Abstract
Objective
To explore the effects of patellectomy on the bony and cartilaginous morphology of the trochlear groove in growing rabbits.
Methods
Forty‐eight 4‐week‐old New Zealand white rabbits were randomly assigned to two groups. The control group underwent a sham surgical procedure, whereas the patellectomy group underwent patella excision surgery. Half of the rabbits in each group were sacrificed 3 months postoperatively; the rest were sacrificed 6 months postoperatively. Hematoxylin and eosin staining was performed on collected samples. Measurements included the bony and cartilaginous sulcus angles of the trochlear groove. In addition, the thickness of the articular cartilage at the deepest sulcus position (central thickness) and at the mid‐position of the medial and lateral facets was measured and compared between groups.
Results
Three months after surgery, histological images revealed significant differences between the control group and the patellectomy group in cartilaginous sulcus angle (144.2° ± 1.5° vs 151.9° ± 2.4°, respectively; P < 0.001). No obvious difference in bony sulcus angle was found between the groups. Six months after surgery, significant between‐group differences were observed in cartilaginous sulcus angle (136.3° ± 2.5° in control group vs 160.7° ± 3.0° in patellectomy group, P < 0.001) and bony sulcus angle (136.2° ± 2.2° in control group vs 160.4° ± 2.6° in patellectomy group, P < 0.001). However, there were no significant intra‐group differences between cartilaginous and bony sulcus angles in either group. Three months after surgery, significant between‐group differences were detected in articular cartilage thickness at the three different positions (medial facet: 324.3 ± 14.0 μm in control group vs 391.7 ± 98.8 μm in patellectomy group, P = 0.029; central position: 362.1 ± 13.6 μm in control group vs 730.3 ± 76.8 μm in patellectomy group, P < 0.001; lateral facet: 324.6 ± 12.7 μm in control group vs 358.5 ± 38.7 μm in patellectomy group, P = 0.009). No between‐group differences in cartilage thickness were found at 6 months.
Conclusions
Abnormal mechanical stress (patellectomy) during a rabbit's development can cause flattening of the femoral trochlear cartilage, followed by changes in the subchondral osseous layer. Abnormal mechanical stress is a crucial factor in the development of trochlear groove dysplasia.
Keywords: Abnormal stress, Femoral trochlear groove, Patellectomy, Rabbit, Sulcus angle
Introduction
Trochlear dysplasia (TD) is a common anatomical abnormality in patellofemoral instability1, 2. It has been reported that 96% of patients with symptomatic patellar instability have some degree of TD3. Numerous studies have indicated that TD is a main cause of patellofemoral instability4, 5, 6, 7. In 1994, H. Dejour classified TD into three types according to findings on true lateral radiographs3. D. Dejour et al. later described four types based on a true lateral view with the addition of a computed tomography scan7. That group defined TD as a sulcus angle of more than 150° and concluded that patellofemoral instability was highly associated with trochlear groove dysplasia7.
Li et al.8 investigated the effects of patellofemoral instability on trochlear groove development in growing rabbits. That study found that the patellar dislocation model of growing rabbits could lead to femoral TD. Huri et al.9 also reported that lateral patellar subluxation or instability (loss of restraint function of medial patellofemoral ligament) early in a rabbit's development caused femoral TD or flattening. Kaymaz et al.10 documented that simulated patella alta (patellar malposition) via patellar tendon Z‐plasty lengthening resulted in a flatter distal femoral groove. These studies used computed tomography to assess only the bony sulcus angle and did not evaluate the relationship between cartilage and subchondral bone.
The patellofemoral joint is one of the three compartments of the knee joint. The characteristic concavo‐convex matching between the femoral trochlea and patella is the structural basis for normal patellofemoral joint function11. TD is the malformation of the shape and depth of the femoral trochlear groove. Previous studies9, 10, 11 have demonstrated that loss of the soft tissues of the medial patella can lead to TD. Computed tomography has been used in some studies to evaluate the sulcus angle of the trochlear groove8, 9, 10. However, human computed tomography produces low‐resolution images in experimental animals, with accompanying measurement discrepancies. In addition, the effect of abnormal mechanical stress during development of the femoral trochlear groove remains unclear.
Li et al.8 established an animal model of patellar dislocation by plicating the lateral patellar capsule. Another patellar instability model was created by releasing the medial retinaculum, medial patellofemoral ligament, medial patellotibial ligament, and medial patellomeniscal ligament, without plicating the lateral retinaculum9. Wang et al.11 reported a rabbit model of patellar subluxation that was similar to that of Li8, but with medial capsule release of about 2 cm. Different mechanisms of abnormal mechanical stress in different animal models can result in TD; however, function and degree of dysplasia may differ among models. These results may explain why there are different types of TD. Our model of patellectomy creates a relatively mild abnormal mechanical stress compared with the stress created in previous studies.
