Abstract
Background:
The introduction of continuous glucose monitoring (CGM) implies new challenges for diabetes care. As CGM systems are often directly linked to a web-based software solution, structured telemedicine care using a video-consultation may be a new option for families who care for children with type 1 diabetes mellitus (T1DM).
Methods:
“ViDiKi” (Virtual Diabetes Outpatient Clinic for Children and Youth) is a multicenter controlled trial carried out in Northern Germany. ViDiKi will examine if monthly telemedical consultations, in addition to regular care, will improve glycemic control and psychosocial outcomes. The primary outcome is glycemic control as measured by a change in glycated hemoglobin (HbA1c). A total of 240 participants aged between one year and 16 years using a CGM with multiple daily injections (MDI) or insulin pump therapy were recruited and assigned to a starter group or a six-month waiting control group. The sample size is designed to detect a between-group difference of 0.5% in HbA1c change at six months. Secondary outcomes are variability of blood glucose, health-related quality of life, self-efficacy, and satisfaction with telemedicine. To gain deeper insight into the experience of using telemedicine, qualitative interviews will be conducted. In a health-economic analysis, the costs of telemedicine and a cost-of-care analysis will be calculated.
Conclusions:
The results from the ViDiKi study shall give important information on the feasibility and putative benefits of telemedicine in children with T1DM and their caregivers.
German Clinical Trails Register (DRKS):
DRKS00012645
Keywords: telemedicine, video-consultation, type 1 diabetes, children and adolescents, continuous glucose monitoring (CGM), health-economic analysis
Introduction
With the development of continuous glucose monitoring (CGM) as the basis for diabetes treatment, the previous standard of a blood glucose log-book with the written records of patients’ self-monitored blood glucose (SMBG) and also software analysis of SMBG has shifted in favor of CGM data stored and preanalyzed in cloud-based software. CGM can provide near real-time information on blood glucose levels and trends, and insulin adjustments can be made very swiftly. However, this new technology requires a high level of skill in dealing with a variety of information that directly affects insulin therapy.1 To get the greatest benefit from CGM data, patients and caregivers may need more frequent contact with the diabetes team to learn how to use the new technology to its full potential. Structured telemedicine care using a video-consultation may be a feasible, efficient, and cost-effective option to enhance diabetes care. ViDiKi (Virtual Diabetes Outpatient Clinic for Children and Youth) is a large multicenter German study that is designed to assess the impact of telemedicine as an additional service to regular care for those pediatric type 1 diabetes mellitus (T1DM) patients who already use either a real-time CGM (rtCGM) or intermittent scanning CGM (iscCGM) system. In the following we combine both rtCGM and iscCGM here as “CGM.”
Methods
Study Design and Study Phases
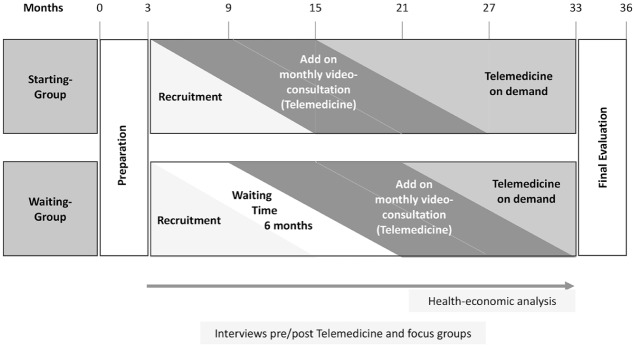
The study is divided into three phases: the first is a six-month, quasiexperimental trial with a control waiting group (WG) to evaluate the effects of monthly telemedicine (video-consultation) in addition to regular care. A quasiexperimental study design does not use random assignment. We have used the place of living as the criterion of randomization for the state of Schleswig-Holstein. The second phase, following the controlled trial, will investigate the impact of telemedicine over 12 months using an observational design. In the subsequent third phase, participants can either chose to end the trial or continue with telemedicine on a freely chosen interval. This “telemedicine on demand” can last between one month and 18 months until the end of the study (December 2019), depending on when the patients were enrolled. The qualitative interviews with teenagers and parents in the intervention group (IG) will be performed after one year of telemedicine experience and in the WG after six months regular care and 12 months telemedicine experience.
