Abstract
Objectives
There is no consensus on whether giving adjuvant concurrent chemoradiotherapy (CCRT) is more effective than adjuvant radiotherapy (RT) alone in patients with early stage cervical cancer and intermediate-risk factor(s). The purpose of this study was to evaluate survival difference according to adjuvant treatment in the intermediate-risk group.
Methods
From 2000 to 2014, the medical records of patients with stage IB–IIA cervical cancer and a history of radical hysterectomy with pelvic lymph node dissection, followed by pelvic RT at a dose ≥40 Gy were retrospectively reviewed. Among these, 316 patients with one or more intermediate-risk factor(s) and no high-risk factors were included. The criteria defined the intermediate-risk group as those patients with any of the following intermediate-risk factors: lymphovascular space involvement, over one-half stromal invasion, or tumor size ≥4 cm.
Results
The median follow-up duration was 70 months (range: 3–203 months). According to adjuvant treatment (adjuvant RT alone vs. adjuvant CCRT), the 5-year recurrence-free survival rates (90.8% vs. 88.9%, p=0.631) and 5-year overall survival rates (95.9% vs. 91.0%, p=0.287) did not show a significant difference in patients with any of the intermediate-risk factors. In multivariate analysis, a distinct survival difference according to adjuvant treatment was not found regardless of the number of risk factors.
Conclusion
The present study showed that giving RT together with chemotherapy is not more effective than RT alone for stage IB–IIA cervical cancer patients with intermediate-risk factor(s).
Trial Registration
ClinicalTrials.gov Identifier: NCT01101451
Keywords: Cervical Cancer, Adjuvant Radiotherapy, Risk Factor, Survival Analysis
INTRODUCTION
Cervical cancer is the fourth most frequent cancer in women and the eighth most commonly occurring type of cancer overall. Most patients with stage IB–IIA cervical cancer, according to the International Federation of Gynecology and Obstetrics (FIGO) staging system, are treated by radical hysterectomy with pelvic lymph node dissection (PLND). After surgical treatment, adjuvant radiotherapy (RT) or concurrent chemoradiotherapy (CCRT) is recommended according to the presence of risks factors on histopathologic examination. Among these, several risk factors, including parametrial invasion, positive resection margin, and lymph node metastasis are defined as high-risk factors. These factors are associated with a higher rate of recurrence (35%–40%), requiring adjuvant CCRT [1,2]. Conversely, isolated intermediate-risk factors such as lymphovascular space involvement (LVSI), large tumor size, or deep stromal invasion (DSI) do not significantly increase recurrence rate. However, when combined, the risk of recurrence increases to 15%–20% [3,4,5,6,7,8]. In consequence, the prognostic significance of intermediate-risk factors and the appropriate management of these patients remain controversial [9,10,11,12].
In order to solve this controversy, several aspects warrant revision [10,11,12,13,14,15]. First, whether CCRT is beneficial for the intermediate-risk group. Second, whether the eligibility criteria used to define intermediate-risk accurately select those patients with the highest risk of relapse. Furthermore, since no randomized prospective trials have been reported that compare outcomes of adjuvant RT with those of CCRT in patients with intermediate-risk factor(s), no standard criteria are universally accepted to define distinct risk groups among these patients.
In this background, the Gynecologic Oncology Group (GOG) has started randomized phase III trial studies to compare the benefit of adjuvant RT combined with chemotherapy with that of RT alone in patients with stage I–II cervical cancer, who have previously undergone surgery (NCT01101451). The purpose of this study was to determine whether the combination of RT with chemotherapy can significantly improve survival outcomes when compared with RT alone in stage IB–IIA cervical cancer patients with intermediate-risk factor(s).
METERIALS AND METHODS
1. Patients
Medical records of 897 patients registered from 2000 to 2014 in the Samsung Medical Center (n=409) and the Asan Medical Center (n=478), the largest general hospitals in South Korea that cover a nation-wide representative population, were retrospectively reviewed. Inclusion criteria were as follows: patients with histological diagnosis of squamous cell carcinoma, adenocarcinoma, or adenosquamous cell carcinoma of the uterine cervix; FIGO stage IB–IIA disease; no history of neoadjuvant chemotherapy; and a history of radical hysterectomy with PLND, followed by pelvic RT at a dose ≥40 Gy. Among these, patients with high-risk factors including pelvic lymph node metastasis, parametrial invasion, and/or positive resection margin were excluded. Finally, 316 patients with intermediate-risk factor(s) were analyzed.
