Abstract
Precision cancer surgery is a system that integrates the accurate evaluation of tumor extension and aggressiveness, precise surgical maneuvers, prognosis evaluation, and prevention of the deterioration of quality of life (QoL). In this regard, nerve-sparing radical hysterectomy has a pivotal role in the personalized treatment of cervical cancer. Various types of radical hysterectomy can be combined with the nerve-sparing procedure. The extent of parametrium and vagina/paracolpium excision and the nerve-sparing procedure are tailored to the tumor status. Advanced magnetic resonance imaging technology will improve the assessment of the local tumor extension. Validated risk factors for perineural invasion might guide selecting treatment for cervical cancer. Type IV Kobayashi (modified Okabayashi) radical hysterectomy combined with the systematic nerve-sparing procedure aims to both maximize the therapeutic effect and minimize the QoL impairment. Regarding the technical aspect, the preservation of vesical nerve fibers is essential. Selective transection of uterine nerve fibers conserves the vesical nerve fibers as an essential piece of the pelvic nervous system comprising the hypogastric nerve, pelvic splanchnic nerves, and inferior hypogastric plexus. This method is anatomically and surgically valid for adequate removal of the parametrial and vagina/paracolpium tissues while preserving the total pelvic nervous system. Local recurrence after nerve-sparing surgery might occur due to perineural invasion or inadequate separation of pelvic nerves cutting through the wrong tissue plane between the pelvic nerves and parametrium/paracolpium. Postoperative management for long-term maintenance of bladder function is as critical as preserving the pelvic nerves.
Keywords: Cervical Cancer, Hysterectomy, Personalized, Quality of Life
INTRODUCTION
Cervical cancer is the fourth most common malignancy in women. An estimated 570,000 new cases worldwide occurred in 2018 [1] and 11,293 new cases were diagnosed in Japan in 2014 [2]. The peak incidence of cervical cancer occurs in young women, with two peaks in some countries: 25-29 years followed by 65+ years in the UK [3], 35–44 years in the US [4], 35–39 years followed by 85+ years in Australia [5], and 35–44 years in Japan [2]. For cervical cancer patients, quality of life (QoL) issues are essential for their posttreatment life over the long term.
Precision medicine is a personalized medical approach for better healthcare, taking into account individual differences in the genes, environment, and lifestyle of each person [6]. Prevention of cervical cancer by targeting the underlying biology of causal human papillomavirus (HPV) infection/integration and HPV-related precancer is a critical part of precision medicine for cervical cancer. However, this topic is not a part of this review. The concept of personalized medicine applied to cervical cancer surgery highlights the need for accurate assessment of the extent of the tumor, prediction of the tumor virulence, technical precision, and prognostic evaluation to tailor adjuvant therapy. Our goal in the surgical treatment of cervical cancer is to maximize the oncological outcome while minimizing the deterioration of a patient's QoL. Ovarian preservation became a standard practice in early-stage cervical cancer after the risk factors for ovarian metastasis was clarified [7,8,9,10]. Some authors have reported surgical methods for the prevention of vaginal shortening [11,12]. It has been suggested that we can reduce the postoperative risk of lower-limb lymphedema by preserving the suprafemoral nodes/circumflex iliac nodes distal to the external iliac nodes [13,14] or by using sentinel node navigated lymphadenectomy [15]. Metastasis to this nodal site is quite rare [16]. The detection rate of the sentinel node in this region is only 0.9% [17]. Removal of this lymph node should not be justified in terms of the oncological and QoL point of view.
Bladder dysfunction was regarded as an almost unavoidable complication after radical hysterectomy. Classical radical hysterectomy by the Latzko method [18] and Okabayashi method [19] result in complete resection of the cardinal ligament including the pelvic splanchnic nerves (PSNs). The Querleu-Morrow type C2 operation [20] might be included in this category of surgical approach. The Kobayashi method (modified Okabayashi radical hysterectomy) [21,22] and type C1 operation are the preferred surgical methods for cervical cancer, in which the neural portion of the cardinal ligament is preserved. In this review, we focus on the tailored use of parametrial/paracolpium excision and pelvic nerve preservation, as well as the possible mechanism of local recurrence after nerve-sparing radical hysterectomy.
PRECISION SURGERY IN CERVICAL CANCER
1. Prediction of pelvic nerve involvement of the tumor
Tumor cells infiltrate the pelvic autonomic nerves via perineural invasion and direct invasion outside the cervix. International Federation of Gynecology and Obstetrics (FIGO) adopted a new 2018 staging system, which allows the preoperative imaging for tumor size, parametrial invasion, and lymph node metastasis and assigning the stage [23]. Modern magnetic resonance imaging (MRI) gives the 3D anatomical information of the hypogastric nerve (HN), the inferior hypogastric plexus (IHP; the pelvic plexus), and even the PSNs [24,25]. MRI techniques for defining the topographical relationship between the tumor and the adjacent pelvic nervous system may be used to guide nerve-sparing radical hysterectomy [26].
Of the recent studies on the biology of the tumor, perineural invasion should be the most relevant for nerve-sparing radical hysterectomy. Perineural invasion is related to lymphovascular space invasion, lymph node metastasis, metastasis to central nervous tissues, and poor prognosis in many cancers, including cervical cancer [27,28,29]. The reported incidence of the perineural invasion in cervical cancer ranges from 7.5% to 35.1% [27]. Perineural invasion is not a passive phenomenon but occurs through the mechanism connecting tumor cells and nerve cells. There is a crosstalk mechanism between tumor cells and nervous cells (tumor nervous connections). Tumor cells and nervous cells influence each other reciprocally and can interact directly or through the signal transduction pathway and the recognition and response of the ligands and receptors [30,31]. A case-series of 17 pelvic malignancies with perineural tumor spread, including one case of cervical cancer, suggested that cancers of the pelvic organs may use splanchnic nerves as conduits and can continue to spread along osseous and muscle nerve branches resulting in muscle and bone metastases [32]. If the preoperative prediction of the presence of perineural invasion were possible, this information will guide selecting the type of treatment. We do not have a validated serum or tissue biomarker for prediction of perineural invasion in cervical cancer. The role of positron emission tomography/computed tomography (PET/CT) scans in the assessment of perineural tumor spread in head and neck cancers has been suggested [33,34]. We need further research on the effectiveness of the combined use of MRI and PET/CT in assessing the perineural spread in cervical cancer.
2. Radical hysterectomy in cervical cancer
Indications of radical hysterectomy for cervical cancer are divergent. National Comprehensive Cancer Network (NCCN) Guidelines 2019 recommends a radical hysterectomy for the new 2018 FIGO stage IB1 (≤2 cm), IB2 (2–4 cm), and stage IIA1, and selected stage IB3 (>4 cm) and IIA2 disease [35]. The Japan Society of Gynecologic Oncology (JSGO) Guidelines 2017 [36] recommends two treatment modalities of radical hysterectomy and concurrent chemoradiotherapy (CCRT) as choices of treatment for stage IB–IIB cervical cancer. It also recommends a nerve-sparing radical hysterectomy for 2008 FIGO stage IB1 (≤4 cm) and IIA1 cervical cancer. A nerve-sparing radical hysterectomy is recommended for selected cases of stage IB2 (>4 cm) and IIA2 disease under the condition that the nerve-sparing procedure does not impair the radicality of surgery.
We need to describe the method of radical hysterectomy that is combined with nerve-sparing procedures. Inadequate surgical removal of cancer increases the risk of tumor recurrence. On the other hand, the radicality of the surgery is related to the risk of postoperative morbidity. The method of radical hysterectomy commonly used in Western countries is the Wertheim operation modified by Meigs [37], and that used in Japan is the Okabayashi operation. The Okabayashi and the Latzko methods are similar in the creation of paravesical and pararectal spaces to separate three components (ventral, lateral, and dorsal part) of parametrial/paracervical tissue. The Okabayashi method uniquely identifies the paravaginal space after the excision of the anterior layer of the vesicouterine ligament (we use the term vesicouterine ligament to indicate the anterior layer of the vesicouterine ligament, and the term vesicovaginal ligament to denote the posterior layer of the vesicouterine ligament throughout this review). We develop the paravaginal space connecting to the paravesical space to dissect the vesicovaginal ligament. This procedure results in the extensive excision of the vagina/paracolpium, conferring high radicality to the surgery. Gitsch and Palmrich have described a modified Latzko method, which uses the resection of the vesicovaginal ligament [38].
