Abstract
Purpose
The clinical behavior of prostate cancer differs by race and ethnicity; however, data on the Korean population are scarce. We assessed the long-term oncologic outcomes of clinically localized prostate cancer after radical prostatectomy in Korean men.
Materials and Methods
We analyzed 786 clinically localized prostate cancer patients who underwent radical prostatectomy, from June 1993 to June 2008. Kaplan–Meier survival curve analysis and log-rank test were used to assess the oncologic outcomes.
Results
The mean age of the patients was 64.9±6.6 years. Pelvic lymph node dissection was performed in 373 patients. Pathologic T and N stage cancer with local advancement and invasion were detected by radical prostatectomy in 307 and 22 patients, respectively. In total, 38 patients who underwent adjuvant therapy were excluded from the analysis of progression after biochemical recurrence (BCR), which occurred in 261 men. In total, 219 patients underwent salvage treatment. Local recurrence and distant metastasis occurred in 109 and 42 patients, respectively; 36 patients experienced metastasis with local recurrence. Castration-resistant prostate cancer developed in 22 patients, and overall and disease-specific mortality was noted in 148 and 23 patients, respectively. The median duration from operation to BCR, BCR to metastasis, and metastasis to disease-specific death was 25, 40, and 22 months, respectively.
Conclusions
We demonstrated the long-term prognosis of localized prostate cancer after radical prostatectomy among Koreans. Our results differ from those reported in the Western literature, with a lower prevalence of distant metastasis and shorter time to metastasis after BCR.
Keywords: Neoplasms, Prognosis, Prostate, Prostatectomy
INTRODUCTION
Currently available treatment options for clinically localized prostate cancer include radical prostatectomy with multiple approaches, radiation therapy, active surveillance, and energy ablative techniques. Each method has unique benefits and complications. Prostate cancer has a heterogeneous natural history and may be indolent without management; it also has a substantial risk for treatment-induced side effects. However, recent data from the Cancer of the Prostate Strategic Urological Research Endeavor (CaPSURE) database showed that radical prostatectomy is the most common option for managing clinically localized prostate cancer [1]. In a randomized trial, radical prostatectomy was reported to have a survival benefit compared with watchful waiting [2]. Furthermore, a recent study suggested that radical prostatectomy can be considered a treatment of choice, although multidisciplinary challenge should be considered in patients with high-risk prostate cancer [3].
Radical prostatectomy has been proven to provide excellent oncologic outcomes in most cases of clinically localized prostate cancers and may be associated with a reduced mortality rate in early prostate cancer of approximately 50% according to a previous randomized trial [4]. Although disease-specific and metastasis-free survival after radical prostatectomy have already been reported [5,6], these previous studies reported on a variety of patient characteristics, reflected the experience bias of individual physicians, and also primarily assessed data from Western countries. It is difficult to provide clinical counseling without a proper understanding of the clinical course of the disease. Therefore, the present study assessed the oncologic outcomes of clinically localized prostate cancer at about 10 years of follow-up after radical prostatectomy in Korea.
MATERIALS AND METHODS
In total, 786 patients with initially untreated, clinically localized, T1 and T2 stage prostate cancer underwent radical prostatectomy at our tertiary medical institution from June 1993 to June 2008. The 7th American Joint Committee on Cancer TNM classification system was used to classify the cases, and based on the review of core biopsy specimens, a Gleason grade was assigned according to the International Society of Urological Pathology (ISUP) grading [7]. Patients were stratified into three groups by biochemical recurrence (BCR) risk according to the D'Amico risk classification [8]. Men who received neoadjuvant hormone or radiation therapy were excluded. All patients preoperatively underwent a physical examination, chest radiography, routine blood tests, and a bone scan. All men included in this study had a negative bone scan result. Patients were postoperatively followed every 3 months for the first year and every 6 months thereafter with digital rectal examinations and serum prostate-specific antigen (PSA) tests. Ultrasonography, magnetic resonance imaging, or bone scans were optionally conducted to assess recurrence or distant metastasis. Medical records were reviewed to obtain information on the history of prostate cancer.
Isolated BCR is commonly used as a measure of cancer control after radical prostatectomy and was defined as PSA elevation and a serum level of at least 0.2 ng/mL with an increase over two consecutive measurements. Local recurrence was defined as a visible lesion on ultrasonography or magnetic resonance imaging with elevated serum PSA, or a histologically confirmed mass on a transrectal biopsy. Distant metastasis was confirmed as a visible metastatic lesion on an imaging study with an elevated serum PSA level, or a histologically confirmed tissue of a metastatic lesion.
