Abstract
Published in 2009, the Saturation Model suggested that there were limits to which androgen encouraged growth of the prostate. This was, in particular, applied to prostate cancer, where conventional wisdom since Huggins has considered it almost taboo for a patient being treated with cancer to receive testosterone replacement therapy (TRT). Since then, several studies began to investigate the application of TRT in, at first, mild and stable prostate cancer patients. While early reports seem promising, the validity of the Saturation Model had not been addressed. The current review investigates the evidence synthesis behind the Saturation Model, based on its original publication where it was presented. The evidence reviewed includes in vitro, in vivo and clinical studies that were referenced as the basis when the model was presented. Despite promising associations, the evidence employed were troublingly taken out of context in many cases and applied freely in cases where it would be unwise to do so. In light of some shortcomings in evidence synthesis, we advise some caution when applying the Saturation Model in prostate cancer.
Keywords: Androgen receptor, Androgens, Hypogonadism, Prostatic neoplasms, Testosterone
INTRODUCTION
In 2009, with the paper titled “Shifting the paradigm of testosterone and prostate cancer: the Saturation Model and the limits of androgen-dependent growth”, Abraham Morgentaler laid siege against the long-held belief that testosterone replacement therapy was harmful to prostate cancer [1]. The proposal, a rallying cry, to investigate the potential benefits in treating hypogonadism in the setting of the prostate cancer patient was bold, audacious, and questioned the foundations of prostate cancer, established by the seminal studies of Charles Huggins. Huggins's study had been the pioneering work describing the hormonal nature of prostate cancer [2]. As a study no less laureated by the Nobel Prize, it served as an anchor for subsequent hormonal treatment of prostate cancer. Yet here, Morgentaler boldly proposed a treatment direction almost counter intuitive to modern urologists.
Further questioning the conventionally held belief, Morgentaler [3] suggested that there was insufficient proof to consider androgens in the setting of prostate cancer harmful. In lectures since, and especially in his recounting of his long career-wide quest to destigmatize testosterone replacement therapy, he described in detail how he had first re-examined Huggins's paper [4]. Indeed, to modern scientists accustomed to more rigorous statistical methods, Huggins's finding against the use of androgens in prostate cancer patients was based upon a single patient.
Emboldened by this lack of evidence within the cornerstone of modern hormonal prostate cancer treatment, Morgentaler and Traish [1] gathered convincing evidence in in vivo studies describing the limited availability of androgen receptors (AR), in animal models that measured prostate growth that mirrored this limited availability, and finally in human studies that also seemed to corroborate these findings. Finally, in this important review, Morgentaler presented the Saturation Model, which detailed how at low androgen concentrations, tumor growth seemed to increase with serum testosterone concentration, as per conventional wisdom, yet beyond a certain threshold—which encompasses physiologically normal androgen states—cancer growth does not follow this correlation, but instead, becomes ‘Saturated’, no longer responding to trophic signals commanded by serum testosterone. Since then, several studies have come forward, cautiously collecting evidence, supporting the Saturation Model [5,6,7].
However, concerns also arose against the Saturation Model [8,9]. While most would seem cautionary in nature against any such bold statement that contests conventional wisdom, neither did those studies which had since supported the Saturation Model sufficiently address these concerns as well [10,11,12,13,14,15]. While all these studies finished with a caveat suggesting the need for the usual long term, large scale follow-up, they still did not address whether or not the Saturation Model itself could be considered valid as a true oncological model upon which serum androgen interacts with prostate cancer.
Here we look back on the evidence which Morgentaler used to present the Saturation Model, specifically, those which were used in the 2009 paper which first proposed this model.
