Abstract
Objectives
To investigate the course of in vivo blood metal ion levels in patients undergoing primary total knee arthroplasty (TKA) and to investigate potential risk factors associated with metal ion release in these patients.
Methods
Twenty‐five patients with indication for TKA were included in this prospective study. Whole blood metal ion analysis was performed pre‐operatively and at 1 week, 6 weeks, 3 months, 6 months, and 12 months postoperatively. Clinical scores were obtained using the American Knee Society Score (AKSS) and the Oxford Knee Score (OKS) at each follow‐up and patientsʼ activity levels were assessed by measuring the mean annual walking cycles at 12 months follow‐up. Anteroposterior and lateral radiographs of the operated knee were evaluated postoperatively and at 12‐month follow‐up with regard to implant position and radiological signs of implant loosening. Correlation analysis using multivariate linear regression was performed to investigate the influence of different variables (age, gender, functional scores, number of walking cycles, and body mass index [BMI]) on blood cobalt ion concentrations.
Results
Mean metal ion levels of cobalt, chromium, molybdenum, and titanium were 0.28 μg/L (SD, 0.14), 0.43 μg/L (SD, 0.49), 0.62 μg/L (SD, 0.45), and 1.96 μg/L (SD, 0.98), respectively at 12‐month follow‐up. Mean cobalt ion levels significantly increased 1‐year after surgery compared to preoperative measurements. There was no statistically significant increase of mean metal ion levels of chromium, titanium, and molybdenum at 1‐year follow‐up. Overall, metal ion levels were low and no patient demonstrated cobalt ion levels above 1 μg/L. Postoperative radiographs demonstrated well‐aligned TKAs in all patients and no signs of osteolysis or implant loosening were detected at 1‐year follow‐up. Both the AKSS and OKS significantly improved during the course of the study up to the final follow‐up. Multivariate regression analysis did not show a statistically significant correlation between the tested variables and blood cobalt ion concentrations.
Conclusion
A statistically significant increase of mean cobalt ion concentration at 1‐year follow‐up was found in this cohort of patients with well‐functioning TKA, although overall blood metal ion levels were relatively low. Despite low systemic metal ion concentrations seen in this cohort, the local effects of increased metal ion concentrations in the periprosthetic environment on the long‐term outcome of TKA should be further investigated.
Keywords: Metal ions, Metal wear, Total knee arthroplasty (TKA)
Introduction
Total knee arthroplasty (TKA) is a successful treatment option for advanced osteoarthritis of the knee with reported long‐term survival rates of 91% after 20 years1. Aseptic component loosening remains the main reason for revision surgery in the long term which accounts for up to 32% of all TKA revisions according to registry data2. Although the pathomechanisms of implant failure are multifactorial, wear‐related complications play a major role in the development of periprosthetic osteolysis and late implant loosening.
Periprosthetic osteolysis can be considered a consequence of either increased bone resorption or decreased bone formation around the orthopedic implant. Accumulating metal wear particles and metal ions are able to stimulate both bone metabolism and the immune system through different pathways hereby contributing to the pathomechanisms of implant loosening3. As part of a foreign body reaction, the role of macrophages and their activation through phagocytosis of wear particles is well understood4, 5, 6. However, less is known about the effects of accumulating metal ions on periprosthetic bone metabolism and their interactions with the immune system. With regard to bone metabolism, metal ions are able to recruit and activate osteoclast precursor cells and have the potential to inhibit osteoblasts at the same time7, 8, 9. Both mechanisms play an important role in the development of periprosthetic osteolysis. For example, Cadosch et al. and others could show that metal ions are able to induce the expression of different chemotactic cytokines in macrophages and osteoclasts, such as CCL17/TARC (thymus and activation‐regulated chemokine) and CCL22/MDC (macrophage‐derived chemokine), that are involved in the recruitment of osteoclast precursor cells which might ultimately stimulate periprosthetic osteolysis and implant loosening7, 10, 11, 12, 13, 14. Other studies have demonstrated that nontoxic concentrations of metal ions are able to suppress the differentiation and function of osteoblastic cells in vitro which could contribute to implant loosening by inhibition of periprosthetic bone formation8, 9.
Due to their size, metal ions are able to pass into the systemic blood circulation where they form complexes with serum proteins15. These hapten‐like metal‐protein‐complexes can be recognized by T‐lymphocytes as an antigen, which leads to their proliferation and the production of chemotactic cytokines, such as interleukin (IL)‐6 and tumor necrosis factor‐alpha (TNF‐a) that might, in turn, trigger a specific immune response and activate osteoclastogenesis3, 16, 17, 18. In this context, delayed type IV hypersensitivity reactions to metal ions have become of increasing interest, and orthopaedic surgeons are facing a growing demand for coated, so‐called hypoallergenic total knee prosthesis19, 20, 21.