Patients who underwent immediate adjuvant hormone or radiation therapy were excluded in disease progression assessment after BCR. Therefore, this study had no impact on adjuvant therapy during the time course of the progression. Among patients with castration-resistant metastatic cancer, various experimental therapies have been performed in some men but are considered to have an insignificant effect on survival [9]. All patients with disease progression had BCR without exception. Since antiandrogens were introduced in 2003, physicians have started to use hormone therapy in patients with elevated serum PSA levels, signs of disease progression, or in cases where it is believed that hormone treatment would be beneficial to patients. The cause of death was determined according to the medical records or mortality data extracted from the National Statistics Office.
Perioperative clinical features and pathologic features were analyzed. Kaplan–Meier survival curves were used to assess oncologic outcomes, and the statistical significance of the Kaplan–Meier survival curves was measured by using the log-rank test. The Cox regression model was fit to predict significant factors associated with disease progression. The primary outcomes were BCR, metastasis-free duration, castration-resistant prostate cancer (CRPC), disease-specific survival, and overall survival. All statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM Corp., Armonk, NY, USA). The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (IRB no. H-1805-069-946). As this was a retrospective study with anonymization of data, the IRB waived the requirement for informed consent from patients. All experiments were performed in accordance with relevant guidelines and regulations.
RESULTS
1. Overall findings
The 786 patients had a mean age of 64.9 years (Table 1). Open surgery was performed in 736 patients (93.6%), and the laparoscopic approach was used in 50 patients (6.4%). Pelvic lymph node dissection and nerve-sparing surgery were performed in 373 (47.5%) and 412 (52.4%) patients, respectively. More than half of the patients had serum PSA levels between 4 and 10 ng/mL, while approximately half of the patients had ISUP grade I at preoperative biopsy. Pathologic T stage was locally advanced at radical prostatectomy in 307 patients (39.1%), and lymph nodes were pathologically invaded in 22 patients (2.8%). In contrast to the clinical stage, approximately one-quarter of the patients were in the ISUP grade I category. The median follow-up duration was 117 months (interquartile range [IQR], 70–139 months).
Table 1. Baseline characteristics of the patients and pathologic outcomes (n=786).
| Variable | Value |
|---|---|
| Age (y) | 64.9±6.6 |
| PSA (ng/mL) | 12.4±12.9 |
| PSA group | |
| <4 | 60 (7.6) |
| ≥4 and <10 | 418 (53.2) |
| ≥10 and <20 | 200 (25.4) |
| ≥20 | 108 (13.7) |
| Clinical T stage | |
| ≤T1c | 186 (23.7) |
| T2a, T2b | 580 (73.8) |
| T2c | 20 (2.5) |
| ISUP grade group | |
| I | 372 (47.3) |
| II | 122 (15.5) |
| III | 115 (14.6) |
| IV–V | 177 (22.5) |
| D'Amico risk classification | |
| Low | 266 (33.8) |
| Intermediate | 274 (34.9) |
| High | 246 (31.3) |
| PLND | |
| None | 413 (52.5) |
| Yes | 373 (47.5) |
| Nerve saving | |
| None | 374 (47.6) |
| Unilateral | 115 (14.6) |
| Bilateral | 297 (37.8) |
| Pathologic ISUP grade group | |
| I | 209 (26.6) |
| II | 295 (37.5) |
| III | 153 (19.5) |
| IV–V | 119 (15.1) |
| Vanishing | 10 (1.3) |
| Pathologic outcomes | |
| T stage | |
| Vanishing | 10 (1.3) |
| T2a,b | 160 (20.4) |
| T2c | 309 (39.3) |
| T3a | 197 (25.1) |
| T3b | 109 (13.9) |
| T4 | 1 (0.1) |
| EPE | 268 (34.1) |
| SVI | 109 (13.9) |
| Surgical margin | 291 (37.0) |
| N stage | |
| N0 | 359 (45.7) |
| N1 | 22 (2.8) |
| Nx | 405 (51.5) |
Values are presented as mean±standard deviation or number (%).