EVIDENCE ACQUISITION
1. The saturation of AR
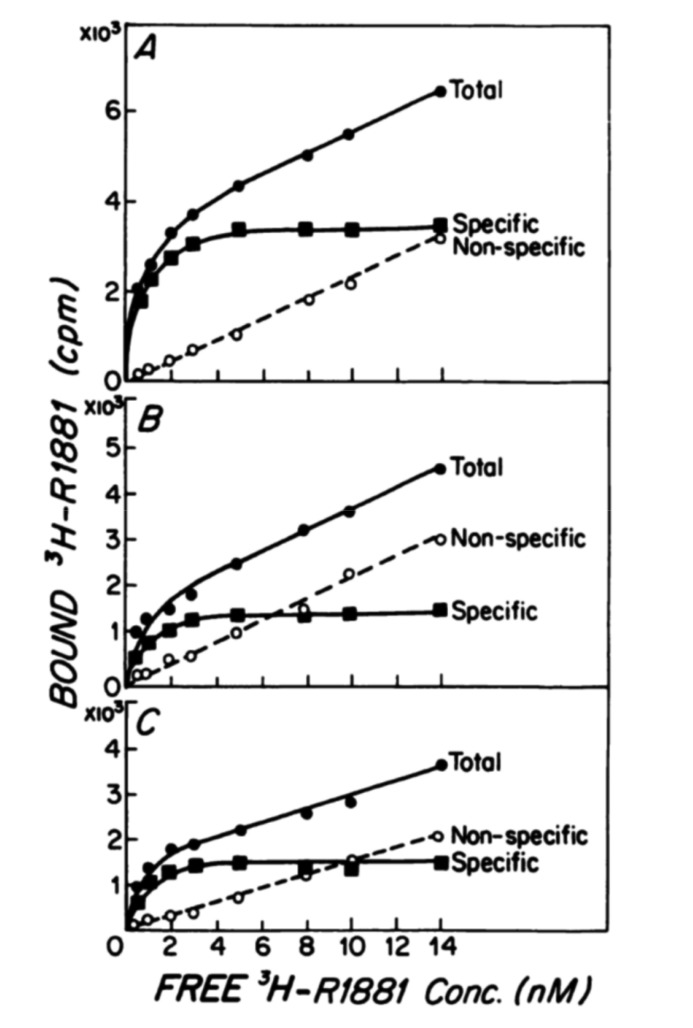
In the 2009 paper, Morgentaler and Traish [1] describes the experiments of Ho et al. [16] published in 1985 on AR levels pertaining to each prostate lobe of the Noble Rat by radioimmune assay (RIA). Fig. 1, reproduced as is from the original article with little to no changes, was described by Morgentaler as the binding of androgen to AR demonstrating a saturation curve [16].
Fig. 1. Original graph from Ho et al. [16] published in 1985 describes the binding capacity for cytosolic androgen receptors (ARs) from three differently designated lobes of the rat prostate, as assessed by radio immune assay. Thus, these values represent the AR capacity within the cytosol in three different lobes, as the aim of the study was to assess the distribution of AR in the prostate. The saturating Gompertzian curve shown is what is naturally expected in such experiments, as new AR formation has ceased in the fixed and minced supernatant that was investigated. Original graph from Ho et al. J Androl 1985;6:279-90 [16], with permission of John Wiley & Sons.
This is the first problem with the evidence synthesis of the paper. While in the original 1985 paper by Ho et al. [16], Fig. 1 does illustrate the binding of androgen to AR, it is not the binding of an active cell system. The experiments described detail that animals were implanted with silastic testosterone pellets, after which the lobes were harvested, then the tissue was minced, homogenized and centrifuged for RIA. The methods further described how the experiments were performed in ice-water and the efforts to further centrifuge the extracts to separate cytosolic and nuclear extracts. Simply put, this is not the response of a live cell.
This may not seem, at first, to contradict its use as evidence for the Saturation Model, as the premise employed that the AR does saturate seems unviolated. However, this takes the experiment out of context, in which the purpose of the experiment itself was not to identify whether or not saturation occurs, but to identify the levels of AR produced in different lobes of the prostate from each level of testosterone implanted. The experiment, in fact, depended on the biologically inert system to be saturated in its frozen state to identify the produced equilibrium of binding.
In a live system, cells would be preserved, not fixed. An experiment would, then, apply testosterone to a live plate of prostate cells cultured and the changes in AR documented. However, even this experiment would be naïve in representing the actual interaction of the AR and its complex dynamics within the prostate cell. Numerous factors act upon several different dimensions of factors, from cofactors, CAG repeats, epigenetics, cross signaling with other steroid hormones—to say nothing of testosterone, or even androgens alone, as well as post translational modifications and degradation [17,18,19,20]. As such, a simple Gompertzian model would be insufficient to express the relationship between testosterone and AR [21,22].
But pertaining to this issue at hand, saturation of AR was never the issue of the paper by Ho et al. [16]. The experiment was designed to be saturated, not because such relationship accurately portrayed the dynamics between androgens and their receptor, but because it was an experiment designed to find the fixed amount of AR within a frozen tissue.