The aims of this experimental study were: (i) to explore the effects of patellectomy (abnormal mechanical stress) on the bony and cartilaginous morphology of the trochlear groove 3 months postoperatively; (ii) to explore the effects of patellectomy on the bony and cartilaginous morphology of the trochlear groove 6 months postoperatively; and (iii) to assess the trochlear cartilage thickness at three different positions. Previous studies12, 13 have indicated that the bony anatomy does not match the cartilaginous anatomy of the trochlea in either normal knees or dysplastic knees. We hypothesized that abnormal mechanical stress (patellectomy) would disturb normal physiological growth and biomechanics of the patellofemoral structures, which would result in abnormal development of the femoral groove with flattening of the trochlea. We predicted that changes in the cartilage and subchondral bone layers would occur simultaneously.
Methods
Animal Model
This study was approved by the Animal Ethical Committee of Hebei Medical University (Z2017‐015‐1). Forty‐eight 4‐week‐old New Zealand white rabbits (provided by the Animal Test Center of Hebei Medical University, Hebei, China), weighing from 280 to 300 g, were randomly assigned to two groups. The control group (n = 24) underwent a sham surgical procedure, whereas the patellectomy group (n = 24) underwent patella excision surgery. Half of the rabbits in each group were sacrificed 3 months after surgery; the remaining rabbits were sacrificed 6 months after surgery (Fig. 1). Rabbits completed skeletal growth by 28 weeks of age14.
Figure 1.
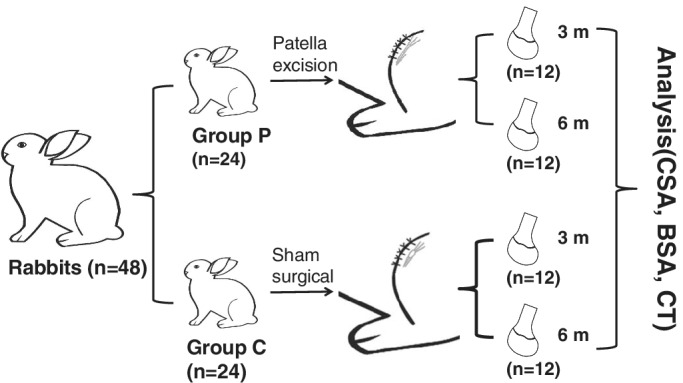
Experimental flowchart. Forty‐eight 4‐week‐old New Zealand white rabbits were randomly assigned to two groups. The control group (Group C, n = 24) underwent a sham surgical procedure, whereas the patellectomy group (Group P, n = 24) underwent patella excision surgery. Half of the rabbits in each group were sacrificed 3 and 6 months after surgery. The analysis included bony sulcus angle (BSA), cartilaginous sulcus angle (CSA) and cartilage thickness (CT).
Surgery
All surgical procedures were performed under intravenous anesthesia with ketamine (20 mg/kg) and xylazine (5 mg/kg). Prior to surgery, the left knee of each rabbit was shaved and sterilized according to standard protocol. For surgical patellectomy (patellectomy group), a 3‐cm midline skin incision was made over the knee, followed by dissection of subcutaneous tissues to expose the patella and patellar tendon. A 7‐ to 10‐mm longitudinal incision was made along the midline of the patella. The dissection was performed from the superior patellar pole to the inferior patellar pole, from the medial to lateral aspects of the patellar tendon, until the anterior surface of the patella was fully exposed. All aspects of the tendon were then freed. The entire patella was excised slowly and gently to minimize injury to the patellar tendon (Fig. 2). The inner patellar tendon was checked to ensure no hard tissue had dropped. The dissected patellar tendon was not sutured. For sham surgery (control group), a 3‐cm midline skin incision was made over the knee. The surgical incision was closed with interrupted sutures.
Figure 2.