In joint replacements, metal ions are generated by corrosive degradation of metal wear debris and by tribocorrosion at the exposed metal surfaces of the implant. In vivo, a passive oxide film is spontaneously formed on the metallic surface which protects the implant material from corrosion and inhibits the release of metal ions22. However, repetitive mechanical stress leads to the disruption of this protective oxide film hereby facilitating corrosion damage and metal ion release23. In addition, galvanic corrosion can occur at taper connections of modular implants, when mixed metal components with different electrochemical potentials are combined24, 25. The two dissimilar metals then act as anode and cathode in the presence of an electrolyte, which facilitates metal ion generation26. Besides these aforementioned electrochemical corrosive mechanisms, in vitro studies could demonstrate that osteoclasts are able to directly corrode metallic biomaterials and release metal ions by leaving resorption pits on the metal surface3, 27.
Modular junctions and large metal surface areas, which are prone to fretting damage and corrosion, are both commonly found in TKAs, which might lead to the generation and accumulation of metal ions in total knee replacements28. Although simulator studies have shown that relevant amounts of metal wear products and metal ions are continuously released from total knee prosthesis29, little is known about in vivo metal ion levels in patients following well‐functioning primary total knee replacement.
Therefore, the aim of this prospective study was: (i) to investigate in vivo blood metal ion concentrations of cobalt, chromium, molybdenum, and titanium over time in patients undergoing primary total knee replacement; (ii) to assess clinical and radiological outcome; and (iii) to investigate the influence of potential risk factors on blood metal ion concentrations in these patients.
Materials and Methods
Inclusion and Exclusion Criteria
The inclusion criteria were: (i) patients aged between 35 and 85 years; and (ii) advanced primary or secondary osteoarthritis of the knee requiring TKA. Exclusion criteria were: (i) patients with existing metal implants or occupational exposure to toxic metals in order to rule out additional sources of metal ion exposure; (ii) patients with a history of hypersensitivity reaction to metals; and (iii) patients with severe renal insufficiency or any other medical condition that would impair their participation in this study.
Patients
Between March 2010 and December 2010, we included 25 patients who were allocated for primary TKA at our institution in this prospective single‐center study. Patients were recruited of a consecutive cohort of 32 patients who were allocated for primary TKA at our institution and who fulfilled the inclusion criteria. Figure 1 illustrates patient enrolment. Seven patients denied participation in the study before study enrolment. From the remaining 25 patients, three patients had to be excluded from metal ion analysis due to incomplete data as they denied blood sampling at one of the follow‐up visits. One patient had to be excluded from clinical analysis due to a missing questionnaire. The study was approved by the local ethics committee (No S‐056/2010) and performed in accordance with the Helsinki Declaration. Written informed consent was obtained by every patient before study inclusion.
Figure 1.
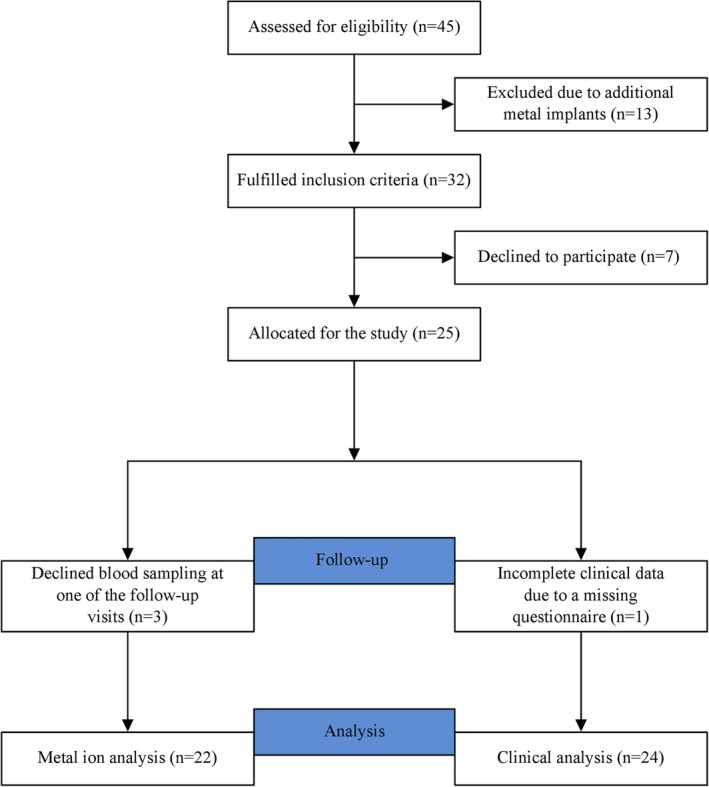
The flowchart illustrates patient enrolment.
