Abstract
High-quality performance of medical devices for glucose monitoring is important for a safe and efficient usage of this diagnostic option by patients with diabetes. The mean absolute relative difference (MARD) parameter is used most often to characterize the measurement performance of systems for continuous glucose monitoring (CGM). Calculation of this parameter is relatively easy and comparison of the MARD numbers between different CGM systems appears to be straightforward on the first glance. However, a closer look reveals that a number of complex aspects make interpretation of the MARD numbers provided by the manufacturer for their CGM systems difficult. In this review, these aspects are discussed and considerations are made for a systematic and appropriate evaluation of the MARD in clinical trials. The MARD should not be used as the sole parameter to characterize CGM systems, especially when it comes to nonadjunctive usage of such systems.
Keywords: continuous glucose monitoring, diabetes therapy, quality of measurement, glucose measurement, blood glucose
Many patients with diabetes routinely use systems for continuous glucose monitoring (CGM) as the diagnostic cornerstone of their diabetes treatment and the number is assumed to increase massively in the next years. CGM systems have seen considerable improvements in their performance after their market introduction some 15 years ago. Their analytical performance, that is, the accuracy with which CGM systems can measure glucose concentrations in interstitial fluid (ISF) in the subcutaneous adipose tissue was also improved. Such improvements have tremendous importance for clinical utility of CGM systems, mainly in view of their use with a nonadjunctive claim, that is, that therapeutic decisions can be based on the glucose values presented without exposing patients to too high risks. CGM data are also used in other clinical applications like bolus calculators (for patients on multiple daily insulin injections), in insulin pumps (with or without automatic changes in insulin infusion rates depending on the current glucose measurement results), and—last but definitively not least—are indispensable in automated insulin delivery (AID) systems.
Time in range(s) (TIR) has been suggested by several working groups as a new “biomarker” to complement HbA1c for glycemic control. There are good arguments that TIR has advantages compared to HbA1c since
(1) it responds faster to treatment changes
(2) it reflects as well glycemic variability, hypo- and hyperglycemia
(3) it is not affected by physiological and pathological factors that affect the HbA1c concentration
In order to establish TIR successfully in clinical practice and in order to make it a useful endpoint in clinical studies it is important that it can be measured accurately. For “time” this is no problem; however, the accuracy of CGM systems strongly affects the ranges. If results differ between different CGM systems, TIR calculated from these results will do so as well. There is a clear need to better assess the analytical accuracy of CGM systems than this can be done by means of the MARD and to establish metrological traceability for CGM measurements. For systems used for self-monitoring of blood glucose (SMBG), an ISO standard was established some decades ago to characterize their analytical performance. However, no such standard was established for CGM systems until now, even if attempts have been made.1 The parameter most often used for description of the analytical performance of CGM systems is the “mean (or median) absolute relative difference” (MARD). Reasons why this parameter is widely used is the relatively ease with which this parameter can be calculated and that a single number if presented that appear to enable a straightforward interpretation of the MARD of a given CGM system and allow comparison of the performance of different CGM systems.
In this review, it will be discussed that in reality a large number of aspects makes the MARD difficult to interpret.
MARD—Background and Properties
CGM systems measure glucose in the interstitial fluid in the subcutaneous tissue while SMBG measures glucose in capillary blood. The assumption is, that glucose levels in both compartments (matrices) are similar; however, this holds true for steady state conditions only, which is not the case most of the day of patients with diabetes. To assess the accuracy of a measurement one has to make sure the “measurand” is identical, that is, (1) same matrix, (2) same substance, (3) same units are used. Otherwise a comparison is metrologically not allowed. For this reason, all manufacturers use algorithms that predict BG values (typically capillary blood values and not venous blood values) from CGM values. This is why CGM values are compared to SMBG values.
The MARD is computed using temporally matched glucose data from CGM systems and comparison glucose measurements (most often obtained by capillary blood glucose (BG) measurements) of all subjects of a clinical study (Figure 1). It is important to note that the MARD is a measurement of the performance of the system (= sensor + algorithm) rather than the sensor element alone. The impact of the algorithm should not be underestimated—its design allows trading-off accuracy against “drop out time“ of the CGM system. For redundant sensor configurations, a “joint” MARD can be obtained as the processed output of the multiple individual sensors with some weight, for example, according to the “health” of the sensor.
Figure 1.
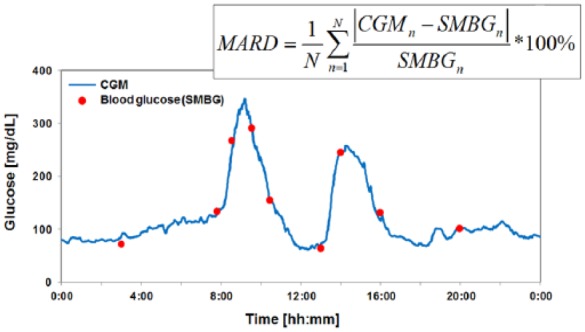
Glucose values continuously monitored (CGM) over 24 hours and corresponding BG values measured by a comparison method in certain time intervals. Based on the absolute differences between the CGM and BG glucose (in this case SMBG, but can also be a different comparison method) the MARD is calculated according to the formula given.
Reported as a percentage, MARD is the average of the absolute difference between these values. The less the MARD is, the closer are the CGM readings to the comparison values. Typically a CGM system with a MARD <10% is regarded to have good analytical performance. The MARD is a statistical approach used in other respects as well; however, it is not used often to characterize the performance of systems for SMBG as it doesn’t distinguish between precision and bias (and is therefore also not mentioned in the respective ISO standard) and in other areas of diabetes research.2 It is worth to note that the MARD does not differentiate between positive and negative errors or between systematic and random errors. In other words, the MARD is influenced by a number of factors (Table 1) and has a number of advantages and disadvantages (Table 2).
Table 1.
Factors That Influence the Assessment of the MARD.
| CGM system-inherent factors (intrinsic performance of the
system) • Calibration • Performance of the CGM sensor over time • Sensor to sensor variation • Algorithms and smoothing filters implemented in the CGM systems Not CGM system-inherent factors • Insertion factors (competency of sensor insertion, body site, movement) • Physiological time delay between CGM and BG measurements • Range and distribution of the paired glucose values • Rate of change in glycemia • Study design / study population • Implementation of the study design (controlled vs uncontrolled environment, time of day, day 1 vs day 2-X, dynamic variation in glucose concentration, number of measurements, synchronization of CGM, and comparison method) • Direction of the deviations from the comparison method |
Table 2.