Cervical cancer was staged according to the updated 2009 FIGO staging system using physical examination, chest X-ray, and abdominopelvic computed tomography (CT), or magnetic resonance imaging (MRI) findings. Cystoscopy or colonoscopy were respectively performed if bladder or rectal involvement was suspected. Tumor size was determined by clinical palpation, inspection, or measurement of the largest diameter of the tumor using imaging modalities such as CT or MRI.
2. Histologic finding
Pathological reports including histological description, DSI, LVSI, number of dissected and positive lymph nodes, parametrial involvement, and tumor invasion of the resection margin were reviewed. DSI was measured in reference to the fractional thickness of the cervix, which was described as a percentage.
The intermediate-risk group was defined as those patients with any of the following intermediate-risk factor(s): LVSI, over one-half stromal invasion, or tumor size ≥4 cm.
3. Adjuvant treatment and surveillance
Adjuvant treatment was determined by the physicians according to the guidelines for cervical cancer treatment of each institution. Postoperative RT was performed in the intermediate risk group, but chemotherapy was added depending on the physician's preference. Adjuvant RT started within 4–6 weeks after surgery using 3-dimensional conformal RT. The gross tumor volume included residual gross tumor or metastatic pelvic lymph nodes and clinical target volume (CTV) including common iliac vessels, external and internal iliac vessels, presacral area, parametrium, and upper vagina, according to the RTOG CTV guideline for whole pelvis RT. And field margin was generated with a 1.5cm expansion of the CTV in all directions, and was then modified considering the block margin (superior; L5/S1 junction, inferior; 2 cm below from stump or lower margin of obturator foramen, lateral; 1.5–2.0 cm margin from the widest portion of pelvic brim, anterior; bisecting line of symphysis pubis, posterior; bisecting line of S2–3). The prescription policy was to deliver at least 97% of the prescribed dose to 95% of the CTVs. Treatment planning for X-ray, Pinnacle treatment planning system, version 9.2 (Royal Phillips Electronics, Miami, FL, USA) was used to calculate the dose distributions. The median radiation dose was 50.4 Gy, ranging from 44.0 Gy in 22 fractions to 50.4 Gy in 28 fractions (daily fractions of 1.8–2.0 Gy over 4.5–6 weeks, 5 fractions per week). Intracavitary brachytherapy was indicated for patients with close (≤2 mm) or positive vaginal resection margin with total dose of 18 Gy in 6 fractions. In current study, 28 patients were treated with intracavitary brachytherapy. For CCRT, the cisplatin-based concurrent chemotherapy regimens consisted of weekly cisplatin for 6 cycles (n=53) or 5-fluorouracil and cisplatin every 3 weeks for 2 or 3 cycles (n=20).
Treatment-related complications were evaluated using the Common Terminology Criteria for Adverse Events version 4.0.
4. Statistical analysis
The primary endpoint of this study was comparison of recurrence-free survival (RFS) and overall survival (OS) according to adjuvant treatment. Loco-regional recurrence was defined as recurrence in the pelvis, including the vaginal stump and the pelvic lymph node area below the aortic bifurcation. RFS was defined as the time from surgery to the date of the first documented recurrence or the latest follow-up. OS was defined as the time from surgery to death from any cause or the latest documented follow-up.
To compare clinicopathologic characteristics and treatment-related complications according to adjuvant treatment, χ2 or Fisher's exact tests were used. Survival rates were estimated using the Kaplan–Meier method and were compared using log-rank tests. Cox proportional hazard regression analysis was used to determine independent prognostic factors; p≤0.05 was considered to be statically significant for 2-tailed tests. Statistical analysis was performed using the SPSS software, standard version 24.0 (IBM Corp., Armonk, NY, USA).