PSA, prostate-specific antigen; ISUP, International Society of Urological Pathology; PLND, pelvic lymph node dissection; EPE, extraprostatic extension; SVI, seminal vesicle invasion.
2. Oncologic outcomes
Adjuvant radiotherapy (RT), RT with androgen deprivation therapy (ADT), and ADT were performed in 3, 28, and 7 patients, respectively. Among these 38 patients, PSA was elevated postoperatively in 22 patients, of whom 5 experienced local recurrence, 8 experienced distant metastases with local recurrence, 4 experienced distant metastases without local recurrence, and 6 experienced CRPC. All-cause mortality and disease-specific mortality was noted for 8 and 5 patients, respectively. These 38 patients were excluded from progression analysis after the BCR. Of the 748 patients, excluding the aforementioned 38, BCR occurred in 261 men (34.9%), and the median duration from radical prostatectomy to BCR was 25 months (IQR, 10.0–50.5 months). A total of 219 patients (29.3%) underwent recovery treatment that comprised RT (n=12), short-term ADT with RT (n=49), long-term ADT with RT (n=41), and ADT (n=117).
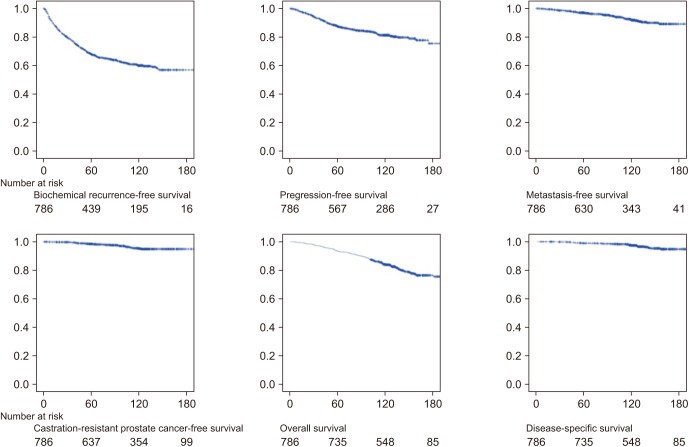
Fig. 1 depicts the Kaplan–Meier survival curves. Disease progression occurred in 115 (15.4%) patients, and the median duration from radical prostatectomy to progression and from BCR to progression was 45 months (IQR, 25.0–72.0 months) and 13 months (IQR, 1.0–36.0 months), respectively. Local recurrence and distant metastasis occurred in 109 (14.6%) and 42 (5.6%) patients, respectively, and 36 (4.8%) of these patients experienced metastasis with local recurrence. CRPC occurred in 22 patients (2.9%), and the median duration from BCR to CRPC was 48 months (IQR, 27.0–68.0 months). All-cause and disease-specific death occurred in 148 (19.8%) and 23 (3.1%) patients, respectively. The median duration from metastasis to disease-specific death was 22.0 months (IQR, 8.0–36.0 months).
Fig. 1. Kaplan–Meier survival curves.
3. Time course to death
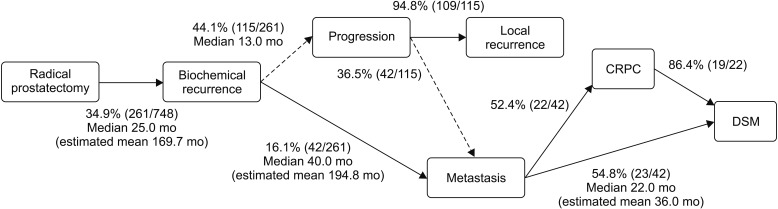
The 10-year BCR-free rate was 59.9% (Table 2), and the mean BCR-free duration was 169.7 months (Fig. 2). Disease progression occurred in 44.1% of patients with BCR. Distant metastasis occurred in 16.1% of patients with BCR, and the median duration from BCR to metastasis was 40.0 months (IQR, 10.0–80.8 months). Of the 42 patients with metastatic cancer, 52.4% experienced CRPC, and 19 (86.4%) of them died as a result of prostate cancer. Of the 42 patients with metastasis, 54.8% died of prostate cancer.
Table 2. Oncologic outcomes.
| Oncologic outcomes | 5-year | 10-year | 15-year |
|---|---|---|---|
| BCR-free survival | 68.10% | 59.9% | 56.8% |
| Progression-free survival | 88.20% | 82.8% | 77.0% |
| Metastasis-free survival | 97.40% | 93.5% | 91.1% |
| CRPC-free survival | 98.70% | 96.2% | 95.9% |
BCR, biochemical recurrence; CRPC, castration-resistant prostate cancer.