Interviews with telemedicine diabetologists and the doctors who refer their patients for cotreatment as part of the study are to be performed after at least six months of experience with telemedicine. The health-economic analysis is to be carried out in the second and third years of the study (Figure 1).
Figure 1.
Study design and study phases.
Study Sites
The study is being performed by three specialized diabetes outpatient clinics in the state of Schleswig-Holstein, Northern Germany: the pediatric diabetes center of the University Clinic of Schleswig-Holstein (UKSH), Campus Luebeck and Campus Kiel, and the pediatric diabetes center of the General Hospital Kiel. The clinic in Luebeck and both clinics in Kiel together take care of approximately 300 children aged 1-18 years with T1DM. All centers have a similar level of experience in MDI, insulin pump, and CGM use and are therefore comparable prior to the study. The diabetes clinic in Luebeck was appointed the IG site, and the two diabetes clinics located in Kiel were appointed the WG sites. Cooperating diabetologists from Schleswig-Holstein referred patients to the study (centers are listed in the acknowledgments). Due to a Europe-wide lack of one specific CGM starter system of a company in 2017, which limited the number of possible participants, the recruitment was extended to the adjacent Federal State of Hamburg with two specialized pediatric diabetes centers. Children from the center in western Hamburg were allocated to the WG, while those from the center in eastern Hamburg were allocated to the starter group.
Participants
Those eligible for the study were children and adolescents aged 1-16 years with T1DM treated in Schleswig-Holstein or Hamburg who used CGM with or without alarm function combined with an MDI or insulin pump.
Recruitment and Treatment Allocation
The study sites informed all families with a child 1-16 years of age with T1DM about the study by an information letter. All diabetologists in Schleswig-Holstein and the two pediatric diabetes centers in Hamburg were asked to inform their patients about ViDiKi. After checking for inclusion and exclusion criteria (Table 1) all eligible participants were invited to enroll in the study.
Table 1.
Inclusion and Exclusion Criteria.
| Inclusion criteria |
|---|
| • Must be one year of age and not reached the 17th
birthday • Diagnosed with type 1 diabetes • Diabetes duration at least six months • Using MDI with at least four injections per day or using an insulin pump • Using a CGM system with alarm or CGM system without alarm as a stand-alone system or as a SAP • Participants must have Internet access at home, and a computer or laptop with a camera and microphone • Sufficient language skills in German, English, or Turkish • Participants must be insured in one of the state health insurances supporting the trial • Get outpatient long-term treatment in the state of Hamburg or Schleswig-Holstein by a diabetologist or pediatric diabetologist |
| Exclusion criteria |
| • Diagnosed with type 2 or type 3
diabetes • Participating in another interventional trial six months before or concurrent to ViDiKi • Participated in a study with the intervention of telemedicine in the last two years • Regular telemedicine support (by phone/video portal) comparable to the intervention for four months prior to the study • Use of a DYS-PSa |
CGM, continuous glucose monitoring; MDI, multiple daily injections; SAP, sensor-augmented pump therapy.
Do-it-yourself (noncommercial) pump system working as a closed-loop insulin pump.
During a first meeting with their study diabetologist, parents and children were informed about the study, data protection, protection of skin irritation, use of email encryption, and use of the certified2 video portal. All participants received a short training session on their CGM system in order to make sure that both the starter group and WG had the same level of knowledge in CGM use. All eligible participants were allocated to the IG (starter) or control WG according to their place of residence in the state of Schleswig-Holstein, defined by postal code. A total of 240 children and teenagers were recruited.
