008, 89: 111–151. [DOI] [PubMed] [Google Scholar]
- 20. Yu JJ, Sheskier S. Total ankle replacement‐evolution of the technology and future applications. Bull Hosp Jt Dis, 2014, 72: 120–128. [PubMed] [Google Scholar]
- 21. Hong CC, Tan KJ. Ankle arthritis: an evolution from arthrodesis to joint and mobility preservation. Orthop Muscular Syst, 2014, 3: 144. [Google Scholar]