Results
1. Patients' characteristics
The clinicopathologic characteristics of the enrolled patients are described in Table 1. The median age of the population was 49 years (range: 16–76 years), and most patients had stage IB disease (88.6%) and squamous cell histologic type (73.4%). Among patients with any of the intermediate-risk factor(s), 243 (76.9%) received adjuvant RT alone and 73 (23.1%), adjuvant CCRT.
Table 1. Clinicopathologic characteristics according to adjuvant treatment.
| Characteristics | Total (n=316) | RT (n=243) | CCRT (n=73) | p-value | |
|---|---|---|---|---|---|
| Age (yr) | 49 (16–76) | 49 (16–75) | 48 (28–76) | 0.775 | |
| Stage | 0.774 | ||||
| IB | 280 | 216 (88.9) | 64 (87.7) | ||
| IIA | 36 | 27 (11.1) | 9 (12.3) | ||
| Histology | 0.355 | ||||
| Squamous cell carcinoma | 232 | 183 (75.3) | 49 (67.1) | ||
| Adenocarcinoma | 68 | 48 (19.8) | 20 (27.4) | ||
| Adenosquamous cell carcinoma | 16 | 12 (4.9) | 4 (5.5) | ||
| Tumor size (cm) | 4.0 (0.1–8.5) | 0.199 | |||
| <4 | 155 | 124 (51.0) | 31 (42.5) | ||
| ≥4 | 161 | 119 (49.0) | 42 (57.5) | ||
| LVSI | <0.001 | ||||
| Negative | 170 | 146 (60.1) | 24 (32.9) | ||
| Positive | 146 | 97 (39.9) | 49 (67.1) | ||
| Depth of invasion | 0.616 | ||||
| <50 | 38 | 28 (11.5) | 10 (13.7) | ||
| ≥50 | 278 | 215 (88.5) | 63 (86.3) | ||
| No. of intermediate-risk factor | <0.001 | ||||
| 1 | 92 | 80 (32.9) | 12 (16.4) | ||
| 2 | 179 | 138 (56.8) | 41 (56.2) | ||
| 3 | 45 | 25 (10.3) | 20 (27.4) | ||
Values are presented as median (interquartile range) or number (%).
CCRT, concurrent chemoradiotherapy; LVSI, lymphovascular space involvement; RT, radiotherapy.
According to adjuvant treatment, clinicopathologic characteristics such as age, stage, histologic type, tumor size, and depth of tumor invasion were not significantly different between the adjuvant RT and the adjuvant CCRT groups. The presence of LVSI of tumor and the number of intermediate-risk factors were significantly different between the 2 groups (p<0.001). Patients with multiple intermediate-risk factors were more likely to receive adjuvant CCRT than adjuvant RT alone.
2. Failure patterns and survival outcomes according to adjuvant treatment
