Abstract
The flash glucose monitoring (FGM) system FreeStyle Libre® is a device that measures interstitial glucose in a very simple way and indicates direction and speed of glucose change. This allows persons with diabetes to prevent hypoglycemic and hyperglycemic events. Scientific evidence indicates that the system can improve glycemic control and quality of life. To obtain the maximum benefit, it is necessary to properly handle glucose values and trends. Due to the generalization of the system use, the purpose of the document is to provide recommendations for the optimal use of the device, not only in the management of glucose values and trends but also in the prevention of hypoglycemia, actuation in exercise, special situations, and retrospective analysis of the glucose data, among others.
Keywords: ambulatory glucose profile, flash glucose monitoring, interstitial glucose, type 1 diabetes, type 2 diabetes
Rationale, Methods, and Evidence
Glucose monitoring is one of the key elements in diabetes management; especially for insulin treated patients. Most of patients rely exclusively on self-monitoring of blood glucose (SMBG) through the use of finger-stick blood samples, test strips, and glucose meters. However, SMBG offers only limited glucose information.
In 2014, the flash glucose monitoring (FGM) system (FreeStyle Libre®; Abbot Diabetes Care, Witney, Oxon, UK), which measures intermittent interstitial glucose (IG), was marketed in Spain. With this system, IG monitoring has become widespread for patients, particularly those on insulin therapy. The need to establish guidelines for optimal use of this system is evident as clinical experience and scientific evidence continue to grow and given its incorporation into health care system’s coverage in many countries including Spain.
Through painless scans, FGM facilitates more glucose readings. This increase in glucose data should in turn:
Improve glycemic control and reduction of hypo-glycemias.
Decrease glucose variability.
Improve quality of life and treatment satisfaction.
All of these benefits could contribute to decrease mortality and chronic complications in the long term, however there is no evidence in this point at present time.
Clinical experience and scientific data suggest that FGM provides clinical benefits in:
Improvement in blood glucose due to more number of glucose measurements.
Reduction of SMBG readings required for glucose control.
Reduction in the number and duration of hypoglycemia and hyperglycemia events.
When FGM is compared to plasma blood glucose (BG) shows adequate accuracy for clinical decision making.1 However, IG is different from BG particularly in situations when glucose changes rapidly. Changes in IG are seen with a delay of ≈5 minutes in relation to BG in the FGM system.2
The system provides predictive (trend arrows) and retrospective information from the last 8 hours. Retrospective information includes estimated glycosylated hemoglobin (HbA1c), monthly pooled profile, glycemic variability, glucose average, proportion of values above, below, and in target (70-180 mg/dL), number and duration of hypoglycemic events, daily profiles, number of SMBGs, number of scans, and so on. This information is very relevant if properly used. Therefore, the purpose of this document is to facilitate the optimal use of this tool through adequate training for patients and professionals.
To prepare this document, the available scientific evidence was reviewed, including similar international recommendations.3,4 Most of these were developed for continuous glucose monitoring (CGM) systems.5-10 This document provides general recommendations adjusted for FGM, which should be adapted to individual patient needs.
Treatment Adjustment Based on Trend Arrows
Prior Considerations
There are situations with a lower correlation between the IG provided by FGM and BG values: first 24 hours of use, in hypoglycemia and hyperglycemia, and when rapid changes of glucose occur (postprandial period, exercise, etc).
Therefore, SMBG measurements are recommended in some situations: when IG readings change rapidly, to confirm hypoglycemia and when symptoms do not correspond with IG value.
Therapeutic diabetes education is mandatory to obtain maximum benefits from the system. Patients must be instructed to scan at least every 8 hours, and to review retrospective data regularly. These actions are essential to detect patterns and to act accordingly. The main objectives of therapeutic diabetes education are addressed:
To establish individual glucose goals
To interpret arrows, adopt preventive actions and evaluate the impact of those actions through a new glucose reading.
To analyze the current situation before making decisions: insulin on board, food intake, postprandial period, exercise, stress, etc.
To make “step-by-step” changes with caution to avoid excessive corrections in both hyperglycemia and hypoglycemia.
To consider insulin administration 15-30 minutes before a meal, if glucose rises rapidly.
To consider insulin administration just before or after a meal, if glucose falls rapidly.
Trend Arrows
FGM system provides the current IG value with a trend arrow. The meaning of trend arrows is shown in Table 1.
Table 1.
Meaning of the Trend Arrows Provided by the FGM System.