Fig. 2. Time courses after radical prostatectomy. CRPC, castration-resistant prostate cancer; DSM, disease-specific mortality.
BCR occurred within 5 years after radical prostatectomy in 206 patients, and over 5 years after the operation in 55 patients. Among these, 38 (18.4%) and 4 (7.3%) patients experienced metastasis that was not significantly different between the timing of BCR within or over 5 years, respectively (log-rank, p=0.172). A lower rate of metastasis occurred in patients whose BCR developed at least 5 years after the operation; however, there was no statistically significant association. Among the 42 patients who experienced metastasis, the median duration from BCR to metastasis was 40 months (IQR, 10.0–80.8 months). Metastasis occurred within 3 years after BCR in 19 patients and at least 3 years after BCR in 23 patients; among these patients, 11 (57.9%) and 12 (52.2%) experienced disease-specific death that showed no significant difference between the two groups (log-rank, p=0.316). Disease-specific mortality was not dependent on the duration to distant metastasis.
4. Predictors of death
We assessed the predictors associated with disease-specific mortality and overall mortality after radical prostatectomy for men with clinically localized prostate cancer. Multivariate analysis showed that the pathologic ISUP Gleason grade (p=0.05; hazard ratio [HR], 1.44) and seminal vesicle invasion (p=0.02; HR, 3.82) were significantly associated with an increased risk of disease-specific mortality. Furthermore, age at diagnosis (p<0.01; HR, 1.07) and seminal vesicle invasion (p<0.01; HR, 2.38) were significantly associated with overall mortality (Table 3).
Table 3. Multivariate analysis of predictors associated with disease-specific mortality and overall mortality.
| Predictors | Disease-specific mortality | Overall mortality | ||
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | HR (95% CI) | |
| Age at diagnosis (continuous) | 0.72 | 1.01 (0.95–1.08) | <0.01 | 1.07 (1.04–1.11) |
| Preoperative PSA (continuous) | 0.80 | 1.00 (0.97–1.03) | 0.67 | 1.00 (0.98–1.01) |
| Pathologic Gleason grade group | 0.05 | 1.44 (1.00–2.06) | 0.85 | 1.02 (0.86–1.20) |
| Extraprostatic extension | 0.08 | 3.40 (0.86–13.35) | 0.06 | 1.54 (0.98–2.41) |
| Seminal vesicle invasion | 0.02 | 3.82 (1.31–11.17) | <0.01 | 2.38 (1.42–4.00) |
| Positive surgical margin | 0.46 | 1.48 (0.53–4.15) | 0.44 | 1.18 (0.78–1.79) |
| Positive lymph nodes | 0.13 | 2.50 (0.77–8.05) | 0.64 | 0.78 (0.28–2.18) |
HR, hazard ratio; CI, confidence interval; PSA, prostate-specific antigen.
DISCUSSION
A small proportion of patients with clinically localized prostate cancer die of the disease 10 to 15 years after their initial diagnosis even without treatment [10,11,12]. In a study with three decades of follow-up with watchful waiting, a prospective cohort study in Sweden reported that localized prostate cancer usually has a silent course; however, progression or distant metastasis can also develop over long-term follow-up [13]. Even though radical prostatectomy reduces the mortality rate, this benefit takes several years to emerge after surgery. Given the heterogeneity of the disease, well-established information about the time course of prostate cancer may allow physicians to give evidence-based management options to patients. We investigated the oncologic outcomes of clinically localized prostate cancer after radical prostatectomy with a 10-year follow-up to identify and obtain evidence of the characteristics of the postoperative course in Korean men.