2. Studies in animal models
The animal model that was the focus of the next segment in Morgentaler and Traish's 2009 paper [1] was based on evidence provided by Wright et al. [23] in a 1999 paper “Androgen-induced regrowth in the castrated rat ventral prostate: role of 5α-reductase”.
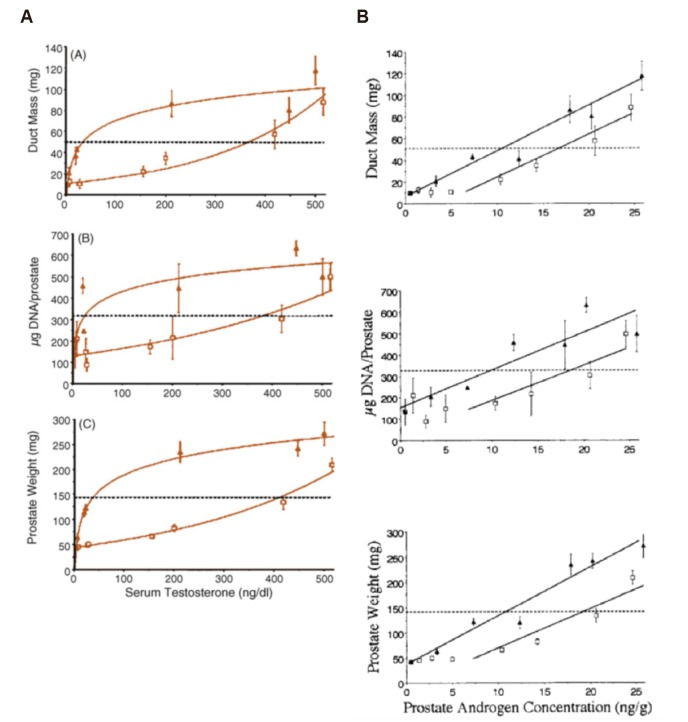
In Morgentaler and Traish's paper [1], the experiment is shown with Fig. 2A and the upper curve, demarked by the filled triangles are described as follows: ‘A steep initial rise is seen at very low T concentrations, followed by minimal further rise over a wide range of increasing T concentrations. Note that a straight, horizontal line can be drawn through most T values >50 ng/dL, suggesting saturation with regard to serum T’, an evidence of saturation in vivo.
Fig. 2. (A) One set of graphs that was reproduced in Morgentaler and Traish [1], originally from Wright et al. [23], shows various experimental prostatic indices plotted against serum testosterone. The filled triangles, which was interpreted as a Gompertzian curve, represent castrated rats implanted with testosterone pellets, while the open squares represent their counterpart rats also given finasteride. Hence, the graph depicts different aspects of androgens on prostatic growth. Considering that rats given finasteride (open squares) still had active testosterone, despite dihydrotestosterone being curtailed does not concur with the Saturation Model. (B) Originally from Wright et al. [23], but neglected in Morgentaler and Traish [1], shows the same prostatic indices for the same rats, now plotted against prostatic androgen concentration. There is no hint of saturation here. Original graphs from Morgentaler and Traish. Eur Urol 2009;55:310–20 [1], with permission of Elsevier, and Wright et al. Endocrinology 1999;140:4509-15 [23], with permission of Oxford University Press.
The original study was an investigation to differentiate the in vivo effect of testosterone versus dihydrotestosterone (DHT), using the 5alpha-reductase inhibitor finasteride. Keen observers will already note that the upper curve is the effect of DHT and testosterone, while the lower curve is that with finasteride. Hence, the difference between the two curves, roughly, denotes the effect of finasteride, and the saturation occurs with DHT, whereas arguably, the lower curve that does not show saturation still represents the effect of unconverted testosterone.
Of primary importance, however, is that this graph shows serum testosterone against prostatic changes. An alternative graph is also presented in the study, not shown in Morgentaler's paper, of prostatic androgen concentration versus the same prostatic changes (Fig. 2B) [1,23]. To be blunt, this does not show saturation.
In fact, from these two sets of graphs, one can already understand that the purpose of this study was to identify the mechanisms of finasteride and 5alpha-reductase enzyme. The saturation that was selected to represent in vivo changes of the prostate from serum testosterone, was merely the outcome of serum androgens, in contrast to intraprostatic androgens unaffected by finasteride. Furthermore, one could venture to say that considering the lower curve, which is also androgen (Testosterone, but not DHT) that saturation, as described by the Saturation Model, is not present.
