Abstract
In the past decade, the number of hip arthroscopy procedures has exponentially increased, primarily for the treatment of femoroacetabular impingement syndrome and labral lesions. As the techniques have evolved, so has the acknowledgment of the potential complications, including iatrogenic instability that may result from soft-tissue laxity, subtle dysplastic morphologies, or residual defects from capsulotomies in which the capsular closure is insufficient. In most cases, direct capsular repair or plication can be performed at the conclusion of the procedure; however, larger defects, poor-quality tissue, or cases of gross ligamentous laxity may require reconstruction or augmentation. In such instances, several options exist. The purpose of this technical note is to describe a capsular repair augmentation with a bioinductive implant during revision hip arthroscopy.
The use of hip arthroscopy and hip joint preservation procedures has increased dramatically in the past decade to treat a wide range of intra-articular pathologies, primarily femoroacetabular impingement syndrome and labral lesions.1, 2, 3 An important procedural step in hip arthroscopy is the capsulotomy, which allows for necessary instrument access and appropriate visualization. Adequate visualization is essential for complete treatment of large cam and more extreme acetabular-sided deformities because insufficient resection and deformity correction remain primary reasons for revision arthroscopy.4
Historically, capsule repair at the end of the procedure was not routinely performed. However, this has evolved with increasing evidence of the microinstability and macroinstability that can occur postoperatively and, in some cases, may exist preoperatively.3,5 Capsular closure and plication have also been shown to improve outcomes and accelerate the return to sports after treatment of intra-articular lesions.2,4, 5, 6 However, in patients with generalized ligamentous laxity, as in cases of Ehlers-Danlos syndrome (EDS), or those with poor tissue quality, which is not uncommonly seen in revision hip arthroscopy, repair alone may not be sufficient to restore adequate stability. In these cases, a capsular reconstruction or, in some cases, an augmentation technique with a bovine bioinductive implant may be used, as described in this article and summarized in Table 1. Similarly, both the advantages and disadvantages of this technique and the clinical indications are described in Tables 2 and 3, respectively.
Table 1.
Key Points to Capsule Closure and Augmentation With Bioinductive Device
| Use of the posterolateral portal can improve the delivery angle of the bioinductive device; however, the distal anterolateral accessory portal can also be used. |
| The midanterior portal should be used as a viewing portal. |
| More extensive soft-tissue debridement should be performed to improve visualization in the extracapsular space when augmentation is performed. |
| Soft-tissue staples can be inserted through the anterolateral portal. |
| After the implant is provisionally secured, the delivery system can be retrieved. |
| The implant is not meant to be structural at time zero, and adequate capsular approximation should be achieved in this setting. |
Table 2.
Advantages and Disadvantages of Augmenting Capsule Repair With Bioinductive Device
| Advantages |
| Native capsular augmentation can improve thickness and strength of repair |
| Can strengthen and thicken lax capsular tissue in carefully selected patients |
| Disadvantages |
| More time-consuming |
| Posterolateral portal may be required to insert bioinductive implant |
| Increases procedural costs |
| Technically demanding |
Table 3.
Indications for Capsule Repair Followed by Augmentation With Bioinductive Device
| Generalized ligamentous laxity |
| Revision hip arthroscopy with capsular disruption and/or poor capsular tissue quality but able to approximate majority of capsule |
| Augmentation of capsular tissue in hip arthroscopic revision for microinstability or macroinstability after hip arthroscopy |
Surgical Technique
Patient Positioning and Portal Placement
The patient is positioned in the modified supine position with the hip positioned such that the pelvis is in a neutral position (10° of flexion, 15° of internal rotation, neutral adduction-abduction, 10° of lateral tilt, and variable degrees of Trendelenburg) on an operative traction table, with or without a well-padded perineal post. General anesthesia is used for muscular relaxation without paralysis. The operative hip is prepared, and traction is applied until 1 cm of joint space is achieved. Alternatively, a spinal needle may be used to release the seal if distraction is not easily obtained. Access to the hip joint is accomplished through 2 standard arthroscopic portals: anterolateral and midanterior portals. In revision cases and in patients with poor tissue quality, the resistance encountered with cannula penetration through the capsule is typically reduced. Intra-articular visualization is achieved with a 70° arthroscope (Smith & Nephew, Andover, MA) (Video 1).