BCR occurred in 34.9% of patients in the present study, which was higher than in a previous study [14] that reported a BCR rate of 15%. Considering that between 27% and 53% of patients who underwent radical prostatectomy experienced a detectable elevation of serum PSA within 10 years after the operation in the era before PSA screening [15,16,17], our results showed a similar, or slightly higher, rate of BCR compared with previous reports. In the current study, 38 patients (4.8%) received immediate adjuvant therapy and 22 of them experienced PSA elevation. Since these men were excluded from the investigation of disease progression after BCR, adjuvant therapy had no impact on the time from BCR to metastasis. BCR preceded disease progression in all cases, and it is well known that patients with BCR are at an increased risk of requiring additional cancer management; therefore, the 219 patients (29.3%) who received recovery treatment because of PSA elevation or disease progression were included in the analysis of disease progression after BCR. Among the 261 patients with BCR, 55 (21.1%) had an unelevated serum PSA level for at least 5 years, and 19 (7.3%) had an unelevated serum PSA level for 10 years before BCR. This result is similar to that of a previous report [14] showing that 23% of patients with BCR had no detectable serum PSA for at least 5 years, and 4% of the patients for at least 10 years, before BCR. Although the other series showed rare PSA progression in patients without detectable serum PSA for the 5 to 6 years after surgery [18], we showed that with extended follow-up, patients experience BCR 10 years or longer after surgery.
In the current study, the rate of progression from BCR to metastasis was higher and the duration was shorter than in the previous study, in that 34% of patients with BCR developed distant metastasis with a median duration of 8 years [14]. The time of onset of BCR appeared to influence distant metastasis in the present study, and among the 54 patients with distant metastasis, 48 had BCR within 5 years of radical prostatectomy. However, the importance of the duration from surgery to BCR on distant metastasis warrants a larger number of BCR cases at least 5 years after the operation. The metastasis-free rate at 5 years after BCR was 87.7%, which was higher than the previously reported rate of 63% [14,19]. The estimated mean metastasis-free duration after BCR was quite long at 16.2 years, and the overall 10-year metastasis-free rate was 93.5%. Zincke et al. [5] reported a 10-year metastasis-free rate of 82% in approximately 3,000 men who underwent radical prostatectomy, whereas Pound et al. [14] reported a rate of 87%. Thus, the present study showed a higher metastasis-free rate than previous reports.
Our study demonstrated that 53.8% of patients with metastasis developed disease-specific mortality with a median duration of 22 months. Pound et al. [14] showed that 43% of patients with metastasis developed disease-specific mortality with a median duration of approximately 5 years, which was longer than in our results. In the current study, the 10-year disease-specific survival rate of 97.5% was slightly higher than the previously reported analysis, which showed rates of 75% to 97% for patients with well-differentiated and moderately differentiated cancer, respectively [6], and 90% for patients who underwent total perineal prostatectomy [20]. In contrast, Hull et al. [21] performed radical retropubic prostatectomy and pelvic lymphadenectomy on 986 patients and demonstrated that the 10-year disease-specific survival rate was 97.6%, which was similar to our results. Our study showed a 10-year disease-specific survival after RP of 100%, 98.2%, and 93.6% for men with D'Amico classification of low-, intermediate-, and high-risk prostate cancer, respectively, which was similar to the rates reported from the Mayo Clinic in 2008 [22].
The predictors associated with disease-specific or overall mortality and the predictors of death have differed for each study. Mitchell et al. [23] reported seminal vesicle invasion and positive lymph nodes as significant predictors of death due to prostate cancer, and seminal vesicle invasion and preoperative PSA level as significant predictors of mortality, regardless of cause. However, investigators from the Mayo Clinic [22] reported the clinical Gleason score as a single significant predictor of disease-specific death. The current study found several predictive measures for disease-specific mortality (pathologic Gleason grade and seminal vesicle invasion) and overall mortality (age at diagnosis, seminal vesicle invasion). The pathologic Gleason grade and seminal vesicle invasion indicate disease aggressiveness and are well-known predictors for disease-specific or overall mortality, as previously described [22,23,24]. Older age is another known associated risk factor for cancer-specific mortality in large Western cohorts [25,26]. In the CaPSURE database [25], older men (≥75 years) were more likely to be diagnosed with higher-risk prostate cancer and were less likely to receive definitive treatment than younger men. In this study, we analyzed localized prostate cancer in definitively treated patients; thus, the hazard of older age on cancer-specific mortality may be obscured by the hazard of adverse pathologic features, such as Gleason grade or seminal vesicle invasion.