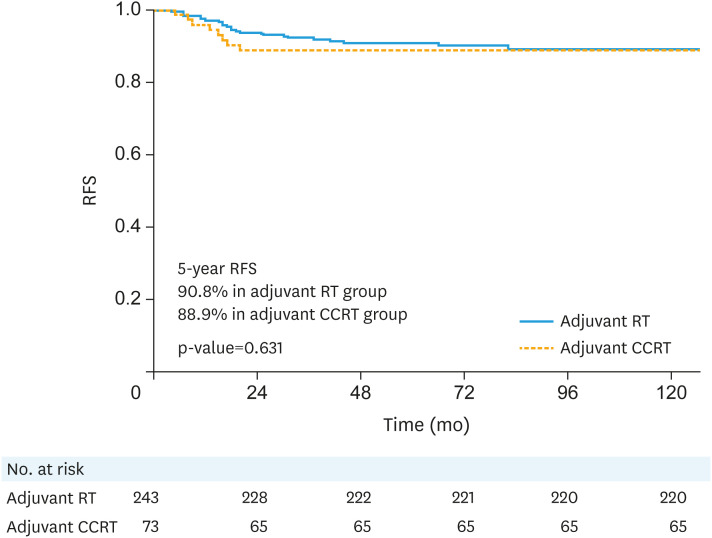
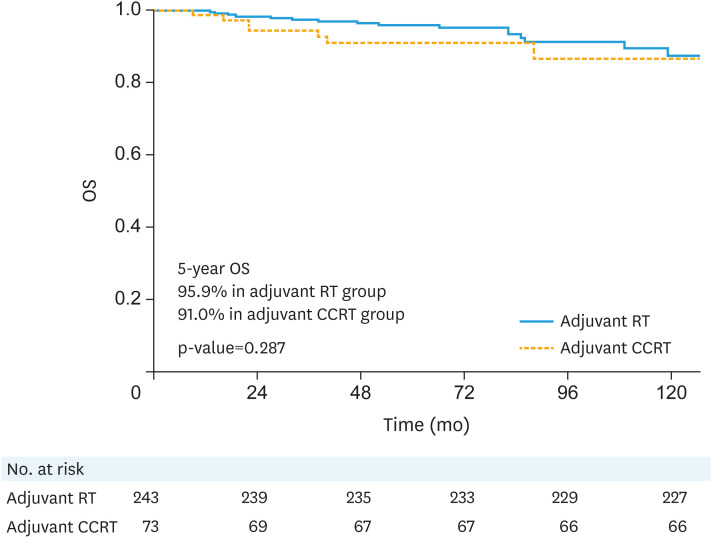
The median follow-up duration was 70 months (range: 3–203 months). Survival outcomes, according to adjuvant treatment, are listed in Table 2. Loco-regional recurrence was found in 3 patients (0.9%); vaginal stump, regional lymph node, and both recurrences were showed in each patient, respectively. And distant metastasis was noted in 28 patients (8.9%), which were the major patterns of failure and the 2 most common sites were the lung (n=14) and para-aortic lymph node (n=6). According to adjuvant treatment, 21 patients (21/243, 8.6%) in adjuvant RT alone group and 7 patients (7/73, 9.6%) in adjuvant CCRT group showed distant metastasis during the follow-up. The 5-year RFS rate was 90.3% and the 5-year OS rate was 94.7% in patients with any of the intermediate-risk factor(s). According to adjuvant treatment (adjuvant RT alone vs. adjuvant CCRT), the 5-year RFS rates (90.8% vs. 88.9%, p=0.631, Fig. 1) and 5-year OS rates (95.9% vs. 91.0%, p=0.287, Fig. 2) of patients with any of the intermediate-risk factor(s) did not show significant differences.
Table 2. Survival outcomes according to adjuvant treatment.
| Characteristics | Total (n=316) | RT (n=243) | CCRT (n=73) | p-value | ||
|---|---|---|---|---|---|---|
| 5-year RFS | 90.3 | 90.8 | 88.9 | 0.631 | ||
| No. of risk factors | ||||||
| 1 (n=92) | 96.6 | 96.1 | 100 | 0.504 | ||
| 2 (n=179) | 86.4 | 86.8 | 85.4 | 0.732 | ||
| 3 (n=45) | 93.0 | 95.8 | 89.5 | 0.734 | ||
| 5-year OS | 94.7 | 95.9 | 91.0 | 0.287 | ||
| No. of risk factors | ||||||
| 1 (n=92) | 97.6 | 98.7 | 90.0 | 0.436 | ||
| 2 (n=179) | 93.1 | 94.3 | 89.4 | 0.239 | ||
| 3 (n=45) | 95.3 | 95.8 | 94.7 | 0.423 | ||
Values are presented as number (%).
CCRT, concurrent chemoradiotherapy; OS, overall survival; RFS, recurrence-free survival; RT, radiotherapy.
Fig. 1. RFS curves according to adjuvant treatment.
CCRT, concurrent chemoradiotherapy; RFS, recurrence-free survival; RT, radiotherapy.
Fig. 2. OS curves according to adjuvant treatment.
CCRT, concurrent chemoradiotherapy; OS, overall survival; RT, radiotherapy.