Intra-articular Assessment
Both the central and peripheral compartments should be inspected for possible labral tears, acetabular and femoral head chondral lesions (Fig 1), loose bodies, adhesions, and residual cam and/or pincer deformities. After intra-articular inspection is performed and all injuries and pathologies are assessed and managed, attention is turned to the capsule.
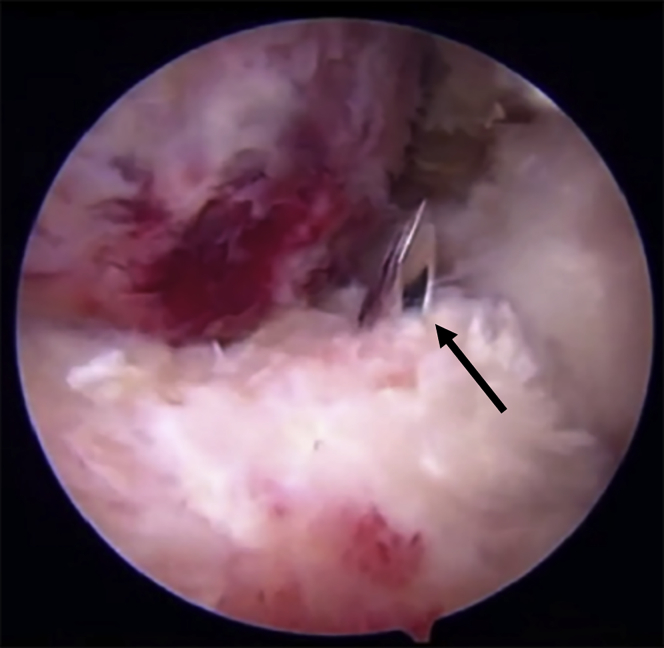
Fig 1.
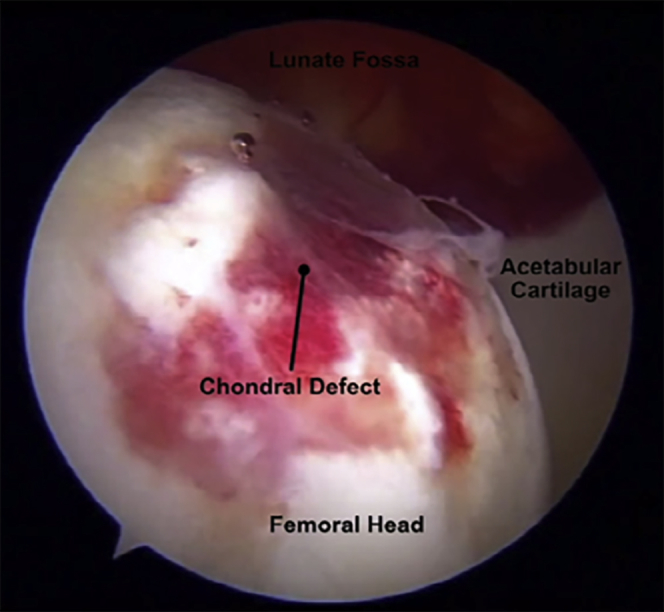
A large femoral head chondral lesion related to instability after hip arthroscopy viewed through an anterolateral viewing portal in the left hip.
