Abstract
Backround and aims
According to the World Obesity Federation, “obesity-related conditions seem to worsen the effect of Covid-19 (SARS-CoV-2)”; additionally the Centres for Disease Control and Prevention reported that “people with heart disease and diabetes are at higher risk of SARS-CoV-2 complications and that severe obesity poses a higher risk for severe illness”. Recent reports have shown elevated levels of cytokines due to increased inflammation in patients with SARS-CoV-2 disease. On the other hand, obesity represents a state of low-grade inflammation, with various inflammatory products directly excreted by adipose tissue. In this concise report we aimed to assess common elements of obesity and SARS-CoV-2 infection.
Methods
Pubmed search on obesity and SARS-CoV-2 infection.
Results
We present “mechanistic” obesity-related problems that aggravate SARS-CoV-2 infection as well as tentative inflammatory/metabolic links between these diseases.
Conclusion
Obesity and SARS-CoV-2 share common elements of the inflammatory process (and possibly also metabolic disturbances), exacerbating SARS-CoV-2 infection in the obese.
Keywords: Human, Coronavirus, Obesity, Glucose, Inflammation, Infection
1. Introduction
Physicians are trying to establish the risk factors that might affect the spread of the novel SARS-CoV-2 virus and those that might worsen the prognosis of hospitalised patients [1]. Among those, it seems that the new pandemic is complicated by an older one, which is already well-defined, that is obesity. Being overweight and obesity are defined as having abnormal or excessive fat accumulation, respectively, that may impair health [2]. Obesity has nearly tripled worldwide since 1975, hence it has been characterised as a pandemic. In 2016, 39% of adults worldwide (more than 1.9 billion people) were overweight and 13% were obese (over 650 million people), whereas in 2018, 40 million children under the age of 5 were overweight or obese. Obesity is a major health concern, mainly because of its side-effects in humans and its associated morbidity and mortality rates [3].
On the other hand, according to the World Obesity Federation, obesity-related conditions seem to worsen the effect of SARS-CoV-2; indeed, the Centres for Disease Control and Prevention (CDC) reported that “people with heart disease and diabetes are at higher risk of Covid-19 (SARS-CoV-2) complications and that severe obesity (body mass index [BMI] of 40 or higher) poses a higher risk for severe illness” [4].
We therefore hypothesised that there might be a pathophysiological link that could explain the fact that obese patients are prone to present with SARS-CoV-2 complications.
Looking back on similar infectious outbreaks, during the 2009 H1N1 pandemic, obesity was recognized as an independent risk factor for complications from influenza [5], thus it is not surprising that obesity is a potential independent risk factor for SARS-CoV-2 as well. Obese individuals have shown diminished protection from influenza immunization, since - despite being vaccinated - obese recipients are 2–3 times more prone to suffer from infection compared to non-obese. Thus, the potential implications for obesity in the SARS-CoV-2 outbreak should be elucidated [6]. We hereby present “mechanistic” obesity-related problems that aggravate SARS-CoV-2 infection as well as tentative molecular links between obesity and SARS-CoV-2 infection.
2. “Mechanistic” problems
Obese patients often need bariatric hospital beds, which may be scarce and are definitely more difficult to position and transport by nursing staff. In these patients proper imaging diagnosis may be compromised (there are weight limits for the beds of imaging equipment). Obese patients are very prone to diminished airway flow, due to limited truncal expansion, making it difficult to ease the airflow (and increasing susceptibility to poor breathing) [7]; oxygen consumption and respiratory potential can be seriously affected and predispose to infection and the need for more oxygen support [7]. Finally these patients pose a serious challenge for intubation (since the additional adipose tissue on the larynx makes intubation more difficult).
3. Molecular pathways
So far, hyperglycemia was noted in 51% of cases with the novel SARS-CoV-2 infection [8]. Hyperglycemia was also observed in patients with SARS in 2003, caused by a different type of coronavirus (SARS-CoV), partly because the virus leads to transient impairment of pancreatic islet cell function [9]. Dipeptidyl peptidase 4 (DPP4; an enzyme responsible for the degradation of incretins such as glucagon like peptide-1, GLP-1) serves as receptor for MERS CoV (Middle eastern respiratory syndrome) and human coronavirus EMC [9]. Moreover, hyperglycemia might also be caused by endogenous stress-induced glucocorticoid hypersecretion [9].