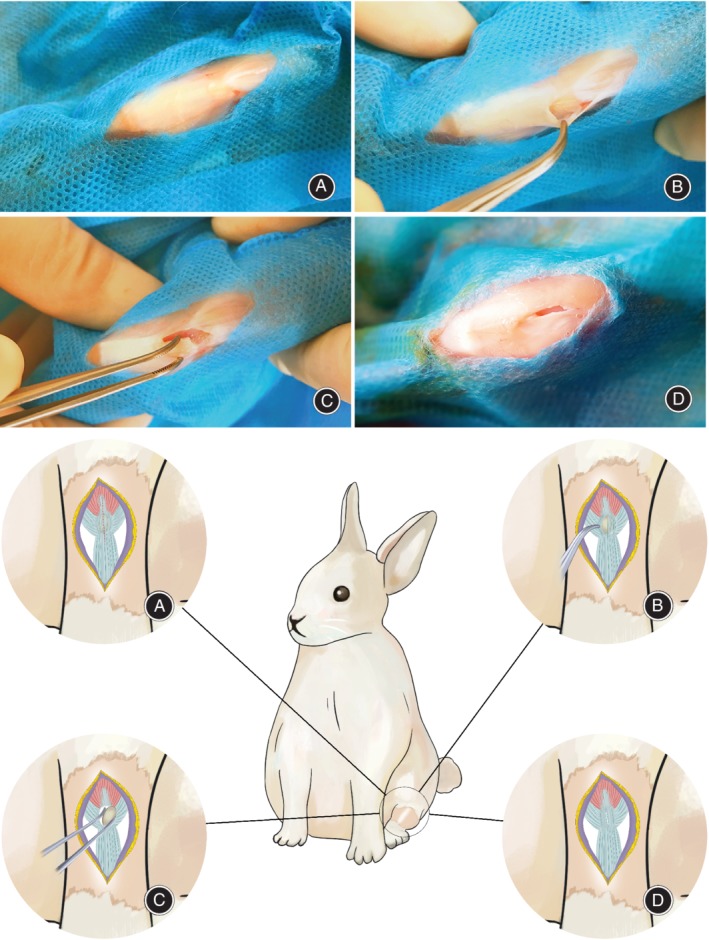
Surgical technique. (A) Patella and patellar tendon exposure. (B) Half of the anterior surface of patella is exposed. (C) Junction of superior patellar pole is dissected. (D) Patella is fully excised.
Postoperative Care
Great care was taken during all surgeries to avoid damaging the femoral articular cartilage. At the completion of surgery, bandages were applied. Oral ciprofloxacin (10 mg/kg) was administered as antibiotic prophylaxis for the initial 3 days after surgery. Intramuscular buprenorphine (0.05 mg/kg) was administered twice daily for 5 days for postoperative analgesia.
Histological Analysis
All rabbits were sacrificed with an overdose of anesthetic (ketamine, 100 mg/kg) at 3 or 6 months after surgery. Distal femurs without soft tissues were collected. Distal femur tissue blocks were fixed in 10% neutral buffered formalin solution for at least 24 h and rinsed in tap water for 2 h. The tissue blocks were subsequently decalcified with 10% ethylenediaminetetraacetic acid (EDTA) for 4 to 5 weeks until completion, followed by alcohol gradient dehydration. Samples were embedded in paraffin wax, cut into 4‐μm sections, and stained with hematoxylin and eosin. Tissue slices were taken 5 mm distal and proximal to the middle of the trochlear groove. Histological analysis of the cartilage and subchondral bone was performed.
Outcome Measures
Sulcus Angle
The prepared tissue slices were scanned with Pannoramic Digital Slide Scanners (Pannoramic MIDI II; 3DHISTECH Ltd., Budapest, Hungary). Measurements included the bony and cartilaginous sulcus angles of the trochlear groove at different growth stages. The sulcus angle is the angle between the medial articular surface and the lateral articular surface of the trochlea. The cartilaginous sulcus angle measures the angle between the medial and lateral trochlear cartilaginous surfaces. The bony sulcus angle measures the angle between the medial and lateral trochlear bony surfaces (Fig. 3). The normal bony and cartilaginous sulcus angles provide normal patellar tracking through knee flexion‐extension.
Figure 3.
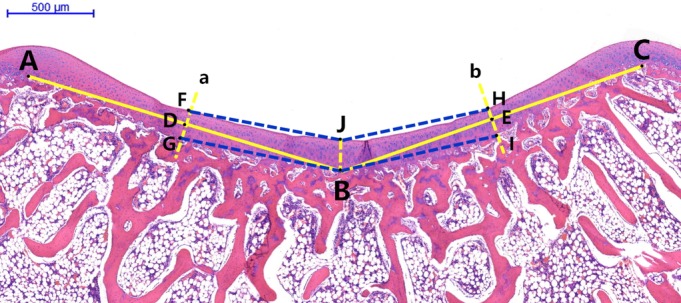
Axial microscopic view of trochlear groove. A and C are highest points of the medial and lateral condyles of the femoral trochlea. B is the deepest point of the bony trochlear groove. D and E are the midpoints of lines AB and BC, respectively. Line a passes through point D perpendicular to line AB. FG is the cartilage thickness of the medial facet. Line b passes through point E perpendicular to line CB. HI is the cartilage thickness of the lateral facet. The distance JB is the thickness of the central cartilage (deepest sulcus position). Angle FJH is the cartilaginous sulcus angle. Angle GBI is the bony sulcus angle. F and H are the points at which the cartilaginous trochlear groove intersects with lines a and b, respectively. G and I are the points at which the bony trochlear groove intersects with lines a and b, respectively.