Kobayashi modified the Okabayashi radical hysterectomy aiming at the preservation of the pelvic nerves. This method preserves the PSNs in the most dorsal part of the cardinal ligament (lateral parametrium). Postoperative bladder dysfunction was significantly prevented. However, many patients still had various disturbances in urination [39,40]. A decrease in bladder function is related to vaginal excision, as well as the lateral parametrial excision. The cut-off value of vaginal resection for bladder dysfunction was 2 cm [41] and 2.7 cm [42]. The length of vaginal cuff removal <2 cm was suggested to be related to increased local recurrence and decreased survival in stage IB–IIA cervical cancer [43]. The prognostic independency of the length of vaginal resection is controversial, and a report found no association between the length of vaginal resection and survival [44]. This inconsistency seems to be related to the difference of FIGO stages of the target patients and the usage of postoperative radiation. The proportion of stage IB2–IIA2 was 73% in the former study, the proportion of stage IB1 was 73.2% in the latter study, and the radiation usage was more frequent in the latter study. We need to be cautious about restricting the length of vaginal removal within <2 cm in cervical cancer. The difference between the Kobayashi operation and the type C1 operation lies in the dissection of the vesicovaginal ligament. The Kobayashi method uses complete excision, and the type C1 operation does not. This technical difference is considered related to the difference in the extent of vaginal/paracolpium tissue resection.
Modification in the resection of the lateral parametrium is not sufficient for preserving bladder function. By definition, the Meigs operation corresponds to the Piver class III operation, which excises only the medial part of the ventral parametrium (the vesicouterine ligament) [45]. The Piver class IV operation excises the entire tissue surrounding the terminal ureter for extended excision of the vagina and the paracolpium, which corresponds to the Okabayashi-Kobayashi operation. What is a sufficient length of upper vaginal removal is a matter of discussion. From the viewpoint of personalized surgical radicality, we face the following contradictory goals that must be achieved: >2 cm of vaginal cuff resection and preservation of the IHP and the vesical nerve fibers in cervical cancer. A small IB1 (<2 cm) tumor might be sufficiently treated with modified radical hysterectomy or the Querleu-Morrow type B1/B2 operation with limited removal of the vagina and the paracolpium.
3. Rationale for nerve-sparing radical hysterectomy
Nerve-sparing radical hysterectomy was developed to preserve the autonomic nerves innervating the bladder in order to maintain postoperative bladder function as well as rectal and vaginal function. The rationale for nerve-sparing radical hysterectomy is based on the assumption that cervical cancer confined to the cervix has little chance to invade the pelvic nervous system outside the uterine cervix. For maintaining the bladder function, the integrity of both the sympathetic and parasympathetic nerve supply is necessary. We need to preserve the HN, the PSNs, the IHP, and the vesical nerve branches (VNB) of the IHP. Kobayashi conceptualized the total preservation of the pelvic nerves, including the vesical nerve fibers, but without showing the method to preserve the vesical nerve fibers. Since then, various nerve-sparing techniques have been put forth with the intention of protecting the pelvic nerves systematically [39,46,47,48]. Many authors have discussed preserving the PSNs and the IHP [49,50,51]. However, the techniques to conserve the VNB innervating the bladder have been little discussed. Japanese gynecologists have tried to develop a method to identify and preserve the vesical nerve fibers based on detailed anatomical studies of the pelvic nerves [39,47,48,52,53]. A detailed topographical and histological study of the pelvic nerves and the vesicouterine ligament was reported [54].
It has been criticized that there is no standardized technique for nerve-sparing radical hysterectomy [55]. The type C1 surgery in the Querleu-Morrow 2008 classification [20] and the TMMR method [50] do not mention the role of dissection of the vesicovaginal ligament in a nerve-sparing radical hysterectomy. The surgical anatomy of the retroperitoneal tissues has been demonstrated in a study using a 3-dimensional anatomic template with a detailed description of parametrial resection [56]. However, separation and protection of the vesical nerve fibers was not mentioned in type C1 surgery. The preservation method of the pelvic nerves varies among the different types of radical hysterectomy. In type III (Piver class III) nerve-sparing radical hysterectomy, it may be hard to identify the uterine nerve branches (UNB) and the VNB. This situation may explain the difficulty in standardizing the nerve-sparing technique [55,57]. There is no qualified method to visualize and identify the uterine nerve fibers and the VNB during the operation even though there are some indirect methods such as electrical stimulation [58]. We aimed to demonstrate the precise surgical procedures to locate these nerve structures using fresh cadavers.
4. Technical aspects of nerve-sparing radical hysterectomy
We carried out cadaver dissection to demonstrate the systematic nerve-sparing Kobayashi radical hysterectomy in the Department of Anatomy II at Sapporo Medical University in July 2005. Cadavers were donated to Sapporo Medical University without inducement, with the family's consent and registration during life for an anatomical gift for fresh cadaver dissection from the person herself. The ethics committee of Sapporo Medical University approved the use of fresh cadavers for research and educational purpose.
The key point of the systematic nerve-preservation is the identification and the selective transection of the UNB of the IHP. The lateral parametrium comprises the pelvic nerve plate (IHP, UNB, and VNB), the deep uterine vein (DUV) of the vascular portion of the cardinal ligament, and the fatty connective tissue baring lymph nodes. We need to separate the DUV, which loosely attaches over the IHP and the UNB to identify the UNB.
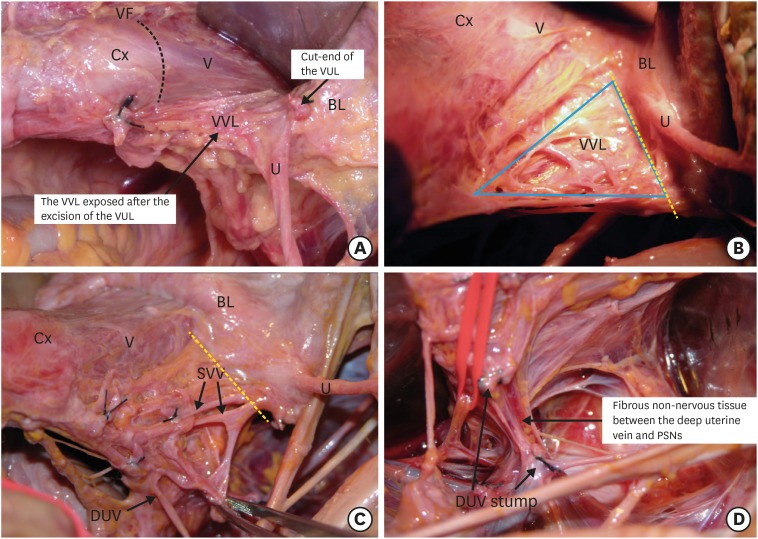
Step 1. Isolation and transection of vesical veins in the vesicovaginal ligament
The vesicovaginal ligament connects the bladder to the vagina at the level of the vaginal fornix (Fig. 1A). The vesicovaginal ligament is a continuum of the paracolpium composed of vesical veins draining into the DUV, fatty connective tissue, and a sheet of the VNB on the dorsolateral aspect. We do not develop the paravaginal space in a nerve-sparing radical hysterectomy (Fig. 1B). We expose the vesical veins. Each vein is transected at the border to the bladder to disconnect the bladder from the cervix and upper vagina (Fig. 1C). The DUV is transected to separate it from the rest of the cardinal ligament, which contains fibrous connective tissue and the PSNs at the most dorsal part (Fig. 1D).
Fig. 1. Isolation and transection of the vesical veins in the vesicovaginal ligament.
(A) The vesicovaginal ligament is exposed after the excision of the vesicouterine ligament. (B) The venous plexus of vesical veins in the vesicovaginal ligament after removing the thin membranous fascia. The vesicovaginal ligament is transected at the yellow dotted line. (C) The vesical veins draining into the DUV. The vesical veins are transected at the yellow dotted line. (D) After cutting the vesical veins, the DUV is transected and separated from the underneath non-nervous connective tissue and, occasionally, the middle rectal artery and vein. This procedure exposes the inferior hypogastric plexus.
BL, bladder; Cx, cervix; DUV, deep uterine vein; U, ureter; V, vagina; VF, vaginal fornix; VUL, vesicouterine ligament; VV, vesical veins; VVL, vesicovaginal ligament.