|
Glucose level increasing rapidly (> 2 mg/dL per minute) or increased by 60-90 mg/dL in 30 minutes |

|
Glucose level increasing more gradually (1-2 mg/dL per minute) or increased by 30-60 mg/dL in 30 minutes |

|
Glucose level stable or with minimal changes (<1 mg/dL per minute) |

|
Glucose level gradually decreasing (1-2 mg/dL per minute) or decreased by 30-60 mg/dL in 30 minutes |

|
Glucose level decreasing rapidly (>2 mg/dL per minute) or decreased by 60-90 mg/dL in 30 minutes |
Prandial Insulin Adjustments
To calculate a prandial bolus based on a trend one must consider the following: insulin on board from previous boluses, exercise, carbohydrate (CH) intake, glycemic index, and patient preferences and skills. Ideally, patients on intensified therapy should be able to calculate doses based on insulin to CH ratio (ICR) and insulin sensitivity factor (ISF), from preprandial IG and CH intake. However, some patients use fixed doses or with minimal changes based on preprandial IG and food intake.
Table 2 shows the proposed adjustments in prandial insulin according to trend. For patients on fixed doses these adjustments are expressed as a percentage of usual doses, or as an increase/decrease in units according to their total daily dose of insulin. This table also shows recommendations for patients who calculate doses with ICR and ISF—both as a percentage and as an increase/decrease in units according to ISF.
Table 2.
Recommendations to Adjust Prandial Insulin Dose Based on Glucose Level and Trend in Subjects Using Fixed Insulin Doses (on a Percentage Basis or Depending on the TDD of Insulin Used) and in Subjects Using ICR and ISF for This Calculation (Usually Supported by Tools for Bolus Calculation).
| Preprandial glucose (mg/dL) | Trend | Bolus adjustment (% vs usual dose) | Bolus adjustment based on TDD (IU/day)*
<35 35-80 >80 |
Bolus adjustment calculated based on ISF
(IU)**
<30 30-70 >70 |
|---|---|---|---|---|
| >180 |

|
+30% | +2 +4 +5 |

|
| 70-180 | + 20% | +1 +2 +3 | +4 +2.5 +1 | |
| <70*** | Do not adjust | −1 –1 –1 |

|
|
| >180 |

|
+20% | +2 +3 + 4 | |
| 70-180 | +15% | +1 +1 +1 | +3 +1.5 +0.5 | |
| <70*** | Do not adjust | −1 –2 –2 |

|
|
| >180 |

|
+10% | +1 +2 +3 | |
| 70-180 | Do not adjust | Do not adjust | Do not adjust | |
| <70*** | −10% | −2 –3 –4 |

|
|
| >180 |

|
+10% | +1 +1 +2 | |
| 70-180 | Do not adjust | −1 –1 –1 | −3 –1.5 –0.5 | |
| <70*** | −20% | −2 –4 –5 |

|
|
| >180 |

|
Do not adjust | +1 +1 +1 | |
| 70-180 | −20% | −1 –2 –3 | −4 –2.5 –1 | |
| <70*** | −30% | −3 –4 –6 |

|
The ranges shown are based on the median TDD used in our setting. **The calculated ranges are based on the median ISF used in our setting. ***Consider starting intake with 10-20 g of fast-absorbing CHs and not administering the insulin bolus until 15-30 minutes after the start of intake. It is recommended to repeat the glucose reading every 30 minutes, until glucose level normalizes and up to two hours after bolus administration.
 If
glucose level <70 mg/dL or >180 mg/dL, add or subtract from
the proposed units by standard calculation, usually provided by a
bolus calculator (therefore incorporating ISF), the IUs indicated in
the table.
If
glucose level <70 mg/dL or >180 mg/dL, add or subtract from
the proposed units by standard calculation, usually provided by a
bolus calculator (therefore incorporating ISF), the IUs indicated in
the table.
In patients using insulin pens that do not allow half units to be administered, round up or down on an individual basis.
Correction Bolus Adjustments
Several factors should be considered to calculate a correction bolus according to the trend including insulin on board, ISF, expected physical activity, and events (stress, prior hypoglycemia, etc).
a) Insulin on board: It is important to prevent overlapping action of insulins that may later lead to hypoglycemia.
b) Postprandial glucose target (2 hours): Values <180 mg/dL are the recommended general objective. Correction bolus must be calculated based on ISF.
c) Trend: It is recommended to increase or decrease the calculated correction bolus in percentages—for patients using ISF—or in insulin units—for those using fixed doses. Table 3 shows the proposed adjustments.
Table 3.
Adjustment of Correction Bolus Insulin Dose, Both for Patients Who Calculate Based on ISF and for Those Using Fixed Insulin Doses.
| Postprandial glucose (mg/dL) | Trend | Adjustment of correction bolus calculated based on ISF | Adjustment of correction bolus depending on
TDD (IU/day) <35 35-80 >80 |
|---|---|---|---|
| 180-250 |