Sample Size
The sample size was designed to detect a true difference of 0.5% in the mean HbA1c level of the IG after six months of telemedicine treatment and the mean HbA1c level of the WG with a power of 80%. A standard deviation of 1.2% was assumed. Alpha was set to 5%. Thus, a sample size of 92 participants per treatment group was needed. A target of 120 patients in each group was set to allow for an anticipated dropout rate of approximately 20%. The enrollment was finished on May 24, 2018. Dropouts are participants who are lost to follow-up, withdraw their consent, cannot wear a sensor regularly, or do not perform telemedicine due to technical or other reasons for more than three consecutive months.
The calculation was conducted with R3 and the function “power.t.test,” which is based on the sample size formula for a two-sided two-sample t-test.4
Intervention
The study diabetologists were trained to use and explain email encryption and use the video-consultation and structured CGM data analysis prior to the study. The intervention consists of a monthly video-consultation additional to regular care. If the participants do not want to use video or cannot use it for technical reasons, the consultation can also be conducted by telephone.
Participants upload their CGM and pump data every month in the software solution of their choice one to two days before their planned video contact. The diabetologists then analyze the data, write a recommendation for insulin treatment, and send it back via encrypted email to the participant. The time and duration of the telemedicine contact, and changes in therapy and the participating family members, are documented. On a quarterly basis the study diabetologist must get in contact with the diabetes team responsible for regular care to discuss the results and adjust treatment aims and choices if needed.
Ethical Issues and Data Protection
The study protocol was approved by the Ethics Committee of the University of Luebeck and is in compliance with the Declaration of Helsinki. A data protection plan was developed and approved by the independent data protection officer of the university hospital according to German federal and state data protection laws and European data protection regulations. Written informed consents of parents and children older than 12 years of age were obtained. School-aged children younger than 12 years gave their oral assent.
The Quantitative Study
The controlled part of the study analyzes the effect of six months of additional telemedical care compared with regular care only. Surveys after 12 and 18 months were conducted to ensure the stability of the observed effects.
Data Collection
The primary outcome of the study is the mean change in glycated hemoglobin (HbA1c) from baseline to six months. HbA1c values are measured locally. To adjust for different laboratory methods, the multiple of the mean method is applied to mathematically standardize HbA1c values to the Diabetes Control and Complications Trial reference range: 20.7-42.6 mmol/mol (4.05%-6.05%).5
Secondary medical outcomes are blood glucose variability (VC%/Time-in-Range) depending on the technical facilities of the CGM software, acute complications, and in-patient days. The target range in the software is set by 70-180 mg/dL (3.9-10.0 mmol/L) for CGM data analysis.
Secondary nonmedical outcomes: Patient-reported outcomes are measured using standardized and validated patient-reported outcome measures and measures of patient-reported experiences. Patients aged eight years and older and the main caregivers complete questionnaires on health-related quality of life (HRQOL), diabetes burden, self-efficacy, and treatment satisfaction. The parent questionnaire also comprises some basic demographic information: family status, parents’ educational level, and migration background.
HRQOL and diabetes burden: Generic and diabetes-related HRQOL of the patients are measured using the KINDL-R modular HRQOL questionnaire, which provides age-appropriate self-report versions of a generic core-measure, combined with a diabetes-specific module (KINDL-DM) for school-aged children and adolescents and a proxy-report version for younger children. Satisfactory psychometric properties and convergent and discriminative validity of the generic core measure have been reported.6,7 We used the 12-items short form of the generic measure, and we adapted the KINDL-DM to capture aspects of HRQOL related to the use of a CGM system. The CGM-adapted version comprises 26 items for children eight years and older (self-report version) and 13 items for children four to seven years (parent-proxy-report version), which are summarized for a diabetes-specific KINDL-DM Total Score, respectively. Items are five-Likert-scaled and scores are transformed into a 0-100 scale. Higher scores indicate better HRQOL in all versions.