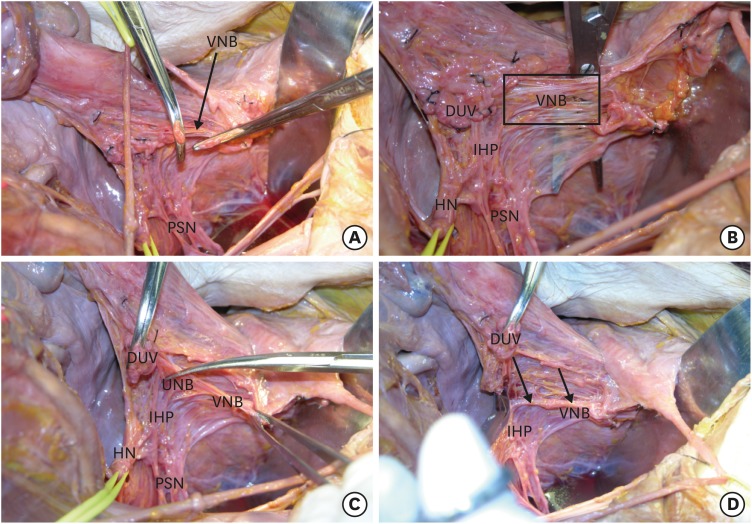
Step 2. Identification of the IHP and the UNB to separate the VNB from the paracervix and paracolpium
We dissect the remaining fatty connective tissue, trying to leave the dorsolateral membranous tissue containing the vesical nerve fibers. Some nerve fibers cross over the distal end of the ureter to the bladder [59,60]. These fibers might be sacrificed (Fig. 2A). The cut-end of the DUV is rubbed off the PSNs and the IHP. We can recognize the vesical nerve fibers on the upper lateral surface of the paracolpium. There is a plane that separates the vesical nerve fibers from the paracolpium (Fig. 2B). The HN is separated from the rectovaginal ligament, and the separation of the HN is extended to just below the lifted DUV. We can identify the UNB of the IHP. Next, we locate the UNB and VNB, both posteriorly and anteriorly (Fig. 2C). Then, the uterine nerve fibers are transected selectively. This procedure makes the IHP and VNB retract dorsolaterally (Fig. 2D).
Fig. 2. Identification of the IHP and the UNB to separate the VNB from the paracervix and paracolpium.
(A) The remaining connective tissue of the vesicovaginal ligament is excised, leaving the dorsolateral tissue containing a portion of the vesical nerve fibers. (B) We demonstrated the plane that separates the vesical nerve fibers from the paracolpium. (C) Identification and selective transection of the UNB. (D) The IHP and VNB move away from the paracervix, paracolpium, and the rectovaginal ligament.
DUV, deep uterine vein; HN, hypogastric nerve; IHP, inferior hypogastric plexus; PSN, pelvic splanchnic nerve; UNB, uterine nerve branches; VNB, vesical nerve branches.
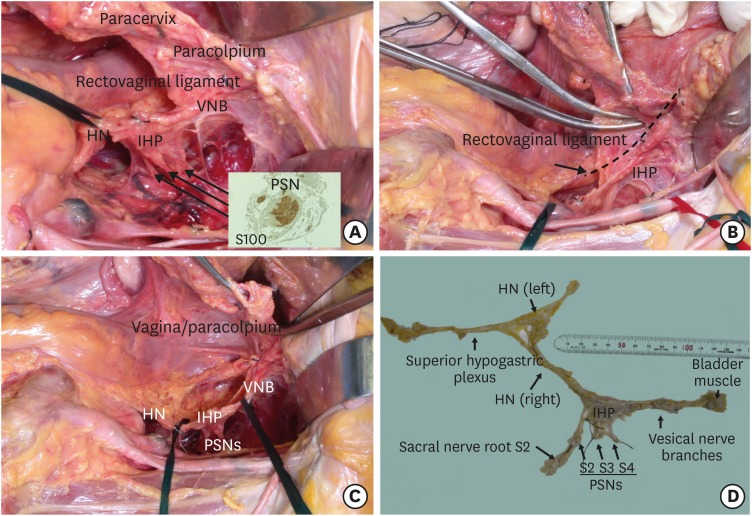
Step 3. Excision of the parametrial tissue and the upper vagina/paracolpium without damaging the pelvic nerves
We can preserve a fence-like structure of the pelvic autonomic nervous system (Fig. 3A). We then excise the rectovaginal ligament (Fig. 3B) and the vagina/paravaginal tissue (paracolpium) to a sufficient extent to achieve local control of the tumor without sacrificing the pelvic nervous system. The T-shaped pelvic nerve sheet is preserved (Fig. 3C). The whole pelvic autonomic nervous system preserved after the systematic nerve-sparing procedure was demonstrated by removing the nerve structures from the cadaver (Fig. 3D).
Fig. 3. Excision of the parametrial tissue and the upper vagina/paracolpium without sacrificing the pelvic nerves.
(A) The pelvic nervous system separated from the rectovaginal ligament, paracervix, and paracolpium. (B) Excision of a sufficient amount of the paracervical tissue without sacrificing the pelvic nerves. (C) T-shaped pelvic nerve structure after the systematic nerve-sparing procedures. (D) The total pelvic autonomic nervous system preserved by the systematic nerve-sparing radical hysterectomy.
HN, hypogastric nerve; IHP, inferior hypogastric plexus; PSN, pelvic splanchnic nerve; VNB, vesical nerve branches.
5. Tailored surgery for parametrial and paravaginal tissue dissection in early and locally advanced cervical cancer
A small stage IB1 cervical tumor (<2 cm) has been shown by many studies to have a very low risk of pathological parametrial invasion [61,62,63,64] and, therefore, modified radical hysterectomy with pelvic lymphadenectomy should have a sufficient radicality for this group. Because of high disease-free survival and overall survival rates, a randomized controlled trial (RCT) comparing modified radical hysterectomy and nerve-sparing radical hysterectomy would not be realizable.
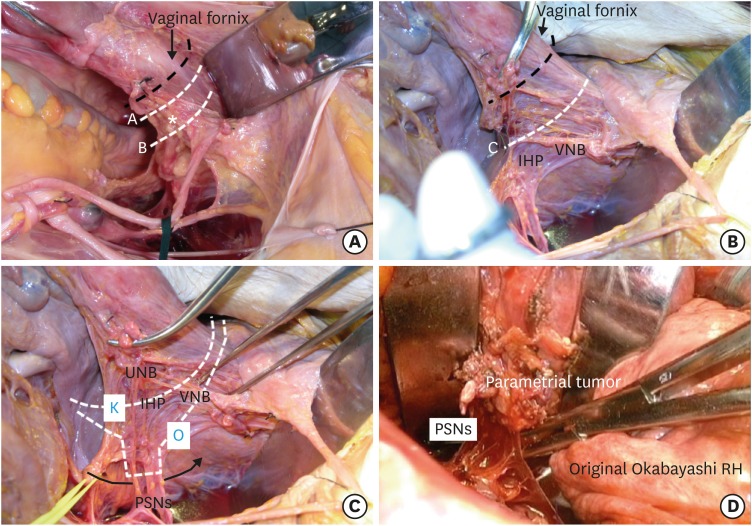
We can compare the views of the paracervical and paravaginal area with or without dissection of the vesicovaginal ligament (Fig. 4A). We can also recognize that modified radical hysterectomy with the removal of approximately 1 cm of the vagina will not damage the IHP or VNB (Fig. 4A, line A). However, in type III radical hysterectomy, removal of the upper 1/3 (2–3 cm) of the vagina/paracolpium will damage the IHP and VNB (Fig. 4A, line B). In contrast, the systematic nerve-sparing method preserves the pelvic nervous system while we remove the upper 1/3 or more of the vagina and paracolpium (Fig. 4B, line C). The nerve-sparing procedure should not be appropriate for the side with parametrial tumor invasion. For such a case, we use partial nerve-sparing (the PSNs and some portion of the IHP are conserved) Kobayashi radical hysterectomy (K in Fig. 4C). In a rare instance, we use the original Okabayashi operation, which removes the entire cardinal ligament, including the PSNs on the affected side (O in Fig. 4C and D). In the Okabayashi operation, the paravesical and the pararectal spaces are interconnected between the PSNs and lateral aspect of the rectum (Fig. 4C, curved arrow line). The PSNs arise from the anterior sacral surface running ventrally along the lateral surface of the rectum. When we pull the uterus upwards ventrally, the PSNs stand up almost vertically towards the IHP. The DUV arises from the internal iliac vein on the pelvic sidewall running to the lateral paracervix horizontally or upwards obliquely. There is a space between the DUV and the PSNs, which is filled with fibrous tissue. It is not rare that these fibers are misrecognized as the PSNs [65].
Fig. 4. The method of parametrial and paravaginal/paracervical tissue dissection in tailored surgery for cervical cancer.
(A) The modified radical hysterectomy with 1 cm of vaginal resection will not damage the IHP and the VNB (line A). Radical hysterectomy removing 2–3 cm of the vagina/paracolpium without excision of the vesicovaginal ligament and the systematic nerve-preservation will damage the IHP and the VNB (line B). The asterisk indicates the location of the IHP underneath the VVL. (B) Radical hysterectomy excising 2–3 cm or more using the systematic nerve-preservation will not damage the pelvic nerves (line C). (C) Nonsystematic nerve-sparing Kobayashi radical hysterectomy may partially preserve the pelvic nerves (K), and the original Okabayashi radical hysterectomy extirpates the pelvic nervous system (O). (D) The total excision of the cardinal ligament, including the PSNs performed in the original Okabayashi radical hysterectomy for stage IIB cervical cancer.