Surgery
TKA was performed with a cemented total knee replacement (P.F.C. ® SIGMA® Total Knee System, DePuy Orthopedics Inc., Warsaw, USA). A posterior cruciate ligament retaining femoral component was used in 19 patients and a cruciate substituting femoral component was used in four patients. A cemented all‐polyethylene resurfacing of the patella was performed in six patients. A fixed bearing, highly cross‐linked polyethylene insert was used in all patients. In two patients an intra‐operative decision was made to use a semi‐constrained total knee replacement (P.F.C. ® SIGMA® TC3 Knee System DePuy Orthopedics Inc., Warsaw, USA) due to insufficiency of the lateral collateral ligament. The femoral components were made of cast CoCr28Mo6 alloy and the tibial components consisted of wrought Ti6Al4V alloy. TKA was performed according to the manufacturer's instructions using a medial parapatellar approach.
Outcome Measures
Metal Ion Measurements
The primary outcome measures were whole blood metal ion concentrations of cobalt, chromium, molybdenum, and titanium that were measured at each follow‐up interval. Whole blood metal ion analysis was performed preoperatively and at 1 week, 6 weeks, 3 months, 6 months, and 12 months postoperatively. Metal ion concentrations were measured using high‐resolution inductively coupled plasma‐mass spectrometry (HR‐ICP‐MS, Element 2, Thermo Fisher Scientific, Bremen, Germany). Blood samples were collected using a blood collection system intended for trace metal ion analysis (Sarstedt, Nuembrecht, Germany; Refs. 58.1162.600 and 01.1604.400). All blood samples were stored at −20°C and analyzed at the same time in order to minimize calibration errors of the spectrometer. Before analysis, the samples were digested with high‐purity nitric acid (HNO3) and hydrogen peroxide under clean‐room conditions in a high‐pressure microwave autoclave (UltraClave II, Milestone, Bergamo, Italy)30, 31. Metal ion measurements were repeated three times in every sample and mean values of metal ion concentrations for each sample were calculated. Detection limits of 0.005 μg/L for cobalt, 0.02 μg/L for chromium, 0.01 μg/L for molybdenum, and 0.06 μg/L for titanium have been established for this method30. In addition, serum creatinine values were measured for each patient in order to rule out severe renal insufficiency.
American Knee Society Score (AKSS) and the Oxford Knee Score (OKS)
Secondary outcome parameters were the clinical and functional outcome after TKA that were assessed using patient reported outcome measures. The clinical and the functional AKSS and the OKS were evaluated at each follow‐up. Both the clinical and the functional AKSS are ranging from 0 (worst) to 100 (best) while the OKS is ranging from 0 (worst) to 48 (best)32, 33.
Patient Activity Levels
Patients’ estimated annual walking cycles were assessed with a StepWatch™ Activity Monitor (Orthocare Innovations LLC, Edmonds, WA, USA) 1‐year after surgery by measuring the number of walking cycles per day over a period of 2 weeks. The estimated annual walking cycles were obtained by multiplying the measured mean daily walking cycles for each patient by 365.
Radiological Outcome Measures
Anteroposterior and lateral radiographs of the operated knee were evaluated postoperatively and at 12‐month follow‐up with regard to implant alignment and signs of periprosthetic radiolucent lines and osteolysis. Mean postoperative femorotibial angle and mean varus/valgus orientation of the tibial component were measured on anteroposterior radiographs of the knee.
Statistical Methods
Statistical analysis was performed using the software SPSS® (version 23.0; SPSS IBM Corp., Chicago, IL, USA). Data were evaluated descriptively as arithmetic mean, standard deviation, minimum, and maximum. Analysis of variance for repeated measures (ANOVA) with post hoc Bonferroni‐correction for multiple comparisons was performed, in order to compare the differences in mean metal ion concentrations and clinical scores at each follow‐up. Correlation between blood cobalt ion levels and clinical variables (age, gender, AKSS, OKS, body mass index [BMI], and number of walking cycles) was assessed using the Spearman rank correlation coefficient and multiple linear regression analysis. All tests were two‐sided and a P‐value < 0.05 was considered significant.
Results
General Results
A total of 25 patients (13 male and 12 female patients) were included in the present study. The mean age of the patients at time of surgery was 64.7 years (range, 42–81 years). Indication for TKA was primary osteoarthritis in 22 patients (88%) and post‐traumatic osteoarthritis in three patients (12%). Mean BMI before surgery was 31.6 kg/m2 (range, 19–46 kg/m2). Mean creatinine values were 0.99 mg/dl (range, 0.8–1.6 mg/dl). No patient of the study cohort had to be excluded due to severe renal insufficiency.