|
+25% | +2 +3 +5 |
| >250 | +30-40% | +2 +4 +6 | |
| 180-250 |

|
+20% | +1 +2 +3 |
| >250 | +25% | +2 +3 +5 | |
| 180-250 |

|
+15% | +1 +2 +3 |
| >250 | +20% | +1 +2 +3 | |
| 180-250 |

|
Do not adjust | Do not adjust |
| >250 | Repeat reading at 15 min: glucose >250 mg/dL, give
bolus –10% |
Repeat reading at 15 minutes: glucose >250 mg/dL, give
bolus: –1 –2 –3 |
|
| 180-250 |

|
Do not adjust | Do not adjust |
| >250 | Repeat reading in 15 minutes: glucose >250 mg/dL, give
bolus –15% |
Repeat reading in 15 minutes, and if glucose >250 mg/dL
give bolus: –1 –2 –3 |
To sum up, when elevated postprandial (2-4 h) IG is treated with a correction bolus:
- Avoid corrections before 2 hours since prandial bolus administration.
- Consider a correction if IG 2 hours after meal is ≥180 mg/dL (in individual cases, consider it with lower IG).
- If a correction bolus has been administered, it is advised not to administer another one in the next 2 hours, monitoring IG during this time.
Hypoglycemia Prevention and Treatment
It is necessary to check glucose levels by SMBG both for diagnosis of hypoglycemia and after recovery due to the physiological delay between IG and BG.
Table 4 shows recommendations for prevention and management of hypoglycemia based on trend, in postprandial conditions and at baseline or interprandial periods.
Table 4.
Prevention of Hypoglycemia in Both the Postprandial (2-4 h after Intake) and the Baseline or Interprandial (>4 hours after intake) Periods.
| Glucose <2-4 h after intake (mg/dL) | Trend | Action |
|---|---|---|
| 100-150 |


|
Repeat reading in 10-15 minutes. If trend persists: intake of 10-15g CH* |
| <100 |


|
Intake of 15-10 g CH* |
| Glucose >4 hours after intake (mg/dL) | Trend | Action |
| 100-150 |

|
No action |


|
Repeat reading in 15 minutes | |
| 70-100 |

|
Repeat reading in 10 minutes |


|
Intake of 15 g CH and repeat reading in 10 minutes: <70
mg/d and trend persists: -Confirm with SMBG -New intake of 20 g CH* |
|
| <70 |
 or or

|
Confirm with CG and take 20 g CH*
Repeat CG in 10 minutes**: -If <70 mg/dL, intake of 15 g CH* -If >70 mg/dL, intake of 15 g of slow-absorbing CHs |

|
Confirm with CG and take 30 g CH* . Repeat SMBG
in 10 minutes**: -If <70 mg/dL, intake of 15 g CH* -If >70 mg/dL, intake of 15 g of slow-absorbed CH |
It is important to ensure the resolution of hypoglycemia with a SMBG measurement. Recovery may seem to be delayed if only IG is used. Thus, using IG only may lead to error and overcorrection of hypoglycemia.
Special Populations and Situations
Children
The pediatric population has special characteristics, requiring particular insulin adjustments. Young children are characterized by very high insulin sensitivity, especially in the second half of the morning with a marked anti-dawn phenomenon. By contrast, high insulin resistance and a marked dawn phenomenon characterize adolescents.11
As there are no methods specifically designed for insulin adjustments in children using FGM, recommendations from the Endocrine Society5 can be extrapolated. This is possible given the analogy of trend arrows between the FGM system and the system for which such methods were designed (adapted).6 Table 5 shows recommendations for pediatric populations.
Table 5.
Insulin Dose Adjustment Based on Trend and ISF in Children.
| Trend | Prandial bolus dose adjustment (IU) by ISF* | ||||
|---|---|---|---|---|---|
| <25 | 25-50 | 50-75 | 75-125 | ≥125 | |