Capsular Repair and Augmentation
The capsular repair is performed using a suture passer device and a tissue penetrator to restore the watertight capsule with a combination of multiple ultrahigh-molecular-weight-polyethylene sutures (No. 2 Ultrabraid [Smith & Nephew] and No. 2 Vicryl [Ethicon, Cincinnati, OH]). By use of a midanterior viewing portal and anterolateral working portal, a monofilament suture passer is inserted through a partially threaded, 8.5 × 11–cm cannula (Smith & Nephew), passed through the distal leaflet of the capsule with the Accupass Suture Shuttle (Smith & Nephew), and then deployed and captured with a suture retriever (Smith & Nephew), as shown in Figures 2 and 3. The braided suture is passed through the loop of the monofilament suture, which is retrieved, carrying the braided suture through the capsule, and a standard extracapsular knot is tied (Fig 4). This process is repeated as the stitches are sequentially passed and tied from posterolateral to anteromedial (Fig 5). To place the more anterior sutures, the anterolateral portal is repositioned more anteriorly to have a better angle for suture passing and tying. In cases with large capsular deficiency or laxity and revision cases, more sutures are typically required beyond the usual 3 to 4 used to close the interportal capsulotomy.
Fig 2.
By use of a midanterior viewing portal and anterolateral working portal in the left hip, the Accupass Suture Shuttle (arrow) is inserted through the distal leaflet of the capsule to allow for suture passage and closure of the interportal capsulotomy.
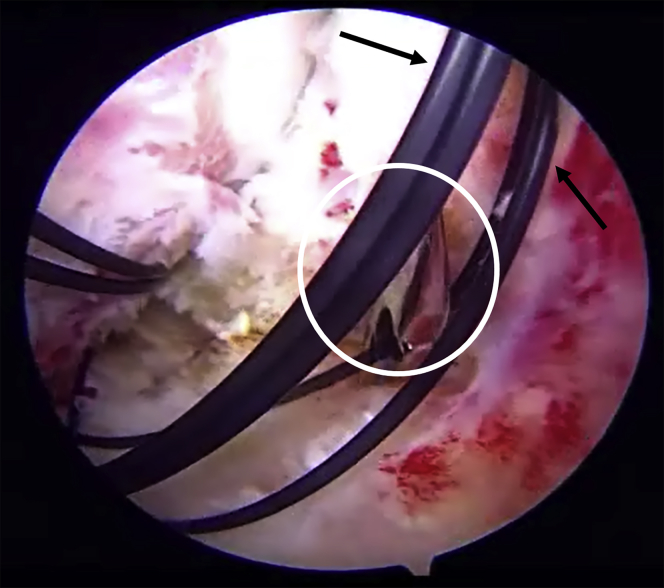
Fig 3.
Through the anterolateral working portal, the suture retriever (circle) is used to pass the monofilament suture (arrows) through the proximal leaflet of the capsule while viewing from the midanterior portal in the left hip. The monofilament suture is subsequently used to pass nonabsorbable No. 2 braided and No. 2 Vicryl sutures for closure of the interportal capsulotomy.
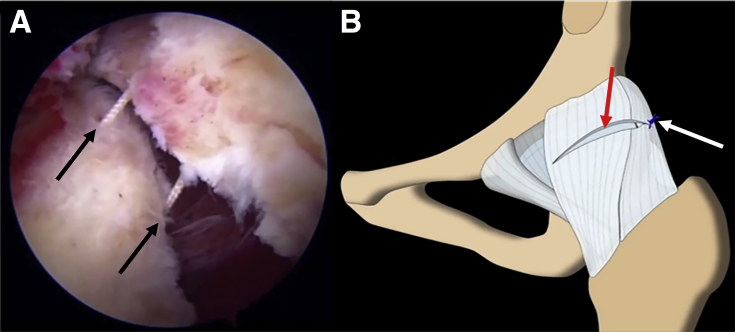
Fig 4.
(A) Capsular repair (arrows) in a left hip is accomplished through a midanterior viewing portal and anterolateral working portal. (B) A combination of nonabsorbable No. 2 braided and No. 2 Vicryl sutures is used to repair the interportal capsulotomy (red arrow) with a series of simple sutures (blue thread), beginning posterolaterally (white arrow).
Fig 5.