Recent reports have shown an increased inflammatory environment, leading to a exacerbated cytokine profile (cytokine storm) in patients with SARS-CoV-2 disease [10], mainly manifested by increased interleukin (IL)-2, IL-7, granulocyte-colony stimulating factor, interferon-γ inducible protein 10, monocyte chemo-attractant protein 1 (MCP1), macrophage inflammatory protein 1-α, and tumour necrosis factor-α (TNFα). Earlier investigations of SARS-CoV infection, showed that it mediated its actions via suppression of NF-kB (nuclear factor kappa-light-chain-enhancer of activated B cells) activity, resulting in lower Cox-2 (cyclooxygenase-2) expression (thus easing inflammation) [11]. In MERS-CoV infection, translocation of NF-κB to the nucleus leads to a cascade of pro-inflammatory cytokines [12]. Among all interleukins, IL-6 was found to be associated with a highly pathogenic SARS-CoV-2 infection, due to enhanced virus replication mainly in the lower respiratory tract [13]. Moreover, levels of ferritin and IL-6 were statistically significantly higher in non-survivors compared to survivors in the recent outbreak of SARS-CoV-2 in China [14].
Obesity represents a state of low-grade inflammation, with various inflammatory products directly secreted by adipose tissue. Hyperplastic or hypertrophied adipose tissue releases inflammatory cytokines (TNFα, IL-1, IL-6, IL-10), transforming growth factor-b (TGF-b), adipokines (leptin, resistin, adiponectin), MCP-1 (monocyte chemoattractant protein-1), CXCL5 (C-X-C motif chemokine ligand 5), hemostatic proteins (plasminogen activator inhibitor-1; PAI-1), proteins affecting blood pressure, (angiotensinogen) and angiogenic molecules (vascular endothelial growth factor; VEGF) [15]. The main adipose tissue-derived inflammatory cytokines are TNFα, IL-1, IL-6, which altogether comprise an “inflammatory triad”. Levels of TNFα are increased in obesity, indicating a role for this cytokine in the obesity-associated inflammation and particularly in insulin resistance and diabetes. Interleukin-1 can lead to the activation of transcription factors such as NF-kB, promoting inflammatory signalling overexpression of the angiogenic factor VEGF (vascular endothelial growth factor), while increased levels of IL-6 in obesity play a key role in inflammation-associated carcinogenesis, via the JAK/STAT (Janus kinase signal transducer and activator of transcription) signalling pathway [16]. Features of inflammation are consequent hypoxia and ischemia (hypoxia is the lack of oxygen in the blood or in adipose tissue, while ischemia is caused by an inadequate blood flow). Both hypoxia and ischemia drive to a state of oxidative stress, further stimulating the secretion of inflammatory proteins and reactive oxygen radicals (radical oxygen species, ROS) that damage mitochondrial functionality and DNA [17]. Thus, the hypertrophic, and at the same time hypoxic, white adipocytes change their normal protein synthesis and shift towards the production of cytokines and inflammatory proteins, leading to insulin resistance, type 2 diabetes mellitus (T2DM), metabolic syndrome, atherosclerosis and arterial hypertension, while recent evidence favours their implication in various types of cancer [18].
Among the various products of adipose tissue, is leptin. Leptin is a cytokine that serves as an alarm (inhibition signal) to the body, in order to reduce caloric consumption and return to a steady state. Initially thought as the cure for obesity in a recombinant form, leptin was found to face serious resistance in the body of the obese. Although in obesity leptin levels are increased, the action of leptin per se is reduced, in a analogous way to insulin’s action in patients with T2DM. Zhang et al. suggested that leptin resistance could aggravate the outcome of the patients during the 2009 A (H1N1) pandemic influenza, since leptin exerts positive effects in B cell maturation, development and function, along with alterations of lymphocytes and inhibition of CD8+ T cell response and impaired memory T cell response, seen in obesity, which both under normal circumstances would act against the virus [19].
Hyperglycaemia or established T2DM (usually associated with obesity) have shown to be independent predictors of mortality and morbidity in patients with SARS [9]. A proposed mechanism is that in SARS the noted enhanced release of cytokines leads to a state of increased metabolic inflammation. Especially in the case of SARS-CoV-2, a cytokine storm (elevated levels of inflammatory cytokines) has been suggested to be implicated in the multi-organ failure in patients with severe disease.