List of Advantages and Disadvantages of the MARD.
| Advantages - Provide information about analytical performance of CGM systems in one number - Widely used - Perceived as a parameter enabling comparison of the performance of different CGM systems Disadvantages - Only a small portion of CGM data is used for calculation of MARD - Addition information provided by CGM systems like trend and rate-of change are not taken into account - The same holds true for frequency and relevance of artifacts (eg, signal dropouts) that most often will not be recognized by relatively seldom performed comparison measurements - Although MARD is influenced by both accuracy metrics, bias and precision of CGM measurements, it does not allow to distinguish between the two - MARD does not differentiate between a positive or negative bias to the comparison measurements - Overall MARD does not provide information during the time between the sporadic comparison measurements - MARD does not provide specific information during time periods with dynamic changes in glycemia or during episodes of hypo- or hyperglycemia - MARD does not provide information about transient large sensor inaccuracies (induced by a calibration issue or movement artifacts) - MARD does not reflect durability of the sensor |
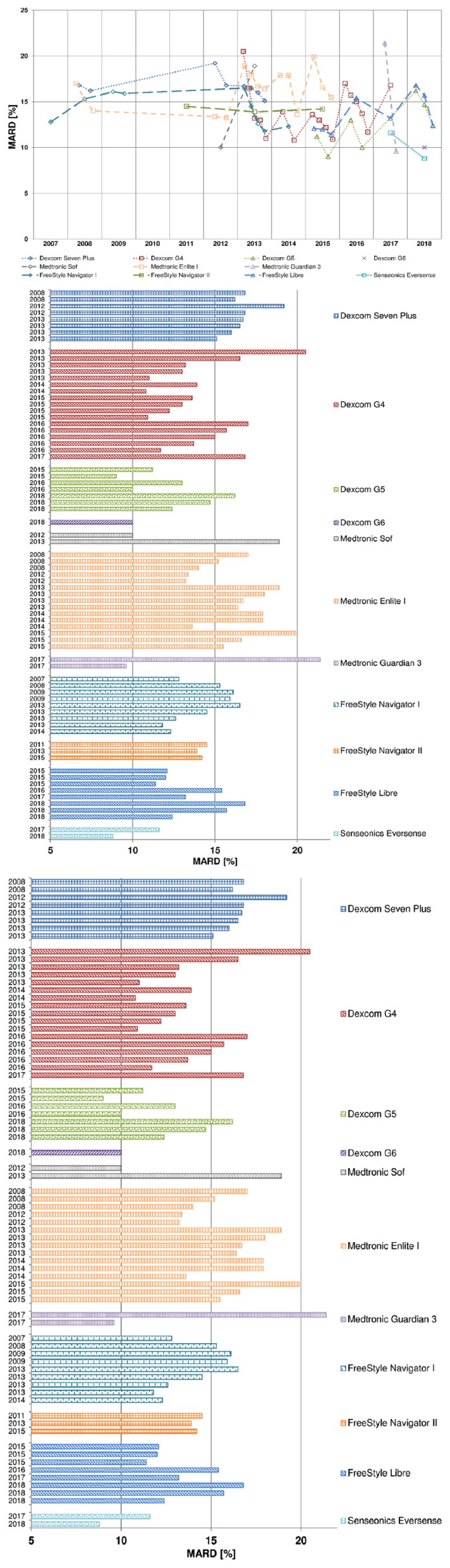
In the last years practically in each publication about CGM systems, a MARD value was reported. However, an attempt to find publications about MARD and its properties itself—with a focus on diabetes—was not very successful: A literature search in PubMed for this term revealed a limited number of such publications. Nevertheless, some of our own publications3-8 and others9,10 do discuss this topic. It is of interest to note that different studies with the same CGM system provide impressively different MARD results (Table 3 and Figure 2). For example, the MARD of the Dexcom G4 has been reported as everything between 11% and 21%, without any regularity over time which could be explained as an improvement of the sensor (eg, the first batch being worse than subsequent versions). In the following different aspects will be discussed that are of relevance for interpreting the MARD and that explain why such differences in MARD have to be expected.
Table 3.
Listing of Clinical Trials With Different CGM Systems Summarizing Key Study Information and the MARD Obtained.
| Reference | Study setting | Number of subjects | T1/T2 | Study duration | Comparison method | Calibration system | Number of calibrations per day | Overall MARD | MARD during hypos | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Dexcom seven plus | ||||||||||
| Garg11 | Home use, with 3 in- clinic visits | 14 | T1 | 15 days (5 days/sensor) | Venous measurements (YSI); SMBG | BG meter | 2 h after sensor insertion; every 12 h thereafter | vs YSI: 16.8%, vs BG: 16.2% | Hypo (<80 mg/dL), YSI: 21.5% | |
| Luijf12 | Both (1 day in clinic and rest is home use) | 12 | T1 | 7 days | In clinic: plasma glucose (YSI); Home use: SMBG (Aviva Nano) | Accu-Chek Aviva Nano | Twice per day | In clinic: 19.2%; Home use: 16.8% | In clinic: 23.9%; Home use: 41.6 | |
| Freckmann13 | In clinic | 12 | T1 | 9 days (7 days for Dexcom Seven Plus) | Capillary BG and venous BG (YSI) | Navigator BG meter | 2 h after sensor insertion and then every 12 h | 16.7% | 31.7% | |
| Facchinetti14 | In clinic | 24 (12 from each database) | T1 | 7 days | BG samples measurements (YSI) | Not reported | According to the manufacturer’s instructions | 15.1% (15.6% study 1 & 14.7% study 2) | Study looked into two databases from 2 studies | |
| Damiano15 | In clinic | 6 | T1 | 2 days | Venous plasma glucose (GlucoScout and YSI) | Not reported | 4 calibrations with venous blood | 16.5% | ||
| Christiansen16 | In clinic | 53 | T1 (81%) & T2 (19%) | 7 days | Venous measurements (YSI) | OneTouch Ultra 2 meter | Twice daily | 16.0% | 27.0% | |
| Facchinetti17 | In clinic | 9 | T1 | 1 day | BG samples measurements (YSI) | Not reported | Twice per day | 14.1% | Median MARD | |
| Dexcom G4 platinum | ||||||||||
| Luijf18 | Clinic (from 8 am until noon) and home use | 20 | T1 | Clinic (1 day) and Home use (7 days) | Venous measurements (YSI) | Abbott Freestyle BG meter | According to manufacturer’s recommendation | Clinic: 20.5%, Home use: 16.5% | Dexcom G4 version A sensor (G4A) | |
| Christiansen16 | In clinic | 72 | T1 (83%) & T2 (17%) | 7 days | Venous measurements (YSI) | OneTouch Ultra 2 meter | Twice daily | 13.0% | 18.0% | |
| Pleus19 | In clinic | 10 | T1 | 7 days | Capillary BG measurements | Accu-Chek Aviva | 2 h after sensor insertion and then every 12 h | 11.0% | 13.7% | |
| Garcia20 | In clinic | 72 | Not reported | 7 days | Venous measurements (YSI) | Not reported | Twice daily | 13.2% | ||
| Damiano21 | In clinic | 24 (12 adults & 12 children) | T1 | 2 days | Venous measurements (GlucoScout) | GlucoScout meter | One calibration before the start of the experiment, and during the experiment | 10.8% | ||
| Matuleviciene22 | Home use | 38 | T1 | 4-6 days | Capillary and venous BG measurements | HemoCue | every 12 h | 13.9% | 20.0% | |
| Kropff23 | In clinic phase (6 h) and home use phase | 24 | T1 | 6 days | Capillary BG measurements & 6 h on the 3rd day compared to venous blood (YSI) | Accu-Chek Aviva | Twice a day after initial calibration | Clinic: 13.6%, Home: 12.2% | Clinic: 17.6%, Home: 21.2% | |
| Facchinetti17 | In clinic | 36 | T1 (32) & T2 (4) | 7 days | BG samples measurements (YSI) | Not reported | Twice daily | 11.2% | Median MARD | |
| Pleus24 | In clinic | 10 | T1 | 7 days | Capillary BG measurements | Accu-Chek Aviva | 2 h after sensor insertion and then every 12 h | 10.9% | ||
| van Beers25 | In clinic (3 sessions) and the rest Home use | 72 | T1 & T2 | Not reported | YSI for in clinic and SMBG for home use | Not reported | YSI for in clinic and SMBG for home use | 13% | ||
| Laffel26 | Both | 176 | T1 or T2 (only 1 patient) | 7 days | BG measurements or venous measurements (YSI) | LifeScan OneTouch Verio IQ | Twice daily | 17% vs YSI. & 15% vs BG | 18% vs YSI. & 21% vs BG | Pediatric subjects, two CGMs with one receiver and masked data. |
| Andelin27 | In clinic | 41 | T1 | 6 days | Capillary and venous blood samples (HemoCue) | HemoCue | Capillary glucose | Capillary: 11.7%; Venous: 13.7 | Capillary: 16.6%; Venous: 31.8% | |
| Bonora28 | Home use | 8 | T1 | 14 days | BG capillary blood | Not reported | At least twice daily | vs SMBG: 15.7%, vs FSL: > Day 1-10: 17.7%; > Day 11-14: 22.2% | FSL vs DG4P: 18.7% | Goal: Determine agreement between FSL and DG4P |
| Aberer29 | In clinic | 12 | T1 | 12 h | CGM and analyzing venous plasma glucose values every 5 min (Super GL Glucose Analyzer) | Capillary BG | every 12 h | 16.8% | 23.8% | |