HN, hypogastric nerve; IHP, inferior hypogastric plexus; PSN, pelvic splanchnic nerve; UNB, uterine nerve branches; VNB, vesical nerve branches.
The survival of patients with locally advanced cervical cancer is still suboptimal. We need to enhance the progress of research and practice to improve outcomes of early locally advanced (2008 FIGO stage IB2–IIB) cervical cancer. NCCN Guidelines recommend CCRT for 2018 FIGO stage IB3, IIA2, and IIB as the treatment of choice. Radical hysterectomy is recommended for 2018 FIGO stage IB1 and IB2, and it is an optional treatment for 2018 FIGO stage IB3 and IIA1. As mentioned earlier, JSGO Guidelines recommend two options for stage IB1–IIB with upfront radical hysterectomy or CCRT. Radical hysterectomy and CCRT/radiotherapy (RT) was used almost equally for stage II disease in 2015 in Japan [66].
Upfront surgery +/− adjuvant therapy for early locally advanced cervical cancer in stage IB2 to IIB has increased in the urban/rural area compared to the metropolitan area in the United States from 2004 to 2012 according to the data derived from a National Cancer Database file. Between 2004 and 2012, the rate of upfront surgery alone and upfront surgery + adjuvant radiation increased from 8.8% to 12.1% and from 13.8% to 19.1%, respectively (total upfront surgery from 22.6% to 31.2%), while chemoradiation decreased from 77.3% to 68.8%. This increase caused criticism with concern for both higher rates of treatment-related morbidity and greater health care costs without the evidence of survival benefit [67]. There have been attempts to clarify the role of surgery for early locally advanced cervical cancer. Neoadjuvant chemotherapy + radical hysterectomy may be an option for early locally advanced cervical cancer. A systematic review article showed that the available RCTs comparing surgery +/− adjuvant therapy and RT found insufficient evidence that surgery +/− adjuvant therapy improves the survival of patients with locally advanced cervical cancer over RT or CCRT alone [68]. Surgery in some trials included even simple hysterectomy but was not restricted to radical hysterectomy. There raised a concern about adding RT after neoadjuvant chemotherapy plus radical hysterectomy, which brought trimodality therapy, which in turn may increase toxicity. A recent RCT conducted in a single institution showed inferior 5-year disease-free survival in the neoadjuvant chemotherapy + surgery group compared to the CCRT group [69]. The surgery used in the protocol was Piver class III radical hysterectomy. An RCT (EORTC 55994) comparing neoadjuvant chemotherapy followed by radical hysterectomy to CCRT for locally advanced stage IB2-IIB cervical cancer has been conducted [70]. The type of radical hysterectomy used was not reported. With a median follow-up time of 8.2 years, the 5-year overall survival for neoadjuvant chemotherapy followed by radical hysterectomy and CCRT was 72% and 76%, respectively. There was no significant difference. While short-term adverse events (≥G3) were more frequent in neoadjuvant chemotherapy + radical hysterectomy, long-term toxicities were less frequent in neoadjuvant chemotherapy + radical hysterectomy. We have to wait for the final results and information about the patterns of recurrence, especially local and regional events, according to the treatment.
SURVIVAL OUTCOMES AFTER NERVE-SPARING RADICAL HYSTERECTOMY
Precise assessment for the risk of recurrence is essential to judge the necessity and select adjuvant treatment to avoid unnecessary morbidity. It is not clear whether the nerve-sparing procedure is associated with a specific pattern of recurrence. Local recurrence is of concern in a nerve-sparing radical hysterectomy. RT is used for pelvic (local and regional) control after surgery, and chemotherapy is used for reducing distant recurrence. Sufficiently extensive surgery combined with systematic regional lymphadenectomy causes less need for local treatment by RT. Cases with a risk of distant failure will need systemic therapy. Chemotherapy showed a similar survival effect to RT for patients with intermediate or high-risk for recurrence after radical hysterectomy [71,72]. Chemotherapy seems to reduce the risk of distant recurrence [73]. A subgroup of patients with certain risk factors was likely to obtain insufficient local control by chemotherapy alone compared to RT [71]. We need to take the adverse effect of adjuvant RT into account in selecting the type of adjuvant therapy after radical hysterectomy [72]. The histologic type, tumor size, depth of invasion, close margin, and presence of lymphovascular invasion are indicators of the biological aggressiveness and the recurrent risk of cervical cancer [74,75,76,77]. In addition to these established prognostic factors, perineural invasion has been reported as an important prognostic factor. The relevance of perineural invasion in nerve-sparing radical hysterectomy needs to be investigated by a large prospective study.
There still exist controversies about the oncological safety of nerve-sparing radical hysterectomy [78,79,80,81]. Several RCTs have compared nerve-sparing radical hysterectomy to conventional radical hysterectomy. These studies suggested that nerve-sparing radical hysterectomy has an equivalent survival outcome to conventional radical hysterectomy. Available RCTs compared type III nerve-sparing radical hysterectomy with type III conventional radical hysterectomy and did not include overall survival or a sufficient number of events to calculate overall survival. Local recurrence after nerve-sparing radical hysterectomy may occur through preserving the nerves already affected by cancer cells via the perineural invasion, and inappropriate separation of pelvic nerves through cutting the tumor-involved parametrium, transitional zone [82]/parametrial initial zone [83] or paracolpium.
An RCT (Laparoscopic Approach to Cervical Cancer [LACC] trial) showed the inferior oncological outcome of laparoscopic radical hysterectomy compared to open radical hysterectomy [84]. The possible explanation for the inferior survival and loco-regional control may include inadequate parametrial and paracolpium dissection, usage of the uterine manipulator, and tumor cell spillage into the abdominal cavity under CO2 insufflation [85,86]. The difference in the usage of the nerve-sparing procedure between the two groups was not included in the study design. Accordingly, European Society of Gynecological Oncology (ESGO) issued a statement, which says that the open approach is the gold standard for radical hysterectomy. They believe it is hasty to ban minimum-access surgery; however, its use must be strictly monitored, and it must not be forgotten that the key issue is radicality [87]. The robotic approach improves technical accuracy. A nationwide population-based cohort study showed that open and robotic radical hysterectomy for early-stage cervical cancer had equivalent long-term survival and the patterns of recurrence [88]. An RCT (Robot-assisted Approach to Cervical Cancer [RACC] trial) comparing abdominal radical hysterectomy and robot-assisted radical hysterectomy for early-stage cervical cancer is ongoing (NCT03719547).
FUNCTIONAL OUTCOMES AFTER NERVE-SPARING RADICAL HYSTERECTOMY
The function of the bladder is the storage and voluntary voiding of urine. In voiding, the bladder sphincters relax and the detrusor muscle contracts, permitting micturition. In the storage phase, the bladder sphincters contract, and the detrusor muscles relax.
Parasympathetic nerves trigger bladder contraction during voiding. Bladder relaxation is maintained by the effect of the sympathetic neurons. Somatic afferent fibers in the pudendal nerve innervate the external urethral sphincter. The pelvic nerves and HNs carry sensory information about bladder fullness, and the pudendal nerve and iliohypogastric nerve transmit sensory information from the bladder neck and urethra [89,90]. Our goal of preservation of bladder function after radical hysterectomy should be the long-term maintenance of bladder compliance and voiding function [91]. Preoperative assessment of bladder function is necessary because a proportion of patients with uterine cervical cancer already have some degree of bladder dysfunction before undergoing surgery [92].
The method used for the assessment of bladder function after nerve-sparing radical hysterectomy varies among studies. Some of them used the first day of urination, duration of the catheter dwelling, and the residual urine volume, which are indicators of the short-term functional outcome. Subjective symptoms alone are not an indicator of long-term bladder function. The indicators of successful nerve-preservation are bladder compliance, contraction of the detrusor muscle, maximum urinary flow rate, and urinary sensation. Patients with bladder dysfunction should be instructed on the usage of clean intermittent catheterization until the recovery of bladder function [93,94]. The risk factors for the impaired restoration of the bladder compliance after a nerve-sparing radical hysterectomy include voiding with abdominal strain during the acute and subacute postoperative phases within three months after surgery [95] and the usage of postoperative RT [95,96].