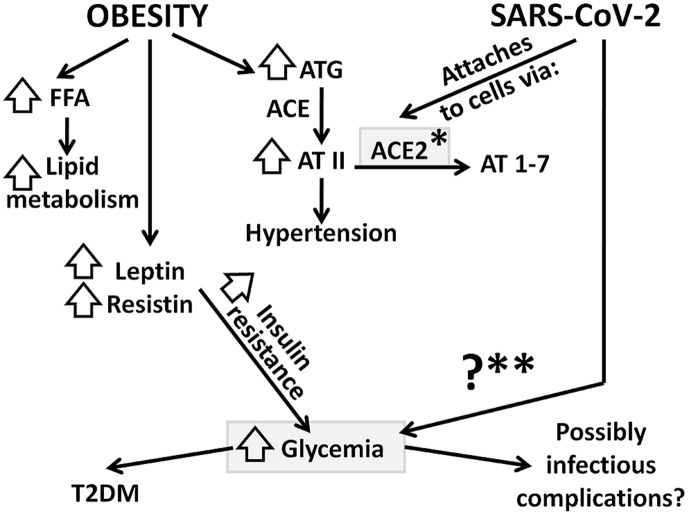
Adipose tissue expresses most of the components of the renin angiotensin aldosterone system (RAAS), such as angiotensinogen (AGT), angiotensin converting enzymes (ACE and ACE2) and their receptors, their mRNA being reduced in starvation and increased in overfeeding. Angiotensin (AT) II promotes prostacyclin synthesis, differentiation of the adipocytes and lipogenesis. Inside the adipose cell, ACE is stimulated towards the conversion of angiotensin I to angiotensin II, thus further stimulating the RAAS axis, the production of aldosterone and the rise in blood pressure [20]. Various reports have included arterial hypertension as a risk factor for severity of SARS-CoV-2, possibly related to ACE2 via the actions of ACE-inhibitors and AT receptor blockers (ARB), given for the treatment of hypertension (ACE2 acts as receptor that indeed seems to facilitate the entry of coronavirus into cells) [21]. Of note, the percentage of patients with hypertension suffering from SARS-CoV-2 is roughly the same as the prevalence of hypertension in the same age group, regardless of SARS-CoV-2 infection, which means that hypetension is not a risk factor per se, but rather a pre-existing disease at that age group [21]. Overall data do not fully support the notion that discontinuation of these medications is beneficial, since other reports in animals show that elevated ACE2 expression might exert potentially protective pulmonary and cardiovascular effects [21] (Fig. 1 ).
Fig. 1.
Selected metabolic pathways for obesity and SARS-CoV-2 infection; their common elements are shown in grey boxes. In obesity, resistance to leptin (along with resistin) leads to insulin resistance (both in the brain and in peripheral tissues) and eventually to hyperglycemia and T2DM. Moreover, adipose tissue releases ATG, which, via ACE is converted to AT II and increases blood pressure. SARS-CoV-2 attaches to cells via ACE2 and may provoke hyperglycemia (see text for more details); FFA: free fatty acids, ATG: angiotensinogen, ACE: angiotensin converting enzyme, AT II: angiotensin II, AT 1–7: angiotensin 1-7 (vasodilatory), NO: Nitric Oxide, T2DM: type 2 diabetes mellitus, ACE2: angiotensin converting enzyme 2, ∗: is usually upregulated in subjects with hypertension on ACE-inhibitors and AT receptor blockers (ARB), ∗∗hyperglycemia has been reported in patients with SARS-CoV-2 infection – the specific mechanisms have not been elucidated.
Among the limitations of this work we have to note that although we presented possible and plausible links among the inflammatory and metabolic aspects of obesity and SARS-CoV-2 infection (based on the available - and rapidly evolving - literature), further relevant research is warranted.
4. Conclusion
Both SARS-CoV-2 infection and obesity seem to share some common metabolic and inflammatory reaction pathways. On the one hand, obesity causes hyperglycemia via insulin resistance whereas SARS-CoV-2 may cause hyperglycemia as well (via mechanisms that are not elucidated yet). On the other hand, obesity represents a state of low grade inflammation, sharing many common molecules and pathways with those observed in SARS-CoV-2 infection.
Authors’ contributions
Both KM and II conceived the idea for this review, searched the available literature and wrote the draft and final form of this article.
Declaration of competing interest
The authors do not have any conflicts of interest and sources of funding to declare. The authors received no funding for this work.