Cartilage Thickness
The thickness of the articular cartilage at the deepest sulcus position and at the mid‐position of the medial and lateral facets was measured with Case Viewer 1.4 software (3DHISTECH Ltd., Budapest, Hungary). The thickness of the cartilage indicates cartilage hyperplasia in different parts of the trochlea. The thickness of the articular cartilage at the deepest sulcus position is the lowest position cartilage thickness of trochlea. The thickness of the articular cartilage at the mid‐position of the medial and lateral facets is the middle position of the medial and lateral articular surfaces (Fig. 3). The thickness of the cartilage at different positions can explain the change of cartilaginous sulcus angle. Our measurement methods had an accuracy of 0.1° for the sulcus angles and 0.1 μm for articular cartilage thickness.
Statistical Analysis
Bony and cartilaginous sulcus angles and cartilage thickness at the three positions were compared between the two groups with Student's t tests. Data (CSA, BSA, CT) were analyzed with SPSS 21.0 statistical software (IBM, Chicago, IL, USA). P < 0.05 was considered to indicate significance.
Results
Sulcus Angle
At 3 Months
At 3 months after operation, the mean cartilaginous sulcus angle (CSA) in the control group (Group C) was 144.2° ± 1.5°, whereas that in the patellectomy group (Group P) was 151.9° ± 2.4°; this increase (5.3%) was statistically significant (P < 0.001). The mean bony sulcus angle (BSA) was 144.2° ± 1.4° in Group C and 143.8° ± 2.3° in Group P. There was no significant difference between groups in the mean BSA. In Group C, the CSA and the BSA did not differ significantly. In Group P, there was a significant difference (P < 0.001) between mean CSA (151.9° ± 2.4°) and mean BSA (143.8° ± 2.3°) 3 months postoperatively (Fig. 4 and Table 1).
Figure 4.
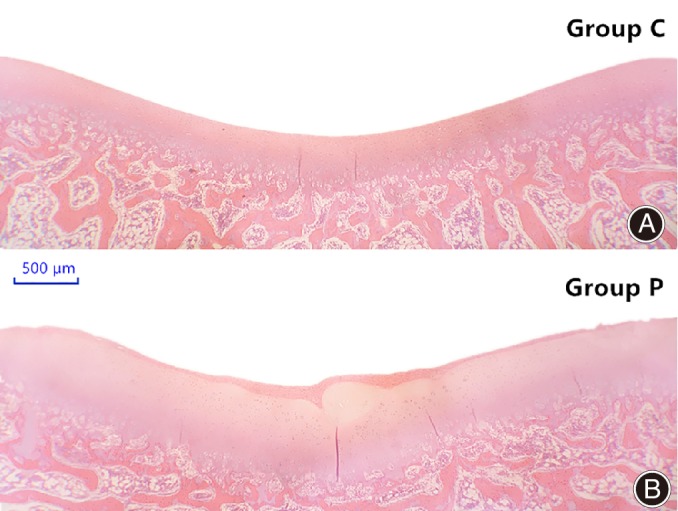
Microscopic view of femoral trochlear groove in the two groups 3 months after surgery. (A) control group; (B) patellectomy group (HE staining).
Table 1.
Comparison of cartilaginous and bony sulcus angles
| 3 months post‐op | 6 months post‐op | |||||
|---|---|---|---|---|---|---|
| Group C | Group P | P value | Group C | Group P | P value | |
| CSA (°) | 144.2 ± 1.5 | 151.9 ± 2.4 | 0.000 | 136.3 ± 2.5 | 160.7 ± 3.0 | 0.000 |
| BSA (°) | 144.2 ± 1.4 | 143.8 ± 2.3 | n.s. | 136.2 ± 2.2 | 160.4 ± 2.6 | 0.000 |
| P value | n.s. | 0.000 | ‐ | n.s. | n.s. | ‐ |
BSA, bony sulcus angle; CSA, cartilaginous sulcus angle; n.s., not significant.
At 6 Months
At 6 months after operation, the mean CSA was 136.3° ± 2.5° in Group C and 160.7° ± 3.0° in Group P; this increase (17.9%) was statistically significant (P < 0.001). The mean BSA was 136.2° ± 2.2° in Group C and 160.4° ± 2.6° in Group P; this increase (17.8%) was also significant (P < 0.001). No significant intra‐group differences between CSA and BSA were observed in either group 6 months postoperatively (Fig. 5 and Table 1).
Figure 5.