|
+3 | +2 | +1 | +0.5 | No adjustment |

|
+2 | +1 | +0.5 | No adjustment | No adjustment |

|
No adjustment in any case | ||||

|
−2 | −1 | −0.5 | No adjustment | No adjustment |

|
−3 | −2 | −1 | −0.5 | No adjustment |
*Five ISF ranges are given. Younger patients usually have higher insulin sensitivity (higher ISF), while older patients have lower insulin sensitivity (lower ISF).
Children aged 2-6 years: often use ISF ≥125; children aged 7-12 years: highly variable ISFs; adolescents/young adults aged 13-22 years: often use ISF 25-50 or <50.
Adjustments may also be made at bedtime, but with caution when insulin administration is required. A bedtime blood glucose level of 130 mg/dL is suggested when glucose is stable (→) or changing slowly, increasing by 1-2 mg/dL per minute.
Continuous Subcutaneous Insulin Infusion (CSII) Users
As an alternative to modify prandial or correction boluses based on the trend, patients on CSII therapy can use a temporary basal rate (TBR), with the advantage that it may be discontinued or adjusted if trend changes. TBR duration as a general rule will be of at least two hours. The trend will be checked in 15-30 minutes, and if there is a sudden change in it, TBR will be modified or discontinued. Table 6 proposes TBR based on trend in prandial and postprandial conditions.
Table 6.
Recommendations for CSII Users With TBR: Preprandial Insulin Dose Adjustments and Corrections in Postprandial Hyperglycemia (<2-4 hours after intake).
| Preprandial glucose (mg/dL) | Trend | TBR |
|---|---|---|
| >180 |

|
+30-40% |
| 70-180 | +20-25% | |
| <70 | +10% | |
| >180 |

|
+20-30% |
| 70-180 | +10-15% | |
| <70 | Usual action | |
| Any glucose level |

|
Usual action |
| >180 |

|
Usual action |
| 70-180 | −10-15% | |
| <70 | −20-30% | |
| >180 |

|
−10% |
| 70-180 | −20-25% | |
| <70 | −30-40% | |
| Postprandial glucose (mg/dL) | Trend | TBR |
| 180-250 |

|
+25% |
| >250 | +30-40% | |
| 180-250 |

|
+20% |
| >250 | +25% | |
| 180-250 |

|
+15% |
| >250 | +20% | |
| 180-250 |

|
Usual action |
| >250 | Repeat reading in 15 minutes and if trend persists: –10% | |
| 180-250 |

|
Usual action |
| >250 | Repeat reading in 15 minutes and if trend persists: –15% |
To prevent hypoglycemia in the postprandial period (2-4 hours), use of TBR is proposed as follows:
- When IG is 100-150 mg/dL and tends to gradually decrease, repeat reading in 15-30 minutes.If the trend persists use a TBR (80%).
- When IG is <100 mg/dL and tends to gradually decrease, consider intake of 10g of CH.
- When IG is 100-150 mg/dL and tends to rapidly decrease, repeat reading in 15 minutes. If the trend persists, use a TBR (70%).
- When IG is <100 mg/dL and tends to rapidly decrease, consider intake of 10-20 g of CH and stop CSII.
Other Situations
a) Exercise
Exercise increases the risk of hypoglycemia both during and after its finalization. The type, intensity, duration, time of day, and individual response are variables affecting glucose response. Therefore, recommendations should be tailored. In all cases, a glucose reading should be performed before exercise. Table 7 includes general and initial recommendations for exercise.
Table 7.
Actions Recommended in Case of Physical Exercise Depending on Glucose Level and Trend.
| Glucose (mg/dL) | Trend | Action |
|---|---|---|
| >180 |
 or or

|
Correction bolus with lower than usual exercise dose reduction, postpone exercise and repeat reading in 30-60 minutes |

|
Correction bolus with the usual exercise dose reduction (in CSII users, use TBR with usual reduction) and repeat reading in 15-30 minutes | |
 or or

|
Correction bolus with exercise dose reduction greater than usual (in CSII users, use TBR with greater than usual reduction) and repeat reading in 15-30 minutes | |
| 70-180 |
 or or

|
Intake of 10g supplement of CH (in CSII users, TBR could be reduced by a lower than usual percentage) |

|
Intake of 15-20g supplement of CH (in CSII users, reduce TBR by the usual percentage) | |
 or or

|
Intake of 25-30g supplement of CHs (in CSII users, TBR could be reduced by a greater percentage than usual) | |
| <70 | Any trend | Postpone exercise until hypoglycemia resolves (see section “Hypoglycemia Prevention and Treatment”) |
b) Driving
A glucose reading is advisable before driving, looking at the trend:
-IG on target with stable or slowly increasing trend: Do not take any action.
-IG on target but decreasing: A CH supplement should be taken. TBR with a percent reduction is an option in CSII users, or basal rate discontinuation.
-IG below target (hypoglycemia): Treatment before driving is advised. IG and trend should be checked again before driving.
c) Illness or stress
Concomitant diseases or emotional stress tend to raise BG. Table 8 shows the recommendations for action facilitating the management of these situations.
Table 8.
Actions Recommended in Disease and/or Stress Situations Based on Glucose Level and Trend.
| Glucose | Trend | Action |
|---|---|---|
| High glucose |
 or or

