Abstract
Background:
Point-of-care (POC) glucometers are commonly used in intensive care units (ICUs). The Centers for Medicare & Medicaid Services have called into question the accuracy of POC glucometers in critically ill patients. This study sought to identify specific characteristics within our facility’s ICU patients that were associated with inaccuracies in POC glucose measurements.
Methods:
We conducted a prospective cohort study that compared POC capillary blood glucose samples with venous samples collected in our ICU. All nonpregnant patients >18 years old admitted to the ICU with orders for daily laboratory testing that included blood glucose were eligible for inclusion.
Results:
A total of 46 patients were enrolled and 85 samples were collected. The mean difference between venous and POC samples was 5.23 mg/dL (95% CI, 3.16-7.3 mg/dL). Measurement inaccuracies would have altered treatment in 7/85 instances (8.2%). The only clinically significant inaccuracy found was the omission of 2 units of insulin in 1 hyperglycemic patient. Measurement inconsistencies generally underestimated low blood glucose values (2/2 instances) and overestimated high blood glucose values (4/5 instances).
Conclusions:
In our study, the mean difference between venous and POC glucose samples was small. Similarly, measurement inaccuracies that would have altered treatment were rare and only one instance was deemed clinically significant. We conclude that POC capillary glucose testing within our cohort and in similar critically ill patients is likely safe and effective.
Keywords: point of care, blood glucose, intensive care unit, critical care
Introduction
Point-of-care (POC) glucometers are commonly used in the intensive care unit (ICU) to assist practitioners in making rapid treatment decisions regarding glycemic management. The Centers for Medicare & Medicaid Services (CMS) govern the use of POC glucometers under the Clinical Laboratory Improvement Amendments. To date, CMS has not certified the vast majority of these devices to be marketed and used in critically ill patients likely due to a perceived lack of clinical reliability in this population.1 Despite this, POC glucometers continue to be used in ICUs because they are easy to use and provide timely results.2
In November 2014, CMS issued a memorandum that deemed that any change in a manufacturer’s intended use or a disregard of a device’s limitations constituted “off-label use.”1 As the vast majority of POC glucometers are not labeled for use in critically ill patients, their use in this population is considered off-label. If a facility desires to use POC glucometers in an off-label fashion, it must acquire a certificate of analysis, establish performance specifications, and comply with high-complexity testing standards. CMS did not define what characteristics constitute a “critically ill” patient, so which patient samples are subject to these standards is largely unknown.
The purpose of this study was to identify specific characteristics within our facility’s ICU patients that were associated with inaccuracies in POC blood glucose measurements in order to define which patients should be considered “critically-ill” in the context of POC glucose testing within our institution.
Methods
This was a prospective cohort study in critically ill adult patients between November 1, 2015 and June 30, 2016. The study site is a 28-bed mixed ICU serving patients in the following service lines: neurosurgery, neurology, cardiothoracic surgery, colorectal surgery, general surgery, and medicine. The study was approved by the SSM St. Louis Institutional Review Board.
Patients were included in the study if they were admitted to the ICU, were ≥18 years old, and had orders for daily laboratory testing of venous blood glucose (ie, basic or comprehensive metabolic panels) during the study period. Pregnant patients were not included in the study.
The primary endpoint of the study was the mean difference between venous and POC glucose measurements across the cohort. Secondary endpoints included the amount of insulin and dextrose that would have been administered or omitted due to POC measurement inaccuracies and a description of the clinical significance of POC measurement inaccuracies. A POC measurement inaccuracy was defined as an alteration in protocolized insulin or dextrose treatment if only the POC result was to be considered to be true. An inaccuracy was deemed clinically significant if appropriate insulin or dextrose therapy would have been omitted (ie, hypoglycemia was overestimated or hyperglycemia was underestimated by POC measurements). Hyperglycemia was defined as a venous glucose value >180 mg/dL and hypoglycemia as defined as a venous glucose value <70 mg/dL. Volume status was defined as the net difference in fluid intake and output in milliliters recorded within the chart at the time of the index POC measurement. This value assumed a euvolemic state at ICU admission.
Patients eligible for inclusion in the study were approached by one of the study authors, were introduced to the study, and were asked to sign an informed consent form. Legal representatives were asked to provide consent for incapacitated or unresponsive patients. The patients who were consented were then enrolled in the study. Study testing commenced on the next calendar day after enrollment.