The present study is limited by its nonrandomized and retrospective design. We compared the oncologic outcomes with previously reported retrospective studies. However, radical prostatectomy during the PSA era showed an improved 15-year disease-specific mortality rate of 7% in previously published reports, which is similar to our result of 4.8%. Stephenson et al. [27] stated that the low fatality or effectiveness of radical prostatectomy resulted in the relatively improved prognosis from modern series. Furthermore, after the introduction of antiandrogens in 2003, additional hormone treatments might have impacted prognosis, while the regimen and timing of additional treatments were dependent on the discretion of the physician. Although laparoscopic radical prostatectomy has been reported to provide favorable cancer control [28], we did not assess the impact of the laparoscopic approach because of the small number (n=50) of cases. We will assess the impact of laparoscopic and robotic surgery in future studies. This study focused on oncologic outcomes without functional outcomes, and the precise analysis of the benefit and cost of radical prostatectomy need to be performed as a prospective clinical trial. Nevertheless, the excellent cancer control of radical prostatectomy should be noted, and this is the first study to assess long-term oncologic outcomes after radical prostatectomy in Asia, especially in Korea.
CONCLUSIONS
In conclusion, radical prostatectomy provided excellent long-term cancer control in clinically localized prostate cancer. The rate of BCR was not higher than previously reported in studies involving Western countries. Distant metastasis developed less frequently but within a shorter duration from BCR compared with Western patients, whereas patients with distant metastasis died of prostate cancer earlier than did Western patients. This study showed the differences in longterm prognosis between Korean and Western patients with clinically localized prostate cancer.
Footnotes
CONFLICTS OF INTEREST: The authors have nothing to disclose.
- Research conception and design: Chang Wook Jeong and Cheol Kwak.
- Data acquisition: Jae Hyun Jung and Jungyo Suh.
- Statistical analysis: Jae Hyun Jung and Chang Wook Jeong.
- Data analysis and interpretation: Jae Hyun Jung and Jungyo Suh.
- Drafting of the manuscript: Jae Hyun Jung.
- Critical revision of the manuscript: Chang Wook Jeong, Cheol Kwak, and Jungyo Suh.
- Administrative, technical, or material support: Cheol Kwak.
- Supervision: Sang Eun Lee, Eunsik Lee, Ja Hyeon Ku, and Hyeon Hoe Kim.
- Approval of the final manuscript: all of the authors.
References
- 1.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117–1123. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011;364:1708–1717. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 3.Joniau S, Van der Eeckt K, Briganti A, Gontero P, Van Bruwaene S, Jeffrey Karnes R, et al. European Multicenter Prostate Cancer Clinical and Translational research Group (EMPaCT) Current role of surgery for high risk prostate cancer. Arch Esp Urol. 2013;66:259–273. [PubMed] [Google Scholar]
- 4.Holmberg L, Bill-Axelson A, Helgesen F, Salo JO, Folmerz P, Häggman M, et al. Scandinavian Prostatic Cancer Group Study Number 4. A randomized trial comparing radical prostatectomy with watchful waiting in early prostate cancer. N Engl J Med. 2002;347:781–789. doi: 10.1056/NEJMoa012794. [DOI] [PubMed] [Google Scholar]
- 5.Zincke H, Oesterling JE, Blute ML, Bergstralh EJ, Myers RP, Barrett DM. Long-term (15 years) results after radical prostatectomy for clinically localized (stage T2c or lower) prostate cancer. J Urol. 1994;152(5 Pt 2):1850–1857. doi: 10.1016/s0022-5347(17)32399-6. [DOI] [PubMed] [Google Scholar]
- 6.Krongrad A, Lai H, Lai S. Survival after radical prostatectomy. JAMA. 1997;278:44–46. [PubMed] [Google Scholar]
- 7.Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA Grading Committee. The 2014 International Society of Urological Pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 8.D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Blank K, Broderick GA, et al. Biochemical outcome after radical prostatectomy, external beam radiation therapy, or interstitial radiation therapy for clinically localized prostate cancer. JAMA. 1998;280:969–974. doi: 10.1001/jama.280.11.969. [DOI] [PubMed] [Google Scholar]