3. Human studies: effect of endogenous testosterone concentration on prostate-specific antigen
Bhasin et al. [24,25] published two studies describing two separate groups of patients, old and young, and their respective responses to varying doses of muscle injected testosterone. The protocol of both studies were identical with 4-week control period, a 20-week treatment period, and a 16-week recovery period, with treatment consisting of monthly injections of long acting gonadotropin releasing hormone agonist to suppress endogenous testosterone production, along with varying doses of testosterone enanthate. The study was aimed to identify the effects in each subgroup of men in their various phenotypical changes, mostly pertaining to anabolic effects, from testosterone enanthate injection. As a safety factor, serum prostate-specific antigen (PSA) was also measured.
The Saturation Model paper shows two graphs that were adapted from these studies, as neither graphs were present in the original papers (Fig. 3) [1,25]. Hence, while the data was from the papers by Bhasin et al. [24,25] the decision in composition of each graph was up to the authors of the Saturation Model. In the graphs we see a significant increase in serum testosterone, despite what seems to be apparently no change, or, arguably even a decrease in serum PSA, affirming the Saturation Model even in the clinical setting.
Fig. 3. (A) Original graphs from Bhasin et al. [25] show increase in change in prostate-specific antigen (PSA) with higher serum testosterone doses. These patients were maintained on gonadotropin releasing hormone agonists while treated with IM testosterone enanthate injections. While only injection doses of 300 mg were shown to have statistically significant changes compared to 25 mg injections (p<0.05), it must be kept in mind that these are different patients measured at the same time point on different doses. Further maintenance of doses beyond 20 weeks of treatment, or even long term follow-up is not presented here. (B) Despite the drastically different scales of means and variances presented by serum testosterone and PSA, and despite the original authors having presented graphs in their original paper, Morgentaler and Traish [1] reconstructed these measurements on questionable scales. *p<0.0001. Original graph from Bhasin et al. J Clin Endocrinol Metab 2005;90:678-88 [25], with permission of Oxford University Press. Original graph from Morgentaler and Traish. Eur Urol 2009;55:310–20 [1], with permission of Elsevier.
Both graphs, however, show a curious choice in how they were composed. The left-hand y-axis depicting levels of serum testosterone at week 20 of treatment in ng/dL, while the right-hand y-axis depicts serum PSA at this same period in ng/mL. These are two different measurements and are not directly comparable. If one were to compare these changes, i.e. change of serum testosterone versus PSA, one must consider the means and variances of these variables. In the study on young men, dose that produced equivalent to baseline was at 125 mg with baseline testosterone at 553±53 ng/dL, becoming 570±75 ng/dL at week 16 when it was last given (p=0.7425) [24]. In contrast, the highest dose, 600 mg, showed a change from a baseline testosterone of 632±63 ng/dL to 2,370±150 ng/dL (p=0.0001). In contrast, serum PSA at 125 mg injection also showed no significant change from 0.7±0.1 ng/mL to 0.8±0.1 ng/mL at week 20 (p=0.1721), while at the highest dose of 600 mg, it showed a change from 0.5±0.1 ng/mL to 0.7±0.1 ng/mL (p=0.001).
Thus, with a high level of serum testosterone, serum PSA also showed significant increase. The results were not duplicated in the older group, with baseline serum PSA levels varying more drastically in this case [25]. However, at 300 mg injections, the same reinterpretation as was made with younger men were also observed, with serum PSA levels increasing from 1.7±0.2 ng/mL to 2.6±0.4 ng/mL (p<0.001) at this dose.
CHARLES HUGGINS AND THE STUDIES ON PROSTATIC CANCER
The original studies by Charges Huggins were published in 1941 in a series of three papers, with Huggins as the primary author, and variably included William Scott and Clarence Hodges [2,26,27]. The first paper, “The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate” is often the most cited, but the second paper, subtitled “The effects of castration on advanced carcinoma of the prostate gland”, and the third paper, subtitled “The effects of fever, of desoxycorticosterone and of estrogen on clinical patients with metastatic carcinoma of the prostate” constitute the whole of the study.