In univariate analysis, RFS was associated with DSI (p=0.036) and the presence of multiple risk factors (p=0.012), while there was no significantly associated factors for OS. In multivariate analysis including the variables associated with RFS on univariate analysis, DSI (p=0.973) and the presence of multiple risk factors (p=0.083) were not significant prognostic factors for RFS. After adjustment for confounding factors, a distinct survival difference according to adjuvant treatment was not found, regardless of the number of risk factors (Table 3).
Table 3. Survival outcomes in the intermediate-risk group.
| Variable | RFS | OS | |||||
|---|---|---|---|---|---|---|---|
| 5-year | HR (95% CI) | p-value* | 5-year | HR (95% CI) | p-value* | ||
| Age | p=0.990 | 0.997 (0.964–1.031) | 0.866 | p=0.165 | 1.032 (0.991–1.076) | 0.126 | |
| Stage | 0.200 | 0.543 | |||||
| IB | 90.9 | Reference | 94.8 | Reference | |||
| IIA | 86.1 | 1.801 (0.732–4.428) | 94.4 | 0.634 (0.146–2.748) | |||
| Histology | 0.168 | 0.357 | |||||
| Squamous cell carcinoma | 91.5 | Reference | 95.4 | Reference | |||
| Adenocarcinoma/adenosquamous cell carcinoma | 87.5 | 1.685 (0.803–3.535) | 93.1 | 1.507 (0.630–3.606) | |||
| Tumor size (cm) | 0.262 | 0.701 | |||||
| <4 | 89.7 | Reference | 94.9 | Reference | |||
| ≥4 | 91.0 | 0.524 (0.169–1.621) | 94.7 | 0.790 (0.236–2.641) | |||
| Lymphovascular space involvement | 0.605 | 0.950 | |||||
| Negative | 91.5 | Reference | 95.6 | Reference | |||
| Positive | 89.0 | 0.736 (0.230–2.356) | 93.8 | 1.042 (0.291–3.727) | |||
| Depth of invasion | 0.973 | 0.642 | |||||
| <50 | 96.9 | Reference | 96.9 | Reference | |||
| ≥50 | 89.0 | 1.290 (0.333–5.101) | 94.4 | 1.778 (0.157–20.120) | |||
| No. of risk factor(s) | 0.083 | 0.453 | |||||
| Single | 96.6 | Reference | 97.6 | Reference | |||
| Multiple | 87.7 | 4.541 (0.821–25.112) | 93.6 | 1.982 (0.333–11.809) | |||
| Adjuvant treatment | 0.879 | 0.374 | |||||
| Radiotherapy alone | 90.8 | Reference | 95.9 | Reference | |||
| Concurrent chemoradiotherapy | 88.9 | 1.067 (0.464–2.451) | 91.0 | 1.525 (0.601–3.868) | |||
Values are presented as number (%).
CI, confidence interval; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival.
*Multivariate Cox proportional hazard regression test.
3. Toxicities according to adjuvant treatment
During follow-up, 67 patients (21.2%) showed grade 3 or higher treatment-related complications (Table 4). Specifically, 30 patients (12.3%) in RT alone group showed grade 3 or higher treatment-related complications, while 37 patients (50.7%) showed grade 3 or higher treatment-related complications in CCRT group (p<0.001). And hematologic toxicity (16.5%) was the most common type of complication in both RT alone (9.1%) and CCRT (41.1%) groups, respectively (p<0.001). In addition, grade 3 or higher gastrointestinal and genitourinary toxicities were more frequently observed in CCRT group (p<0.001 and p=0.001, respectively).
Table 4. Toxicities according to adjuvant treatment.
| Characteristics | Total (n=316) | RT (n=243) | CCRT (n=73) | p-value | |
|---|---|---|---|---|---|
| ≥Grade 3 toxicities | 67 (21.2) | 30 (12.3) | 37 (50.7) | <0.001 | |
| Hematologic toxicity | 52 (16.5) | 22 (9.1) | 30 (41.1) | <0.001 | |
| Gastrointestinal toxicity | 20 (6.3) | 8 (3.3) | 12 (16.4) | <0.001 | |
| Genitourinary toxicity | 15 (4.7) | 6 (2.5) | 9 (12.3) | 0.001 | |
Values are presented as number (%).
CCRT, concurrent chemoradiotherapy; RT, radiotherapy.