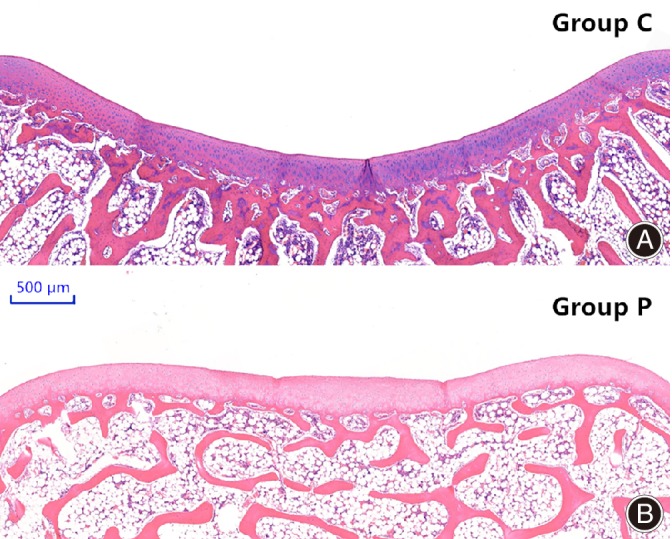
Microscopic view of femoral trochlear groove in the two groups 6 months after surgery. (A) control group; (B) patellectomy group (HE staining).
The Cartilage Thickness of Femoral Trochlea
At 3 Months
Table 2 shows the cartilage thickness of the femoral trochlea at three positions. At 3 months after operation, significant between‐group differences were observed in the mean cartilage thickness at the mid‐position of the medial facet (324.3 ± 14.0 μm in control group vs 391.7 ± 98.8 μm in patellectomy group, the CTM significantly increased [20.8%], P = 0.029), at the mid‐position of the lateral facet (324.6 ± 12.7 μm in control group vs 358.5 ± 38.7 μm in patellectomy group, the CTL significantly increased [10.4%], P = 0.009), and at the deepest sulcus position (362.1 ± 13.6 μm in control group vs 730.3 ± 76.8 μm in patellectomy group, the CTC significantly increased [101.7%], P < 0.001).
Table 2.
Comparison of cartilage thickness at three different positions
| Group C | Group P | P value | |
|---|---|---|---|
| 3 Months Post‐op | |||
| CTM (μm) | 324.3 ± 14.0 | 391.7 ± 98.8 | 0.029 |
| CTC (μm) | 362.1 ± 13.6 | 730.3 ± 76.8 | 0.000 |
| CTL (μm) | 324.6 ± 12.7 | 358.5 ± 38.7 | 0.009 |
| 6 Months Post‐op | |||
| CTM (μm) | 217.5 ± 6.6 | 215.1 ± 4.6 | n.s. |
| CTC (μm) | 234.4 ± 8.3 | 240.8 ± 9.3 | n.s. |
| CTL (μm) | 215.7 ± 5.2 | 216.3 ± 4.5 | n.s. |
CTC, Cartilage thickness at center (deepest sulcus position); CTL, Cartilage thickness at lateral facet (mid‐position); CTM, Cartilage thickness at medial facet (mid‐position); n.s., not significant.
The four layers (the surface layer, the transition layer, the radiation layer, and the calcification layer) of articular cartilage were thicker than for the patellectomy group when compared to the control group at 3 months. In particular, the thicknesses (cells and cartilage matrix) of transition layer and radiation layer are significant. The cells in the transition layer and radiation layer were mildly irregular and sparsely dispersed. The structure of the four layers were clearly distinguishable (Fig. 4).
At 6 Months
At 6 months after operation, the cartilage thickness at the three positions was comparable between groups. No significant between‐group differences were observed in the mean cartilage thickness at the mid‐position of the medial facet (217.5 ± 6.6 μm in control group vs 215.1 ± 4.6 μm in patellectomy group, n.s.), at the mid‐position of the lateral facet (215.7 ± 5.2 μm in control group vs 216.3 ± 4.5 μm in patellectomy group, n.s.), and at the deepest sulcus position (234.4 ± 8.3 μm in control group vs 240.8 ± 9.3 μm in patellectomy group, n.s.) (Fig. 6 and Table 2).
Figure 6.