Morgentaler often lectures the Saturation Model, citing the work of Huggins, making note that the notion of testosterone as ‘food for a hungry tumor’ was based on a single anecdotal case described in the first paper above [28,29]. The entire work itself was primarily focused on identifying how a decrease in testosterone (by castration) could lead to a decrease in prostatic cancer (if p, then q), and not the logical inverse statement, i.e. increase in testosterone leading to an increase in testosterone (if ~p, then ~q). As Morgentaler rightly assumed, these statements are not logically equivalent.
While the methods employed by Huggins during his active years indeed do not fit the statistical standards employed today, nevertheless, it is worth noting that the description of the single patient in the article encompassed an aside in the entire work of ‘Studies in Prostatic Cancer’, an addendum to bolster the thesis of the hormonal nature of prostate cancer.
However, regardless of the speculation as to whether it was the focus of Charles Huggins himself, what is similar in other respects to the aforementioned evidence in the second section of this article is that when employed to support the Saturation Model these results were taken out of context.
CURRENT STATE OF TESTOSTERONE REPLACEMENT THERAPY IN PROSTATE CANCER
Since its publication and widespread advocacy, the Saturation Model has slowly gained several adherents. At first tentatively, and then more robustly, this proposed supplement in prostate cancer patients has been slowly gaining ground. At the present several randomized control trials (RCTs) have been published compiling these outcomes.
A meta-analysis by Cui et al. [7] reviewed 22 RCTs involving 2,351 patients. The cited studies consisted of 11 studies the authors determined were ‘short-term’ which denoted they were less than 12 months, as well as 11 studies which were designated ‘long-term’, meaning they were observed for 12 to 36 months. While this seems like a significant number of cases that evaluated the equivalence between testosterone replacement therapy (TRT) and placebo in the prostate cancer patient, it is important to note that of these 22 studies included in the results, only 3 studies of 379 patients (191 receiving TRT and 188 in the control group) were involved in generating the most important outcome of prostate cancer recurrence in the long term. While individual studies all deserve merit, it must be stressed that the results are based on only 3 out of 191 patients who received TRT following prostate cancer, and while cases propounding a positive treatment for its effect could be established with such meta-analysis, a conclusion that suggests safety against an agent may be considered premature.
Similarly, a more recent publication, by Boyle et al. [6] applying the more modern convention of PRISMA guidelines to their meta-analysis also suggested that exogenous testosterone did not increase the risk of prostate cancer. However, as with Cui et al. [7], in the study by Boyle et al. [6] the study populations were small, with the number of patients receiving exogenous testosterone ranging from 6 to 234 patients. Curiously enough, the studies with 234 patients of transdermal testosterone versus 40 cases treated with placebo were included twice in this meta analysis [30,31]. A duplicate patient base is usually screened for in meta-analysis, but the authors might have missed this aspect of the two studies. However, despite these technical issues, we suggest that the population for comparing raw incidence of prostate cancer occurring is somewhat underpowering.
To put it in context, the incidence of prostate cancer is estimated as 73.7 per 100,000 as an age standardized rate in North America. If not even one of the individual studies that were summarized by the meta analysis allowed for even 1 per 1,000 naturally occurring cases of prostate cancer to compare with statistically, then one must conclude that the meta analysis is underpowered. Rare incidence meta analysis is a subject onto itself, requiring statistical considerations that might not have been applied in these instances [32].
This is a crucial weakness of meta analyses, and should be doubly cautious when investigating whether some particular treatment does not show some outcome. We are too often used to meta analyses with positive outcomes. These studies do not require such a strong power of investigation. Any likely candidate treatment under investigation that has already accumulated multiple RCTs would likely have a positive outcome that does not threaten the sensitivity of the study itself, regardless of whether such treatment is better than the alternative.
The question arises how one can investigate potential benefits or harm in the setting of prostate cancer. Simply, in comparison, a study to identify the potential harms or benefit of aspirin in prostate cancer would be a good model of meta analysis on which to model future investigations [33].
However, as such level of evidence is unavailable at this time, it would be wise to refrain from definitive statements. As the primary focus of this article was to review the evidence employed in constructing the Saturation Model, we conclude merely that there seems to be insufficient evidence to support TRT in prostate cancer based on the Saturation Model alone.
Further studies, of course, would be required to evaluate whether TRT is safe for patients with prostate cancer. But in the context of the evidence supporting the Saturation Model, it is questionable.