Enrolled patients received a supplemental capillary POC glucose measurement within 30 minutes of their venous glucose measurement during the study period. These measurements were facilitated by ICU nurses using available, quality-controlled glucometers (Precision Xceed Pro, Abbott, Chicago, IL, USA). These glucometers are not specifically labeled for use in “critically ill” patients. Patients did not eat, receive dextrose-containing fluids, or receive insulin or antidiabetic medication between the index venous and POC glucose sampling. Patients were tested once per day for a total of 3 days to yield up to 3 study samples. Of note, ICU nurses were instructed to treat patients based on the venous value collected to avoid potential adverse events during the study period. POC values were drawn only for comparison’s sake. After the study period was completed, relevant data were extracted from the medical records of enrolled patients by the study coordinator.
The mean difference between venous and POC glucose measurements across the cohort and the corresponding limits of agreement were determined using the Bland-Altman method. The amount of insulin, dextrose, and the clinical significance of POC measurement inaccuracies were determined by way of descriptive analysis. Statistical testing was completed using Minitab 17 software (Minitab Inc., State College, PA, USA).
Results
Forty-six patients were enrolled in the study and 85 POC samples were obtained for comparison. The main reason for the exclusion of patient samples was discharge from the ICU (53.8%) (Figure 1). The average time between the capillary POC glucose collection and the venous glucose collection was 3 minutes and 30 seconds. The enrolled patients were predominantly medical patients (80%) with a mean age of 65 years, a mean hemoglobin level of 10.6 mg/dL, and a mean hematocrit of 31.9%. Thirty-six percent of patients in the study were on the ventilator at the time of sample collection and 18.8% of patients were receiving vasopressor medication (Table 1).
Figure 1.
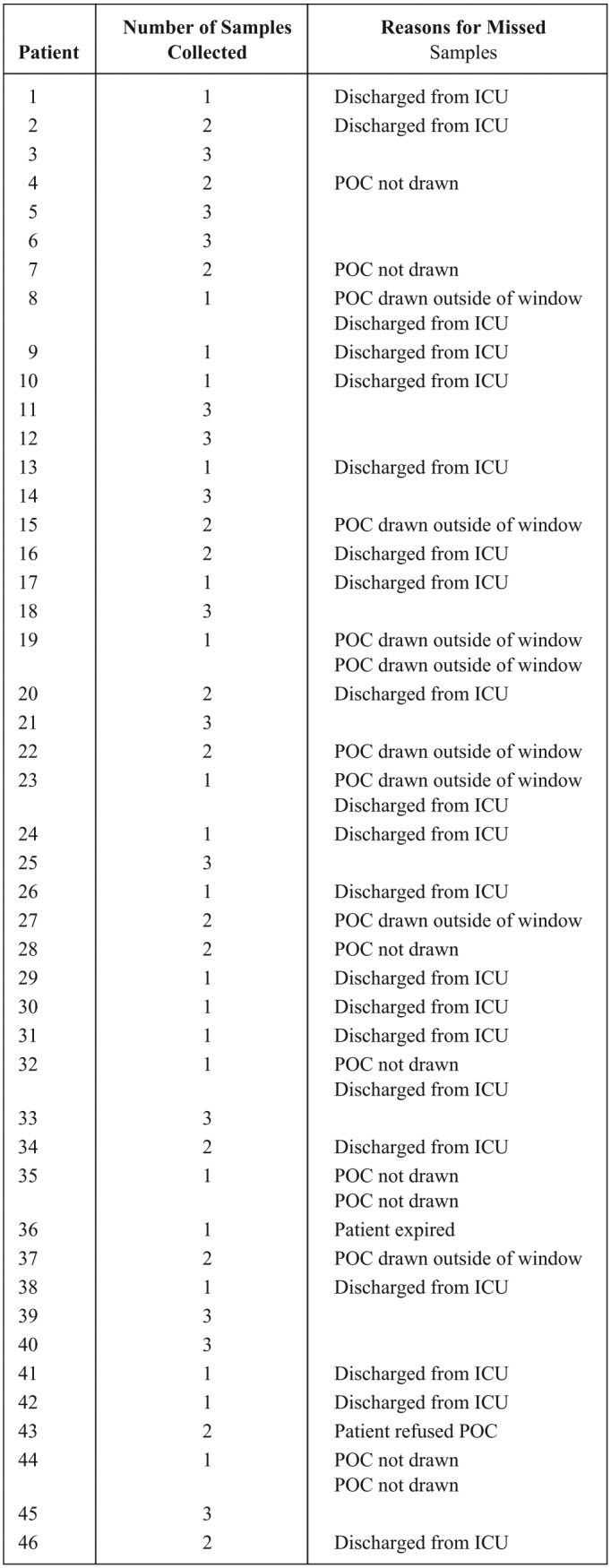
Sample collection.