Discussion
Although no universally accepted criteria are available to define distinct risk patterns among cervical cancer patients with intermediate-risk factor(s), GOG's randomized clinical trials define the intermediate-risk group as follows [10]: category 1, positive capillary lymphatic space, deep one-third stromal invasion, and tumor of any size; category 2, positive capillary lymphatic space, middle one-third stromal invasion, and tumor size ≥2 cm; category 3, negative capillary lymphatic space, deep or middle one-third stromal invasion, and tumor size ≥4 cm; and category 4, positive capillary lymphatic space, superficial one-third stromal invasion, and tumor size ≥5 cm. In the current study, we defined the intermediate-risk group as patients with any of the following intermediate-risk factors: LVSI, over one-half stromal invasion, or tumor size ≥4 cm. Even though the GOG's criteria define the intermediate-risk group more strictly, no difference was found between the recurrence rate of the group defined using our criteria (31/316, 9.8%) and that of the GOG (27/230, 11.7%, data not shown), favoring the use of more simple and practical criteria in the clinical setting.
A GOG's randomized clinical trial has already shown that adjuvant RT reduces the risk of recurrence and the risk of progression or death in patients with early stage cervical cancer with intermediate-risk factors [10,11]. Until now, many physicians are reluctant to treat intermediate-risk patients with adjuvant CCRT because of concerns regarding overtreatment. In addition, combined modalities are more likely to result in serious complications. However, as shown in the GOG 92 trial, although recurrence rate was reduced with the use of adjuvant RT, the rate of recurrence was still significant (15%, 21 out of 137 cases) [11]. Ryu et al. [16] showed that a recurrence rate of 17.6% in the RT group was further decreased to 2.2% using cisplatin-based chemotherapy concurrently with RT. The incidence of grade 3–4 hematologic and gastrointestinal toxicities was not significantly different between the RT and cognitive remediation therapy groups (6.1% and 13.4%, respectively; p>0.05). In this study, the definition of intermediate-risk group was determined using the 2-factor model (defined as any 2 or more of the following 3 intermediate-risk factors: LVSI, DSI, and tumor size ≥2 cm). The differences in recurrence rates were higher when they included only those patients who met the criteria (27.3%, 11.4%, and 2.5% in the no-further-treatment, RT, and CCRT group, respectively; p<0.001). However, the statistical significance of the difference in RFS between CCRT and RT was marginal (p=0.09), considering the tendency to prescribe CCRT to patients with relatively higher risk. This result was also supported by a retrospective study that showed better outcomes prescribing CCRT than RT to cervical cancer patients with intermediate-risk factors [17]. But, in this study, 30% of the patients were treated with neoadjuvant chemotherapy, which might have confounded the pathologic findings and ranked some patients with FIGO stage IIB in the intermediate-risk group, when they might actually belong to the high-risk group. Although several studies showed the effectiveness of CCRT over RT alone for the intermediate-risk group, suggesting improved survival with concurrent chemotherapy, the issues on the side effects still remains [18,19]. In retrospective studies which showed that addition of concurrent chemotherapy to postoperative RT might improve survival outcomes, acute grade 3 and 4 hematologic toxicities were more frequently observed in CCRT group (p<0.001), while acute grade 3 and 4 gastrointestinal and chronic toxicities did not differ between the groups [18].
Conversely, other previous studies demonstrated that CCRT did not improve survival outcomes when compared to RT alone in the intermediate-risk group [20,21]. In a retrospective analysis of a nation-wide cohort study examining 6,003 women with stage IB–IIB cervical cancer who underwent radical hysterectomy between 2004 and 2008 in Japan [21], women who received systemic chemotherapy had disease-free survival (DFS; 5-year rate=88.1% vs. 90.2%; adjusted-hazard ratio (HR)=0.98; 95% confidence interval (CI)=0.52–1.83; p=0.94) and cause-specific survival (95.4% vs. 94.8%; adjusted-HR=0.85; 95% CI=0.34–2.07; p=0.71) similar to those who received CCRT on multivariable analysis. Similar results were seen among 329 women with multiple intermediate-risk factors (5-year rates for DFS, chemotherapy vs. concurrent chemo-radiotherapy, 87.1% vs. 90.2%, p=0.86; and cause-specific survival 94.6% vs. 93.4%, p=0.82).