Results of Metal Ion Analysis
Results of metal ion analysis are summarized in Table 1. We found no statistically significant difference in mean metal ion concentrations of chromium, titanium, and molybdenum at 1‐year follow‐up compared to preoperative metal ion levels. Mean cobalt ion concentrations significantly increased at all follow‐up intervals compared to preoperative measurements, with a slight decrease between the 3‐month and 6‐month follow‐ups. In order to rule out the influence of a higher constrained implant design (PS and TC3) on metal ion release, a subgroup analysis of the cruciate‐retaining implants (n = 17) was performed, which demonstrated similar results regarding the statistically significant differences of cobalt ion concentrations at 1‐year follow‐up compared to preoperative measurements (P < 0.001). No statistically significant increase of chromium (P = 1.00), molybdenum (P = 1.00), and titanium ion concentrations (P = 0.295) was found in the subgroup analysis at 1‐year follow‐up.
Table 1.
Metal ion measurements in μg/L at the different follow‐up intervals (n = 22)
| Pre‐operation (T0) | 6 weeks post‐op (T1) | 3 months post‐op (T2) | 6 months post‐op (T3) | 1 year post‐op (T4) | P‐value | ||||
|---|---|---|---|---|---|---|---|---|---|
| T0 vs T1 | T0 vs T2 | T0 vs T3 | T0 vs T4 | ||||||
| Cobalt | 0.023 ± 0.04 | 0.110 ± 0.20 | 0.172 ± 0.16 | 0.060 ± 0.07 | 0.279 ± 0.14 | 0.035 | <0.001 | 0.026 | <0.001 |
| 0.006 (0.005–0.141) | 0.037 (0.005–0.896) | 0.105 (0.006–0.603) | 0.025 (0.005–0.229) | 0.243 (0.122–0.615) | |||||
| Chromium | 0.318 ± 0.28 | 0.255 ± 0.18 | 0.123 ± 0.15 | 0.167 ± 0.12 | 0.430 ± 0.49 | 0.384 | 0.008 | 0.024 | 0.353 |
| 0.251 (0.052–1.297) | 0.206 (0.022–0.671) | 0.069 (0.005–0.469) | 0.126 (0.056–0.486) | 0.268 (0.000–1.984) | |||||
| Molybdenum | 0.489 ± 0.26 | 0.545 ± 0.27 | 0.555 ± 0.59 | 0.451 ± 0.27 | 0.622 ± 0.45 | 0.327 | 0.534 | 0.598 | 0.190 |
| 0.421 (0.225–1.221) | 0.489 (0.244–1.479) | 0.404 (0.168–2.967) | 0.355 (0.174–1.164) | 0.456 (0.212–1.827) | |||||
| Titanium | 1.417 ± 1.37 | 1.831 ± 0.84 | 2.346 ± 1.03 | 1.177 ± 0.80 | 1.960 ± 0.98 | 0.244 | 0.020 | 0.549 | 0.133 |
| 1.077 (0.006–4.517) | 1.680 (0.797–3.142) | 2.446 (0.006–3.752) | 1.137 (0.006–4.058) | 1.837 (0.598–4.28) | |||||
Note: Data were expressed as mean, SD (upper row) and median, range (lower row). Analysis of variance for repeated measures (ANOVA) with post hoc Bonferroni‐correction was used for comparison between the intervals. A P‐value < 0.05 was considered statistically significant.
No patient demonstrated cobalt ion levels higher than 1μg/L. Chromium ion levels significantly decreased between the 3‐month and 6‐month follow‐ups compared to preoperative measurements. Two patients showed chromium ion levels above 1μg/L at the 1‐year follow‐up visit. In these patients, there was no evidence of component malposition or signs of mechanical failure on plain radiographs.
Outcome of AKSS and the OKS
Both the AKSS and OKS significantly improved during the course of the study up to the final follow‐up (Table 2).
Table 2.
American Knee Society score (AKSS) and the Oxford Knee score (OKS) as measured at the different follow‐up intervals (n = 24)
| Mean ± SD (Range) | Pre‐operation (T0) | 6 weeks post‐op (T1) | 3 months post‐op (T2) | 6 months post‐op (T3) | 1 year post‐op (T4) | P‐value | |||
|---|---|---|---|---|---|---|---|---|---|
| T0 vs T1 | T0 vs T2 | T0 vs T3 | T0 vs T4 | ||||||
| Clinical (AKSS) | 32.5 ± 11.8 (13–55) | 70.6 ± 18.9 (32–92) | 78.3 ± 15 (37–99) | 82.6 ± 15.1 (28–95) | 84.8 ± 13.5 (30–100) | <0.001 | <0.001 | <0.001 | <0.001 |
| Functional (AKSS) | 41.7 ± 14.2 (5–80) | 45.8 ± 19.7 (15–90) | 64.8 ± 14.5 (35–90) | 74.4 ± 20.9 (15–100) | 78.6 ± 21.1 (15–100) | 0.394 | <0.001 | <0.001 | <0.001 |
| Oxford knee Score (OKS) | 18.9 ± 6.9 (9–34) | 31.3 ± 9 (7–44) | 33 ± 8.7 (7–44) | 39.1 ± 8 (6–48) | 39.7 ± 8.5 (5–40) | <0.001 | <0.001 | <0.001 | <0.001 |
Note: Data were expressed as mean, standard deviation (SD) and range. Analysis of variance for repeated measures (ANOVA) with post hoc Bonferroni‐correction was used for comparison between the intervals. A P‐value < 0.05 was considered statistically significant.