(A) Arthroscopic view of the monofilament suture (black arrow) being pulled back and passing a No. 2 Vicryl suture or No. 2 nonabsorbable braided suture (white arrow) through the capsule leaflets in the left hip. (B) Continuation of the capsular repair and closure of the interportal capsulotomy using the same midanterior viewing portal and anterolateral working portal.
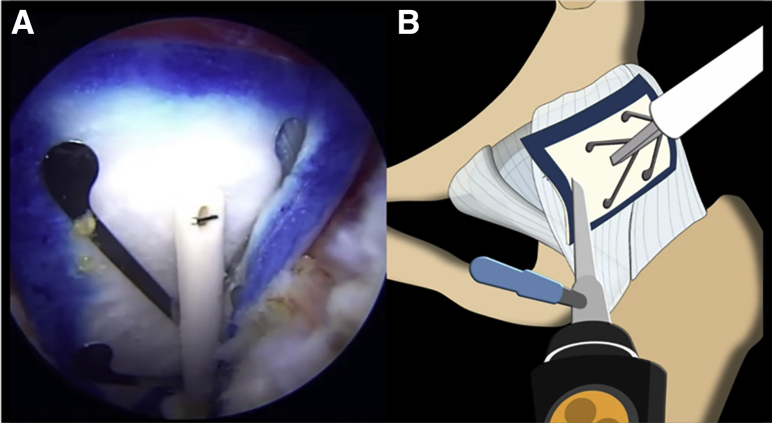
After complete capsule repair is performed, if the capsule is approximated but somewhat tenuous, a 25 × 31–mm bovine bioinductive implant (Regeneten; Smith & Nephew) is introduced through a new posterolateral portal with a delivering device and is placed over the site of repair and tenuous capsule (Fig 6). A tendon stapler is inserted through the anterolateral portal, and the implant is provisionally secured to the capsule with absorbable polylactic acid copolymer staples (Smith & Nephew). It is important to note that these staples should be placed within the footprint of the bioinductive implant indicated by the blue border. After the implant is provisionally secured to the capsule, the delivering device is removed and additional staples are placed to achieve adequate stability of the implant, with 7 to 9 staples typically being sufficient to secure the implant to the capsule (Fig 7).
Fig 6.
(A) Arthroscopic view of the Regeneten bioinductive implant placed over the capsular repair and site of the interportal capsulotomy closure in the left hip. (B) The scope is placed in the midanterior viewing portal, while the implant is introduced through a new posterolateral working portal, leaving the anterolateral working portal open for subsequent staple fixation of the implant.
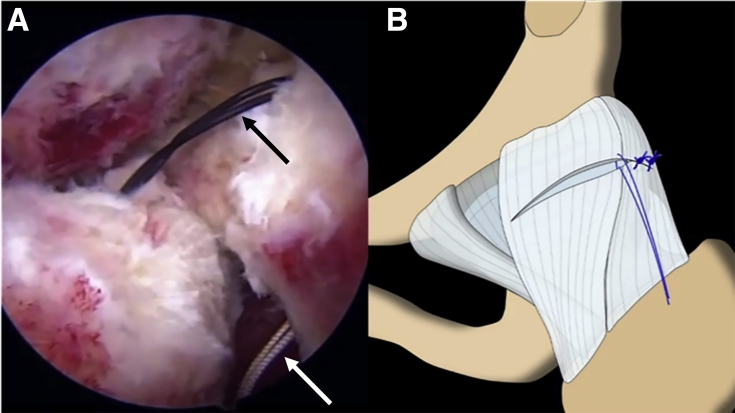
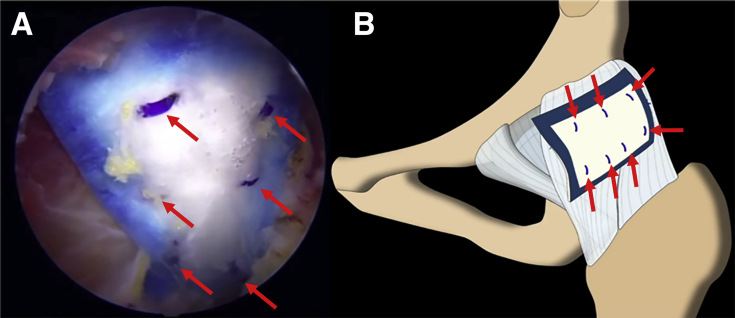
Fig 7.