| Dexcom G5 (or G4 Platinum sensor + Software 505) | ||||||||||
| Bailey30 | Both | 51 | T1 (86%) & T2 (14%) | 7 days | During clinic session: YSI, during home use: BG | Bayer Contour Next USB | Twice daily | vs YSI: 9.0% vs BG: 11.2% | ||
| Laffel26 | Both | 79 | T1 | 7 days | BG measurements or venous measurements (YSI) | Bayer Contour Next USB | Twice daily | vs YSI 10% & vs BG 13% | vs YSI 17% & vs BG 19% | Pediatric subjects, single unmasked sensor |
| Freckmann31 | Home use with 3×48 hours in-clinic visits | 20 | T1 | 14 days (7 days/sensor) | SMBG (14 days) / SMBG + venous BG (Hexokinase (HK)) during 3x 6 hours induced glucose excursions | FreeStyle Freedom Lite | 2 h after sensor insertion and then every 12 h | 14 days: 12.4%; Glucose excursions: vs HK: 16.2% vs SMBG: 14.7% | ||
| Dexcom G6 | ||||||||||
| Wadwa32 | In clinic phases for CGM assessment, otherwise patients were at home | 262 | T1 (99%) & T2 on insulin (1%) | 10 days | YSI | None (glucose values were computed retrospectively based rely on measured sensor currents) | None | 10.0% | ||
| Medtronic SofSensor | ||||||||||
| Welsh33 | Not reported | 71 adults (19-72) & 61 children (7-17) | T1 | 3 days | Not reported | Paradigm Link BG monitor | Not reported | Adults: 9.9%; Children: 10.1% | ||
| O’Neal34 | Home use | 10 | T1 | 3 days | (YSI) | Contour Link Meter, Bayer | Not reported | 18.9% | Study is only looking into nocturnal hypoglycemia, no overall MARD | |
| Christiansen16 | In clinic | 72 | T1 | 4 nights (from 18:00 until the next morning) | venous BG (Roche Hitachi 912 chemistry analyzer) | Not reported | Twice every night | 45.0% | Using the iPro algorithm | |
| Medtronic Enlite I | ||||||||||
| Kovatchev35 | In clinic | 14 | T1 | Not reported | YSI | YSI BG analyzer | According to manufacturer’s recommendation | 15.2% | 16.1% | |
| DirectNet Study Group36 | Most at home and 18 h in clinic | 30 | T1 | 7 days | BG at home and Laboratory serum glucose in clinic | One Touch® Ultra® Meter (at home) | Every 12 h at least | 14% (in clinic); 17% (at home) | Children and youth (4-17 years); Median MARD | |
| Keenan37 | In clinic | 98 | T1 (79) & T2 (18) | 7 days | Plasma glucose measurements (YSI) | Not reported | Not reported | G: 13.1% & 14.6%; V: 12.9% & 14.7% | Data were processed using the calib. Algo. of Guardian (G) and the Veo real time (V) | |
| Calhoun38 | In clinic | 54 | T1 | 69 nights | 19% YSI analyzer, 66% GlucoScout monitor, and 15%; HemoCue analyzer | Not reported | Manufacturer-recommended intervals | 15% | Median MARD | |
| Luijf18 | In clinic phase (from 8 am until noon) and a home use phase | 20 | T1 | Clinic phase (1 day) and Home use phase (6 days) | (YSI glucose analyzer) | Abbott Freestyle BG meter | According to manufacturer’s recommendation | Clinic: 16.4%; Home use: 18.9% | Clinic: 21.5% | |
| Damiano15 | In clinic | 6 | T1 | 2 days | Venous BG measurements (GlucoScout and YSI) | No reported | Average of 4.7 calibrations with venous blood | 18.0% | ||
| Freckmann39 | In clinic | 12 | T1 | 9 days (6 days for Enlite) | capillary BG measurements and venous BG (YSI) | Navigator BG meter | 1, 2, 10, 24, 72 hours after sensor insertion | 16.7% | 34.3% | |
| Mahmoudi40 | In clinic | 10 | T1 | Not reported | Plasma glucose (HemoCue glucose analyzer) | Not reported | At least three times daily | 32.1% | 62.8% | Glucose data were restricted to nighttime measurements |
| Damiano21 | In clinic | 24 (12 adults & 12 children) | T1 | 2 days | Venous BG measurements (GlucoScout) | GlucoScout meter | One calibration before the start of the experiment, during the experiments | 17.9% | ||
| Bailey41 | In clinic (3 sessions) and the rest Home use | 90 | T1 & T2 | 6 days | YSI | Bayer Contour NEXT LINK meter | Different frequencies | 1.2 calibrations daily: 14.7%; 2.8 calib. daily: 13.6% | ||
| Matuleviciene22 | Home use | 38 | T1 | 4-6 days | Capillary and Venous BG measurements | HemoCue | According to manufacturer’s recommendation | 17.9% | 34.7% | |
| Kropff23 | In clinic phase (6 h) and a home use phase | 26 | T1 | 6 days | Capillary BG fingersticks & 6 h on the 3rd day compared to venous blood (YSI) | Accu-Chek Aviva | Twice a day after initial calibration | In clinic phase: 16.6%, Home phase: 19.9% | In clinic phase: 24.6%, Home phase: 36.5% | |
| Nørgaard42 | Home use | 45 | T1 | 15 days (3 days/device) | BG capillary blood | Not reported | Mean number of 4.7 per day | 15.5% | ||
| Medtronic Guardian Sensor 3 | ||||||||||
| Aberer29 | In clinic | 12 | T1 | 12 hours | CGM and analyzing venous BG values every 5 min (Super GL Glucose Analyzer) | Capillary BG calibration | At least twice a day | 21.4% | 26.9% | Guardian Sensor 3 as part of the Medtronic MiniMed 640G |
| Christiansen43 | In clinic (3 sessions / 12-14h) and the rest Home use | 88 | T1 (62) & T2(26) | 7 days | YSI FST; using 2300 STAT Plus | Home: not reported; During FST: sensors were calibrated based on prompts from the MiniMed 640G | FST: 40-120 min after sensor insertion, 6 h and 12 h after first calibration | Minimum calibrations: 10.6%; one additional calibration: 9.6% | Guardian Sensor 3 as part of the Medtronic MiniMed 640G | |
| Abbott Navigator I | ||||||||||
| Weinstein44 | In clinic | 58 | T1 | 5 days | Venous YSI measurements | Integrated FreeStyle meter | Initially at 10 h post insertion; 12, 24, and 72h after insertion | 12.8% | 19.8% | |
| Kovatchev35 | In clinic | 14 | T1 | Not reported | YSI | YSI r | According to manufacturer’s recommendation | 15.3% | 10.3% | |
| Garg11 | Home use with 3 in- clinic visits | 14 | T1 | 15 days (5 days/sensor) | Venous YSI; SMBG | FreeStyle integrated BG meter | Initially at 10 h post insertion; 12, 24, and 72h after insertion | vs YSI:16.1%; vs BG: 15.9% | hypo (<80 mg/dL) vs YSI: 29.8% | |
| Luijf18 | In clinic phase (from 8 am until noon) and a home use phase | 20 | T1 | Clinic (1 day) and Home use (5 days) | (YSI) | Abbott Freestyle BG meter | According to manufacturer’s recommendation | Clinic: 16.5%; Home: 14.5% | Clinic: 17.4% | |
| Damiano15 | In clinic | 6 | T1 | 2 days | Venous BG measurements (GlucoScout and YSI) | No reported | One calibration with venous blood | 11.8% | ||
| Freckmann13 | In clinic | 12 | T1 | 9 days (5 days use for Navigator) | Capillary BG and venous BG (YSI) | Navigator BG meters | 1, 2, 10, 24, 72 hours after sensor insertion | 12.6% | 23.3% | |
| Damiano21 | In clinic | 24 (12 adults & 12 children) | T1 | 2 days | Venous BG (GlucoScout) | GlucoScout meter | Two calibrations before the start of the experiment, and during the experiment an average of 6.4 | 12.3% | ||
| Abbott Navigator II | ||||||||||
| McGarraugh45 | In clinic | 47 | T1 | 10 h | Venous BG vs YSI | Not reported | Not reported | 14.5% | 17.5% | |