Education of patients and nursing staff about the natural course of bladder function recovery after radical hysterectomy is essential. We cannot avoid decreased bladder compliance in the acute phase of the postoperative period, as manifested by the impaired storage of urine. Decreased bladder compliance occurs irrespective of the usage of the nerve-sparing method. This decrease in bladder compliance is due to extensive surgical separation of the bladder and postoperative tissue edema. During the acute postoperative phase after radical hysterectomy, we need to keep the bladder at rest. Patients should be instructed to refrain from abdominal strain and to avoid bladder overdistension. Otherwise, the bladder muscle will be damaged, resulting in fibrosis of the muscle with weakness in the contraction of the muscle. In this context, we should exclude bladder training from postoperative urination management after radical hysterectomy.
TOWARDS A CONSENSUS FOR THE NERVE-SPARING RADICAL HYSTERECTOMY FOR CERVICAL CANCER
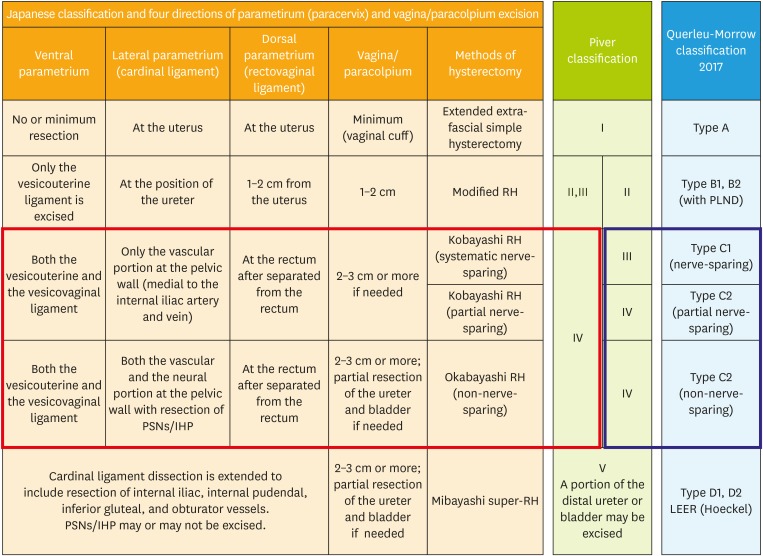
The recent update of the Querleu-Morrow classification [97] proposed that the proximal part of the vesicovaginal ligament is excised in type C1 radical hysterectomy. This statement was not in the original classification published in 2008 [20]. The necessity of the excision of the vesicovaginal ligament and the optimal extent of vaginal resection was a primary focus of discussion in attempts to modernize and standardize the classification of radical hysterectomy at the International Symposium on Radical Hysterectomy Dedicated to Hidekazu Okabayashi organized by Professor Shingo Fujii held in Kyoto in 2007. There seems to be room for review. The role of excision of the vesicovaginal ligament in nerve-sparing radical hysterectomy has not reached consensus, and the technique to preserve the vesical nerve fibers has not been standardized. Recognition of the difference between type III (Piver class III) nerve-sparing radical hysterectomy and type IV nerve-sparing radical hysterectomy will be a prerequisite to discussing the standardization of nerve-sparing radical hysterectomy. We consider that this difference needs to be mentioned in the article and the study protocol on nerve-sparing radical hysterectomy. Based on these discussions, we tried to compare various classifications of radical hysterectomy and nerve-sparing radical hysterectomy in Fig. 5, which needs to be critically reviewed.
Fig. 5. Comparison of various method of hysterectomy. The hysterectomy used for cervical cancer in Japan is defined by the extent of 4 directions of the tissue surrounding the cervix: three components of the parametrium/paracervix and the paracolpium. Type C2 surgery in Querleu-Morrow classification is equivalent to the Okabayashi radical hysterectomy if it removes the PSNs in the lateral parametrial excision.
IHP, inferior hypogastric plexus; PLND, pelvic lymphadenectomy; PSN, pelvic splanchnic nerve; RH, radical hysterectomy.
CONCLUSION
Nerve-sparing radical hysterectomy has an essential role in precision surgery for cervical cancer. We reviewed various types of radical hysterectomy and clarified the anatomical and surgical validity of type IV systematic nerve-sparing radical hysterectomy. Preservation of the vesical nerve fibers is the most challenging part. It is necessary to gain a three-dimensional topographic recognition of the complicated relationship between the pelvic nervous system and the parametrium and paracolpium. In addition, the precise maneuver to separate the nerve structure from the parametrium/paracolpium is required in this surgery. The surgical technique is necessary but not sufficient by itself. We need to improve patient care to maximize the survival effect and maintain the QoL level in the personalized surgical treatment of cervical cancer.
ACKNOWLEDGMENTS
The authors wish to thank Springer Nature Author Services for English Language editing a draft of the manuscript.
Footnotes
Presentation: Presented in part at the 51st Annual Meeting of Taiwan Association of Obstetrics and Gynecology (TAOG) with the title of “What is the role of tailored surgery in gynecologic oncology? – Modification, Intensification, Individualization -, Taipei, Taiwan, March 3–4, 2012; the 100th Annual Congress of Korean Society of Obstetrics and Gynecology (KSOG) with the title of “QoL-oriented surgery for patients with cervical cancer”, Seoul, Korea, September 26–27, 2014; the 20th Workshop of Korean Gynecologic Oncology Group (KGOG) with the title of “Updates on nerve-sparing radical hysterectomy”, April 23–24, 2015; the 2nd International Symposium on Gynecologic Oncology with the title of “Nerve-sparing radical hysterectomy: indications and outcomes”, Seoul, February 10–11, 2017; and the 56th Annual Congress of Taiwan Association of Obstetrics and Gynecology (TAOG) with the title of “Nerve-sparing radical hysterectomy: its indications and outcomes”, Taipei, Taiwan, March 18–19, 2017.
- Conceptualization: S.N., M.G., W.H.
- Data curation: S.N., K.Y.
- Writing - original draft: S.N., K.M.
- Writing - review & editing: S.N.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Registry and Statistics. Cancer incidence (1975–2015) [Internet] Tokyo: Cancer Information Service, National Cancer Center; 2016. [cited 2019 Sep 1]. Available from: https://ganjoho.jp/reg_stat/statistics/dl/index.html. [Google Scholar]
- 3.Cancer Research UK. Cervical cancer incidence statistics [Internet] London: Cancer Research UK; 2019. [cited 2019 Sep 1]. Available from: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/cervical-cancer/incidence. [Google Scholar]
- 4.American Cancer Society. Key statistics for cervical cancer [Internet] New York (NY): American Cancer Society; c2020. [cited 2019 Sep 1]. Available from: https://www.cancer.org/cancer/cervical-cancer/about/key-statistics.html. [Google Scholar]
- 5.Cancer Australia. Cervical cancer in Australia statistics [Internet] Surry Hills: Cancer Australia; c2020. [cited 2019 Sep 1]. Available from: https://cervical-cancer.canceraustralia.gov.au/statistics. [Google Scholar]
- 6.U.S. Department of Health & Human Services. What is precision medicine? [Internet] Bethesda (MD): U.S. Department of Health & Human Services; c2020. [cited 2019 Sep 1]. Available from: https://ghr.nlm.nih.gov/primer/precisionmedicine/definition. [Google Scholar]
- 7.Tabata M, Ichinoe K, Sakuragi N, Shiina Y, Yamaguchi T, Mabuchi Y. Incidence of ovarian metastasis in patients with cancer of the uterine cervix. Gynecol Oncol. 1987;28:255–261. doi: 10.1016/0090-8258(87)90170-3. [DOI] [PubMed] [Google Scholar]
- 8.Sutton GP, Bundy BN, Delgado G, Sevin BU, Creasman WT, Major FJ, et al. Ovarian metastases in stage IB carcinoma of the cervix: a Gynecologic Oncology Group study. Am J Obstet Gynecol. 1992;166:50–53. doi: 10.1016/0002-9378(92)91828-x. [DOI] [PubMed] [Google Scholar]
- 9.Sakuragi N, Takeda N, Hareyama H, Fujimoto T, Todo Y, Okamoto K, et al. A multivariate analysis of blood vessel and lymph vessel invasion as predictors of ovarian and lymph node metastases in patients with cervical carcinoma. Cancer. 2000;88:2578–2583. [PubMed] [Google Scholar]
- 10.Landoni F, Zanagnolo V, Lovato-Diaz L, Maneo A, Rossi R, Gadducci A, et al. Ovarian metastases in early-stage cervical cancer (IA2–IIA): a multicenter retrospective study of 1965 patients (a Cooperative Task Force study) Int J Gynecol Cancer. 2007;17:623–628. doi: 10.1111/j.1525-1438.2006.00854.x. [DOI] [PubMed] [Google Scholar]