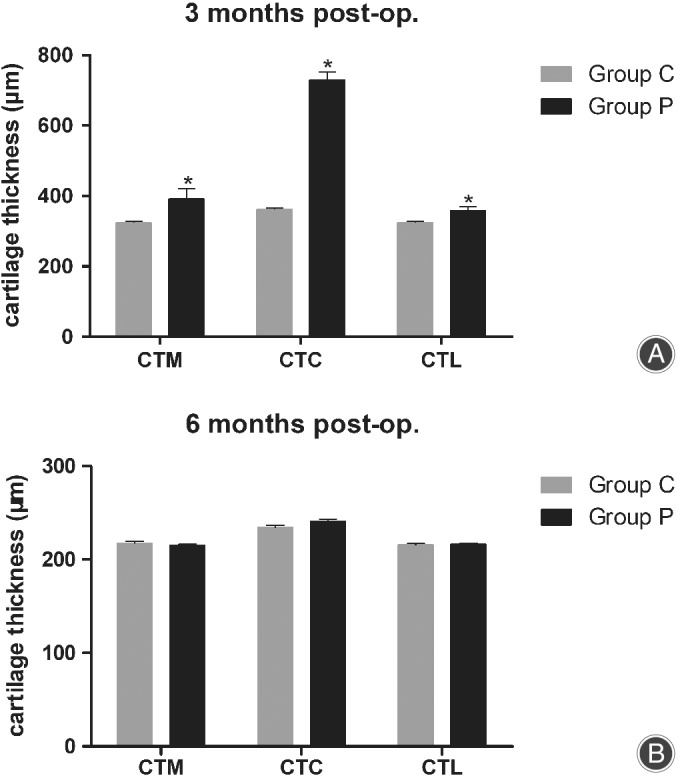
Comparison of cartilage thickness at three different positions. (A) At 3 months post‐operation. (B) At 6 months post‐operation. CTM, cartilage thickness at medial facet (mid‐position); CTC, cartilage thickness at center (deepest sulcus position); CTL, cartilage thickness at lateral facet (mid‐position).
No obvious differences in articular cartilage thickness at the three different positions were seen in the patellectomy group compared with the control group at 6 months. However, the four layers of cells were irregular and sparsely dispersed. The orientation of the transition layer and radiation layer cells lacked a pattern, and the four structural layers could not be clearly identified (Fig. 5).
Discussion
The most important finding of this animal study was that abnormal mechanical stress caused femoral TD (flat trochlear groove) in growing rabbits. This finding is consistent with previous reports8, 9, that patellar subluxation or instability early in a rabbit's development causes femoral TD or flattening. In addition, we found initial changes in the cartilage layer and subsequent changes in the subchondral osseous layer in the progression of trochlear groove flattening. Stäubli et al.15 reported that the osseous anatomy of the patellofemoral joint did not match the corresponding surface geometry of the articular cartilage. Therefore, we analyzed both bony and cartilaginous sulcus angles of the trochlear groove with histology.
Animal studies have found that abnormal mechanical stress (patellar subluxation or instability) may lead to patellofemoral TD and patellofemoral osteoarthritis8, 9. However, both animal and clinical investigations have revealed that early restoration of the patella can minimize such dysplasia11, 16. Therefore, the development of the femoral trochlea may not only be determined by genetics, but may also be altered by acquired conditions. Above all, it is clinically vital to ensure early surgical correction for children and adolescents with patellar instability and/or TD.
The patellofemoral joint needs normal mechanical stress stimulus to develop and maintain normal morphology. Li et al.8 and Wang et al.11 found that patellar dislocation resulted in TD in an animal model. In the present study, histological investigations showed that the patellectomy group had a larger CSA than the control group at 3 months after surgery (151.9° ± 2.4° vs 144.2° ± 1.5°, respectively; P < 0.001). There were no significant between‐group differences in the BSA at 3 months. These results suggest that abnormal mechanical stress on the patellar tendon stimulates the cartilage layer, causing an increase in the CSA. In Group C, the CSA (mean ± standard deviation) was similar to the BSA 3 months postoperatively (n.s.). However, in Group P, the CSA was larger than the BSA (151.9° ± 2.4° vs 143.8° ± 2.3°, respectively; P < 0.001) 3 months postoperatively. These results indicate that changes occur in the cartilage layer separately from changes in the subchondral osseous layer. At 6 months after operation, both the BSA and CSA were larger in the patellectomy group than in the control group (P < 0.001) (Table 1). No significant differences were observed between groups in CSA or BSA 6 months postoperatively (Table 1).
No patellofemoral joint osteoarthritis was observed in any of the study animals. However, in our pre‐experiment, nonabsorbable suture was used to close the capsule. When the cartilage was exposed, we realized this had been a mistake. There was a cartilage ridge on the femoral trochlear groove. The gross and histological images are shown in Figs 7 and 8, respectively. The suture knot produced abnormal friction that led to formation of a cartilage ridge and dysplasia of the trochlear groove. This friction was a relatively severe abnormal mechanical stress. Therefore, the changes in the cartilage layer were comparatively pronounced. The cartilage of the femoral trochlea was thick and rough, and the cartilage cells were irregular. Histopathological examination revealed disordered arrangement of cells on the surface and in the transition layer, forming a spherical chondrocyte mass under the ridge of the trochlea. Changes in the congruence of the trochlear groove and patella will affect mechanical stress and lead to abnormalities in distribution of weight‐bearing in the joint8. In the first classification of Dejour, TD was divided into three types3. After improvements, a more precise four‐type classification of TD was proposed7, 17. In addition, three different patellar types were described by Wiberg; a fourth type (the “Jaegerhut” patella) was later described by Baumgartl. TD often accompanies patellar dysplasia. There is no precise definition of the perfect geometrical shape of the trochlear groove and patella. However, it is clear that a normal trochlear groove will never match a type IV patella.