|
Correction bolus +25-30% (CSII users can use TBR +25-30%) |

|
Correction bolus +15-20% (CSII users can use TBR +15-20%) | |
 or or

|
Repeat glucose reading in 15-30 minutes | |
| Goal glucose |
 or or

|
Correction bolus +20-25% (CSII users can use TBR +20-25%) |

|
Usual action as recommended by your health care team | |
 or or

|
Repeat glucose reading in 15-30 minutes | |
| Low glucose | Treat hypoglycemia (please see section “Hypoglycemia Prevention and Treatment”) |
Treatment Adjustment Based on Retrospective Data Analysis
Blood Glucose Measurement
General Considerations
Health care professionals should be familiar with a broader definition of optimal glycemic control including more than just HbA1c—a measure of central tendency. A combination of other variables reflects more realistically glucose control, especially regarding variability and risk of hyperglycemia and hypoglycemia.12
Recommendations
Different consensuses have been published to standardize collection of glucometric data obtained with CGM.13-15 Table 9 presents a proposal for recording these data, most of which can be obtained with the FGM system and Libreview® platform. Information that should be included in the clinical reports and considered in clinical decision making appears in bold.
Table 9.
Blood Glucose Parameters to be Assessed from a CGM/FGM Record.
| Glucose reading variables | Specific measure | Definition | Available at Freestyle Libre/Libreview |
|---|---|---|---|
| Data quality |
-Data capture period
-Minimum % of readings -Average daily readings |
≥2 weeks 70-80% (10 of 14 days) At least 1 every 8 hoursa |
Freestyle Libre/Libreview Freestyle Libre/Libreview Freestyle Libre/Libreview |
| Glucose exposure |
-Mean blood glucose
-Estimated HbA1c -Area under the 24-h glucose curve |
Primary measure Primary measure Research measure |
Freestyle Libre/Libreview Freestyle Libre/Libreview Unavailable |
| Time in range | -% time in range | General: 70-180 mg/dLb
Strict: 70-140 mg/dL |
Freestyle Libre/Libreviewc |
| Glycemic variability |
-Coefficient of variation (CV)
-Standard deviation -Interquartile range |
Primary measure: ideal 36%d
Secondary measure Research measure |
Libreview Libreview Libreview |
| Hypoglycemiae |
-% time in hypoglycemia level 1
-% time in hypoglycemia level 2 -Number of hypoglycemic episodes -Hypoglycemia risk index (LBGI) |
Blood glucose <70 mg/dLe
Blood glucose <54 mg/dLf(optional) Number and mean duration (min)g Secondary measureh |
Freestyle Libre/Libreviewg
Freestyle Libre/Libreviewg Freestyle Libre/Libreviewg Libreview |
| Hyperglycemia |
-% time in hyperglycemia level 1
-% time in hyperglycemia level 2 -Hyperglycemia risk index (HBGI) |
Blood glucose >180 mg/dLi
Blood glucose >250 mg/dLj (optional) Secondary measurel |
Freestyle Libre/Libreviewk
Freestyle Libre/Libreviewk Unavailable |
One reading is required every 8 hours to capture 100% of the data. The number of daily readings correlates with improved glycemic control, less hypoglycemia, and longer time in the target range.
A time in the 70-180 mg/dL range >50% usually corresponds to HbA1c levels ≤7%.
The ranges in the system should be changed to obtain both times.
CVs <36% indicate low variability. CVs >36% indicate high variability.
Hypoglycemia level 1 is defined as a warning value for hypoglycemia.
Level 2 hypoglycemia is defined as clinically significant. Level 3 hypoglycemia (severe hypoglycemia) is clinically diagnosed and should be recorded in patient history. A hypoglycemic episode starts with a reading below the hypoglycemic threshold, maintained for at least 15 minutes, and ends with a reading above 70 mg/dL for at least 15 minutes.
Hypoglycemic thresholds in the system should be changed to obtain the different percentages or numbers of level 1 and 2 hypoglycemia.
LBGI is a value predicting the risk of severe hypoglycemia, and the system gives a qualitative value—high, moderate, or low.
Hyperglycemia level 1 is defined as a warning value for hyperglycemia.