The main caregiver (usually a parent) reports on their overall diabetes burden using a one-dimensional five-point intensity scale, which has proved sensitive to treatment change.8 Higher scores indicate a higher degree of diabetes burden. We assess psychological well-being of the main caregiver using the World Health Organization Five Well-Being Index (WHO-5). Higher scores indicate greater well-being.9
Self-efficacy: In the Maternal Self-Efficacy for Diabetes Management Scale (MSED), the primary caregivers rate their confidence in independently managing diabetes-related tasks on a five-point scale.10
The revised version comprises 11 items on three scales: MSED-M describes the perceived ability of parents to manage their child’s diabetes (two items), MSED-P comprises problem-solving issues surrounding glycemic control (six items), and MSED-T comprises the perceived ability to teach their child about diabetes care (three items). The subscales are summarized to a total score for good internal consistency as well as convergent and discriminative validity.11 Higher scores indicate a higher perceived self-efficacy. The German version of the MSED is being validated in the ViDiKi study.
Adolescents complete the Pediatric Self-Efficacy or Diabetes Type 1 Scale (PSEDT-1), a modified German version of the Self-Efficacy for Diabetes questionnaire.12 The 20-item questionnaire measures how much a child believes that they can or cannot handle situation-specific challenges of their current diabetes regimen. Responses are given on a six-point Likert-scale.
The four scales—insulin management competence (PSEDT-1-M), diabetes self-management skills (PSEDT-D), self-assertiveness (PSEDT-1-G), and autonomous self-regulation (PSEDT-1-S)—are combined to give a total score. The PSEDT-1 has shown good reliability and construct validity.13
Adolescents also completed the General Self-Efficacy Scale14 to capture a general sense of perceived self-efficacy. Responses to the ten-item scale are made on a four-point scale and summed up to give the total score.
Treatment satisfaction: Adolescents and their main caretaker complete the Diabetes Treatment Satisfaction Questionnaire, status version (DTSQs Parent and DTSQs Teen). Responses on treatment satisfaction (TS, ten items) and perceived metabolic control (PMC, two items) are given on a seven-point scale and summarized for the TS+C-Parent and the TS+C-Teen summary scores (11/9 items). Good psychometric properties, validity, and sensitivity to change are reported.15,16 Higher scores indicate greater satisfaction with diabetes treatment.
Telemedical care utilization: Frequency, duration, and time of day are assessed in order to analyze preferred time slots and time consumption with telemedicine. Other medical variables (comorbidity, medication, unexpected adverse events), major life-events, and technical-related variables are collected every six months in order to describe the study sample and allow for subgroup analyses and the inclusion of moderating variables. Demographic variables, diabetes onset, and diabetes duration were collected at baseline. Technical problems with data upload, PDF creation, email encryption, and use of the video-consultation are recorded for every study contact.
Data Analysis
Descriptive statistics are presented as frequencies or means and standard deviation. The primary outcome, the mean HbA1c level at six months, is compared between the two groups with a linear regression, adjusted for baseline HbA1c. The secondary outcomes are also compared after six months. In order to assess the stability of the telemedicine effect on the outcomes, time trends over the intervention phase are observed and analyzed with statistical methods for repeated measurements. Neither adaptations nor interim analyses are planned.
The Interview Study
The interview study focuses on barriers and benefits in using telemedicine, which consists of the preparation of the telemedicine appointment (CGM-software upload, email encryption contact) and the video-consultation process.
The interview study includes telephone interviews with families (substudy I), focus groups with parents and teenagers (substudies II, III), and one-to-one interviews with diabetologists performing telemedicine (substudy IV) and those referring their patients to the study (substudy V).
Interviews
The problem-centered interviews focus on the acceptance, implementation, and effectiveness of telemedicine counseling for children and adolescents with T1DM and are conducted using semistructured interview guidelines. The interview guidelines differ in their complexity, but include the same key topics as acceptance, feasibility, learned behaviors, barriers, and supportive factors. All interviews are recorded, completely transcribed, and anonymized as part of the transcription process.
Data Collection
Substudy I includes 15 participants from each center (Kiel, Luebeck) who are selected on the basis of age and study site. The parents are interviewed by phone shortly after enrolment in the study and again after 12 and 18 months.
In substudies II and III, parents and teenagers are invited to take part in eight focus groups after 12 months of telemedicine contact. In substudies IV and V, seven study diabetologists and eight referring diabetologists are interviewed.