CONCLUSIONS
The Saturation Model which proposed that clinically relevant normalization of serum testosterone levels do not contribute to prostate cancer, since the effects on the prostate are saturated, is questionable. The evidence used in generating the model might have been taken out of context in some cases. Caution should be practiced when reducing something as complex as that between Testosterone and the Prostate to such generalization. As such, further evidence regarding the safety of TRT in prostate cancer must be acquired with much more caution.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1C1B5076536).
Footnotes
CONFLICTS OF INTEREST: The author has nothing to disclose.
- Conceptualization: Jin Wook Kim.
- Data curation: Jin Wook Kim.
- Formal analysis: Jin Wook Kim.
- Funding acquisition: Jin Wook Kim.
- Investigation: Jin Wook Kim.
- Methodology: Jin Wook Kim.
- Project administration: Jin Wook Kim.
- Resources: Jin Wook Kim.
- Supervision: Jin Wook Kim.
- Validation: Jin Wook Kim.
- Visualization: Jin Wook Kim.
- Writing—original draft: Jin Wook Kim.
- Writing—review & editing: Jin Wook Kim.
References
- 1.Morgentaler A, Traish AM. Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol. 2009;55:310–320. doi: 10.1016/j.eururo.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 3.Morgentaler A. Testosterone and prostate cancer: an historical perspective on a modern myth. Eur Urol. 2006;50:935–939. doi: 10.1016/j.eururo.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 4.Morgentaler A. Controversies and advances with testosterone therapy: a 40-year perspective. Urology. 2016;89:27–32. doi: 10.1016/j.urology.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 5.Bell MA, Campbell JD, Joice G, Sopko NA, Burnett AL. Shifting the paradigm of testosterone replacement therapy in prostate cancer. World J Mens Health. 2018;36:103–109. doi: 10.5534/wjmh.170007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyle P, Koechlin A, Bota M, d'Onofrio A, Zaridze DG, Perrin P, et al. Endogenous and exogenous testosterone and the risk of prostate cancer and increased prostate-specific antigen (PSA) level: a meta-analysis. BJU Int. 2016;118:731–741. doi: 10.1111/bju.13417. [DOI] [PubMed] [Google Scholar]
- 7.Cui Y, Zong H, Yan H, Zhang Y. The effect of testosterone replacement therapy on prostate cancer: a systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2014;17:132–143. doi: 10.1038/pcan.2013.60. [DOI] [PubMed] [Google Scholar]
- 8.Gleave ME, Klotz L. Testosterone therapy can be given to men with no concern that it will promote prostate cancer development or progression: con. J Urol. 2016;196:985–988. doi: 10.1016/j.juro.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Morales A. Testosterone and prostate health: debunking myths demands evidence, caution, and good clinical judgment. Eur Urol. 2006;50:895–897. doi: 10.1016/j.eururo.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 10.Rhoden EL, Morgentaler A. Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol. 2003;170(6 Pt 1):2348–2351. doi: 10.1097/01.ju.0000091104.71869.8e. [DOI] [PubMed] [Google Scholar]
- 11.Dobs AS, Morgentaler A. Does testosterone therapy increase the risk of prostate cancer? Endocr Pract. 2008;14:904–911. doi: 10.4158/EP.14.7.904. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, et al. Clinical review 1: adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:2560–2575. doi: 10.1210/jc.2009-2575. [DOI] [PubMed] [Google Scholar]
- 13.Kacker R, Hult M, San Francisco IF, Conners WP, Rojas PA, Dewolf WC, et al. Can testosterone therapy be offered to men on active surveillance for prostate cancer? Preliminary results. Asian J Androl. 2016;18:16–20. doi: 10.4103/1008-682X.160270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaplan AL, Hu JC, Morgentaler A, Mulhall JP, Schulman CC, Montorsi F. Testosterone therapy in men with prostate cancer. Eur Urol. 2016;69:894–903. doi: 10.1016/j.eururo.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yassin A, AlRumaihi K, Alzubaidi R, Alkadhi S, Al Ansari A. Testosterone, testosterone therapy and prostate cancer. Aging Male. 2019;22:219–227. doi: 10.1080/13685538.2018.1524456. [DOI] [PubMed] [Google Scholar]