In this current study, we also proved that the 5-year RFS rates (90.8% vs. 88.9%, p=0.631) and 5-year OS rates (95.9% vs. 91.0%, p=0.287) of patients with any of the intermediate-risk factor(s) did not show significant differences according to adjuvant treatment. A distinct survival difference according to adjuvant treatment was not found, regardless of the number of risk factors, in multivariate analysis. Additionally, we showed that distant metastasis (28 patients, 8.9%) was the major patterns of failure in early-stage cervical cancer with intermediate-risk factor(s). According to adjuvant treatment, 21 patients (21/243, 8.6%) in adjuvant RT alone group and 7 patients (7/73, 9.6%) in adjuvant CCRT group showed distant metastasis during the follow-up. There was no role of chemotherapy to prevent disease progression. In aspect of toxicity, 30 patients (12.3%) in RT alone group showed grade 3 or higher treatment-related complications, while 37 patients (50.7%) showed grade 3 or higher treatment-related complications in CCRT group. And hematologic toxicity was the most common type of complications in both groups. Overall grade 3 or higher treatment-related complications were more frequently observed in CCRT group (p<0.001).
Our study had several limitations. First, it was a retrospective study, which might have resulted in selection bias. Second, adjuvant treatment varied according to the physician's preference. Until now, some debates in clinical practice remain regarding whether giving RT together with chemotherapy is more effective than RT alone in patients with early-stage cervical cancer with intermediate-risk factor(s).
In conclusion, the present study showed that giving RT together with chemotherapy is not more effective than RT alone in stage IB–IIA cervical cancer patients with intermediate-risk factor(s). However, to confirm the effect of CCRT in the intermediate-risk group, we must await the results of a prospective study from the GOG (NCT01101451).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: P.W., K.Y.S.
- Data curation: K.H., K.Y.J.
- Formal analysis: K.H.
- Investigation: K.H., K.Y.J.
- Methodology: P.W., K.Y.S.
- Resources: P.W.
- Supervision: P.W.
- Validation: P.W., K.Y.S., K.Y.J.
- Writing - original draft: K.H.
- Writing - review & editing: P.W., K.Y.S.
References
- 1.Lahousen M, Haas J, Pickel H, Hackl A, Kurz C, Ogris H, et al. Chemotherapy versus radiotherapy versus observation for high-risk cervical carcinoma after radical hysterectomy: a randomized, prospective, multicenter trial. Gynecol Oncol. 1999;73:196–201. doi: 10.1006/gyno.1999.5343. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG. Advances in the management of cervical cancer. J Reprod Med. 2000;45:971–978. [PubMed] [Google Scholar]
- 3.Rotman M, John M, Boyce J. Prognostic factors in cervical carcinoma: implications in staging and management. Cancer. 1981;48:560–567. doi: 10.1002/1097-0142(19810715)48:1+<560::aid-cncr2820481320>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 4.Inoue T. Prognostic significance of the depth of invasion relating to nodal metastases, parametrial extension, and cell types. A study of 628 cases with stage IB, IIA, and IIB cervical carcinoma. Cancer. 1984;54:3035–3042. doi: 10.1002/1097-0142(19841215)54:12<3035::aid-cncr2820541236>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990;38:352–357. doi: 10.1016/0090-8258(90)90072-s. [DOI] [PubMed] [Google Scholar]