- 11.Saito M, Kumasaka T, Kato K, Yazawa K. Vaginal repair in the radical operation for cervical carcinoma. Acta Obstet Gynecol Scand. 1976;55:151–154. doi: 10.3109/00016347609156804. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto R, Okamoto K, Ebina Y, Shirato H, Sakuragi N, Fujimoto S. Prevention of vaginal shortening following radical hysterectomy. BJOG. 2000;107:841–845. doi: 10.1111/j.1471-0528.2000.tb11080.x. [DOI] [PubMed] [Google Scholar]
- 13.Ohba Y, Todo Y, Kobayashi N, Kaneuchi M, Watari H, Takeda M, et al. Risk factors for lower-limb lymphedema after surgery for cervical cancer. Int J Clin Oncol. 2011;16:238–243. doi: 10.1007/s10147-010-0171-5. [DOI] [PubMed] [Google Scholar]
- 14.Hareyama H, Hada K, Goto K, Watanabe S, Hakoyama M, Oku K, et al. Prevalence, classification, and risk factors for postoperative lower extremity lymphedema in women with gynecologic malignancies: a retrospective study. Int J Gynecol Cancer. 2015;25:751–757. doi: 10.1097/IGC.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 15.Niikura H, Okamoto S, Otsuki T, Yoshinaga K, Utsunomiya H, Nagase S, et al. Prospective study of sentinel lymph node biopsy without further pelvic lymphadenectomy in patients with sentinel lymph node-negative cervical cancer. Int J Gynecol Cancer. 2012;22:1244–1250. doi: 10.1097/IGC.0b013e318263f06a. [DOI] [PubMed] [Google Scholar]
- 16.Sakuragi N, Satoh C, Takeda N, Hareyama H, Takeda M, Yamamoto R, et al. Incidence and distribution pattern of pelvic and paraaortic lymph node metastasis in patients with stages IB, IIA, and IIB cervical carcinoma treated with radical hysterectomy. Cancer. 1999;85:1547–1554. doi: 10.1002/(sici)1097-0142(19990401)85:7<1547::aid-cncr16>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Takeshita S, Todo Y, Okamoto K, Sudo S, Yamashiro K, Kato H. Incidence of metastasis in circumflex iliac nodes distal to the external iliac nodes in cervical cancer. J Gynecol Oncol. 2016;27:e42. doi: 10.3802/jgo.2016.27.e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latzko W, Schiffmann J. Klinisches und anatomisches zur radikaloperation des gebarmutterkrebses. Zentralbl Gynakol. 1919;43:715–719. [Google Scholar]
- 19.Okabayashi H. Radical abdominal hysterectomy for cancer of the cervix uteri. Modification of the Takayama operation. Surg Gynecol Obstet. 1921;33:335–341. [Google Scholar]
- 20.Querleu D, Morrow CP. Classification of radical hysterectomy. Lancet Oncol. 2008;9:297–303. doi: 10.1016/S1470-2045(08)70074-3. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T. Abdominal radical hysterectomy with pelvic lymphadenectomy for cancer of the cervix. 1st ed. Tokyo: Nanazando; 1961. [Google Scholar]
- 22.Sakamoto S, Takizawa K. An improved radical hysterectomy with fewer urological complications and with no loss of therapeutic results for invasive cervical cancer. Baillieres Clin Obstet Gynaecol. 1988;2:953–962. doi: 10.1016/s0950-3552(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 23.Bhatla N, Berek JS, Cuello Fredes M, Denny LA, Grenman S, Karunaratne K, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynaecol Obstet. 2019;145:129–135. doi: 10.1002/ijgo.12749. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Liu P, Chen C, Duan H, Qiao W, Ognami OH. The 3D reconstructions of female pelvic autonomic nerves and their related organs based on MRI: a first step towards neuronavigation during nerve-sparing radical hysterectomy. Eur Radiol. 2018;28:4561–4569. doi: 10.1007/s00330-018-5453-8. [DOI] [PubMed] [Google Scholar]
- 25.Wijsmuller AR, Giraudeau C, Leroy J, Kleinrensink GJ, Rociu E, Romagnolo LG, et al. A step towards stereotactic navigation during pelvic surgery: 3D nerve topography. Surg Endosc. 2018;32:3582–3591. doi: 10.1007/s00464-018-6086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haldorsen IS, Lura N, Blaakær J, Fischerova D, Werner HM. What is the role of imaging at primary diagnostic work-up in uterine cervical cancer? Curr Oncol Rep. 2019;21:77. doi: 10.1007/s11912-019-0824-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu Y, Zhang GN, Shi Y, Cui L, Leng XF, Huang JM. Perineural invasion in cervical cancer: pay attention to the indications of nerve-sparing radical hysterectomy. Ann Transl Med. 2019;7:203. doi: 10.21037/atm.2019.04.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang M, Liu Q, Yang X, Chen L, Yu J, Qi X, et al. Perineural invasion as a prognostic risk factor in patients with early cervical cancer. Oncol Lett. 2019;17:1101–1107. doi: 10.3892/ol.2018.9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vural C, Bayrak BY, Muezzınoglu B, Yucesoy I. Perineural invasion is a valuable prognostic factor in advanced stage and/or node (+) cervical cancer. Indian J Pathol Microbiol. 2017;60:27–32. doi: 10.4103/0377-4929.200021. [DOI] [PubMed] [Google Scholar]
- 30.Arese M, Bussolino F, Pergolizzi M, Bizzozero L, Pascal D. Tumor progression: the neuronal input. Ann Transl Med. 2018;6:89. doi: 10.21037/atm.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen SH, Zhang BY, Zhou B, Zhu CZ, Sun LQ, Feng YJ. Perineural invasion of cancer: a complex crosstalk between cells and molecules in the perineural niche. Am J Cancer Res. 2019;9:1–21. [PMC free article] [PubMed] [Google Scholar]
- 32.Capek S, Howe BM, Amrami KK, Spinner RJ. Perineural spread of pelvic malignancies to the lumbosacral plexus and beyond: clinical and imaging patterns. Neurosurg Focus. 2015;39:E14. doi: 10.3171/2015.7.FOCUS15209. [DOI] [PubMed] [Google Scholar]
- 33.Dercle L, Hartl D, Rozenblum-Beddok L, Mokrane FZ, Seban RD, Yeh R, et al. Diagnostic and prognostic value of 18F-FDG PET, CT, and MRI in perineural spread of head and neck malignancies. Eur Radiol. 2018;28:1761–1770. doi: 10.1007/s00330-017-5063-x. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Lazor JW, Assadsangabi R, Shah J. An imager's guide to perineural tumor spread in head and neck cancers: radiologic footprints on 18F-FDG PET, with CT and MRI correlates. J Nucl Med. 2019;60:304–311. doi: 10.2967/jnumed.118.214312. [DOI] [PubMed] [Google Scholar]
- 35.National Comprehensive Cancer Network. NCCN Guidelines version 4. 2019. Cervical cancer [Internet] Plymouth Meeting (PA): National Comprehensive Cancer Network; c2020. [cited 2019 Sep 1]. Available from: ( https://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf. [Google Scholar]
- 36.Ebina Y, Mikami M, Nagase S, Tabata T, Kaneuchi M, Tashiro H, et al. Japan Society of Gynecologic Oncology guidelines 2017 for the treatment of uterine cervical cancer. Int J Clin Oncol. 2019;24:1–19. doi: 10.1007/s10147-018-1351-y. [DOI] [PubMed] [Google Scholar]
- 37.Meigs JV. Radical hysterectomy with bilateral pelvic lymph node dissections; a report of 100 patients operated on five or more years ago. Am J Obstet Gynecol. 1951;62:854–870. doi: 10.1016/0002-9378(51)90175-5. [DOI] [PubMed] [Google Scholar]
- 38.Gitsch E, Palmrich AH. Gynecological Operative Anatomy: The simple and radical hysterectomy. Berlin: Walter de Gruyter & Co.; 1977. [Google Scholar]
- 39.Kuwabara Y, Suzuki M, Hashimoto M, Furugen Y, Yoshida K, Mitsuhashi N. New method to prevent bladder dysfunction after radical hysterectomy for uterine cervical cancer. J Obstet Gynaecol Res. 2000;26:1–8. doi: 10.1111/j.1447-0756.2000.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 40.Oguchi K, Kuwabara M, Sakuragi N, et al. Urodynamics study for bladder dysfunction following radical hysterectomy. Acta Obstet Gynaecol Jpn. 1999;51:325–334. [Google Scholar]
- 41.Ralph G, Winter R, Michelitsch L, Tamussino K. Radicality of parametrial resection and dysfunction of the lower urinary tract after radical hysterectomy. Eur J Gynaecol Oncol. 1991;12:27–30. [PubMed] [Google Scholar]