Table 1.
Baseline Characteristics.
| Characteristic | Total (n = 85) |
|---|---|
| Age (years)a | 65 ± 15 |
| Weight (kg)a | 97 ± 35 |
| Hemoglobin (mg/dL)a | 10.6 ± 1.8 |
| Hematocrit (%)a | 31.9 ± 5.9 |
| Body mass index (kg/m2)a | 33 ± 11 |
| Net fluid balance (mL)b | 1717 (−14 280 to 20 353) |
| Medical patient | 68 (80%) |
| Surgical patient | 17 (20%) |
| Ventilator | 31 (36.5%) |
| Vasopressors | 16 (18.8%) |
| Ventilator + vasopressors | 10 (11.8%) |
| Diagnoses | |
| Acute hypoxic respiratory failure | 20 (23.5%) |
| Coronary artery bypass graft | 15 (17.6%) |
| Septic shock | 11 (12.9%) |
| Cardiac arrest | 8 (9.4%) |
| Sepsis | 5 (5.9%) |
| Severe sepsis | 3 (3.5%) |
| Diabetic ketoacidosis | 3 (3.5%) |
| Atrial fibrillation with rapid ventricular rate | 2 (2.4%) |
| Non-ST-segment elevation myocardial infarction | 2 (2.4%) |
| Intraparenchymal hemorrhage | 2 (2.4%) |
| Urosepsis | 2 (2.4%) |
| Seizures | 1 (1.2%) |
| Drug overdose | 1 (1.2%) |
| Subdural hematoma | 1 (1.2%) |
| Gastrointestinal bleed | 1 (1.2%) |
| Obstructed colon | 1 (1.2%) |
| Status epilepticus | 1 (1.2%) |
| Cerebral vascular accident | 1 (1.2%) |
| Hypotension | 1 (1.2%) |
| Mitral value replacement | 1 (1.2%) |
| Cardiogenic shock | 1 (1.2%) |
| Pericardial effusion | 1 (1.2%) |
| Subarachnoid hemorrhage | 1 (1.2%) |
Unless otherwise stated, values are n (%).
Mean ± SD.
Median (range).
The mean difference between venous and POC glucose measurements across the cohort was 5.2 mg/dL (95% CI, 3.2-7.3). The upper limit of agreement for the mean difference was 42.7 mg/dL and the lower limit of agreement was −32.2 mg/dL (Figure 2). Seven POC glucose results (8.2%) were deemed to be inaccurate by the study authors. Four POC measurement inaccuracies (4/7, 57.1%) overestimated high glucose values and resulted in the excessive use of a total of 10 units of insulin aspart. Two POC measurement inaccuracies (2/7, 28.6%) underestimated low glucose values and resulted in the excessive use of a total of 25 g of dextrose (Table 2).
Figure 2.
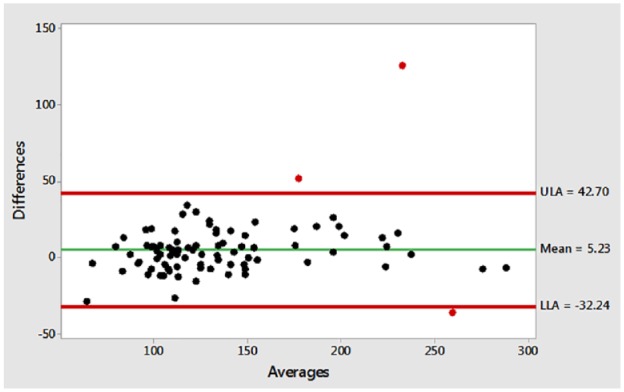
Bland-Altman plot.
Table 2.