(A) Arthroscopic view through the midanterior portal in the left hip showing the final construct after staple fixation (arrows) of the Regeneten bioinductive implant. (B) All fixation staples (arrows) are within the footprint of the implant as outlined by the blue border.
Discussion
In this technical note, we have presented a safe and reproducible technique for capsule repair and augmentation with a bioinductive implant to stimulate adequate capsule healing after hip arthroscopy and a postoperative subluxation event. Recent data have shown that residual microinstability due to an inadequately repaired iliofemoral ligament defect or capsulotomy can negatively impact outcomes, despite adequate treatment of intra-articular pathology.2,5 Patients typically reported vague and persistent pain in the hip, as well as a sensation of laxity or uncontrolled translation in the joint, resulting in inferior patient-reported outcome measures (PROMs).2 In contrast, complete capsule closure resulted in improved athletic performance and reduced rates of revision surgery compared with patients treated with partial capsular closure. In more rare but more severe cases, patients can present with frank dislocation of the hip.3 In a systematic review, Yeung et al.3 reported 10 cases of hip dislocation after hip arthroscopy, which correlated with capsular deficiency and laxity including unrepaired capsulotomy (77.8%), female sex (77.8%), iliopsoas release (33.3%), acetabular dysplasia (22.2%), and ligamentous laxity (11.1%). In patients with borderline dysplasia undergoing hip arthroscopy, Larson et al.7 reported a higher percentage of good or excellent outcomes (73% vs 53%) and lower failure rates (18% vs 40%) for patients who underwent capsular plication versus patients who did not. Regarding EDS, Larson et al.8 reported on a series of 16 hips, showing increased instability evidenced by an ability to distract the hip with manual traction under fluoroscopy. In these patients, PROM assessment showed significant improvements in modified Harris Hip Scores, 12-Item Short Form Health Surveys, and Visual Analog Scales in EDS patients undergoing arthroscopy with capsular plication.8
These clinical findings are supported by prior anatomic investigations. During hip arthroscopy, the interportal capsulotomy is usually performed between the 10- and 2-o’clock positions, 1 cm distal to the labrum, followed by the longitudinal limb of the T-capsulotomy starting at the 2-o’clock position when a T-capsulotomy is performed.5,6 Philippon et al.6 showed, in an anatomic study, that the thickest portion of the capsule is at the 2-o’clock position, between 0 and 15 mm from the labrum, consistent with the origin of the iliofemoral ligament, which has a primary function in the restriction of extension and external rotation, preventing anterior instability of the hip. Given that this is potentially the thickest portion of the iliofemoral ligament, it is essential that this be adequately repaired to restore stability.
In cases of generalized ligamentous laxity, insufficient capsular tissue, or postoperative capsular disruption, primary repair with the addition of a bovine bioinductive graft can be used to augment the repair when the tissue can be approximated but is somewhat tenuous. The graft does not mechanically strengthen the repair but instead improves the biological environment surrounding the capsule by enhancing local vascularity. Cases that require mechanical support or those with large irreparable defects require capsular reconstruction when significant osseous dysplastic features are not present. This patch has been used previously for other indications such as in the technique described by Gulledge and Makhni,9 who successfully used the patch to augment open gluteus medius repair, with good outcomes, suggesting that the patch may be useful in the repair of other soft tissues. Similarly, Bokor et al.10 and Schlegel et al.11 have shown promising results with this type of implant in partial rotator cuff tears, after arthroscopic subacromial decompression without repair of the tears, with magnetic resonance imaging showing new tissue formation, no tear progression, and improvements in PROM scores. More recently, Thon et al.12 reported a 96% healing rate, confirmed by ultrasound and magnetic resonance imaging, with no reported complications in patients undergoing large or massive rotator cuff tear repair augmented with this bioinductive implant.