Data Analysis
The data will be evaluated by the Qualitative Content Analysis method and software for Qualitative Content Analysis (QCAmap). The exploratory data analysis involves an inductive approach where the categories are developed from the data material. To ensure quality control and greater analysis objectivity, methods of both intracoder-reliability and intercoder-reliability are used.17
The Healthcare-Economic Analysis
The costs of medical care for children with diabetes in Germany have so far only been estimated in individual aspects of inpatient care18 or on the basis of list prices for medical aids.19 Detailed data, including modern therapy options such as CGM-sensors or sensor-augmented pump therapy, are not yet available. This study will therefore collect real cost data.
Data Collection
The cost data are made available by the health insurance companies of the study participants. This disclosure of data takes place after an extensive approval process by the patients and supervisory authorities of the health insurance companies. For all participating children in the study, the medical treatment costs will be collected over a period of six months and compared between the WG and IG (n = 120/n = 120). The aim is to compare the medical treatment costs with and without telemedicine. At the same time, the costs for telemedicine are calculated.
Data Analysis
A cost-of-care analysis associated with T1DM in childhood is performed. Here, the actual costs of care for outpatient and hospital treatment as well as costs for insulin, CGM systems, pumps, pump supplies, and pens are to be analyzed. The second focus is the detailed cost analysis of the telemedicine appointments including the time required for preparation and follow-up.
Dropout Analysis
Those participants who dropped out of the study are asked to fill in a questionnaire at the next outpatient appointment.
Discussion
Telemedicine is rapidly increasing in many medical fields, and digitization is especially advancing in diabetology, creating a pressing need for new communication channels and care models. Studies published so far, however, either had a relatively small number of cases or were published before the introduction of CGM in standard care.20-25 Since September 2016, in Germany every child with T1DM with a diabetes-related medical problem (e.g., hypoglycemia) can receive an rtCGM system with the cost reimbursed by their health insurance, which allows for more representative sampling in the ViDiKi study. To further reduce the selection bias we invited all potential participants in the federal state Schleswig-Holstein to the study. In order to meet real-life conditions, we allowed participants to use every available CGM system with or without an alarm function in combination with insulin pumps and MDI. The inclusion of children with a wide range of modern diabetes technologies reflects the diversity of diabetes outpatient clinics and makes it easier to transfer the findings into daily diabetes care. A further strength of the ViDiKi study is the mixed methods approach, combining surveys on a large number of patients, and open-ended information gathered through interviews and focus groups of patients and healthcare providers (HCPs).
Subgroup and nonresponder analyses can help to identify which groups of study participants benefit particularly, and why. The quasiexperimental design may be considered as a weakness of the study. However, we have chosen this option to prevent patients from changing diabetes outpatient departments in order to start telemedicine immediately. Furthermore, we judged the disadvantages of possible transfer effects within the institutions as more profound than a possible institutional bias. In the near future, telemedical care may replace some outpatient appointments. In the ViDiKi study, telemedicine was deliberately chosen as an add-on service, because in Germany, financing of diabetes treatment is based on regular visits of the patient in an outpatient clinic. Since it is not yet clear whether telemedicine can in fact replace outpatient contacts, we considered the discontinuation to be ethically questionable. ViDiKi will provide new evidence of how telemedical care may impact the metabolic control and day-to-day living of young patients with T1DM and their families, and also give insight into the experiences of HCPs with using telemedicine, as well as the costs of this new model of diabetes care.
Acknowledgments
We thank all ViDiKi consortium partners:
The health insurance AOK NORDWEST, General hospital Kiel and University of Luebeck, and cooperating health insurances (in alphabetical order): Barmer, BKK Atlas-Ahlmann, BKK Deutsche Bank, BKK Diakonie, BKK Energie, BKK Melitta Plus, BKK Mobil Oil, BKK Novitas, BKK Verbund Plus, BKK Viactiv, DAK, IKK Berlin-Brandenburg, IKK Nord, SVLFG, and TK.
We thank all diabetes centers and diabetologists from Schleswig-Holstein and the two referring specialized pediatric diabetes centers from Hamburg, who referred their patients to the study and allowed telemedical cotreatment 003A.