Hyperglycemia level 2 is defined as clinically significant. Hyperglycemia level 3 (ketoacidosis) is clinically diagnosed and should be recorded in patient history.
Hyperglycemic thresholds in the system should be changed to obtain the different percentages or numbers of hyperglycemia levels 1 and 2.
HBGI is a value predicting poor glycemic control and wide glycemic excursions.
The standardized summary of glucose data provided by CGM/FGM should be a part of the assessment of patients. To expedite data collection, in future updates of the software it would be desirable to have a summary similar to the one proposed in automatically generated reports.
AGP Analysis
Aspects to Consider
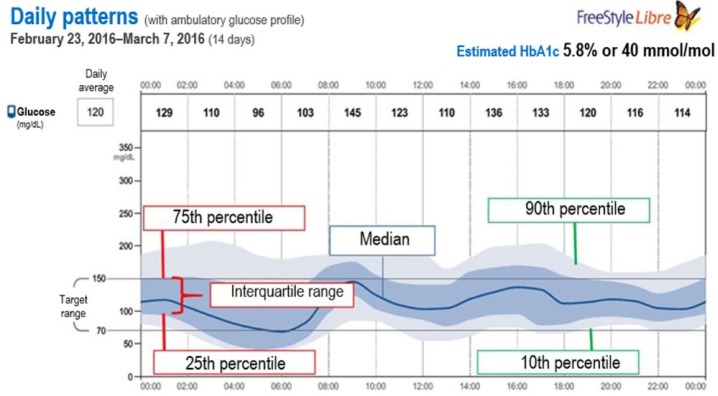
The AGP integrates changes in IG levels recorded over a given period of time into a graph. AGP groups all recordings by time of day-modal day. These data are represented using the median, 25th-75th, and 10th-90th percentiles (Figure 1).16,17
Figure 1.
Plot of 14-day ambulatory glucose profile (AGP). This plot was obtained using the FreeStyle Libre download software. The dark blue line represents the median (intermediate value) glucose reading. The dark blue shadow represents the 25th-75th percentile range of sensor readings (50% of data), while the light blue shadow represents the 10th-90th percentile range (80% of data). These shaded areas help assess glycemic variability.
AGP is very useful to identify patterns, quantify variability, and stratify the risk of hypoglycemia at different time intervals.18
Systematic Review of Results
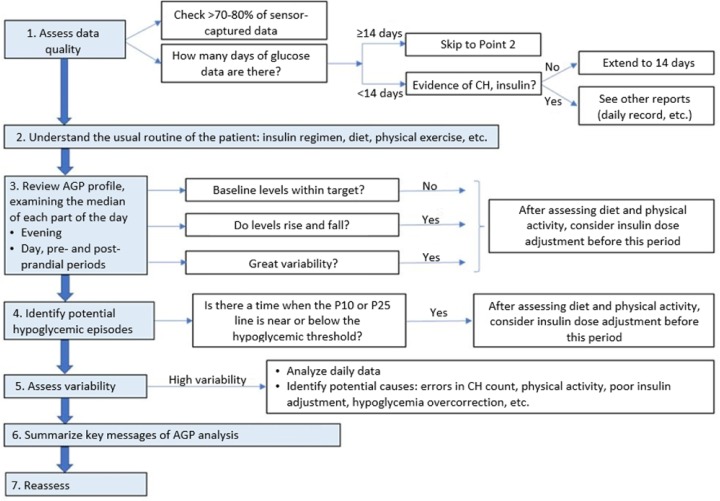
It is recommended to follow an established order for AGP interpretation19 (Figure 2):
Figure 2.
Consensus recommendations for the use of ambulatory glucose profile in clinical practice. Modified from Matthaei et al.19
- Assess data quality:
- To have 70%-80% of the measurements is necessary for data interpretation.
- At least 14 days are needed to generate an AGP for analysis and decision making.20
- Understand the usual routine of the patient:
- Treatments, diet, exercise, and so on. Entry of these data into the system by the patient facilitates its interpretation.
- Review the AGP profile for each part of the day:
- Interpret the median IG, its stability throughout the day and its behavior, above or below the target at different times (day, night, postprandial period).
- Identify hypoglycemic patterns:
- Identify when the 10th or 25th percentiles are close to the hypoglycemia limit—usually established at 70 mg/dL. A 10th percentile close to or crossing the cut-off represents a risk of hypoglycemia, being higher when is the 25th percentile close to or crossing the limit.
- Identify potential causes: diet and exercise, or consider reduce insulin dose before this period.
- Analyze glycemic variability:
- Glucose variability is measured by the interquartile range (between the 25th and 75th percentiles) (Figure 1) preferably assessed at different times of the day.
- Identify potential causes: inadequate CH estimation or exercise management, omission of boluses, overcorrection of hypoglycemia, and so on.
- Day-to-day analysis:
- Identify potential causes of variability, hypoglycemia, or hyperglycemia in different days.
The essential message(s) of data analysis should be summarized, as well as recommended actions.
Reassess after a given period of time depending on the actions suggested.
Summary
AGP is a very useful tool for analysis of FGM data because facilitates visual, accurate, and consistent identification of trends at different times of day, complementing information provided by HbA1c21. AGP allows professionals and patients to identify the effects of treatment adjustments or any given nonpharmacological intervention.
Footnotes