Figure 7.
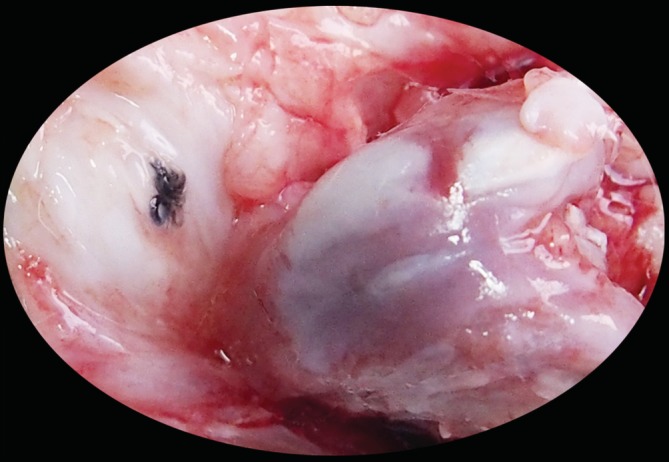
Patellofemoral joint osteoarthritis specimen from pre‐experiment. A cartilage ridge is present on the femoral trochlear groove.
Figure 8.
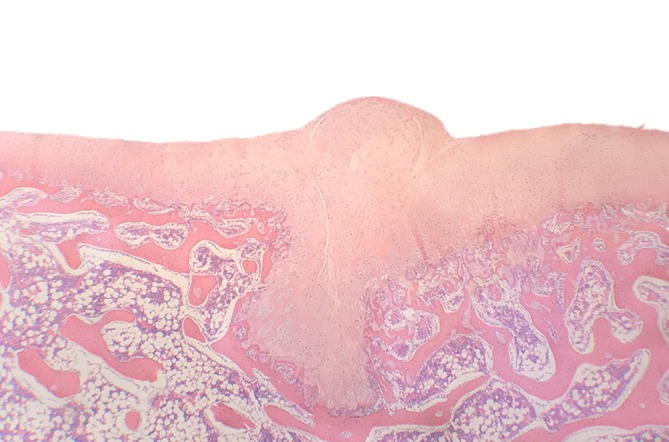
Histological view of cartilage ridge. The normal “V” shape of the trochlear groove was altered, with an abnormal cartilage ridge. Hematoxylin and eosin staining.
The stability of the patellofemoral joint depends on the smooth and normal interplay of complex geometries18. The stimulation between the femoral trochlea and the patella is key in promoting normal patellofemoral bone and cartilage development11. Especially in children and adolescents, if a mildly abnormal mechanical stress (for example, partial medial patellofemoral ligament injury) is applied to a patella that is well matched by the trochlea, the forces remain well distributed and the stresses remain within a tolerable range. Thus, a trochlea that is slightly steeper or more shallow than usual can be tolerated, as long as the patella matches this variation18.
In the current study, an immature rabbit model was established to simulate abnormal mechanical stress on the femoral trochlear groove. Our animal model simulated a type of abnormal mechanical stress without damage to the medial or lateral restrictive structures of the patella. The patellectomy group underwent patella excision surgery. At 3 months after operation, Group P had a larger CSA and thicker trochlear cartilage than Group C (P < 0.05); however, the BSA did not differ between groups (Table 1 and 2). These findings indicate that cartilage remodeling was active in the first 3 months after patellectomy. At 6 months after operation, the cartilage thickness in Group P was similar to that in Group C at each measurement point (n.s.). Only the CSA and BSA differed significantly between the groups. Moreover, bone and cartilage remodeling were shown to be completed.
This study has several limitations. First, the established rabbit model did not undergo the same dissection as the human patellofemoral joint. Secondly, the molecular mechanisms of bone and cartilage remodeling in this study remain unclear. Thirdly, a small number of animals were included, which might have affected the experimental results. More in‐depth studies with a larger sample size and multiple measurement time points are required to confirm our findings. Finally, the length of observation may have been too short; therefore, longer observation time is needed. The short study period may explain why no obvious degenerative changes were seen in either group.
Conclusion
Abnormal mechanical stress (patellectomy) during a rabbit's development can cause flattening of the femoral trochlear cartilage, followed by changes in the subchondral osseous layer. Abnormal mechanical stress is a crucial factor in the development of trochlear groove dysplasia.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. This study was approved by the Animal Ethical Committee of Hebei Medical University (Z2017‐015‐1).