- 42.Benedetti-Panici P, Zullo MA, Plotti F, Manci N, Muzii L, Angioli R. Long-term bladder function in patients with locally advanced cervical carcinoma treated with neoadjuvant chemotherapy and type 3–4 radical hysterectomy. Cancer. 2004;100:2110–2117. doi: 10.1002/cncr.20235. [DOI] [PubMed] [Google Scholar]
- 43.Zuo N, Hu H, Thapa N, Li Z, Jiang D, Meng X, et al. Vaginal cuff length during radical hysterectomy is a prognostic factor for stage IB–IIA cervical cancer: a retrospective study. Cancer Manag Res. 2018;10:5927–5935. doi: 10.2147/CMAR.S175726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K, Cho SY, Park SI, Kim BJ, Kim MH, Choi SC, et al. Vaginal and pelvic recurrence rates based on vaginal cuff length in patients with cervical cancer who underwent radical hysterectomies. Eur J Surg Oncol. 2011;37:824–827. doi: 10.1016/j.ejso.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 45.Piver MS, Rutledge F, Smith JP. Five classes of extended hysterectomy for women with cervical cancer. Obstet Gynecol. 1974;44:265–272. [PubMed] [Google Scholar]
- 46.Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Satou N. A new proposal for radical hysterectomy. Gynecol Oncol. 1996;62:370–378. doi: 10.1006/gyno.1996.0251. [DOI] [PubMed] [Google Scholar]
- 47.Sakuragi N, Todo Y, Kudo M, Yamamoto R, Sato T. A systematic nerve-sparing radical hysterectomy technique in invasive cervical cancer for preserving postsurgical bladder function. Int J Gynecol Cancer. 2005;15:389–397. doi: 10.1111/j.1525-1438.2005.15236.x. [DOI] [PubMed] [Google Scholar]
- 48.Fujii S, Takakura K, Matsumura N, Higuchi T, Yura S, Mandai M, et al. Anatomic identification and functional outcomes of the nerve sparing Okabayashi radical hysterectomy. Gynecol Oncol. 2007;107:4–13. doi: 10.1016/j.ygyno.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 49.Possover M, Stöber S, Plaul K, Schneider A. Identification and preservation of the motoric innervation of the bladder in radical hysterectomy type III. Gynecol Oncol. 2000;79:154–157. doi: 10.1006/gyno.2000.5919. [DOI] [PubMed] [Google Scholar]
- 50.Höckel M, Horn LC, Hentschel B, Höckel S, Naumann G. Total mesometrial resection: high resolution nerve-sparing radical hysterectomy based on developmentally defined surgical anatomy. Int J Gynecol Cancer. 2003;13:791–803. doi: 10.1111/j.1525-1438.2003.13608.x. [DOI] [PubMed] [Google Scholar]
- 51.Trimbos JB, Maas CP, Deruiter MC, Peters AA, Kenter GG. A nerve-sparing radical hysterectomy: guidelines and feasibility in Western patients. Int J Gynecol Cancer. 2001;11:180–186. doi: 10.1046/j.1525-1438.2001.01023.x. [DOI] [PubMed] [Google Scholar]
- 52.Sato K, Sato T. The vascular and neuronal composition of the lateral ligament of the rectum and the rectosacral fascia. Surg Radiol Anat. 1991;13:17–22. doi: 10.1007/BF01623135. [DOI] [PubMed] [Google Scholar]
- 53.Ercoli A, Delmas V, Gadonneix P, Fanfani F, Villet R, Paparella P, et al. Classical and nerve-sparing radical hysterectomy: an evaluation of the risk of injury to the autonomous pelvic nerves. Surg Radiol Anat. 2003;25:200–206. doi: 10.1007/s00276-003-0137-7. [DOI] [PubMed] [Google Scholar]
- 54.Niikura H, Katahira A, Utsunomiya H, Takano T, Ito K, Nagase S, et al. Surgical anatomy of intrapelvic fasciae and vesico-uterine ligament in nerve-sparing radical hysterectomy with fresh cadaver dissections. Tohoku J Exp Med. 2007;212:403–413. doi: 10.1620/tjem.212.403. [DOI] [PubMed] [Google Scholar]
- 55.Kietpeerakool C, Aue-Aungkul A, Galaal K, Ngamjarus C, Lumbiganon P. Nerve-sparing radical hysterectomy compared to standard radical hysterectomy for women with early stage cervical cancer (stage Ia2 to IIa) Cochrane Database Syst Rev. 2019;2:CD012828. doi: 10.1002/14651858.CD012828.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cibula D, Abu-Rustum NR, Benedetti-Panici P, Köhler C, Raspagliesi F, Querleu D, et al. New classification system of radical hysterectomy: emphasis on a three-dimensional anatomic template for parametrial resection. Gynecol Oncol. 2011;122:264–268. doi: 10.1016/j.ygyno.2011.04.029. [DOI] [PubMed] [Google Scholar]
- 57.Muallem MZ, Diab Y, Sehouli J, Fujii S. Nerve-sparing radical hysterectomy: steps to standardize surgical technique. Int J Gynecol Cancer. 2019;29:1203–1208. doi: 10.1136/ijgc-2019-000410. [DOI] [PubMed] [Google Scholar]
- 58.Nagai T, Niikura H, Kurosawa H, Tanaka S, Otsuki T, Utunomiya H, et al. Individualized radical hysterectomy procedure using intraoperative electrical stimulation for patients with cervical cancer. Int J Gynecol Cancer. 2012;22:1591–1596. doi: 10.1097/IGC.0b013e31826fd684. [DOI] [PubMed] [Google Scholar]
- 59.Kraima AC, Derks M, Smit NN, van de Velde CJ, Kenter GG, DeRuiter MC. Careful dissection of the distal ureter is highly important in nerve-sparing radical pelvic surgery: a 3D reconstruction and immunohistochemical characterization of the vesical plexus. Int J Gynecol Cancer. 2016;26:959–966. doi: 10.1097/IGC.0000000000000709. [DOI] [PubMed] [Google Scholar]
- 60.Takenaka A, Soga H, Murakami G, Niikura H, Tatsumi H, Yaegashi N, et al. Understanding anatomy of “hilus” of detrusor nerves to avoid bladder dysfunction after pelvic surgery: demonstration using fetal and adult cadavers. Urology. 2009;73:251–257. doi: 10.1016/j.urology.2008.09.039. [DOI] [PubMed] [Google Scholar]
- 61.Steed H, Capstick V, Schepansky A, Honore L, Hiltz M, Faught W. Early cervical cancer and parametrial involvement: is it significant? Gynecol Oncol. 2006;103:53–57. doi: 10.1016/j.ygyno.2006.01.027. [DOI] [PubMed] [Google Scholar]
- 62.Strnad P, Robova H, Skapa P, Pluta M, Hrehorcak M, Halaska M, et al. A prospective study of sentinel lymph node status and parametrial involvement in patients with small tumour volume cervical cancer. Gynecol Oncol. 2008;109:280–284. doi: 10.1016/j.ygyno.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Al-Kalbani M, McVeigh G, Nagar H, McCluggage WG. Do FIGO stage IA and small (≤2 cm) IB1 cervical adenocarcinomas have a good prognosis and warrant less radical surgery? Int J Gynecol Cancer. 2012;22:291–295. doi: 10.1097/IGC.0b013e3182339fff. [DOI] [PubMed] [Google Scholar]
- 64.Xiong Y, Liu JH, Zheng M, Cao LP, Liang LZ. Use of preoperative clinicopathologic characteristics to identify patients with low-risk cervical cancer suitable for Piver class II radical hysterectomy. Int J Gynaecol Obstet. 2013;122:52–56. doi: 10.1016/j.ijgo.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 65.Kato T, Murakami G, Yabuki Y. A new perspective on nerve-sparing radical hysterectomy: nerve topography and over-preservation of the cardinal ligament. Jpn J Clin Oncol. 2003;33:589–591. doi: 10.1093/jjco/hyg107. [DOI] [PubMed] [Google Scholar]
- 66.Nagase S, Ohta T, Takahashi F, Enomoto T 2017 Committee on Gynecologic Oncology of the Japan Society of Obstetrics and Gynecology. Annual report of the committee on gynecologic oncology, the Japan Society of Obstetrics and Gynecology: annual patients report for 2015 and annual treatment report for 2010. J Obstet Gynaecol Res. 2019;45:289–298. doi: 10.1111/jog.13863. [DOI] [PubMed] [Google Scholar]
- 67.Amini A, Robin TP, Stumpf PK, Rusthoven C, Schefter TE, Shinde A, et al. Rising rates of upfront surgery in early locally advanced cervical cancer: what factors predict for this treatment paradigm? Int J Gynecol Cancer. 2018;28:1560–1568. doi: 10.1097/IGC.0000000000001323. [DOI] [PubMed] [Google Scholar]
- 68.Kokka F, Bryant A, Brockbank E, Powell M, Oram D. Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer. Cochrane Database Syst Rev. 2015:CD010260. doi: 10.1002/14651858.CD010260.pub2. [DOI] [PubMed] [Google Scholar]