References
- 1.World Health Organisation (WHO) Coronavirus disease (Covid-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- 2.World Health Organisation (WHO) Obesity. https://www.who.int/topics/obesity/en/
- 3.Michalakis K., Goulis D.G., Vazaiou A., Mintziori G., Polymeris A., Abrahamian-Michalakis A. Obesity in the ageing man. Metabolism. 2013;62:1341–1349. doi: 10.1016/j.metabol.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Centres for Disease Control and Prevention (CDC) Coronavirus (Covid-19) https://www.cdc.gov/coronavirus
- 5.Sun Y., Wang Q., Yang G., Lin C., Zhang Y., Yang P. Weight and prognosis for influenza A(H1N1)pdm09 infection during the pandemic period between 2009 and 2011: a systematic review of observational studies with meta-analysis. Inf Disp. 2016 Nov-Dec;48(11–12):813–822. doi: 10.1080/23744235.2016.1201721. Epub 2016 Jul 6. [DOI] [PubMed] [Google Scholar]
- 6.Neidich S.D., Green W.D., Rebeles J., Karlsson E.A., Schultz-Cherry S., Noah T.L. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41:1324–1330. doi: 10.1038/ijo.2017.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryan D.H., Ravussin E., Heymsfield S. COVID 19 and the patient with obesity - the editors speak out. Obesity. 2020 Apr 1 doi: 10.1002/oby.22808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ilias I., Zabuliene L. Hyperglycemia and the novel Covid-19 infection: possible pathophysiologic mechanisms. Med Hypotheses. 2020;139 doi: 10.1016/j.mehy.2020.109699. 109699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X., Wu K., Wang D., Yue X., Song D., Zhu Y. Nucleocapsid protein of SARS-CoV activates interleukin-6 expression. through cellular transcription factor NF-κB. Virology. 2007;365:324–335. doi: 10.1016/j.virol.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang X., Gao J., Zheng H., Li B., Kong L., Zhang Y. The membrane protein of SARS-CoV suppresses NF-kappaB activation. J Med Virol. 2007;79:1431–1439. doi: 10.1002/jmv.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canton J., Fehr A.R., Fernandez-Delgado R., Gutierrez-Alvarez F.J., Sanchez-Aparicio M.T., García-Sastre A. MERS-CoV 4b protein interferes with the NF-κB-dependent innate immune response during infection. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1006838. e1006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ulhaq Z.S., Soraya G.V. Interleukin-6 as a potential biomarker of COVID-19 progression. Med Maladies Infect. 2020 Apr 4 doi: 10.1016/j.medmal.2020.04.002. pii: S0399-077X(20)30088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Divella R., De Luca R., Abbate I., Naglieri E., Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Canc. 2016;7:2346–2359. doi: 10.7150/jca.16884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Michalakis K., Venihaki M., Mantzoros C., Vazaiou A., Ilias I., Gryparis A. In prostate cancer, low adiponectin levels are not associated with insulin resistance. Eur J Clin Invest. 2015;45:572–578. doi: 10.1111/eci.12445. [DOI] [PubMed] [Google Scholar]
- 17.Ellulu M.S., Patimah I., Khaza’ai H., Rahmat A., Abed Y. Obesity and inflammation: the linking mechanism and the complications. Arch Med Sci. 2017;13:851–863. doi: 10.5114/aoms.2016.58928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone T.W., McPherson M., Gail Darlington L. Obesity and cancer: existing and new hypotheses for a causal connection. EBioMedicine. 2018;30:14–28. doi: 10.1016/j.ebiom.2018.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang A.J., To K.K., Li C., Lau C.C., Poon V.K., Chan C.C. Leptin mediates the pathogenesisiof severe 2009 pandemic influenza A (H1N1) infection associated with cytokine dysregulation in mice with diet-induced obesity. J Infect Dis. 2013;207:1270–1280. doi: 10.1093/infdis/jit031. [DOI] [PubMed] [Google Scholar]
- 20.Lelis D.F., Freitas D.F., Machado A.S., Crespo T.S., Santos S.H.S. Angiotensin-(1-7), adipokines and inflammation. Metabolism. 2019;95:36–45. doi: 10.1016/j.metabol.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Danser A.H.J., Epstein M., Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is No evidence to abandon renin-angiotensin system blockers. Hypertension. 2020 doi: 10.1161/HYPERTENSIONAHA.120.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]