- 9.Scher HI, Curley T, Yeh S, Iversen JM, O'Dell M, Larson SM. Therapeutic alternatives for hormone-refractory prostatic cancer. Semin Urol. 1992;10:55–64. [PubMed] [Google Scholar]
- 10.Chodak GW, Thisted RA, Gerber GS, Johansson JE, Adolfsson J, Jones GW, et al. Results of conservative management of clinically localized prostate cancer. N Engl J Med. 1994;330:242–248. doi: 10.1056/NEJM199401273300403. [DOI] [PubMed] [Google Scholar]
- 11.Adolfsson J, Steineck G, Hedlund PO. Deferred treatment of clinically localized low-grade prostate cancer: actual 10-year and projected 15-year follow-up of the Karolinska series. Urology. 1997;50:722–726. doi: 10.1016/S0090-4295(97)00320-8. [DOI] [PubMed] [Google Scholar]
- 12.Johansson JE, Holmberg L, Johansson S, Bergström R, Adami HO. Fifteen-year survival in prostate cancer. A prospective, population-based study in Sweden. JAMA. 1997;277:467–471. [PubMed] [Google Scholar]
- 13.Popiolek M, Rider JR, Andrén O, Andersson SO, Holmberg L, Adami HO, et al. Natural history of early, localized prostate cancer: a final report from three decades of follow-up. Eur Urol. 2013;63:428–435. doi: 10.1016/j.eururo.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 14.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–1597. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 15.Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 16.Trapasso JG, deKernion JB, Smith RB, Dorey F. The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol. 1994;152(5 Pt 2):1821–1825. doi: 10.1016/s0022-5347(17)32394-7. [DOI] [PubMed] [Google Scholar]
- 17.Catalona WJ, Smith DS. 5-year tumor recurrence rates after anatomical radical retropubic prostatectomy for prostate cancer. J Urol. 1994;152(5 Pt 2):1837–1842. doi: 10.1016/s0022-5347(17)32397-2. [DOI] [PubMed] [Google Scholar]
- 18.Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50:93–99. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 19.Sgrignoli AR, Walsh PC, Steinberg GD, Steiner MS, Epstein JI. Prognostic factors in men with stage D1 prostate cancer: identification of patients less likely to have prolonged survival after radical prostatectomy. J Urol. 1994;152:1077–1081. doi: 10.1016/s0022-5347(17)32507-7. [DOI] [PubMed] [Google Scholar]
- 20.Gibbons RP, Correa RJ, Jr, Brannen GE, Weissman RM. Total prostatectomy for clinically localized prostatic cancer: long-term results. J Urol. 1989;141:564–566. doi: 10.1016/s0022-5347(17)40895-0. [DOI] [PubMed] [Google Scholar]
- 21.Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002;167(2 Pt 1):528–534. doi: 10.1016/S0022-5347(01)69079-7. [DOI] [PubMed] [Google Scholar]
- 22.Boorjian SA, Karnes RJ, Rangel LJ, Bergstralh EJ, Blute ML. Mayo Clinic validation of the D'amico risk group classification for predicting survival following radical prostatectomy. J Urol. 2008;179:1354–1360. doi: 10.1016/j.juro.2007.11.061. discussion 1360–1. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell CR, Boorjian SA, Umbreit EC, Rangel LJ, Carlson RE, Karnes RJ. 20-year survival after radical prostatectomy as initial treatment for cT3 prostate cancer. BJU Int. 2012;110:1709–1713. doi: 10.1111/j.1464-410X.2012.11372.x. [DOI] [PubMed] [Google Scholar]
- 24.Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178(3 Pt 1):864–870. doi: 10.1016/j.juro.2007.05.048. discussion 870–1. [DOI] [PubMed] [Google Scholar]
- 25.Bechis SK, Carroll PR, Cooperberg MR. Impact of age at diagnosis on prostate cancer treatment and survival. J Clin Oncol. 2011;29:235–241. doi: 10.1200/JCO.2010.30.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandaglia G, Karakiewicz PI, Abdollah F, Becker A, Roghmann F, Sammon JD, et al. The effect of age at diagnosis on prostate cancer mortality: a grade-for-grade and stage-forstage analysis. Eur J Surg Oncol. 2014;40:1706–1715. doi: 10.1016/j.ejso.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson AJ, Kattan MW, Eastham JA, Bianco FJ, Jr, Yossepowitch O, Vickers AJ, et al. Prostate cancer-specific mortality after radical prostatectomy for patients treated in the prostatespecific antigen era. J Clin Oncol. 2009;27:4300–4305. doi: 10.1200/JCO.2008.18.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Touijer K, Secin FP, Cronin AM, Katz D, Bianco F, Vora K, et al. Oncologic outcome after laparoscopic radical prostatectomy: 10 years of experience. Eur Urol. 2009;55:1014–1019. doi: 10.1016/j.eururo.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]