- 6.Sevin BU, Lu Y, Bloch DA, Nadji M, Koechli OR, Averette HE. Surgically defined prognostic parameters in patients with early cervical carcinoma. A multivariate survival tree analysis. Cancer. 1996;78:1438–1446. doi: 10.1002/(SICI)1097-0142(19961001)78:7<1438::AID-CNCR10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Ho CM, Chien TY, Huang SH, Wu CJ, Shih BY, Chang SC. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol. 2004;93:458–464. doi: 10.1016/j.ygyno.2004.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Van de Putte G, Lie AK, Vach W, Baekelandt M, Kristensen GB. Risk grouping in stage IB squamous cell cervical carcinoma. Gynecol Oncol. 2005;99:106–112. doi: 10.1016/j.ygyno.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 9.Zaino RJ, Ward S, Delgado G, Bundy B, Gore H, Fetter G, et al. Histopathologic predictors of the behavior of surgically treated stage IB squamous cell carcinoma of the cervix. A Gynecologic Oncology Group study. Cancer. 1992;69:1750–1758. doi: 10.1002/1097-0142(19920401)69:7<1750::aid-cncr2820690717>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 11.Rotman M, Sedlis A, Piedmonte MR, Bundy B, Lentz SS, Muderspach LI, et al. A phase III randomized trial of postoperative pelvic irradiation in stage IB cervical carcinoma with poor prognostic features: follow-up of a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 2006;65:169–176. doi: 10.1016/j.ijrobp.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Sartori E, Tisi G, Chiudinelli F, La Face B, Franzini R, Pecorelli S. Early stage cervical cancer: adjuvant treatment in negative lymph node cases. Gynecol Oncol. 2007;107:S170–4. doi: 10.1016/j.ygyno.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Schorge JO, Molpus KL, Koelliker D, Nikrui N, Goodman A, Fuller AF., Jr Stage IB and IIA cervical cancer with negative lymph nodes: the role of adjuvant radiotherapy after radical hysterectomy. Gynecol Oncol. 1997;66:31–35. doi: 10.1006/gyno.1997.4691. [DOI] [PubMed] [Google Scholar]
- 14.Lai CH, Hong JH, Hsueh S, Ng KK, Chang TC, Tseng CJ, et al. Preoperative prognostic variables and the impact of postoperative adjuvant therapy on the outcomes of stage IB or II cervical carcinoma patients with or without pelvic lymph node metastases: an analysis of 891 cases. Cancer. 1999;85:1537–1546. doi: 10.1002/(sici)1097-0142(19990401)85:7<1537::aid-cncr15>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Yang K, Park W, Huh SJ, Bae DS, Kim BG, Lee JW. Clinical outcomes in patients treated with radiotherapy after surgery for cervical cancer. Radiat Oncol J. 2017;35:39–47. doi: 10.3857/roj.2016.01893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryu SY, Park SI, Nam BH, Cho CK, Kim K, Kim BJ, et al. Is adjuvant chemoradiotherapy overtreatment in cervical cancer patients with intermediate risk factors? Int J Radiat Oncol Biol Phys. 2011;79:794–799. doi: 10.1016/j.ijrobp.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Morris M, Eifel PJ, Lu J, Grigsby PW, Levenback C, Stevens RE, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 18.Song S, Song C, Kim HJ, Wu HG, Kim JH, Park NH, et al. 20 year experience of postoperative radiotherapy in IB-IIA cervical cancer patients with intermediate risk factors: impact of treatment period and concurrent chemotherapy. Gynecol Oncol. 2012;124:63–67. doi: 10.1016/j.ygyno.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 19.Okazawa M, Mabuchi S, Isohashi F, Suzuki O, Yoshioka Y, Sasano T, et al. Impact of the addition of concurrent chemotherapy to pelvic radiotherapy in surgically treated stage IB1-IIB cervical cancer patients with intermediate-risk or high-risk factors: a 13-year experience. Int J Gynecol Cancer. 2013;23:567–575. doi: 10.1097/IGC.0b013e31828703fd. [DOI] [PubMed] [Google Scholar]
- 20.Qin AQ, Liang ZG, Ye JX, Li J, Wang JL, Chen CX, et al. Significant efficacy of additional concurrent chemotherapy with radiotherapy for postoperative cervical cancer with risk factors: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2016;17:3945–3951. [PubMed] [Google Scholar]
- 21.Matsuo K, Shimada M, Yokota H, Satoh T, Katabuchi H, Kodama S, et al. Effectiveness of adjuvant systemic chemotherapy for intermediate-risk stage IB cervical cancer. Oncotarget. 2017;8:106866–106875. doi: 10.18632/oncotarget.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]