Abbreviations: AGP, ambulatory glucose profile; BG, blood glucose; CGM, continuous glucose monitoring; CH, carbohydrate; CSII, continuous subcutaneous insulin infusion; CV, coefficient of variation; FGM, flash glucose monitoring; HbA1c, glycosylated hemoglobin; HBGI, high blood glucose index; ICR, insulin to carbohydrate ratio; IG, interstitial glucose; ISF, insulin sensitivity factor; LBGI, low blood glucose index; SMBG, self-monitoring of blood glucose; TBR, temporary basal rate; TDD, total daily dose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AC has received support from Roche, Lilly, MSD, Esteve, Sanofi, Medtronic, Novalab, Novo Nordisk and has taken part in advisory boards for Abbott, Lilly, Novo Nordisk, Sanofi. EA has received honorary from Astra Zeneca, Sanofi, and Roche and participated in Advisory Boards for Abbott, Roche, Sanofi, and Novo. FJAB has served on advisory panels for Abbott, Astra Zeneca, Boehringer Ingelheim, Lilly, GlaxoSmithKline, LifeScan, Medtronic, Merck, Novartis, Novo Nordisk, Pfizer, Roche, and Sanofi and has received research support from Abbott, Astra Zeneca, Boehringer Ingelheim, Bayer, Lilly, GlaxoSmithKline, LifeScan, Merck, Novo Nordisk, Pfizer, Sanofi, and Servier. VB has received speaker honoraria and advisory from Abbott, Astra Zeneca, Boehringer-Ingelheim, Esteve, Lilly, MSD, Novo Nordisk, Roche, Sanofi; Clinical researcher: Abbott, Novo Nordisk, Sanofi. RCH has received speaker honoraria and has consulted for Abbott, Dexcom, Lilly, Medtronic, Novo Nordisk, and Roche and has received research grants from Lilly, Novo Nordisk, and Sanofi. FJE has received support from Abbott, Boehringer I gelheim, Esteve, Janssen, Lilly, MSD, Novo Nordisk, Sanofi and advisory for Sanofi, Novo Nordisk, MSD. DF reports grants and personal fees from Novo Nordisk, MSD, Lilly, Janssen, Astra Zeneca,Sanofi, outside the submitted work. FGP has received research support from Sanofi, Novo Nordisk, Boehringer Ingelheim, and Lilly; has taken part in advisory boards for Sanofi, Novo Nordisk, and Astra Zeneca ; and has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, Novartis, Lilly, and Astra Zeneca. NGPV has acted as a speaker for Sanofi, Novo Nordisk, Boehringer Ingelheim, Lilly, and Astra Zeneca, Abbott, and MSD. JJG has the following financial relationships: advisor on scientific boards for Astra Zeneca, Janssen, Lilly,Merck Sharp & Dohme, Mundipharma, Novo Nordisk, and Pfizer; lectures for Abbott, AbbVie Inc, Astra Zeneca, Boehringer Ingelheim, Esteve, Janssen, Lilly, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Roche, and Sanofi, and research activities for Astra Zeneca, NovoNordisk, and Sanofi. PMR has received research support from Amgen, Astra Zeneca, Bristol-Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck Sharp & Dohme, Novo Nordisk, Pfizer, Roche, Sanofi, Theracos; has taken part in advisory boards for Ascensia, Astra Zeneca, Bristol-Myers Squibb, FAES, Janssen, Lifescan, MSD, Novo Nordisk; and has acted as a speaker for Abbott, Astra Zeneca, Bristol-Myers Squibb, Esteve, FAES, GlaxoSmithKline, Lifescan, Merck, Sharp & Dohme, Novartis, Novo Nordisk, Sanofi. CM Advisor and speaker from Novo Nordisk, MSD, Lilly, Janssen, Astra Zeneca, Sanofi, Theracos, Lexicon, Hanmi, Intarcia, Novartis, Takeda outside the submitted work. PPV has received a research support from Sanofi, Boehringer Ingelheim, Astra Zeneca, and Lilly; has taken part in advisory boards for Abbot . RP has taken part in advisory boards for Boehringer Ingelheim, Novartis, Sanofi, Novo Nordisk, Abbot, and Astra Zeneca; and has acted as a speaker for Astra Zeneca, Abbott, Almirall, Bayer, Boehringer Ingelheim, Bristol-Myers, Esteve, Faes Farma, Ferrer, GSK, Janssen, Lacer, Lifescan, Lilly, Menarini, Merck, MSD, Novartis, Novo Nordisk, Pfizer, Roche, Sanofi, Servier and Takeda. JP reports grants and personal fees from Abbott, Novo Nordisk, Lilly, Janssen, Astra Zeneca andSanofi, outside the submitted work. MTR has acted as a speaker for Sanofi, Novo Nordisk, MSD, Lilly, Janssen, Astra Zeneca, Roche, and Abbot. CGB has received support from Roche, Lilly, MSD, Esteve, Sanofi, Novo Nordisk, Astra Zeneca and has taken part in advisory boards for Abbott, Sanofi, and Roche.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Bianchi C, Aragona M, Rodia C, et al. Freestyle Libre trend arrows for the management of adults with insulin-treated diabetes: a practical approach. J Diabetes Complications. 2019;33:6-12. [DOI] [PubMed] [Google Scholar]