There are some limitations and inherent risks to the described technique. The risks of surgery are similar to those of other hip arthroscopy procedures, including infection, persistent pain and instability, and the potential need for subsequent revision surgery. The bioinductive implant does not have any inherent strength, so in some cases, when a gap in the capsular repair is present, a capsular reconstruction may be more biomechanically appropriate than this bioinductive patch. In addition, this technique does require a third portal such as a posterolateral portal or distal anterolateral portal, requiring greater operative time and technical demand. Finally, the polylactic acid staples can sometimes become dislodged, requiring retrieval, which can also lengthen the operative time.
We have described the successful use of this bioinductive patch to repair and augment the closure of the interportal capsulotomy in the context of poor tissue quality and a postoperative hip subluxation event. However, more extensive research and long-term outcomes are required to further characterize the preoperative and intraoperative indications for the use of this bioinductive implant to enhance the healing capacity of the capsule in revision cases and a subset of primary cases.
Footnotes
The authors report the following potential conflicts of interest or sources of funding: C.M.L. is a consultant for Smith & Nephew. S.F. is a paid consultant for and receives research support from AlloSource; is a board or committee member of the American Orthopaedic Society for Sports Medicine; is on the editorial or governing board of Arthroscopy; is a paid consultant for and receives IP royalties from Artoss; is a paid consultant for and receives IP royalties and research support from Smith & Nephew; is a paid presenter or speaker for Ossur; is a paid consultant for Trice; and receives IP royalties from Trice Orthopaedics. J.C. is a consultant for and receives research support from Smith & Nephew, ConMed, and Arthrex. Full ICMJE author disclosure forms are available for this article online, as supplementary material.
Supplementary Data
Intraoperatively, the central compartment of the left hip is inspected for possible recurrent labral tears, chondral defects, adhesions, and loose bodies, which are adequately removed. The capsule repair is achieved with a midanterior viewing portal and anterolateral working portal. An Accupass Suture Shuttle is inserted through a partially threaded, 8.5 × 11–cm cannula and passed through the distal leaflet of the capsule to allow passage of a monofilament suture, which is deployed in the proximal leaflet with a suture retriever. A braided suture is then passed through the loop of the monofilament suture, which is retrieved, carrying the braided suture through the capsule, and a standard extracapsular knot is tied. This process is repeated as the stitches are sequentially passed and tied from posterolateral to anteromedial. The working portal may be repositioned to reach very medial aspects of the capsulotomy. A total of 6 No. 2 Ultrabraid sutures and 4 No. 2 Vicryl sutures are used to close the capsule. A Regeneten bioinductive implant is inserted through a new posterolateral portal to augment the capsule repair while viewing from the midanterior portal. The implant is placed over the capsule repair and provisionally secured with a tendon stapler, inserted through the anterolateral portal. The staples are placed within the blue footprint of the implant. After adequate fixation of the implant onto the capsule, the insertion device is removed, and further staples are placed to achieve good stability of the implant over the capsule repair.