Georg Schenkluhn, Cordula Burghoff, Dorothea van Carnap, Thomas Borrmann, Urte Büssen, Ljubov Ott, Thorsten Wygold, Nadine Scheffler, Thomas Brinkmeier; Meike Femerling, Niko Lorenzen, Paul-Martin Holterhus; Inka Baus, Stefan Nissen, Dorothée Schmidt, Julia Hoppmann, Ulrike Menzel, Esther Schulz, Ulla Döhnert, Ulrike Duvigneau, Birgit Schipper, Elke Hammer, Anne Böhle, Benita Momm, and Sandra Wenzel.
The ViDiKi study group:
Institute of Social Medicine and Epidemiology at the University of Luebeck: Fabian-Simon Frielitz, Jana Dördelmann, Sabine Brehm, Joachim Hübner, Nora Eisemann, Alexander Katalinic.
University Medical Center, Campus Kiel: Paul-Martin Holterhus, Jessica Bokelmann, Miriam Krasmann, Tanja Ottersberg.
General Hospital Kiel: Andreas Claaß, Patrizia Kaczmarczyk, Bettina Bertram, Nicole Ahrendt, Christel Dziallias.
University Medical Center, Campus Luebeck: Olaf Hiort, Ingo Menrath, Alev Erdem, Tanja Meinsen, Renate Wagner, Simone von Sengbusch.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: F-S.F. worked as a consultant for Medtronic; S.v.S. is a consultant for Medtronic, Abbott, Lilly, Dexcom, Novo Nordisk, and Roche and received speaker honoraria from Medtronic, Abbott, Lilly, Novo Nordisk, Berlin-Chemie, Merck, and Sanofi. I.M. received speaker honoraria from Merck. O.H. is a consultant for Novo Nordisk, Infectopharm, Merck Serono, and UpToDate. A.K., J.D., N.E., E.M.G., and J.H. declared no conflicts of interest. All authors contributed to the design of the study and all have approved this manuscript.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the German Federal Joint Committee. Funding code 01NVF16023.
ORCID iDs: Fabian-Simon Frielitz  https://orcid.org/0000-0002-9414-347X
https://orcid.org/0000-0002-9414-347X
Nora Eisemann  https://orcid.org/0000-0002-8521-6497
https://orcid.org/0000-0002-8521-6497
References
- 1. Bomba F, Müller-Godeffroy E, von Sengbusch S. Experiences in sensor-augmented pump therapy in families with two children with type 1 diabetes: a qualitative study. Exp Clin Endocrinol Diabetes. 2018;126:162-167. [DOI] [PubMed] [Google Scholar]
- 2. Kassenärztliche Bundesvereinigung. Anbieter für Videosprechstunde zertifiziert. https://www.kbv.de/html/1150_30410.php. Accessed January 29, 2019.
- 3. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: 2018; https://www.r-project.org/. Accessed April 2, 2019. [Google Scholar]
- 4. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: L. Erlbaum Associates; 1988. [Google Scholar]
- 5. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977-986. [DOI] [PubMed] [Google Scholar]
- 6. Ravens-Sieberer U, Bullinger M. Assessing health-related quality of life in chronically ill children with the German KINDL: first psychometric and content analytical results. Qual Life Res. 1998;7:399-407. [DOI] [PubMed] [Google Scholar]
- 7. Ravens-Sieberer U. Manual - kindl.org. kindl.org. https://www.kindl.org/deutsch/manual/. Accessed April 15, 2019.
- 8. Müller-Godeffroy E, Treichel S, Wagner VM, on behalf of the German Working Group for Paediatric Pump Therapy. Investigation of quality of life and family burden issues during insulin pump therapy in children with Type 1 diabetes mellitus-a large-scale multicentre pilot study. Diabetic Medicine. 2009;26:493-501. [DOI] [PubMed] [Google Scholar]
- 9. WHO. WHO-5 Questionnaires. https://www.psykiatri-regionh.dk/who-5/Pages/default.aspx. Accessed July 31, 2018.