- 16.Ho SM, Damassa D, Kwan PW, Seto HS, Leav I. Androgen receptor levels and androgen contents in the prostate lobes of intact and testosterone-treated Noble rats. J Androl. 1985;6:279–290. doi: 10.1002/j.1939-4640.1985.tb00846.x. [DOI] [PubMed] [Google Scholar]
- 17.Jacob S, Nayak S, Kakar R, Chaudhari UK, Joshi D, Vundinti BR, et al. A triad of telomerase, androgen receptor and early growth response 1 in prostate cancer cells. Cancer Biol Ther. 2016;17:439–448. doi: 10.1080/15384047.2016.1156255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson N, Neuwirt H, Puhr M, Klocker H, Eder IE. In vitro model systems to study androgen receptor signaling in prostate cancer. Endocr Relat Cancer. 2013;20:R49–R64. doi: 10.1530/ERC-12-0401. [DOI] [PubMed] [Google Scholar]
- 19.Kim JW, Oh MM, Yoon CY, Bae JH, Kim JJ, Moon DG. The effect of diet-induced insulin resistance on DNA methylation of the androgen receptor promoter in the penile cavernosal smooth muscle of mice. Asian J Androl. 2013;15:487–491. doi: 10.1038/aja.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vander Griend DJ, Litvinov IV, Isaacs JT. Conversion of androgen receptor signaling from a growth suppressor in normal prostate epithelial cells to an oncogene in prostate cancer cells involves a gain of function in c-Myc regulation. Int J Biol Sci. 2014;10:627–642. doi: 10.7150/ijbs.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park SW, Kim JH, Lee HJ, Shin DH, Lee SD, Yoon S. The expression of androgen receptor and its variants in human prostate cancer tissue according to disease status, and its prognostic significance. World J Mens Health. 2019;37:68–77. doi: 10.5534/wjmh.180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fujita K, Nonomura N. Role of androgen receptor in prostate cancer: a review. World J Mens Health. 2019;37:288–295. doi: 10.5534/wjmh.180040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright AS, Douglas RC, Thomas LN, Lazier CB, Rittmaster RS. Androgen-induced regrowth in the castrated rat ventral prostate: role of 5alpha-reductase. Endocrinology. 1999;140:4509–4515. doi: 10.1210/endo.140.10.7039. [DOI] [PubMed] [Google Scholar]
- 24.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, et al. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 25.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Mac RP, Lee M, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 26.Huggins C, Stevens RE, Jr, Hodges CV. Studies on prostatic cancer. II. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 27.Huggins C, Scott WW, Hodges CV. Studies on prostatic cancer. III. The effects of fever, of desoxycorticosterone and of estrogen on clinical patients with metastatic carcinoma of the prostate. J Urol. 1941;46:997–1006. [Google Scholar]
- 28.Morgentaler A. Guilt by association: a historical perspective on Huggins, testosterone therapy, and prostate cancer. J Sex Med. 2008;5:1834–1840. doi: 10.1111/j.1743-6109.2008.00889.x. [DOI] [PubMed] [Google Scholar]
- 29.European Association of Urology. Re-evaluating conventional wisdom on testosterone therapy [Internet] Arnhem: Uroweb; 2016. Mar 12, Available from: https://uroweb.org/re-evaluating-conventional-wisdom-on-testosterone-therapy/ [Google Scholar]
- 30.Morgentaler A, Benesh JA, Denes BS, Kan-Dobrosky N, Harb D, Miller MG. Factors influencing prostate-specific antigen response among men treated with testosterone therapy for 6 months. J Sex Med. 2014;11:2818–2825. doi: 10.1111/jsm.12657. [DOI] [PubMed] [Google Scholar]
- 31.Kaufman JM, Miller MG, Garwin JL, Fitzpatrick S, McWhirter C, Brennan JJ. Efficacy and safety study of 1.62% testosterone gel for the treatment of hypogonadal men. J Sex Med. 2011;8:2079–2089. doi: 10.1111/j.1743-6109.2011.02265.x. [DOI] [PubMed] [Google Scholar]
- 32.Spittal MJ, Pirkis J, Gurrin LC. Meta-analysis of incidence rate data in the presence of zero events. BMC Med Res Methodol. 2015;15:42. doi: 10.1186/s12874-015-0031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sauer CM, Myran DT, Costentin CE, Zwisler G, Safder T, Papatheodorou S, et al. Effect of long term aspirin use on the incidence of prostate cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol. 2018;132:66–75. doi: 10.1016/j.critrevonc.2018.09.013. [DOI] [PubMed] [Google Scholar]