References
- 1.McCormick F., Slikker W., III, Harris J.D. Evidence of capsular defect following hip arthroscopy. Knee Surg Sports Traumatol Arthrosc. 2014;22:902–905. doi: 10.1007/s00167-013-2591-z. [DOI] [PubMed] [Google Scholar]
- 2.Frank R.M., Lee S., Bush-Joseph C.A., Kelly B.T., Salata M.J., Nho S.J. Improved outcomes after hip arthroscopic surgery in patients undergoing T-capsulotomy with complete repair versus partial repair for femoroacetabular impingement: A comparative matched-pair analysis. Am J Sports Med. 2014;42:2634–2642. doi: 10.1177/0363546514548017. [DOI] [PubMed] [Google Scholar]
- 3.Yeung M., Memon M., Simunovic N., Belzile E., Philippon M.J., Ayeni O.R. Gross instability after hip arthroscopy: An analysis of case reports evaluating surgical and patient factors. Arthroscopy. 2016;32:1196–1204.e1. doi: 10.1016/j.arthro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Westermann R.W., Bessette M.C., Lynch T.S., Rosneck J. Does closure of the capsule impact outcomes in hip arthroscopy? A systematic review of comparative studies. Iowa Orthop J. 2018;38:93–99. [PMC free article] [PubMed] [Google Scholar]
- 5.Nho S.J., Beck E.C., Kunze K.N., Okoroha K., Suppauksorn S. Contemporary management of the hip capsule during arthroscopic hip preservation surgery. Curr Rev Musculoskelet Med. 2019:260–270. doi: 10.1007/s12178-019-09564-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philippon M.J., Michalski M.P., Campbell K.J. A quantitative analysis of hip capsular thickness. Knee Surg Sports Traumatol Arthrosc. 2015;23:2548–2553. doi: 10.1007/s00167-014-3030-5. [DOI] [PubMed] [Google Scholar]
- 7.Larson C.M., Ross J.R., Stone R.M. Arthroscopic management of dysplastic hip deformities: Predictors of success and failures with comparison to an arthroscopic FAI cohort. Am J Sports Med. 2016;44:447–453. doi: 10.1177/0363546515613068. [DOI] [PubMed] [Google Scholar]
- 8.Larson C.M., Stone R.M., Grossi E.F., Giveans M.R., Cornelsen G.D. Ehlers-Danlos syndrome: Arthroscopic management for extreme soft-tissue hip instability. Arthroscopy. 2015;31:2287–2294. doi: 10.1016/j.arthro.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Gulledge C.M., Makhni E.C. Open gluteus medius and minimus repair with double-row technique and bioinductive implant augmentation. Arthrosc Tech. 2019;8:e585–e589. doi: 10.1016/j.eats.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bokor D.J., Sonnabend D., Deady L. Evidence of healing of partial-thickness rotator cuff tears following arthroscopic augmentation with a collagen implant: A 2-year MRI follow-up. Muscles Ligaments Tendons J. 2016;6:16–25. doi: 10.11138/mltj/2016.6.1.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlegel T.F., Abrams J.S., Bushnell B.D., Brock J.L., Ho C.P. Radiologic and clinical evaluation of a bioabsorbable collagen implant to treat partial-thickness tears: A prospective multicenter study. J Shoulder Elbow Surg. 2018;27:242–251. doi: 10.1016/j.jse.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Thon S.G., O'Malley L., III, O'Brien M.J., Savoie F.H., III Evaluation of healing rates and safety with a bioinductive collagen patch for large and massive rotator cuff tears: 2-Year safety and clinical outcomes. Am J Sports Med. 2019;47:1901–1908. doi: 10.1177/0363546519850795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperatively, the central compartment of the left hip is inspected for possible recurrent labral tears, chondral defects, adhesions, and loose bodies, which are adequately removed. The capsule repair is achieved with a midanterior viewing portal and anterolateral working portal. An Accupass Suture Shuttle is inserted through a partially threaded, 8.5 × 11–cm cannula and passed through the distal leaflet of the capsule to allow passage of a monofilament suture, which is deployed in the proximal leaflet with a suture retriever. A braided suture is then passed through the loop of the monofilament suture, which is retrieved, carrying the braided suture through the capsule, and a standard extracapsular knot is tied. This process is repeated as the stitches are sequentially passed and tied from posterolateral to anteromedial. The working portal may be repositioned to reach very medial aspects of the capsulotomy. A total of 6 No. 2 Ultrabraid sutures and 4 No. 2 Vicryl sutures are used to close the capsule. A Regeneten bioinductive implant is inserted through a new posterolateral portal to augment the capsule repair while viewing from the midanterior portal. The implant is placed over the capsule repair and provisionally secured with a tendon stapler, inserted through the anterolateral portal. The staples are placed within the blue footprint of the implant. After adequate fixation of the implant onto the capsule, the insertion device is removed, and further staples are placed to achieve good stability of the implant over the capsule repair.