- 10. Leonard BJ, Skay CL, Rheinberger MM. Self-management development in children and adolescents with diabetes: the role of maternal self-efficacy and conflict. J Pediatr Nurs. 1998;13:224-233. [DOI] [PubMed] [Google Scholar]
- 11. Noser AE, Patton SR, Van Allen J, Nelson MB, Clements MA. Evaluating parents’ self-efficacy for diabetes management in pediatric type 1 diabetes. J Pediatr Psychol. 2017;42:296-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grossman HY, Brink S, Hauser ST. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care. 1987;10:324-329. [DOI] [PubMed] [Google Scholar]
- 13. Sethe D, Büssing A, Hilgard D, Berger B. Validation of the German version of Pediatric Self-Efficacy for Diabetes-Type-1 Scale. PPmP; in press. [DOI] [PubMed] [Google Scholar]
- 14. Schwarzer R, Jerusalem M. Generalized Self-Efficacy scale. In: Weinman J, Wright S, Johnston M. (eds) Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. Windsor: NFER-NELSON; 1995:35-37. [Google Scholar]
- 15. Bradley C, Lewis KS. Measures of psychological well-being and treatment satisfaction developed from the responses of people with tablet-treated diabetes. Diabet Med. 1990;7:445-451. [DOI] [PubMed] [Google Scholar]
- 16. Mueller-Godeffroy E, Vonthein R, Ludwig-Seibold C, et al. Psychosocial benefits of insulin pump therapy in children with diabetes type 1 and their families: the pumpkin multicenter randomized controlled trial. Pediatric Diabetes. 2018;19:1471-1480. [DOI] [PubMed] [Google Scholar]
- 17. Mayring P. Qualitative Inhaltsanalyse: Grundlagen und Techniken. 11th ed. Weinheim: Beltz; Germany, 2010. [Google Scholar]
- 18. Icks A, Strassburger K, Baechle C, et al. Frequency and cost of diabetic ketoacidosis in Germany–study in 12,001 paediatric patients. Exp Clin Endocrinol Diabetes. 2013;121:58-59. [DOI] [PubMed] [Google Scholar]
- 19. Bächle C, Icks A, Straßburger K, et al. Direct diabetes-related costs in young patients with early-onset, long-lasting type 1 diabetes. Postma M, ed. PLoS One. 2013;8:e70567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guttmann-Bauman I, Kono J, Lin AL, Ramsey KL, Boston BA. Use of telehealth videoconferencing in pediatric type 1 diabetes in Oregon. Telemed E Health. 2017;24:86-88. [DOI] [PubMed] [Google Scholar]
- 21. Bertuzzi F, Stefani I, Rivolta B, et al. Teleconsultation in type 1 diabetes mellitus (TELEDIABE). Acta Diabetol. 2018;55:185-192. [DOI] [PubMed] [Google Scholar]
- 22. Esmatjes E, Jansà M, Roca D, et al. The efficiency of telemedicine to optimize metabolic control in patients with type 1 diabetes mellitus: telemed study. Diabetes Technol Therap. 2014;16:435-441. [DOI] [PubMed] [Google Scholar]
- 23. Izquierdo R, Morin PC, Bratt K, et al. School-centered telemedicine for children with type 1 diabetes mellitus. J Pediatrics. 2009;155:374-379. [DOI] [PubMed] [Google Scholar]
- 24. Wood CL, Clements SA, McFann K, Slover R, Thomas JF, Wadwa RP. Use of telemedicine to improve adherence to American Diabetes Association standards in pediatric type 1 diabetes. Diabetes Technol Therap. 2015;18:7-14. [DOI] [PubMed] [Google Scholar]
- 25. Raymond JK, Berget CL, Driscoll KA, Ketchum K, Cain C, “Fred” Thomas JF. CoYoT1 clinic: innovative telemedicine care model for young adults with type 1 diabetes. Diabetes Technol Therap. 2016;18:385-390. [DOI] [PMC free article] [PubMed] [Google Scholar]