| Leelarathna46 | AID and conventional treatment or two AID visits | 32 (12 pregnant) | T1 | Study visits: 18 h to 36 h | (YSI) | Integrated Freestyle BG meter | According to manufacturer’s recommendation | 13.9% | Only median ARD: 21.0% | Analyzed data from five AID studies |
| Thabit47 | Home use | 57 | T1 | 2 periods in the overnight studies, and 1 week for the day-and-night study | Capillary BG fingersticks | Integrated Freestyle BG meter | According to manufacturer’s recommendation | 14.2% | 30.6% | Accuracy was assessed using data from 3 studies |
| Abbott Freestyle Libre | ||||||||||
| Bailey48 | Home use with 3 in-clinic visits | 72 | T1 & T2 | 14 days | Capillary BG / venous BG (YSI) | Factory-calibrated | No calibration during 14 days of wear | vs BG: 11.4%; clinic alone with BG and YSI: 12.1% and 12.0% | ||
| Bonora28 | Home use | 8 | T1 | 14 days | BG capillary blood | Factory-calibrated | No calibration during 14 days of wear | vs BG: 15.4%, vs DG4P: >Day1-10: 17.7%; >Day 11-14: 22.2% | FSL vs DG4P: 18.7% | Determine agreement between FSL and DG4P, >3 sensor scans per day at <8 h intervals |
| Aberer29 | In clinic | 12 | T1 | 12 h | CGM and BG values every 5 min (Super GL Glucose Analyzer) | Factory-calibrated | Not reported—max. wear time: 14 days | 13.2% | 14.6% | Sensor on the back of each upper arm (two sensors total); no significant difference |
| Freckmann31 | Home use with 3×48 hours in-clinic visits | 20 | T1 | 14 days (7 days/sensor) | SMBG (14 days) / SMBG + venous BG (Hexokinase (HK)) during 3x 6 hours induced glucose excursions | Factory-calibrated | No calibration during 14 days of wear | 14 days: 13.0%; Glucose excursions: vs HK: 16.8%; vs SMBG: 15.7% | ||
| Senseonics Eversense | ||||||||||
| Kropff49 | Home use with 11 clinic visits | 71 | T1 (66) & T2 (5) | 180 days | Venous measurements (YSI) | Accu-Chek Aviva | Twice daily | 11.6% | 21.7% | |
| Christiansen50 | Home use with 7 clinic visits | 90 | T1 (61) & T2 (29) | 90 days | Home: BG (Contour next) Clinic: YSI | Contour Next USB | Twice daily | 8.8% | 40-54 mg/dL: 10.7%, 55-70 mg/dL: 9.0% | |
Figure 2.
Graphical representation of the MARD values obtained in the different studies listed in Table 3. (a) MARD values separated for the different CGM systems and year the respective studies were published. (b) MARD values sorted by CGM manufacturer / CGM system and year the respective studies were published.
Median or Mean
Usage of the median instead of the mean absolute relative difference (MARD) has pro and cons: outliers have reduced impact on the median in comparison to the mean value; however, conversely, if a data set has a several outliers, these are somewhat hidden by using the median. Usually the median ARD is lower than the mean ARD, the median ARD is nearly 0.8 multiplied by the MARD. This relationship been observed both empirically for multiple data sets and has been shown theoretically.9 Outliers have essentially no influence on the median ARD and only a small influence on MARD. From a clinical point of view or better from a patient point of view, outliers are of relevance when the patient is adapting his therapy on such a value. In publications, it is often not clearly stated if the given MARD represents the median or mean.
Glucose Range
Due to differences in the analytical performance of the CGM system and the comparison measurement system when it comes to measurement of glucose in the hypo-, eu-, and hyperglycemic range (plus the fact that the CGM system measures glucose in ISF and the comparison system in blood), the MARD of a given CGM system might differ between these ranges, that is, the MARD can be worse in the hypoglycemic range in comparison to the euglycemic range.9 Thus, not only an overall MARD should be reported, but also stratified MARD values for different glucose ranges. As an alternative way to account for this effect, it has recently been proposed to normalize MARD based on some comparison distribution for paired glucose measurements in order to make values from different clinical trials more comparable.8
Precision of CGM Systems
A key advantage of CGM is the high frequency of measurement. However, as MARD requires using temporarily matched comparison values, and comparison values are not available with the same frequency, many CGM values are not used. An alternative metric, the precision of CGM systems, overcomes this limitation by using the data obtained from two CGM systems (of the same make and model!) worn in parallel by the same patient; this enables calculation of the “precision absolute relative difference” (PARD; see below).5,6,19 An additional advantage of the PARD—besides making use of all CGM data available—is that it does not measure glucose in two different compartments (= all physiological differences [see below] are not of relevance).
In general, it is appropriate to assume that both CGM systems have more or less identical errors; however, also different CGM systems can be used (as an attempt to have a continuous comparison measurement signal instead of the relatively sparse BG measurements) (see below), but of course we may not assume that the errors will be the same.
Timing of Comparison Measurements and Quality of such Measurements
For the calculation of the MARD CGM values are brought in relation to the “true” glucose value measured in blood samples. The underlying assumption of this approach is that the “ comparison measurements” (ie, those produced by the comparison method) are inherently error free—which in reality is not true, as also this comparison method has a more or less large measurement error. Thus, the MARD does not only reflect the analytical performance of the CGM system under test, but also that of the comparison measurement. In other words, the calculation of the MARD includes the influence of both bias (= accuracy) and precision. As an example, consider two perfectly accurate but different CGM systems (when using one CGM system but measure with two devices in the same subjects, the PARD can be calculated; see below) with an expected bias of zero across the entire range of measurement, but with some random measurement errors. If these two systems are tested against each other and the MARD is computed from the paired measurements, this MARD will not be zero—it will reflect the presence of the measurement errors. One would need to have both “perfect accuracy” (no bias) and “perfect precision” (no measurement errors) in order to obtain a MARD of zero. It might also be, that two quite different CGM systems have the same MARD, but are quite different with respect to bias and precision: one has a good precision, but a large bias and the other has a small bias, but a bad precision. What is clinically more relevant?