Acknowledgment
The authors gratefully acknowledge funding from the National Natural Science Foundation of China (Grant Number: 81873983). We thank Rebecca Tollefson, DVM, from Liwen Bianji, Edanz Editing China (http://www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
References
- 1. Tscholl PM, Wanivenhaus F, Fucentese SF. Conventional radiographs and magnetic resonance imaging for the analysis of trochlear dysplasia: the influence of selected levels on magnetic resonance imaging. Am J Sports Med, 2017, 45: 1059–1065. [DOI] [PubMed] [Google Scholar]
- 2. Weber AE, Nathani A, Dines JS, et al An algorithmic approach to the management of recurrent lateral patellar dislocation. J Bone Joint Surg Am, 2016, 98: 417–427. [DOI] [PubMed] [Google Scholar]
- 3. Dejour H, Walch G, Nove‐Josserand L, Guier C. Factors of patellar instability an anatomic radiographic study. Knee Surg Sports Traumatol Arthrosc, 1994, 2: 19–26. [DOI] [PubMed] [Google Scholar]
- 4. Lippacher S, Reichel H, Nelitz M. Radiological criteria for trochlear dysplasia in children and adolescents. J Pediatr Orthop B, 2011, 20: 341–344. [DOI] [PubMed] [Google Scholar]
- 5. Nelitz M, Lippacher S. Arthroscopic evaluation of trochlear dysplasia as an aid in decision making for the treatment of patellofemoral instability. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 2788–2794. [DOI] [PubMed] [Google Scholar]
- 6. Nelitz M, Lippacher S, Reichel H, Dornacher D. Evaluation of trochlear dysplasia using MRI: correlation between the classification system of Dejour and objective parameters of trochlear dysplasia. Knee Surg Sports Traumatol Arthrosc, 2014, 22: 120–127. [DOI] [PubMed] [Google Scholar]
- 7. Tecklenburg K, Dejour D, Hoser C, Fink C. Bony and cartilaginous anatomy of the patellofemoral joint. Knee Surg Sports Traumatol Arthrosc, 2006, 14: 235–240. [DOI] [PubMed] [Google Scholar]
- 8. Li W, Wang Q, Wang F, Zhang Y, Ma L, Dong J. Femoral trochlear dysplasia after patellar dislocation in rabbits. Knee, 2013, 20: 485–489. [DOI] [PubMed] [Google Scholar]
- 9. Huri G, Atay OA, Ergen B, Atesok K, Johnson DL, Doral MN. Development of femoral trochlear groove in growing rabbit after patellar instability. Knee Surg Sports Traumatol Arthrosc, 2012, 20: 232–238. [DOI] [PubMed] [Google Scholar]
- 10. Kaymaz B, Atay OA, Ergen FB, et al Development of the femoral trochlear groove in rabbits with patellar malposition. Knee Surg Sports Traumatol Arthrosc, 2013, 21: 1841–1848. [DOI] [PubMed] [Google Scholar]
- 11. Wang S, Ji G, Yang X, et al Femoral trochlear groove development after patellar subluxation and early reduction in growing rabbits. Knee Surg Sports Traumatol Arthrosc, 2016, 24: 247–253. [DOI] [PubMed] [Google Scholar]
- 12. Shih YF, Bull AM, Amis AA. The cartilaginous and osseous geometry of the femoral trochlear groove. Knee Surg Sports Traumatol Arthrosc, 2004, 12: 300–306. [DOI] [PubMed] [Google Scholar]
- 13. van Huyssteen AL, Hendrix MR, Barnett AJ, Wakeley CJ, Eldridge JD. Cartilage‐bone mismatch in the dysplastic trochlea. An MRI study. J Bone Joint Surg Br, 2006, 88: 688–691. [DOI] [PubMed] [Google Scholar]
- 14. Masoud I, Shapiro F, Kent R, Moses A. A longitudinal study of the growth of the New Zealand white rabbit: cumulative and biweekly incremental growth rates for body length, body weight, femoral length, and tibial length. J Orthop Res, 1986, 4: 221–231. [DOI] [PubMed] [Google Scholar]
- 15. Stäubli HU, Dürrenmatt U, Porcellini B, Rauschning W. Anatomy and surface geometry of the patellofemoral joint in the axial plane. J Bone Joint Surg Br, 1999, 81: 452–458. [DOI] [PubMed] [Google Scholar]
- 16. Fu K, Duan G, Liu C, Niu J, Wang F. Changes in femoral trochlear morphology following surgical correction of recurrent patellar dislocation associated with trochlear dysplasia in children. Bone Joint J, 2018, 100‐B: 811–821. [DOI] [PubMed] [Google Scholar]
- 17. Dejour D, Le Coultre B. Osteotomies in patello‐femoral instabilities. Sports Med Arthrosc Rev, 2007, 15: 39–46. [DOI] [PubMed] [Google Scholar]
- 18. Grelsamer RP, Dejour D, Gould J. The pathophysiology of patellofemoral arthritis. Orthop Clin North Am, 2008, 39: 269–274. [DOI] [PubMed] [Google Scholar]