- 2. Rebrin K, Sheppard NF, Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4:1087-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kudva YC, Ahmann AJ, Bergenstal RM, et al. Approach to using trend arrows in the FreeStyle Libre flash glucose monitoring systems in adults. J Endocr Soc. 2018;2:1320-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Haak T, Hanaire H, Ajjan R, Hermanns N, Riveline JP, Rayman G. Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther. 2017;8(1):55-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aleppo G, Laffel LM, Ahmann AJ, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system for the management of adults with diabetes. J Endocr Soc. 2017;1:1445-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laffel LM, Aleppo G, Buckingham BA, et al. A practical approach to using trend arrows on the Dexcom G5 CGM system to manage children and adolescents with diabetes. J Endocr Soc. 2017;1:1461-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Klonoff DC, Kerr D. A simplified approach using rate of change arrows to adjust insulin with real-time continuous glucose monitoring. J Diabetes Sci Technol. 2017;11:1063-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pettus J, Edelman SV. Recommendations for using real-time continuous glucose monitoring (rtCGM) data for insulin adjustments in type 1 diabetes. J Diabetes Sci Technol. 2017;11:138-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ajjan RA, Cummings MH, Jennings P, Leelarathna L, Rayman G, Wilmot EG. Optimising use of rate-of-change trend arrows for insulin dosing decisions using the FreeStyle Libre flash glucose monitoring system. Diab Vasc Dis Res. 2019;16(1):3-12. [DOI] [PubMed] [Google Scholar]
- 10. Ziegler R, von Sengbusch S, Kröger J, et al. Therapy adjustment based on trend arrows using continuous glucose monitoring systems [published online ahead of print January 22, 2019]. J Diabetes Sci Technol. doi: 10.1177/1932296818822539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klinkert C, Bachran R, Heidtmann B, Grabert M, Holl RW; DPV-Initiative. Age-specific characteristics of the basal insulin-rate for pediatric patients on CSII. Exp Clin Endocrinol Diabetes. 2008;116:118-122. [DOI] [PubMed] [Google Scholar]
- 12. Wright LA, Hirsch IB. Metrics beyond hemoglobin A1C in diabetes management: time in range, hypoglycemia, and other parameters. Diabetes Technol Ther. 2017;19(suppl 2):S16-S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7:562-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fonseca VA, Grunberger G, Anhalt H, et al. Consensus conference writing committee. continuous glucose monitoring: a consensus conference of the American Association of Clinical Endocrinologists and American College of Endocrinology. Endocr Pract. 2016;22:1008-1021. [DOI] [PubMed] [Google Scholar]
- 16. Hirsch IB, Verderese CA. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1c: rationale and practical implementation. Endocr Pract. 2017;23:1333-1344. [DOI] [PubMed] [Google Scholar]
- 17. Solá E. ¿De qué nos sirve evaluar las tendencias del perfil glucémico ambulatorio? Av Diabetol. 2014;30:121-130. [Google Scholar]
- 18. Bergenstal RM, Ahmann AJ, Bailey T, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the ambulatory glucose profile. J Diabetes Sci Technol. 2013;7:562-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matthaei S, Antuña De, Alaiz R, Bosi E, Evans M, Geelhoed-Duijvestijn N, Joubert M. Consensus recommendations for the use of ambulatory glucose profile in clinical practice. Br J Diabetes Vasc Dis. 2014;14:153-157. [Google Scholar]
- 20. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40:1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hirsch IB, Verderese CA. Professional flash continuous glucose monitoring with ambulatory glucose profile reporting to supplement A1c: rationale and practical implementation. Endocr Pract. 2017;23:1333-1344. [DOI] [PubMed] [Google Scholar]