Characterization of POC Measurement Inaccuracies.
| Venous (mg/dL) | POC (mg/dL) | Treatment variation |
|---|---|---|
| 216 | 229 | +2 units insulin aspart |
| 166 | 185 | +2 units insulin aspart |
| 152 | 204 | +2 units insulin aspart |
| 170 | 296 | +4 units insulin aspart |
| 79 | 50 | +12 g dextrose |
| 70 | 66 | +12 g dextrose |
| 278 | 242 | −2 units insulin aspart |
One POC measurement inaccuracy (1/7, 14.3%) underestimated a hyperglycemic value and resulted in the omission of 2 units of insulin aspart. This inaccuracy was deemed by study authors to be clinically significant. This was the only clinically significant POC measurement inaccuracy reported during the study (1/46 patients, 2.2%). There were no POC measurement inaccuracies that overestimated hypoglycemic values in the study. Patient characteristics noted to be present in greater than 50% of POC measurement inaccuracies included a net fluid balance of 2000 mL or greater (6/7, 85.7%) and admission to the ICU after coronary artery bypass grafting (CABG) surgery (4/7, 57.1%). The need for vasopressors was less commonly present (1/7, 14.3%) and the need for mechanical ventilation was not present for any recorded POC inaccuracies.
Discussion
Blood glucose monitoring is vital in critically ill patients in order to effectively minimize hyperglycemia, hypoglycemia, and glucose variability. Hyperglycemia has been most associated with impaired immune function, impaired wound healing, and increased overall morbidity in critically ill patients.2 Both hypoglycemia and glucose variability have been associated with an increased risk of mortality with the former having the strongest association with mortality in critically ill patients.3
POC capillary glucose testing is an efficient way to monitor blood glucose in critically ill patients and presents a lower risk of both preanalytical and postanalytical errors than venous testing due to its close proximity to the patient and single operator requirement.3 It also is likely preferable for patients as it requires less blood volume for testing than does venous testing.3 In theory, frequent POC glucose monitoring can help identify harmful blood glucose trends and can help to minimize the occurrence of both hyper and hypoglycemia and glucose variability when coupled with protocolized insulin or dextrose therapy.
Despite the use of POC capillary glucose testing in critically ill patients for decades, the clinical reliability of this testing in this population has often been called into question.1,4-8 Factors commonly thought to impact analytical accuracy of these values that are prevalent in critically ill patients include poor blood flow to peripheral capillaries due to shock and/or vasopressor use, fluid overload or anasarca, and decreased hemoglobin concentration/hematocrit.2,5-8 Therefore, venous sampling has been deemed to be the most appropriate modality for assessing blood glucose in the critically ill as it is less affected by these factors. However, venous sampling is significantly more time-consuming than POC testing, making it a nonideal modality for routine glucose testing in ICUs. Thus, POC testing has remained the mainstay modality used to monitor glucose in ICUs despite perceived inaccuracies.
After the publishing of the aforementioned CMS memorandum, use of unapproved POC glucometers in critically ill patients now requires acquisition of a certificate of analysis, establishment of performance specifications, and compliance with high-complexity testing standards.1 These requirements likely require additional training, personnel hours, and equipment in most ICUs and force ICU staff to decide how best to allocate resources to remain in compliance with CMS requirements.
We conducted a prospective cohort study within our ICU that directly compared POC values to venous values in order to assess clinical reliability of POC glucose testing in critically ill patients. Our major intent in conducting this study was to identify characteristics that predisposed our patients to aberrant POC values to best define which patients were “critically ill” in the context of POC glucose testing and the CMS memorandum. Our hope was our study would yield characteristics within our critically ill population that predisposed patients to clinically significant POC measurement inaccuracies in order to focus our use of high-complexity testing and related resources on those patients. We discovered that the average measurement difference between POC and venous glucose values was small. This finding precluded an analysis of specific characteristics that predicted clinically significant POC measurement inaccuracies. We did note clinically unacceptable limits of agreement between the two measurement modalities however, and thought it necessary to then perform an in-depth analysis of the clinical significance of POC measurement inaccuracies before declaring that the use of these measurements was safe and effective in our cohort. In that analysis, we found that clinically significant measurement differences were extremely rare. Only one patient (2.2% of patients enrolled) in the cohort was considered to have experienced undertreatment of hyperglycemia and should have received 2 additional units of rapid-acting insulin based on the venous glucose value obtained. Of note, that patient had undergone CABG surgery and was volume overloaded (+4,887 mL) but was not on vasopressors or the ventilator. In all other instances of POC measurement inaccuracies, POC testing seemed to overestimate higher glucose values (more insulin was administered than required per protocol if the venous measurement was followed) and underestimate lower blood glucose values (dextrose was administered when not required if the venous measurement was followed). Of note, the study authors felt as though the prevalence of hypoglycemia would be extremely rare if patients received extra insulin based on the POC glucose value (if that value overestimated the venous glucose value) because the small amounts of additional insulin called for by those inaccuracies. Most important to the study authors was that hypoglycemia was never found to have been overestimated by POC testing and was never undertreated during the study. Such a scenario was thought to be the most likely to lead to an increased risk of mortality for study patients.