More precisely, some additional aspects have to be considered:
The error distribution of the test method (= CGM system) and of the comparison method should be similar and the magnitude of bias and random error of the comparison method should be lower. If the comparison method is a good laboratory method, then its error might be <±2%; however, if BG systems are used as comparison systems, as it is frequently done, their errors are in the range of ±5% to ±15%. As most recent CGM systems have a low measurement error, their analytical performance comes close to that of at least some comparison methods, but for reasons of principle can never reach this. Especially at low glucose levels, the random error of the comparison method may be particularly high and, therefore, important to consider.
The number of comparison measurements has a massive impact on the MARD. Therefore, results obtained in clinical studies under highly controlled conditions and frequent comparison measurements differ from that obtained under real-world conditions (with more sparse comparison measurements and most often usage of a comparison method with a lower measurement quality).
As already discussed, the distribution of the comparison measurements in the glucose value range is of high importance.
The MARD does not consider the time periods between comparison measurements, that is, there might be a higher number of parallel measurements during daytime, but only a limited number during the night. It is not clear immediately if the performance of CGM systems is equally good during both times, especially because during night time there may be additional mechanical stresses as the patient may induce a compression artefact when lying on the glucose sensor.
Dynamic Changes in Glycemia
Patients with diabetes exhibit more or less pronounced glucose swings in daily life, that is, glycemia changes over time with different rates. Glucose variability make patients also prone to develop acute glycemic deteriorations into clinical relevant ranges outside the physiological range; however, there are considerable differences in the risk of patients (depending on many other factors) to become hypo- or hyperglycemic.5,19,51
It is important to acknowledge that the measurements for MARD calculation are performed in two different compartments. When glucose levels are more or less constant (rate of glucose change <1 mg/dl/min), the glucose levels in BG and ISF are practically identical. However, in case of swings in glycemia (= rate of change >1 mg/dl/min) physiological differences in glucose levels between both compartments show up. In other words, a rapid increase in glycemia after eating a meal with rapidly absorbable carbohydrates does not result in an immediate increase in CGM recordings; a decrease in peripheral glucose levels during exercise is not directly reflected in a decline in BG levels, a not removable (albeit predictable) time delay shows up.3 Such differences can lead to relevant differences in insulin dose selection depending on which glucose signal the selection is based on.52
The extent of differences in numbers does not only depend on the rate of change in glucose levels and the CGM system studied (Figures 2 and 3), it can also depend on the period of the day and different body sites. The knowledge about how constant this time delay is and to which extent it differs inter- and intraindividually is limited.3,7 An additional “physical” time delay is introduced by the measurement technique and data filtering plus analysis; however, this delay is in the range of seconds or minutes with most CGM systems. The algorithms implemented into the CGM systems try to compensate for this delay. The reported CGM values are an estimated/predicted BG value. This is done since (1) patients are used to interpreting BG values and (2) BG values are used for calibrating and (3) verifying CGM values.
Figure 3.
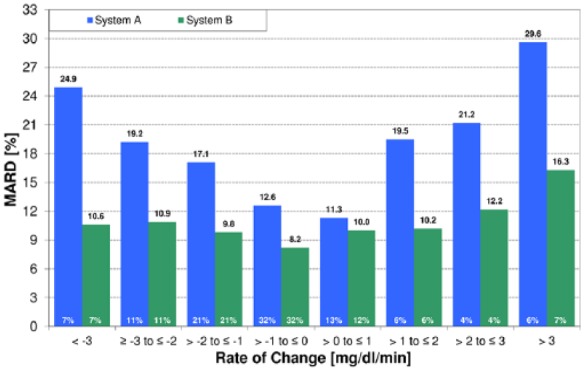
MARD during glucose swings (= periods of rapid glucose changes) for two different CGM systems as a function of the rate of change.19
The impact of the rate of change of glycemia on MARD values has two reasons:
The time delay seen by a CGM system expresses itself as a “wrong” concentration measurement, which is in fact not true.
The additional physical time delay of a given CGM system (with considerable differences between them due to different diffusion times, algorithms used to calculate the glucose values from the current measured by the glucose sensor by using a calibration algorithm while trying to reduce noise at the same time, etc) can make this difference between BG and ISF glucose larger or smaller. In the example shown in Figure 3 the shorter physical time delay of one CGM system leads to a lower MARD than that of the other CGM system with a longer time delay. At the same time, the overall MARD of both CGM systems was relatively similar (9% vs 11%).
Currently therapeutic decision-making is based on SMBG-related thinking, that is, the assumption that a given BG spot measurement provides “reliable” insights into the glucose changes over time. Such spot measurements are made with high accuracy; however, they provide only snap shots of changes over time in comparison to CGM systems which provide a complete picture. The lower accuracy of glucose concentration measurements with CGM systems is compensated by the additional information (trend arrows, alarms) provided. Nevertheless, also these CGM measurements are affected with errors. The MARD only considers the error in glucose concentration measurement and does not take these additional CGM information into account; however, when therapeutic decisions are based on CGM data, they can be taken into account.52
Compensation of the time delay during analysis of clinical trial data leads to lower MARD values. Manufacturers of CGM systems make attempts to do so as well during daily life usage of CGM systems by using “smart” sensors; such CGM systems use algorithms to improve their performance.14
Differences between glucose values measured at the same point in time with a BG system and a CGM system can be disturbing for patients (and diabetologists) if they are not aware of the physiological background of these.52 They might lose confidence in the technology. This can be even worse if alarms are generated by the CGM systems that are not confirmed by BG measurements. Such issues have to be addressed in CGM teaching and training programs.53
Performance of an Individual Glucose Sensor
The MARD does not allow making an accurate statement about the performance of given glucose sensors of one type of CGM system. Relatively high numbers of the standard deviation (as a reflection of the differences in MARD between sensors) often given with the calculated mean (or median) MARD indicate that considerable differences between the performances of glucose sensors show up in reality. However, MARD does not distinguish whether they are due to the analytical performance of the sensors themselves or if they reflect physiological factors (and movement artifacts) at the insertion site of the sensors (see below).
In other words, the MARD provides a number that reflects the total performance of a given CGM system in a clinical study, but is not as accurate when it is used to characterize the specific sensor alone. This in turn has relevance for the clinical usage of CGM systems; the therapeutic decision in a given moment is based on the glucose value provided by the sensor, but the error of this value will not be the same for every patient and sensor of the same make.
Calibration
The performance of a glucose sensor is potentially massively influenced by the calibration procedure; this can induce a systematic deviation with respect to the measurement results obtained with the comparison method. CGM systems that require calibration need BG measurements for this purpose in regular intervals after the glucose sensor was inserted (“in vivo” calibration). Two broadly used systems (FreeStyle Libre and Dexcom G6) are calibrated during the manufacturing process. Patients don’t need to perform any BG measurements when using such CGM systems; however, they have an option to do so in case of the G6. Prerequisite is a homogenous manufacturing of the glucose sensors over time and the assumption that the tissue conditions in the subcutaneous space within a patient and between patients are also “constant”; that is, the ratio between the in vitro and the in vivo sensitivity of a CGM sensor needs to be constant over time both within-patients and between patients
Optimal calibration of CGM systems requires that BG measurements are performed adequately (ie, avoiding user errors) with BG systems with a good quality. Performance of a “quality check” by BG measurements once per day helps ensure that the measurement of a given CGM system is not too far removed from the BG values; even for current CGM systems where calibration is not mandatory. In clinical studies with CGM systems, the same BG system should be used for performing calibration measurements by different patients to avoid additional sources of variability.