Outcome of Patients Activity Levels
Mean annual walking cycles were 1,566,219 (range, 776,776–2,484,920). Multivariate regression analysis showed no correlation between blood cobalt ion concentrations at 12‐months follow‐up and gender (Beta = −0.17, P = 0.552), age at time of surgery (Beta = −0.17, P = 0.613), BMI (Beta = −0.38, P = 0.341), number of walking cycles (Beta = 0.08, P = 0.788), or OKS (Beta = −0.45, P = 0.427).
Radiological Outcome
Postoperative radiographs demonstrated well‐aligned TKAs in all patients. Mean postoperative femorotibial angle was 176.4 degrees (range, 174–179; SD, 1.5 degrees). Mean varus/valgus orientation of the tibial component was 90.2 degrees (range, 89–93; SD, 0.97 degrees). No signs of osteolysis or implant loosening were detected at 1‐year follow‐up (Fig. 2).
Figure 2.
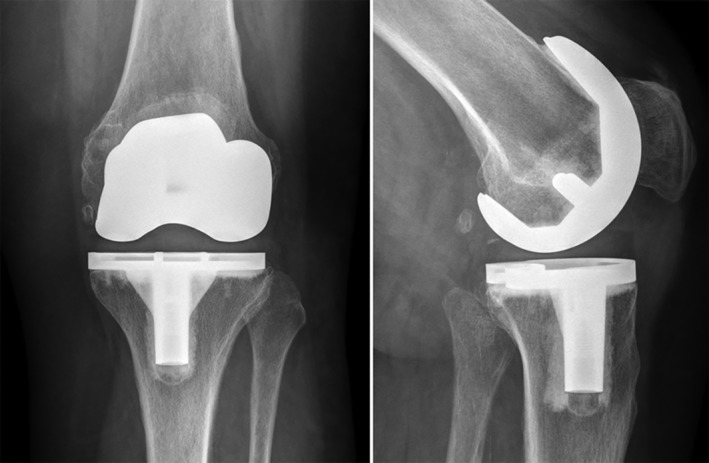
The radiograph shows the x‐ray images of the left knee of a 72‐year‐old female patient at 1‐year follow‐up demonstrating a well‐aligned implant position.
Discussion
Blood metal ion analysis has proved to be a viable tool in the follow‐up of patients with metal‐on‐metal total hip arthroplasties in order to detect excessive metal ion release associated with increased wear or corrosion. In TKA, wear related problems have been typically reported with regard to damage at the polyethylene insert. Although retrieval studies have recently demonstrated that abrasive and corrosive damages commonly occur at the femoral component of total knee replacements, little is known about the metal ion exposure in patients with well‐functioning TKA34. Therefore, the aim of this prospective study was to determine in vivo blood metal ion levels in patients following primary TKA and to investigate potential risk factors for increased metal ion release in these patients.
We found no statistically significant difference in mean metal ion concentrations of chromium, titanium, and molybdenum at 1‐year follow‐up compared to preoperative metal ion levels. Mean cobalt ion concentration significantly increased 1‐year after surgery. However, absolute cobalt ion concentrations were low and no patient demonstrated cobalt ion levels higher than 1 μg/L at the 1‐year follow‐up visit. This is in contrast to the findings of a cross‐sectional study in which elevated metal ion levels were found 5–7 years after primary total knee replacement with median chromium ion concentrations of 0.92 μg/L and cobalt ion concentrations of 3.28 μg/L.35 Postler et al. reported elevated chromium levels 1‐year after TKA with 11% of patients in this cohort demonstrating chromium ion concentrations above 2 μg/L, which is considered to be a potentially critical value for metal‐on‐metal hip arthroplasties according to current guidelines36, 37. In another study by Lons et al., a significant increase in blood metal ion concentrations of cobalt, chromium, and titanium was found 1‐year after primary TKA with mean ion levels of 1.27 μg/L, 1.41 μg/L, and 4.08 μg/L, respectively38. We could not confirm these findings with mean cobalt, chromium, and titanium levels of 0.28 μg/L, 0.43 μg/L, and 1.96 μg/L at 1‐year follow‐up. Clinical results demonstrated significant functional improvement at short‐term follow‐up in our study, which were comparable to those reported in the literature2. No influence of clinical scores, BMI, or patient activity levels on blood metal ion concentrations was observed, which is in accordance to the findings of other studies investigating blood metal ion release of total knee replacements35, 38.