While no specific characteristics predisposing patients to aberrant POC glucose measurements were discovered in the study due to the rarity of measurement inaccuracies between testing modalities, fluid overload and postoperative CABG admissions were the most prevalent characteristics among patients with measurement inaccuracies. Further investigation into POC capillary glucose measurement in patients with these characteristics may be warranted in future studies.
The results of this study must be interpreted in the context of its limitations. First, due to the observational nature of the study, we cannot account for all possible confounding variables that may have affected study results. Second, a relatively small sample size may have limited our ability to provide a more reliable average measurement difference between POC and venous sampling and to detect clinically significant POC measurement inaccuracies. Third, our study was performed at a single center and is representative of the use of one POC glucometer in one ICU population. As such, the results may not be fully translatable to other ICU populations and further study may be warranted. Finally, despite established protocols within our institution to ensure quality control of POC glucometers and test strips and the provision of hands-on training to ICU nurses responsible for drawing POC capillary samples, we cannot rule out the possibility of human error or machine malfunction having an effect on the measurement differences reported in the study.
Conclusions
This study directly compared POC capillary glucose values to venous glucose values in our facility’s ICU population in order to assess the clinical reliability of POC glucose testing and to define which patients should be considered “critically ill” in the context of POC glucose testing. We report a small mean difference in measurements obtained by POC and venous sampling modalities, a small incidence of POC measurement inaccuracies, and only one clinically significant measurement inaccuracy that resulted in omission of insulin for a hyperglycemic value. We conclude that POC capillary glucose testing within our cohort and in similar critically ill patients is likely safe and effective. We do recognize that further study may be warranted to increase the applicability of these results to a more general critically ill population using a wider variety of POC glucometers. We suggest that postoperative CABG patients and those experiencing volume overload be preferentially included in future studies based on our findings.
Acknowledgments
We would like to acknowledge the ICU nursing staff at SSM Health St. Clare Hospital—Fenton for their dedication and making this study a reality. We would also like to acknowledge Kristina Bryowsky, PharmD, for helping the authors see the true value of this study.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chris Carter  https://orcid.org/0000-0003-4723-1853
https://orcid.org/0000-0003-4723-1853
References
- 1. Survey and Certification Group. Reissuance of S&C 15-11 As DRAFT ONLY – FOR COMMENT. CMS. March 2015. Available at: http://www.cms.gov. Accessed July 7, 2018.
- 2. Rajendran R, Rayman G. Point-of-care blood glucose testing for diabetes care in hospitalized patients: an evidence-based review. J Diabetes Sci Technol. 2014;8:1081-1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Balloni A, Lari F, Giostra F. Evaluation and treatment of hyperglycemia in critically ill patients. Acta Biomed. 2016;87(3):329-333. [PMC free article] [PubMed] [Google Scholar]
- 4. Kiechle FL, Main RI. Blood glucose: measurement in the point-of-care setting. Lab Med. 2000;31(5):276-282. [Google Scholar]
- 5. Dungan K, Chapman J, Braithwaite SS, et al. Glucose measurement: confounding issues in setting targets for inpatient management. Diabetes Care. 2007;30(7):403-409. [DOI] [PubMed] [Google Scholar]
- 6. Rebel A, Rice MA, Fahy BG. Accuracy of point-of-care glucose measurements. J Diabetes Sci Technol. 2012;6(2):396-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pitkin AD, Rice MJ. Challenges to glycemic measurement in the perioperative and critically ill patient: a review. J Diabetes Sci Technol. 2009;3(6):1270-1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wahl HG. How accurately do we measure blood glucose levels in intensive care unit (ICU) patients? Best Pract Res Clin Anaesth. 2009;23(4):387-400. [DOI] [PubMed] [Google Scholar]