Consistency of MARD Over Time of CGM Usage
After insertion of the glucose sensor through the skin into the subcutaneous space it usually takes some hours until reliable measurement results are possible (“run-in phase”).19 The MARD in this period of time is high and declines in thereafter, that is, the analytical performance is better after this run-in phase for a number of days before it starts to worsen. Depending on which data are used for MARD calculation (including those of the run-in phase or not) and those of the days before the decline in performance starts (usually the recommended duration of use) the MARD might differ. Not only a summary MARD should be provided, but also calculations for all usage days (ie, MARD per day). Such a stratification over time provides instructive information about the changes in analytical performance of different CGM systems.54
The measurement performance of a given glucose sensor is also influenced by the conditions it is exposed to in the subcutaneous space. If the sensor tip is moved around all the time by movements of the patient (including “micro movements” that are not visible) or pressure is applied on the skin area around the sensor insertion site (eg, during sleep or sitting), this can affect tissue physiology such as exchange rates of glucose between blood and ISF and local blood flow around the sensor tip. Such effects can induce acute changes in the performance of the CGM system that lead to erroneous glucose readings or result in transient signal disruption but can last several hours.55 Nontransient long-term changes (“sensor fouling”) in glucose sensor performance have been described previously in detail.56
Impact of Algorithms and Filters Implemented in CGM Systems
It is important to understand that the CGM data used for MARD calculation are not the raw glucose signals measured by the glucose sensor, but those provided by the CGM system as output (stored data that can be downloaded for subsequent analysis). As the raw data, which are measured truly continuously, are superimposed with considerable amounts of noise and artifacts (with considerable differences between CGM systems depending on the sensor technology used), the data stream provided by the glucose sensor undergoes robust filtering and analysis by algorithms. Thereby the signal output is improved massively; however, it is an inherent feature of, for example, smoothing algorithms to introduce at least some physical time delay. Not much is known about the impact of algorithms and filtering on the MARD as the manufacturers of these devices regard these data and computational improvement opportunities as proprietary intellectual property, and the raw data, the sensor current, is rarely if ever available to outside parties or the end users.3
Estimation of MARD in Clinical Studies
The minimum performance criteria for BG systems are defined in the internationally accepted standard ISO 15197. The requirements for SMBG system accuracy are based on three considerations:
- the effectiveness of current technology for monitoring patients with diabetes mellitus
- recommendations of diabetes researchers as well as existing product standards and regulatory guidelines
- the state-of-the-art of BG monitoring technology
The advantage of having such a standard are:
- manufacturers know how good a newly developed BG system must be
- authorities know how good a BG system has to be in order to approve a new product
- clinicians and patients know how well they can trust the BG measurement results
As long as no internationally accepted standard for the performance of clinical evaluation studies of CGM systems is established and accepted by manufacturers, authorities and clinicians, each manufacturer can design and perform clinical studies according to its own discretion. Such a standard should also include recommendations for a structured and systematic evaluation of MARD; ideally in head-to-head studies. Without such an approach MARD numbers obtained by different studies have to be regarded with great care. The following aspects have to be taken into consideration:
Study Design
If the study design avoids large swings in glycemia, the MARD will be lower in comparison to the situation in which these show up. Ideally, two (or more) CGM systems would be studied in a head-to-head approach, as this neutralizes the potential impact of study cohort or study setting differences.
Patient Groups
Studies with patients with type 1 diabetes most probably will lead to higher MARD values than those with patients with type 2 diabetes as those with type 1 usually have larger swings in glycemia. Additionally, studies with well-controlled patients with a lower level of glycemic variability are expected to result in lower MARDs.
Reporting of Study Results
Besides providing an overall MARD (= mean of interindividual MARD values) as main study outcome, additional analyses should be performed providing MARD for time periods with different rates of glucose changes, different glucose ranges, night and day, duration of CGM usage. The MARD data obtained with all individual patients in a given clinical study should be presented in a sorted manner, listing the MARD by individual patient is one approach, or one can simply use the frequency distribution for the observed MARD within patients. This also provides information about the range of MARD results obtained with a given CGM system in different patients. The effect of compensation of measurement delays of the sensors should also be analyzed. This enables to construct a relation between the clinical study design and the accuracy of the computed MARD value. When two or more different CGM systems were studied in parallel, results of head-to-head assessments should also be presented. At least for some patients also intraindividual MARD data should be presented (if a given CGM system is used with multiple glucose sensors over time).
Such presentation of all MARD data available provide a better understanding of the CGM system performance and supports comparison of different CGM systems plus facilitates understanding the improvement seen with different generations of a given CGM system.
MARD Values of Different CGM Systems Obtained in Clinical Studies
For an optimal usability from a clinical point of view, CGM systems should provide glucose values that accurately reflect BG values, that is, the glucose measurement should be performed with a high accuracy, good precision, and without outliers. One would assume that exactly this is evaluated in the multitude of clinical studies performed over time with different CGM systems (Table 3 and Figure 2). The range of MARD values seen with each of the different CGM systems listed can be attributed to the variability in study design, patient selection, comparison method, and so on used. However, this also implies that if the MARD and the way it is measured in clinical studies were a really reliable parameter, different studies would provide more or less the same MARD. In other words, by selecting certain study conditions (eg, more patients with type 2 diabetes), one can influence the MARD in the preferred direction, which is usually toward lower values. Without more standardized clinical trials, the MARD is not a reliable parameter from an analytical point of view.
Discussion and Conclusion
In view of the rapid improvements seen in the last 15 years with respect to the analytical performance of CGM systems, there is a clear need for a parameter that characterizes it adequately. Despite all its limitations, currently the overall MARD is the most often used parameter to characterize the analytical performance of CGM systems. This was appropriate when the MARD was established, as CGM systems were approved for “adjunctive use” only, that is, therapeutic decisions had to be based on additional (confirmation?) BG measurements. However, nowadays CGM systems are approved as “nonadjunctive”-systems. In this case use of the MARD as sole parameter to characterize the performance of CGM systems is not sufficient. For example, the overestimation of the performance using the MARD of a given CGM obtained if large swings in glycemia are avoided, is one clear example of such critical topics—it is hard to understand why the very same sensor is reported to have any MARD between 11% and 21%. As CGM systems are an essential part of each AID system, an in-depth evaluation of the performance of such CGM systems is critical.
From the regulative side, this has not yet happed, the one approved guideline for the evaluation of CGM systems (POCT-05 from CLSI; https://clsi.org/media/1502/poct05a_sample.pdf) that does exist is from 2008 and describes generic performance metrics and how studies should be designed and the data analyzed, but no acceptance criteria (like for BG systems) are given—a new version of this guideline is currently in preparation. The FDA has recently formulated criteria for a minimal performance of CGM systems that are used in combination with AID (“iCGM”; https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm602870.htm), but those are (so far) not binding for approval, but fulfilling them merely facilitates the approval process.