Although wear in TKA primarily consists of polyethylene debris, metal ions and particles are potentially released by well‐functioning TKAs. A retrieval analysis of 52 total knee replacements demonstrated that metal loss at the articulating surface of the femoral components caused by scratching damage and corrosion was present in 98% of the retrievals34. Kretzer et al. investigated polyethylene and metal wear products released by TKAs in a simulator study and found that 12% by weight of the wear was metallic29. The main mechanisms of metal ion generation in TKA are mechanical wear and corrosion. Abrasive damage at the articulating surface of the femoral component leads to the generation of metal debris, and the electrochemical degradation of these wear particles ultimately results in the formation of metal ions. In addition, metal ions can be released from the implant bulk by different corrosion mechanisms such as inflammatory cell‐induced corrosion (ICIC) or mechanically assisted crevice corrosion (MACC) at modular taper junctions39, 40. The clinical effects of accumulating metal wear products and metal ions in the periprosthetic environment of total knee replacements are still not completely understood. Metal ions are able to influence both the immune system and bone metabolism, which might result in periprosthetic osteolysis, implant loosening, or adverse local tissue reactions41. Reports of pseudotumor formation after total knee replacements are rare and have been usually associated with extensive metal wear due to full‐thickness damage of the polyethylene insert or taper corrosion of modular total knee megaprostheses42, 43, 44.
There are limitations to the present study that have to be mentioned. The relatively small sample size of 25 patients has to be considered as the main limitation of the study. This was mainly attributed to the exclusion criterion regarding additional metal implants or history of metal hypersensitivity reactions, which led to the exclusion of patients during the recruitment period. However, due to the longitudinal study design and the high standardization and accuracy of the methodology used for metal ion analysis, we consider our results to be representative for larger patient cohorts. In addition, the follow‐up period was short and a longer follow‐up duration may influence metal ion concentrations. However, other studies demonstrated that metal ion levels are supposed to be consistent over time in patients with well‐functioning TKA38, 45, 46, and simulator studies have shown that the kinetics of metal ion release of TKA seem to differ from those of metal‐on‐metal total hip arthroplasties29, in which the highest wear rates occur during the running‐in phase in the first postoperative year followed by a decreased steady‐state wear afterwards. In contrast, metal‐on‐polyethylene articulations in well‐functioning TKA are supposed to steadily release metal wear products without alteration in wear progression, which is mainly attributed to a distinct mode of lubrications and the viscoelasticity of the polyethylene, when compared to metal‐on‐metal articulations29. Therefore, we believe that the 1‐year follow‐up of this study design is representative for longer follow‐up periods, although it would be interesting to investigate the kinetics of metal ion release of well‐functioning TKA with longer follow‐up intervals in future studies.
Conclusion
In conclusion, we found that cobalt ion levels significantly increased 1‐year after surgery compared to preoperative measurements in this cohort of patients with well‐functioning primary total knee replacements. However, absolute metal ion levels were relatively low and no patient demonstrated cobalt ion levels above 1 μg/L. Despite low systemic metal ion concentrations seen in this cohort, the accumulation of metal ions in the periprosthetic environment could play a role in the development of hypersensitivity reactions or implant loosening, and the local effects of increased metal ion concentrations on the long‐term outcome of TKA should be further investigated.
References
- 1. Callaghan JJ, Beckert MW, Hennessy DW, Goetz DD, Kelley SS. Durability of a cruciate‐retaining TKA with modular tibial trays at 20 years. Clin Orthop Relat Res, 2013, 471: 109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Joint Registry 16th Annual Report 2019 ‐ National Joint Registry for England, Wales, Northern Ireland and the Isle of Man Available from: https://reports.njrcentre.org.uk/Portals/0/PDFdownloads/NJR%2016th%20Annual%20Report%202019.pdf.