- 69.Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: a randomized controlled trial. J Clin Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 70.Kenter G, Greggi S, Vergote I, Katsaros D, Kobierski J, Massuger L, et al. Results from neoadjuvant chemotherapy followed by surgery compared to chemoradiation for stage Ib2–IIb cervical cancer, EORTC 55994. J Clin Oncol. 2019;37:5503. doi: 10.1200/JCO.22.02852. [DOI] [PubMed] [Google Scholar]
- 71.Matsuo K, Shimada M, Aoki Y, Sakamoto M, Takeshima N, Fujiwara H, et al. Comparison of adjuvant therapy for node-positive clinical stage IB-IIB cervical cancer: systemic chemotherapy versus pelvic irradiation. Int J Cancer. 2017;141:1042–1051. doi: 10.1002/ijc.30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takekuma M, Kasamatsu Y, Kado N, Kuji S, Tanaka A, Takahashi N, et al. The issues regarding postoperative adjuvant therapy and prognostic risk factors for patients with stage I–II cervical cancer: a review. J Obstet Gynaecol Res. 2017;43:617–626. doi: 10.1111/jog.13282. [DOI] [PubMed] [Google Scholar]
- 73.Lee KB, Shim SH, Lee JM. Comparison between adjuvant chemotherapy and adjuvant radiotherapy/chemoradiotherapy after radical surgery in patients with cervical cancer: a meta-analysis. J Gynecol Oncol. 2018;29:e62. doi: 10.3802/jgo.2018.29.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sedlis A, Bundy BN, Rotman MZ, Lentz SS, Muderspach LI, Zaino RJ. A randomized trial of pelvic radiation therapy versus no further therapy in selected patients with stage IB carcinoma of the cervix after radical hysterectomy and pelvic lymphadenectomy: a Gynecologic Oncology Group study. Gynecol Oncol. 1999;73:177–183. doi: 10.1006/gyno.1999.5387. [DOI] [PubMed] [Google Scholar]
- 75.McCann GA, Taege SK, Boutsicaris CE, Phillips GS, Eisenhauer EL, Fowler JM, et al. The impact of close surgical margins after radical hysterectomy for early-stage cervical cancer. Gynecol Oncol. 2013;128:44–48. doi: 10.1016/j.ygyno.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ryu SY, Kim MH, Nam BH, Lee TS, Song ES, Park CY, et al. Intermediate-risk grouping of cervical cancer patients treated with radical hysterectomy: a Korean Gynecologic Oncology Group study. Br J Cancer. 2014;110:278–285. doi: 10.1038/bjc.2013.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nishio S, Mikami Y, Tokunaga H, Yaegashi N, Satoh T, Saito M, et al. Analysis of gastric-type mucinous carcinoma of the uterine cervix - An aggressive tumor with a poor prognosis: a multi-institutional study. Gynecol Oncol. 2019;153:13–19. doi: 10.1016/j.ygyno.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 78.Long Y, Yao DS, Pan XW, Ou TY. Clinical efficacy and safety of nerve-sparing radical hysterectomy for cervical cancer: a systematic review and meta-analysis. PLoS One. 2014;9:e94116. doi: 10.1371/journal.pone.0094116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basaran D, Dusek L, Majek O, Cibula D. Oncological outcomes of nerve-sparing radical hysterectomy for cervical cancer: a systematic review. Ann Surg Oncol. 2015;22:3033–3040. doi: 10.1245/s10434-015-4377-7. [DOI] [PubMed] [Google Scholar]
- 80.Roh JW, Lee DO, Suh DH, Lim MC, Seo SS, Chung J, et al. Efficacy and oncologic safety of nerve-sparing radical hysterectomy for cervical cancer: a randomized controlled trial. J Gynecol Oncol. 2015;26:90–99. doi: 10.3802/jgo.2015.26.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim HS, Kim K, Ryoo SB, Seo JH, Kim SY, Park JW, et al. Conventional versus nerve-sparing radical surgery for cervical cancer: a meta-analysis. J Gynecol Oncol. 2015;26:100–110. doi: 10.3802/jgo.2015.26.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beyer FD, Jr, Murphy A. Patterns of spread of invasive cancer of the uterine cervix. Cancer. 1965;18:34–40. doi: 10.1002/1097-0142(196501)18:1<34::aid-cncr2820180107>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 83.Tabata M, Makinoda S, Yamaguchi T, Sakuragi N, Fujimoto S. Importance of the transitional zone between the cervical stroma and the parametrium in the treatment of cervical carcinoma. J Obstet Gynaecol Res. 1997;23:111–117. doi: 10.1111/j.1447-0756.1997.tb00818.x. [DOI] [PubMed] [Google Scholar]
- 84.Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med. 2018;379:1895–1904. doi: 10.1056/NEJMoa1806395. [DOI] [PubMed] [Google Scholar]
- 85.Kong TW, Chang SJ, Piao X, Paek J, Lee Y, Lee EJ, et al. Patterns of recurrence and survival after abdominal versus laparoscopic/robotic radical hysterectomy in patients with early cervical cancer. J Obstet Gynaecol Res. 2016;42:77–86. doi: 10.1111/jog.12840. [DOI] [PubMed] [Google Scholar]
- 86.Kohler C, Hertel H, Herrmann J, Marnitz S, Mallmann P, Favero G, et al. Laparoscopic radical hysterectomy with transvaginal closure of vaginal cuff - a multicenter analysis. Int J Gynecol Cancer. 2019;29:845–850. doi: 10.1136/ijgc-2019-000388. [DOI] [PubMed] [Google Scholar]
- 87.Querleu D. Laparoscopic radical hysterectomy: an ESGO statement [Internet] place unknown: European Society of Gynaecological Oncology; c2019. [cited 2019 Sep 1]. Available from: https://www.esgo.org/explore/council/laparoscopic-radical-hysterectomy-an-esgo-statement/ [DOI] [PubMed] [Google Scholar]
- 88.Alfonzo E, Wallin E, Ekdahl L, Staf C, Rådestad AF, Reynisson P, et al. No survival difference between robotic and open radical hysterectomy for women with early-stage cervical cancer: results from a nationwide population-based cohort study. Eur J Cancer. 2019;116:169–177. doi: 10.1016/j.ejca.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 89.Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Groat WC, Griffiths D, Yoshimura N. Neural control of the lower urinary tract. Compr Physiol. 2015;5:327–396. doi: 10.1002/cphy.c130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Todo Y, Kuwabara M, Watari H, Ebina Y, Takeda M, Kudo M, et al. Urodynamic study on postsurgical bladder function in cervical cancer treated with systematic nerve-sparing radical hysterectomy. Int J Gynecol Cancer. 2006;16:369–375. doi: 10.1111/j.1525-1438.2006.00345.x. [DOI] [PubMed] [Google Scholar]
- 92.Lin HH, Yu HJ, Sheu BC, Huang SC. Importance of urodynamic study before radical hysterectomy for cervical cancer. Gynecol Oncol. 2001;81:270–272. doi: 10.1006/gyno.2001.6155. [DOI] [PubMed] [Google Scholar]
- 93.Sekido N, Kawai K, Akaza H. Lower urinary tract dysfunction as persistent complication of radical hysterectomy. Int J Urol. 1997;4:259–264. doi: 10.1111/j.1442-2042.1997.tb00183.x. [DOI] [PubMed] [Google Scholar]
- 94.Hikita K, Honda M, Kimura Y, Kawamoto B, Tsounapi P, Morizane S, et al. Evaluation of a program of clean intermittent catheterization for underactive bladder after radical hysterectomy. Yonago Acta Med. 2018;61:156–159. doi: 10.33160/yam.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oda Y, Todo Y, Hanley S, Hosaka M, Takeda M, Watari H, et al. Risk factors for persistent low bladder compliance after radical hysterectomy. Int J Gynecol Cancer. 2011;21:167–172. doi: 10.1097/IGC.0b013e318204c3df. [DOI] [PubMed] [Google Scholar]
- 96.Oh JK, Choo MS, Lee J, Park NH, Oh SJ. Short-term effect of radical hysterectomy with or without adjuvant radiation therapy on urodynamic parameters in patients with uterine cervical cancer. Int Neurourol J. 2012;16:91–95. doi: 10.5213/inj.2012.16.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Querleu D, Cibula D, Abu-Rustum NR. 2017 update on the Querleu-Morrow classification of radical hysterectomy. Ann Surg Oncol. 2017;24:3406–3412. doi: 10.1245/s10434-017-6031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]