Against this background, attempts are going on to retain MARD, but to improve the information provided by it with calculation of additional parameters like MARDs computed for subsets of the entire dataset of the clinical trial (stratified by glucose range, time of sensor wear, etc), MARD reliability index (MRI7), or PARD.5 Another option would be to define parameters that provide information about concentration errors (the MARD does not differentiate between accuracy and precision, see above) and also incorporate the additional information, such as trend arrows, that CGM provides.57 Still, there is not yet a consensus which should be based on clinical relevance and therapeutic concepts into account as well, which is still missing.
Indeed, more insight is needed on several critical issues: what happens when glucose levels are shown x% too high and/or the trend arrow indicates an incorrect rate of change in glycemia in the near future? What are the clinical consequences of a therapeutic decision based on such incorrect information? Do these parameters—in particular MARD—reflect the daily experience of patients and health care professionals? If, say, CGM systems that are reported to have comparable MARD values, lead to a different clinical experience, the practical relevance of the metric is unclear.
The improvement of MARD values makes the picture even more complex. Indeed, the manufacturers proudly announce an improvement in MARD from one generation of their CGM system to the next, and the most recent generations of CGM systems are reported to have MARD values in the single digit range (<10%). How low will the MARD become in the future? 6%? will this analytical performance become as good as that of BG systems? But the key question: it is clear from the list of factors described above, that the minimum MARD that can be achieved will not be zero—if the comparison method is SMBG, MARD values of 5% would never be achievable. Also the other inherent measurement errors will remain with each CGM system and the comparison method. So the key question is: will a very low MARD be indicative at all of the accuracy of the device? Indeed, it is not even clear if a reduction in MARD from 13% to 10% reflects the same improvement in clinical outcome than a reduction from 10% to 7%.58
Summarizing, in view of the recent progress of the CGM systems, MARD needs to evolve as well, and in the meantime caution in its use is needed.
Footnotes
Abbreviations: AID, automated insulin delivery; BG, blood glucose; CGM, continuous glucose monitoring; ISF, interstitial fluid; MARD, mean absolute relative difference; MRI, MARD reliability index; PARD, precision absolute relative difference; SMBG, self-monitoring of blood glucose; TIR, time in range.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH and GF support a number of companies in the development of novel diagnostic and therapeutic options for diabetes treatment. MS, GSR, RH, and AK are employees of Roche Diabetes Care. FR and LDR have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Writing of the manuscript was supported by an unrestricted grant by Roche Diabetes Care.
ORCID iDs: Lutz Heinemann  https://orcid.org/0000-0003-2493-1304
https://orcid.org/0000-0003-2493-1304
Günther Schmelzeisen-Redecker  https://orcid.org/0000-0001-8915-7044
https://orcid.org/0000-0001-8915-7044
Guido Freckmann  https://orcid.org/0000-0002-0406-9529
https://orcid.org/0000-0002-0406-9529
References
- 1. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilmoth DR. The relationships between common measures of glucose meter performance. J Diabetes Sci Technol. 2012;6:1087-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schmelzeisen-Redeker G, Schoemaker M, Kirchsteiger H, Freckmann G, Heinemann L, Del Re L. Time delay of CGM sensors: relevance, causes, and countermeasures. J Diabetes Sci Technol. 2015;9:1006-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kirchsteiger H, Heinemann L, Freckmann G, et al. Performance comparison of CGM systems: MARD values are not always a reliable indicator of CGM system accuracy. J Diabetes Sci Technol. 2015;9:1030-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Obermaier K, Schmelzeisen-Redeker G, Schoemaker M, et al. Performance evaluations of continuous glucose monitoring systems: precision absolute relative deviation is part of the assessment. J Diabetes Sci Technol. 2013;7:824-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoemaker M, Parkin C. CGM: how good is good enough? In: Kirchsteiger H, Jorgensen J, Renard E, del Re L, eds. Prediction Methods for Blood Glucose Concentrations. Cham, Switzerland: Springer; 2016:43-55. [Google Scholar]
- 7. Reiterer F, Polterauer P, Schoemaker M, et al. Significance and reliability of MARD for the accuracy of CGM systems. J Diabetes Sci Technol. 2017;11:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schrangl P, Reiterer F, Heinemann L, Freckmann G, Del Re L. Limits to the evaluation of the accuracy of continuous glucose monitoring systems by clinical trials. Biosensors (Basel). 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodbard D. Characterizing accuracy and precision of glucose sensors and meters. J Diabetes Sci Technol. 2014;8:980-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noujaim SE, Horwitz D, Sharma M, Marhoul J. Accuracy requirements for a hypoglycemia detector: an analytical model to evaluate the effects of bias, precision, and rate of glucose change. J Diabetes Sci Technol. 2007;1:652-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garg SK, Smith J, Beatson C, Lopez-Baca B, Voelmle M, Gottlieb PA. Comparison of accuracy and safety of the SEVEN and the Navigator continuous glucose monitoring systems. Diabetes Technol Ther. 2009;11:65-72. [DOI] [PubMed] [Google Scholar]
- 12. Luijf YM, Avogaro A, Benesch C, et al. Continuous glucose monitoring accuracy results vary between assessment at home and assessment at the clinical research center. J Diabetes Sci Technol. 2012;6:1103-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freckmann G, Pleus S, Link M, Zschornack E, Klotzer HM, Haug C. Performance evaluation of three continuous glucose monitoring systems: comparison of six sensors per subject in parallel. J Diabetes Sci Technol. 2013;7:842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Facchinetti A, Sparacino G, Guerra S, Luijf YM, DeVries JH, Mader JK, et al. Real-time improvement of continuous glucose monitoring accuracy: the smart sensor concept. Diabetes Care. 2013;36:793-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Damiano ER, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors. Diabetes Care. 2013;36(2):251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Christiansen M, Bailey T, Watkins E, et al. A new-generation continuous glucose monitoring system: improved accuracy and reliability compared with a previous-generation system. Diabetes Technol Ther. 2013;15:881-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Facchinetti A, Del Favero S, Sparacino G, Cobelli C. Model of glucose sensor error components: identification and assessment for new Dexcom G4 generation devices. Med Biol Eng Comput. 2015;53:1259-1269. [DOI] [PubMed] [Google Scholar]
- 18. Luijf YM, Mader JK, Doll W, Pieber T, Farret A, Place J, et al. Accuracy and reliability of CGM systems: a head-to head comparison. Diabetes Technol Ther. 2013;15:721-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pleus S, Schmid C, Link M, et al. Performance evaluation of a continuous glucose monitoring system under conditions similar to daily life. J Diabetes Sci Technol. 2013;7:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia A, Rack-Gomer AL, Bhavaraju NC, et al. Dexcom G4AP: an advanced continuous glucose monitor for the artificial pancreas. J Diabetes Sci Technol. 2013;7:1436-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Damiano ER, McKeon K, El-Khatib FH, Zheng H, Nathan DM, Russell SJ. A comparative effectiveness analysis of three continuous glucose monitors: the Navigator, G4 Platinum, and Enlite. J Diabetes Sci Technol. 2014;8:699-708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matuleviciene V, Joseph JI, Andelin M, et al. A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther. 2014;16:759-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kropff J, Bruttomesso D, Doll W, et al. Accuracy of two continuous glucose monitoring systems: a head-to-head comparison under clinical research centre and daily life conditions. Diabetes Obes Metab. 2015;17:343-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pleus S, Schoemaker M, Morgenstern K, et al. Rate-of-change dependence of the performance of two CGM systems during induced glucose swings. J Diabetes Sci Technol. 2015;9:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Beers CA, DeVries JH, Kleijer SJ, et al. Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol. 2016;4:893-902. [DOI] [PubMed] [Google Scholar]