- 3. Cadosch D, Chan E, Gautschi OP, Filgueira L. Metal is not inert: role of metal ions released by biocorrosion in aseptic loosening–current concepts. J Biomed Mater Res A, 2009, 91: 1252–1262. [DOI] [PubMed] [Google Scholar]
- 4. Jiranek WA, Machado M, Jasty M, et al Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am, 1993, 75: 863–879. [DOI] [PubMed] [Google Scholar]
- 5. Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of three human monocytic cell lines to challenge with polyethylene particles of known size and dose. J Mater Sci Mater Med, 2001, 12: 249–258. [DOI] [PubMed] [Google Scholar]
- 6. Stea S, Visentin M, Granchi D, et al Cytokines and osteolysis around total hip prostheses. Cytokine, 2000, 12: 1575–1579. [DOI] [PubMed] [Google Scholar]
- 7. Cadosch D, Gautschi OP, Chan E, Simmen HP, Filgueira L. Titanium induced production of chemokines CCL17/TARC and CCL22/MDC in human osteoclasts and osteoblasts. J Biomed Mater Res A, 2010, 92: 475–483. [DOI] [PubMed] [Google Scholar]
- 8. Thompson GJ, Puleo DA. Effects of sublethal metal ion concentrations on osteogenic cells derived from bone marrow stromal cells. J Appl Biomater, 1995, 6: 249–258. [DOI] [PubMed] [Google Scholar]
- 9. Yao J, Cs‐Szabó G, Jacobs JJ, Kuettner KE, Glant TT. Suppression of osteoblast function by titanium particles. J Bone Joint Surg Am, 1997, 79: 107–112. [DOI] [PubMed] [Google Scholar]
- 10. Lean JM, Murphy C, Fuller K, Chambers TJ. CCL9/MIP‐1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem, 2002, 87: 386–393. [DOI] [PubMed] [Google Scholar]
- 11. Nakamura ES, Koizumi K, Kobayashi M, et al RANKL‐induced CCL22/macrophage‐derived chemokine produced from osteoclasts potentially promotes the bone metastasis of lung cancer expressing its receptor CCR4. Clin Exp Metastasis, 2006, 23: 9–18. [DOI] [PubMed] [Google Scholar]
- 12. Bi Y, Van De Motter RR, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg Am, 2001, 83: 501–508. [DOI] [PubMed] [Google Scholar]
- 13. Greenfield EM, Bi Y, Ragab AA, Goldberg VM, Van De Motter RR. The role of osteoclast differentiation in aseptic loosening. J Orthop Res, 2002, 20: 1–8. [DOI] [PubMed] [Google Scholar]
- 14. Cadosch D, Chan E, Gautschi OP, Meagher J, Zellweger R, Filgueira L. Titanium IV ions induced human osteoclast differentiation and enhanced bone resorption in vitro. J Biomed Mater Res A, 2009, 91: 29–36. [DOI] [PubMed] [Google Scholar]
- 15. Martin SF. T lymphocyte‐mediated immune responses to chemical haptens and metal ions: implications for allergic and autoimmune disease. Int Arch Allergy Immunol, 2004, 134: 186–198. [DOI] [PubMed] [Google Scholar]
- 16. Hallab NJ, Caicedo M, Finnegan A, Jacobs JJ. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg, 2008, 3: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Streich NA, Breusch SJ, Schneider U. Serum levels of interleukin 6 (IL‐6), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) and elastase in aseptic prosthetic loosening. Int Orthop, 2003, 27: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Taki N, Tatro JM, Lowe R, Goldberg VM, Greenfield EM. Comparison of the roles of IL‐1, IL‐6, and TNFalpha in cell culture and murine models of aseptic loosening. Bone, 2007, 40: 1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Faschingbauer M, Renner L, Boettner F. Allergy in total knee replacement. Does it exist?: review article. HSS J, 2017, 13: 12–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Middleton S, Toms A. Allergy in total knee arthroplasty: a review of the facts. Bone Joint J, 2016, 98‐B: 437–441. [DOI] [PubMed] [Google Scholar]
- 21. Munch HJ, Jacobsen SS, Olesen JT, et al The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish knee arthroplasty register. Acta Orthop, 2015, 86: 378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eliaz N. Corrosion of metallic biomaterials: a review. Materials (Basel), 2019, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pourzal R, Lundberg HJ, Hall DJ, Jacobs JJ. What factors drive taper corrosion? J Arthroplasty, 2018, 33: 2707–2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Collier JP, Surprenant VA, Jensen RE, Mayor MB. Corrosion at the interface of cobalt‐alloy heads on titanium‐alloy stems. Clin Orthop Relat Res, 1991: 305–312. [PubMed] [Google Scholar]
- 25. Gilbert JL, Buckley CA, Jacobs JJ. In vivo corrosion of modular hip prosthesis components in mixed and similar metal combinations. The effect of crevice, stress, motion, and alloy coupling. J Biomed Mater Res, 1993, 27: 1533–1544. [DOI] [PubMed] [Google Scholar]
- 26. Koh J, Berger A, Benhaim P. An overview of internal fixation implant metallurgy and galvanic corrosion effects. J Hand Surg Am, 2015, 40: 1703–1710 1710. e1‐4. [DOI] [PubMed] [Google Scholar]
- 27. Cadosch D, Chan E, Gautschi OP, Simmen HP, Filgueira L. Bio‐corrosion of stainless steel by osteoclasts–in vitro evidence. J Orthop Res, 2009, 27: 841–846. [DOI] [PubMed] [Google Scholar]
- 28. Jacobs JJ, Gilbert JL, Urban RM. Corrosion of metal orthopaedic implants. J Bone Joint Surg Am, 1998, 80: 268–282. [DOI] [PubMed] [Google Scholar]
- 29. Kretzer JP, Reinders J, Sonntag R, et al Wear in total knee arthroplasty—just a question of polyethylene? Metal ion release in total knee arthroplasty. Int Orthop, 2014, 38: 335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Krachler MHC, Kretzer JP. Validation of ultratrace analysis of co, Cr, Ni and Mo in whole blood, serum and urine using ICP‐SMS. J Anal at Spectrom, 2009, 24: 605–610. [Google Scholar]
- 31. Omlor GW, Kretzer JP, Reinders J, et al In vivo serum titanium ion levels following modular neck total hip arthroplasty–10 year results in 67 patients. Acta Biomater, 2013, 9: 6278–6282. [DOI] [PubMed] [Google Scholar]
- 32. Dawson J, Fitzpatrick R, Murray D, Carr A. Questionnaire on the perceptions of patients about total knee replacement. J Bone Joint Surg Br, 1998, 80: 63–69. [DOI] [PubMed] [Google Scholar]
- 33. Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the knee society clinical rating system. Clin Orthop Relat Res, 1989: 13–14. [PubMed] [Google Scholar]
- 34. Arnholt CM, MacDonald DW, Malkani AL, et al Corrosion damage and wear mechanisms in long‐term retrieved CoCr femoral components for total knee arthroplasty. J Arthroplasty, 2016, 31: 2900–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Luetzner J, Krummenauer F, Lengel AM, Ziegler J, Witzleb WC. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res, 2007, 461: 136–142. [DOI] [PubMed] [Google Scholar]
- 36. Hannemann F, Hartmann A, Schmitt J, et al European multidisciplinary consensus statement on the use and monitoring of metal‐on‐metal bearings for total hip replacement and hip resurfacing. Orthop Traumatol Surg Res, 2013, 99: 263–271. [DOI] [PubMed] [Google Scholar]
- 37. Postler A, Beyer F, Lutzner C, Tille E, Lutzner J. Similar outcome during short‐term follow‐up after coated and uncoated total knee arthroplasty: a randomized controlled study. Knee Surg Sports Traumatol Arthrosc, 2018, 26: 3459–3467. [DOI] [PubMed] [Google Scholar]
- 38. Lons A, Putman S, Pasquier G, Migaud H, Drumez E, Girard J. Metallic ion release after knee prosthesis implantation: a prospective study. Int Orthop, 2017, 41: 2503–2508. [DOI] [PubMed] [Google Scholar]
- 39. Arnholt CM, MacDonald DW, Tohfafarosh M, et al Mechanically assisted taper corrosion in modular TKA. J Arthroplasty, 2014, 29: 205–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gilbert JL, Sivan S, Liu Y, Kocagöz SB, Arnholt CM, Kurtz SM. Direct in vivo inflammatory cell‐induced corrosion of CoCrMo alloy orthopedic implant surfaces. J Biomed Mater Res A, 2015, 103: 211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cooper HJ, Della Valle CJ, Berger RA, et al Corrosion at the head‐neck taper as a cause for adverse local tissue reactions after total hip arthroplasty. J Bone Joint Surg Am, 2012, 94: 1655–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Craig R, Vlychou M, McCarthy CL, Gibbons CLMH, Athanasou NA. Metal wear‐induced pseudotumour following an endoprosthetic knee replacement for Ewing sarcoma. Skeletal Radiol, 2017, 46: 967–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harvie P, Torres‐Grau J, Beaver RJ. Common peroneal nerve palsy associated with pseudotumour after total knee arthroplasty. Knee, 2012, 19: 148–150. [DOI] [PubMed] [Google Scholar]
- 44. McMaster WC, Patel J. Adverse local tissue response lesion of the knee associated with Morse taper corrosion. J Arthroplasty, 2013, 28: 375.e5–375.e8. [DOI] [PubMed] [Google Scholar]
- 45. Garrett S, Jacobs N, Yates P, Smith A, Wood D. Differences in metal ion release following cobalt‐chromium and oxidized zirconium total knee arthroplasty. Acta Orthop Belg, 2010, 76: 513–520. [PubMed] [Google Scholar]
- 46. Granchi D, Cenni E, Giunti A, Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. J Bone Joint Surg Br, 2012, 94: 1126–1134. [DOI] [PubMed] [Google Scholar]