- 26. Laffel L. Improved accuracy of continuous glucose monitoring systems in pediatric patients with diabetes mellitus: results from two studies. Diabetes Technol Ther. 2016;18(suppl 2):S223-S233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Andelin M, Kropff J, Matuleviciene V, et al. Assessing the accuracy of continuous glucose monitoring (CGM) calibrated with capillary values using capillary or venous glucose levels as a reference. J Diabetes Sci Technol. 2016;10:876-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonora B, Maran A, Ciciliot S, et al. Head-to-head comparison between flash and continuous glucose monitoring systems in outpatients with type 1 diabetes. J Endocrinol Invest. 2016;39:1391-1399. [DOI] [PubMed] [Google Scholar]
- 29. Aberer F, Hajnsek M, Rumpler M, et al. Evaluation of subcutaneous glucose monitoring systems under routine environmental conditions in patients with type 1 diabetes. Diabetes Obes Metab. 2017;19:1051-1055. [DOI] [PubMed] [Google Scholar]
- 30. Bailey TS, Chang A, Christiansen M. Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol. 2015;9:209-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Freckmann G, Pleus S, Grady M, Setford S, Levy B. Measures of accuracy for continuous glucose monitoring and blood glucose monitoring devices [published online ahead of print November 19, 2018]. J Diabetes Sci Technol. doi: 10.1177/1932296818812062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wadwa RP, Laffel LM, Shah VN, Garg SK. Accuracy of a factory-calibrated, real-time continuous glucose monitoring system during 10 days of use in youth and adults with diabetes. Diabetes Technol Ther. 2018;20:395-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Welsh JB, Kaufman FR, Lee SW. Accuracy of the Sof-sensor glucose sensor with the iPro calibration algorithm. J Diabetes Sci Technol. 2012;6:475-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. O’Neal DN, Adhya S, Jenkins A, Ward G, Welsh JB, Voskanyan G. Feasibility of adjacent insulin infusion and continuous glucose monitoring via the Medtronic Combo-Set. J Diabetes Sci Technol. 2013;7:381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kovatchev B, Anderson S, Heinemann L, Clarke W. Comparison of the numerical and clinical accuracy of four continuous glucose monitors. Diabetes Care. 2008;31:1160-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Diabetes Research in Children Network (DirecNet) Study Group; Buckingham B, Xing D, Weinzimer S, et al. Use of the DirecNet Applied Treatment Algorithm (DATA) for diabetes management with a real-time continuous glucose monitor (the FreeStyle Navigator). Pediatr Diabetes. 2008;9:142-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Keenan DB, Mastrototaro JJ, Zisser H, et al. Accuracy of the Enlite 6-day glucose sensor with guardian and Veo calibration algorithms. Diabetes Technol Ther. 2012;14:225-231. [DOI] [PubMed] [Google Scholar]
- 38. Calhoun P, Lum J, Beck RW, Kollman C. Performance comparison of the Medtronic Sof-Sensor and Enlite glucose sensors in inpatient studies of individuals with type 1 diabetes. Diabetes Technol Ther. 2013;15:758-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Freckmann G, Pleus S, Link M, Zschornack E, Klotzer HM, Haug C. Performance evaluation of three continuous glucose monitoring systems: comparison of six sensors per subject in parallel. J Diabetes Sci Technol. 2013;7:842-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahmoudi Z, Jensen MH, Dencker Johansen M, et al. Accuracy evaluation of a new real-time continuous glucose monitoring algorithm in hypoglycemia. Diabetes Technol Ther. 2014;16:667-678. [DOI] [PubMed] [Google Scholar]
- 41. Bailey TS, Ahmann A, Brazg R, Christiansen M, Garg S, Watkins E, et al. Accuracy and acceptability of the 6-day Enlite continuous subcutaneous glucose sensor. Diabetes Technol Ther. 2014;16:277-283. [DOI] [PubMed] [Google Scholar]
- 42. Norgaard K, Shin J, Welsh JB, Gjessing H. Performance and acceptability of a combined device for insulin infusion and glucose sensing in the home setting. J Diabetes Sci Technol. 2015;9:215-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Christiansen MP, Garg SK, Brazg R, et al. Accuracy of a fourth-generation subcutaneous continuous glucose sensor. Diabetes Technol Ther. 2017;19:446-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weinstein RL, Schwartz SL, Brazg RL, Bugler JR, Peyser TA, McGarraugh GV. Accuracy of the 5-day FreeStyle Navigator Continuous Glucose Monitoring System: comparison with frequent laboratory reference measurements. Diabetes Care. 2007;30:1125-1130. [DOI] [PubMed] [Google Scholar]
- 45. Geoffrey M, Brazg R, Richard W. FreeStyle navigator continuous glucose monitoring system with TRUstart algorithm, a 1-hour warm-up time. J Diabetes Sci Technol. 2011;5:99-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leelarathna L, Nodale M, Allen JM, et al. Evaluating the accuracy and large inaccuracy of two continuous glucose monitoring systems. Diabetes Technol Ther. 2013;15:143-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thabit H, Leelarathna L, Wilinska ME, et al. Accuracy of continuous glucose monitoring during three closed-loop home studies under free-living conditions. Diabetes Technol Ther. 2015;17:801-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey T, Bode BW, Christiansen MP, Klaff LJ, Alva S. The performance and usability of a factory-calibrated flash glucose monitoring system. Diabetes Technol Ther. 2015;17:763-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kropff J, Choudhary P, Neupane S, et al. Accuracy and longevity of an implantable continuous glucose sensor in the PRECISE study: a 180-day, prospective, multicenter, pivotal trial. Diabetes Care. 2017;40:63-68. [DOI] [PubMed] [Google Scholar]
- 50. Christiansen MP, Klaff LJ, Brazg R, et al. A prospective multicenter evaluation of the accuracy of a novel implanted continuous glucose sensor: PRECISE II. Diabetes Technol Ther. 2018;20:197-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nielsen JK, Freckmann G, Kapitza C, et al. Glucose monitoring by microdialysis: performance in a multicentre study. Diabet Med. 2009;26:714-721. [DOI] [PubMed] [Google Scholar]
- 52. Siegmund T, Heinemann L, Kolassa R, Thomas A. Discrepancies between blood glucose and interstitial glucose-technological artifacts or physiology. J Diabetes Sci Technol. 2017;11:766-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gehr B, Holder M, Kulzer B, et al. SPECTRUM: a training and treatment program for continuous glucose monitoring for all age groups [published online ahead of print August 20, 2016]. J Diabetes Sci Technol. doi: 10.1177/1932296816661735. [DOI] [Google Scholar]
- 54. Zschornack E, Schmid C, Pleus S, et al. Evaluation of the performance of a novel system for continuous glucose monitoring. J Diabetes Sci Technol. 2013;7:815-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Helton KL, Ratner BD, Wisniewski NA. Biomechanics of the sensor-tissue interface-effects of motion, pressure, and design on sensor performance and foreign body response-part II: examples and application. J Diabetes Sci Technol. 2011;5:647-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. Semin Immunol. 2008;20:86-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Freckmann G, Link M, Westhoff A, Kamecke U, Pleus S, Haug C. Prediction quality of glucose trend indicators in two continuous tissue glucose monitoring systems. Diabetes Technol Ther. 2018;20:550-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kovatchev BP, Patek SD, Ortiz EA, Breton MD. Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther. 2015;17:177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]